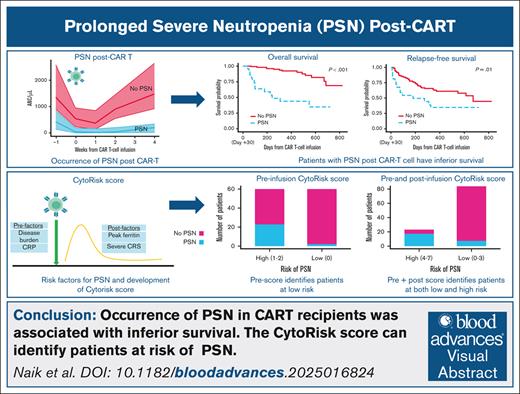
Key Points
PSN occurred in 15% and 21% of CAR-T recipients in training and validation cohorts, respectively, and was associated with inferior survival.
The CytoRisk score predicted PSN with high specificity (93%), sensitivity (74%), positive (74%) and negative predictive values (92%).
Visual Abstract
Hematotoxicity is the most frequent severe toxicity after chimeric antigen receptor T-cell (CAR-T) therapy. However, limited data exist on risk factors and outcomes for hematotoxicity for children and young adults (CAYAs) with B-acute lymphoblastic leukemia treated with tisagenlecleucel. We conducted a multi-institutional study involving 326 CAYAs, with 144 evaluable in an initial training cohort and 141 evaluable in a validation cohort, through the Pediatric Real-World CAR Consortium to characterize the incidence and outcomes of prolonged severe neutropenia (PSN) and to develop a predictive risk score for PSN, tailored for use in this population. The incidence of PSN, defined as an absolute neutrophil count of <0.5 x 103 cells per μL for ≥30 days, was 15.3% in the initial training cohort and 21% in the validation cohort. Development of PSN was associated with inferior overall survival (P < .001), relapse-free survival (P = .01), higher nonrelapse mortality (P = .003), and a greater risk of infections within 30 days (P = .03). Multivariable penalized regression analysis identified key risk factors for PSN, which included preinfusion C-reactive protein and bone marrow disease burden, and postinfusion peak ferritin and occurrence of severe cytokine release syndrome, which were used to create the CytoRisk score. In the validation cohort, the CytoRisk score discriminated between patients with and without PSN (area under the curve, 0.90; specificity, 93%; sensitivity, 71%; positive predictive value, 74%; and negative predictive value, 92%). The CytoRisk score may be used to a priori identify patients at highest risk of PSN and overall worse outcomes.
Introduction
Chimeric antigen receptor (CAR) T cells (CAR-Ts) have revolutionized outcomes for children, adolescents, and young adults (CAYAs) with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL).1,2 CAR-T–associated toxicities such as cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) have been studied extensively, with guidelines in place for grading and management.3-5 However, the most common severe adverse event reported after CAR-T therapy is the occurrence of cytopenias or hematologic toxicity. For adults with lymphoma receiving CAR-T therapy, the incidence of severe cytopenias has been reported to be between 37% and 70%,6-11 with ∼30% having persistent severe neutropenia at 1 month.7,10,12,13 In CAYAs with B-ALL treated with tisagenlecleucel (Kymriah), persistence of severe neutropenia has ranged from 16% with real-world use to 53% in the pivotal ELIANA trial.2,14 Based primarily on data from adult patients, severe and prolonged cytopenias are associated with an increased risk of serious infections, nonrelapse mortality, transfusion requirements, and ultimately increased health care resource use.11,13,15-19
Rejeski et al developed and validated a risk stratification tool, CAR-HEMATOTOX, for severe neutropenia lasting ≥14 days in adults with large B-cell lymphoma, and then further validated this score in adults with multiple myeloma, and mantle cell lymphoma.10,20,21 Recently, a joint effort between the European Society for Blood and Marrow Transplantation and the European Hematology Association led to the development of a consensus grading system for immune effector cell–associated hematotoxicity that classified neutropenia based on its depth and duration, outlined risk factors, and provided recommendations for diagnostic workup and guidelines for management.22
These efforts have not included CAYAs treated with CAR-T therapy for B-ALL. Because there are significant differences in disease biology between lymphoma and B-ALL amongst CAYAs and adults, along with differences in toxicity profiles based on CAR-T products used, it is imperative to identify and validate factors specifically associated with prolonged severe neutropenia (PSN) in CAYAs. Herein, we describe the incidence and outcomes of PSN in CAYAs with B-ALL treated with tisagenlecleucel in the real-world setting. We additionally developed and validated a risk score tailored to predict PSN in CAYAs with B-ALL receiving tisagenlecleucel, the CytoRisk score, and compare its performance to the established CAR-HEMATOTOX score.
Methods
Patient and data collection
We conducted a multi-institutional retrospective study using data collected through the Pediatric Real-World CAR Consortium. Deidentified data were collected using REDCap (research electronic data capture), and research was conducted with approval from site-specific institutional review boards. The study cohort included CAYAs, aged ≤26 years, with B-ALL treated with tisagenlecleucel, who underwent leukapheresis and manufacturing between August 2017 and March 2020. Patients were excluded from analysis for progressive disease, nonresponse by day 35 (defined by detectable bone marrow disease by flow cytometry, extramedullary disease, or peripheral blasts), or mortality before day 35. Data from this group were used as a training data set for development of the predictive score.
We established a validation cohort, consisting of data from 5 Pediatric Real-World CAR Consortium institutions who contributed additional data not included in the original training cohort (St. Jude Children’s Research Hospital, Texas Children’s Hospital, Lurie Children’s Hospital, Lucile Packard Children’s Hospital Stanford, and Children’s Hospital Colorado). Patients in the validation data set included those who received tisagenlecleucel between 2017 and 2024.
Definitions
PSN was defined as absolute neutrophil count (ANC) of <0.5 x 103/μL for ≥30 consecutive days after CAR-T infusion. We previously reported that severe neutropenia (ANC of <0.5 x 103/μL) occurred in more than half of CAYAs with B-ALL receiving tisagenlecleucel with a median duration of 14 days.23 Our definition that extends duration of severe neutropenia (ANC of <0.5 x 103/μL) after CAR-T infusion to ≥30 consecutive days after infusion, was chosen to identify patients with PSN. Complete blood count and differential were graded according to National Cancer Institute common terminology criteria for adverse events, version 4.03. CRS was graded according to American Society of Transplant and Cellular Therapy consensus criteria3 and ICANS was graded according to institutionally adopted grading scales (CAR-T–related encephalopathy syndrome,8 CAR-T-cell-therapy-associated TOXicity (CARTOX),24 National Cancer Institute common terminology criteria for adverse events version 4.03, or American Society of Transplant and Cellular Therapy consensus criteria3). CRS with hemophagocytic lymphohistiocytosis (HLH)-like toxicities were defined using previously defined criteria.25 Severe CRS was defined as grade ≥3.3 High disease burden was defined as ≥5% bone marrow blasts, central nervous system 3 disease, peripheral blasts, or presence of non–central nervous system extramedullary disease. Relapse was defined as new extramedullary disease, progression of marrow involvement to either ≥5% blasts in the marrow by morphology, or new minimal-residual disease for which therapy was altered.
Statistical analyses
Descriptive and inferential analyses
Demographic, preinfusion, and postinfusion variables were descriptively reported using count (%) and median (interquartile range) and tabulated by PSN status. Univariable associations of these variables with PSN outcomes were evaluated with likelihood ratio tests from logistic regression and likelihood ratio test–based 95% confidence intervals for odds ratios.
Time to recovery from severe neutropenia was defined as days between onset and resolution of severe neutropenia (ANC ≥ 0.5 x 103/μL). For those with severe neutropenia (ANC < 0.5 x 103/μL) before CAR-Ts, day 0 of severe neutropenia was considered the day of CAR-T infusion. All other time-to-event analyses used the landmark analysis approach,26 with an index date of 30 days after infusion. Overall survival (OS) was defined as the time until death by any cause and relapse-free survival (RFS) as time until relapse or death by any cause, both analyzed by the Kaplan-Meier method. Nonrelapse mortality (NRM) was defined as time until death by any cause with the competing risk of relapse, analyzed by the Kalbfleisch-Prentice method. Relapse was analyzed similarly with the competing risk of death without previous relapse. Patients who did not experience an event (or competing risk) were censored at last contact date. Inferential analyses for OS and RFS used log-rank tests, and, for NRM, used the Gray test; 95% confidence intervals used the complementary log-log transformation. All analyses were performed using R 4.3.1. Inferential analyses were considered exploratory, and no adjustments were made for multiple comparisons.
Prediction model development
We developed 2 prediction systems by using only predictors available at, or before, infusion (herein labeled “2-point preinfusion model”) or all predictors available within 30 days after infusion (“5-point preinfusion and postinfusion model”). Within each system, we used least absolute shrinkage and selection operator-penalized27 logistic regression to select candidate predictors. For continuous variables selected as predictors, we identified 1 cut point based on the Youden index for each predictor separately. We evaluated 2 cut points for peak ferritin. We assigned 1 point for each grouping with successively higher risk and 0 points to the lowest risk grouping, with the patient’s risk score being the sum of these points across the chosen predictors. We recommended the risk score threshold as the threshold with highest Youden index. After identifying the prediction systems and thresholds, we calculated areas under the receiver operating characteristic curves (AUCs) using validation cohort data. All analyses for model development used only the training data.
After the initial validation analysis, we sought to further optimize the scoring systems by reusing the training data to evaluate the use of 2 cut points each for preinfusion C-reactive protein (CRP) and disease burden. This resulted in 2 scores labeled as “4-point preinfusion model” and “7-point preinfusion and postinfusion model,” for which we repeated the validation analyses.
Among evaluable patients, we used complete-case analysis for variable selection and available-case analysis for univariable inferential analyses and for discretizing continuous predictors selected for inclusion. Further details of methodologies and sensitivity analyses are available in the supplementary methods.
Results
Patterns of neutrophil recovery and incidence of PSN
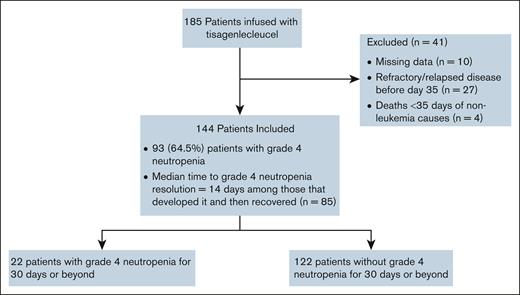
Of 185 patients who received infusion in the training data set, 144 were evaluable: 41 were excluded from analysis because of missing data on severe neutropenia (n = 10), refractory bone marrow disease/death from leukemia (n = 27), or NRM (n = 4) before day 35 after infusion (Figure 1).
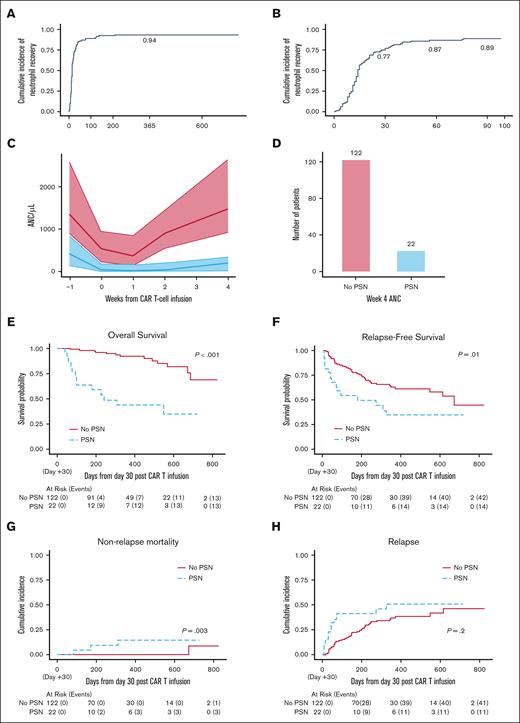
Overall, 93 patients (64.5%) experienced severe neutropenia after CAR-T infusion. Among these, 83 (89%) recovered within 90 days after infusion, and 88 (95%) recovered during study follow-up (Figure 2A-B). Among patients experiencing resolution of grade 4 neutropenia, the median time to resolution was 14 days from onset.
Neutrophil recovery and survival data. (A) Estimate of cumulative incidence of recovery to ANC of >0.5 x 103/μL among patients experiencing grade 4 neutropenia. (B) Estimate of cumulative incidence of recovery to ANC of >0.5 x 103/μL among patients experiencing grade 4 neutropenia restricted to the first 100 days. (C) ANC median (solid lines) and interquartile range (shaded region) by PSN status. (D) Bar plot of number of patients by PSN status. (E) OS probability estimates by PSN status. (F) RFS probability estimates by PSN status. (G) Estimate of cumulative incidence of NRM by PSN status. (H) Estimate of cumulative incidence of relapse by PSN status.
Neutrophil recovery and survival data. (A) Estimate of cumulative incidence of recovery to ANC of >0.5 x 103/μL among patients experiencing grade 4 neutropenia. (B) Estimate of cumulative incidence of recovery to ANC of >0.5 x 103/μL among patients experiencing grade 4 neutropenia restricted to the first 100 days. (C) ANC median (solid lines) and interquartile range (shaded region) by PSN status. (D) Bar plot of number of patients by PSN status. (E) OS probability estimates by PSN status. (F) RFS probability estimates by PSN status. (G) Estimate of cumulative incidence of NRM by PSN status. (H) Estimate of cumulative incidence of relapse by PSN status.
Among 144 evaluable patients, 22 patients (15.3%) met the definition of PSN. Most patients with PSN (20/22) had severe neutropenia before infusion compared with a smaller proportion (40/122) of patients without PSN. Figure 2C demonstrates ANC values over time by medians and interquartile ranges stratified based on occurrence of PSN. Patients without PSN experienced a rapid increase in neutrophil count after week 1 after infusion. Figure 2D shows number of patients in each group.
Disease and treatment characteristics of patients with and without PSN are reported in Tables 1 and 2.
Demographics and preinfusion variables in patients by occurrence of PSN
| Variable . | No PSN (n = 122) . | PSN (n = 22) . | Overall (N = 144) . | P value . |
|---|---|---|---|---|
| Sex | .11 | |||
| Female | 44 (78.6%) | 12 (21.4%) | 56 (38.9%) | |
| Male | 78 (88.6%) | 10 (11.4%) | 88 (61.1%) | |
| Age at CAR-Ts, y | .87 | |||
| Median (IQR) | 13 (8-18) | 10.5 (6.25-18.8) | 12.5 (8-18) | |
| Race/ethnicity | .78 | |||
| Black | 3 (100%) | 0 (0%) | 3 (2.1%) | |
| Hispanic | 43 (84.3%) | 8 (15.7%) | 51 (35.4%) | |
| Other | 13 (86.7%) | 2 (13.3%) | 15 (10.4%) | |
| White | 63 (84%) | 12 (16%) | 75 (52.1%) | |
| No. of relapses before CAR-Ts | .88 | |||
| Median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | |
| No. of lines of therapy before CAR-Ts | .04 | |||
| Median (IQR) | 3 (2-4) | 3 (3-5) | 3 (2-4) | |
| HSCT before CAR-Ts | .26 | |||
| No | 97 (86.6%) | 15 (13.4%) | 112 (77.8%) | |
| Yes | 25 (78.1%) | 7 (21.9%) | 32 (22.2%) | |
| Months from previous HSCT to CAR-Ts | .63 | |||
| Median (IQR) | 19 (10-34.5) | 16 (6.50-18.5) | 16.5 (9-30.8) | |
| Missing | 2 (100%) | 0 (0%) | 2 (6.2%) | |
| HSCT source | .47 | |||
| Cord | 3 (100%) | 0 (0%) | 3 (9.4%) | |
| Haplo-identical | 6 (75%) | 2 (25%) | 8 (25%) | |
| Matched related | 9 (69.2%) | 4 (30.8%) | 13 (40.6%) | |
| Matched unrelated | 7 (87.5%) | 1 (12.5%) | 8 (25.0%) | |
| Intensity of most recent HSCT∗ | .51 | |||
| 1 | 22 (78.6%) | 6 (21.4%) | 28 (87.5%) | |
| 2 | 1 (100%) | 0 (0%) | 1 (3.1%) | |
| 3 | 2 (100%) | 0 (0%) | 2 (6.3%) | |
| Missing | 0 (0%) | 1 (100%) | 1 (3.1%) | |
| Bone marrow disease burden (% morphologic blasts) | <.01 | |||
| Median (IQR) | 1 (0-9) | 36.0 (8-84) | 1.80 (0-15) | |
| Missing | 4 (80%) | 1 (20%) | 5 (3.5%) | |
| High disease burden† | <.01 | |||
| No | 73 (96.1%) | 3 (3.9%) | 76 (52.8%) | |
| Yes | 47 (71.2%) | 19 (28.8%) | 66 (45.8%) | |
| Missing | 2 (100%) | 0 (0%) | 2 (1.4%) | |
| CAR-T dose infused (×106/kg) | .24 | |||
| Median (IQR) | 1.70 (1.30-2.4) | 1.77 (1.52-2.5) | 1.70 (1.31-2.4) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Chemotherapy regimen | .02 | |||
| Fludarabine (30 mg/m2 × 4 days) and cyclophosphamide (500 mg/m2 × 2 days) | 120 (86.3%) | 19 (13.7%) | 139 (96.5%) | |
| Other | 2 (40.0%) | 3 (60.0%) | 5 (3.5%) | |
| Ferritin (ng/mL) | .007 | |||
| Median (IQR) | 998 (572-2120) | 2630 (1210-5500) | 1230 (578-2630) | |
| Missing | 12 (80%) | 3 (20%) | 15 (10.4%) | |
| CRP (mg/dL) | <.01 | |||
| Median (IQR) | 0.49 (0.3-1.4) | 4.56 (0.93-7.3) | 0.50 (0.3-1.79) | |
| Missing | 17 (77.3%) | 5 (22.7%) | 22 (15.3%) | |
| Platelet (×109/L) | <.01 | |||
| Median (IQR) | 134 (84-209) | 24.5 (21-107) | 122 (55.8-206) | |
| ANC (cells per mm3) | <.01 | |||
| Median (IQR) | 590 (250-1090) | 30 (0-195) | 500 (180-1040) | |
| Absolute leukocyte count (cells per mm3) | .20 | |||
| Median (IQR) | 70.0 (30-143) | 40.0 (2.5-90) | 60.0 (20-125) | |
| LDH (U/L) | .01 | |||
| Median (IQR) | 214 (176-309) | 283 (194-504) | 217 (178-319) | |
| Missing | 11 (84.6%) | 2 (15.4%) | 13 (9%) | |
| Creatinine (mg/dL) | .64 | |||
| Median (IQR) | 0.47 (0.34-0.64) | 0.6 (0.31-0.68) | 0.49 (0.34-0.64) |
| Variable . | No PSN (n = 122) . | PSN (n = 22) . | Overall (N = 144) . | P value . |
|---|---|---|---|---|
| Sex | .11 | |||
| Female | 44 (78.6%) | 12 (21.4%) | 56 (38.9%) | |
| Male | 78 (88.6%) | 10 (11.4%) | 88 (61.1%) | |
| Age at CAR-Ts, y | .87 | |||
| Median (IQR) | 13 (8-18) | 10.5 (6.25-18.8) | 12.5 (8-18) | |
| Race/ethnicity | .78 | |||
| Black | 3 (100%) | 0 (0%) | 3 (2.1%) | |
| Hispanic | 43 (84.3%) | 8 (15.7%) | 51 (35.4%) | |
| Other | 13 (86.7%) | 2 (13.3%) | 15 (10.4%) | |
| White | 63 (84%) | 12 (16%) | 75 (52.1%) | |
| No. of relapses before CAR-Ts | .88 | |||
| Median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | |
| No. of lines of therapy before CAR-Ts | .04 | |||
| Median (IQR) | 3 (2-4) | 3 (3-5) | 3 (2-4) | |
| HSCT before CAR-Ts | .26 | |||
| No | 97 (86.6%) | 15 (13.4%) | 112 (77.8%) | |
| Yes | 25 (78.1%) | 7 (21.9%) | 32 (22.2%) | |
| Months from previous HSCT to CAR-Ts | .63 | |||
| Median (IQR) | 19 (10-34.5) | 16 (6.50-18.5) | 16.5 (9-30.8) | |
| Missing | 2 (100%) | 0 (0%) | 2 (6.2%) | |
| HSCT source | .47 | |||
| Cord | 3 (100%) | 0 (0%) | 3 (9.4%) | |
| Haplo-identical | 6 (75%) | 2 (25%) | 8 (25%) | |
| Matched related | 9 (69.2%) | 4 (30.8%) | 13 (40.6%) | |
| Matched unrelated | 7 (87.5%) | 1 (12.5%) | 8 (25.0%) | |
| Intensity of most recent HSCT∗ | .51 | |||
| 1 | 22 (78.6%) | 6 (21.4%) | 28 (87.5%) | |
| 2 | 1 (100%) | 0 (0%) | 1 (3.1%) | |
| 3 | 2 (100%) | 0 (0%) | 2 (6.3%) | |
| Missing | 0 (0%) | 1 (100%) | 1 (3.1%) | |
| Bone marrow disease burden (% morphologic blasts) | <.01 | |||
| Median (IQR) | 1 (0-9) | 36.0 (8-84) | 1.80 (0-15) | |
| Missing | 4 (80%) | 1 (20%) | 5 (3.5%) | |
| High disease burden† | <.01 | |||
| No | 73 (96.1%) | 3 (3.9%) | 76 (52.8%) | |
| Yes | 47 (71.2%) | 19 (28.8%) | 66 (45.8%) | |
| Missing | 2 (100%) | 0 (0%) | 2 (1.4%) | |
| CAR-T dose infused (×106/kg) | .24 | |||
| Median (IQR) | 1.70 (1.30-2.4) | 1.77 (1.52-2.5) | 1.70 (1.31-2.4) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Chemotherapy regimen | .02 | |||
| Fludarabine (30 mg/m2 × 4 days) and cyclophosphamide (500 mg/m2 × 2 days) | 120 (86.3%) | 19 (13.7%) | 139 (96.5%) | |
| Other | 2 (40.0%) | 3 (60.0%) | 5 (3.5%) | |
| Ferritin (ng/mL) | .007 | |||
| Median (IQR) | 998 (572-2120) | 2630 (1210-5500) | 1230 (578-2630) | |
| Missing | 12 (80%) | 3 (20%) | 15 (10.4%) | |
| CRP (mg/dL) | <.01 | |||
| Median (IQR) | 0.49 (0.3-1.4) | 4.56 (0.93-7.3) | 0.50 (0.3-1.79) | |
| Missing | 17 (77.3%) | 5 (22.7%) | 22 (15.3%) | |
| Platelet (×109/L) | <.01 | |||
| Median (IQR) | 134 (84-209) | 24.5 (21-107) | 122 (55.8-206) | |
| ANC (cells per mm3) | <.01 | |||
| Median (IQR) | 590 (250-1090) | 30 (0-195) | 500 (180-1040) | |
| Absolute leukocyte count (cells per mm3) | .20 | |||
| Median (IQR) | 70.0 (30-143) | 40.0 (2.5-90) | 60.0 (20-125) | |
| LDH (U/L) | .01 | |||
| Median (IQR) | 214 (176-309) | 283 (194-504) | 217 (178-319) | |
| Missing | 11 (84.6%) | 2 (15.4%) | 13 (9%) | |
| Creatinine (mg/dL) | .64 | |||
| Median (IQR) | 0.47 (0.34-0.64) | 0.6 (0.31-0.68) | 0.49 (0.34-0.64) |
CNS, central nervous system; IQR, interquartile range; LDH, lactate dehydrogenase.
Intensity of HSCT defined as: (1) myeloablative HSCT with radiation, (2) myeloablative SCT with chemotherapy, and (3) nonmyeloablative.
High disease burden defined as: >5% bone marrow blasts, having CNS3 disease, having peripheral blasts, odds ratio having non-CNS extramedullary disease.
Postinfusion variables in patients by occurrence of PSN
| Variable . | No PSN (n = 122) . | PSN (n = 22) . | Overall (N = 144) . | P value . |
|---|---|---|---|---|
| pRBC transfusions required | <.01 | |||
| No | 86 (97.7%) | 2 (2.3%) | 88 (61.1%) | |
| Yes | 33 (63.5%) | 19 (36.5%) | 52 (36.1%) | |
| Missing | 3 (75%) | 1 (25%) | 4 (2.8%) | |
| Platelet transfusions required | <.01 | |||
| No | 96 (99%) | 1 (1%) | 97 (67.4%) | |
| Yes | 24 (53.3%) | 21 (46.7%) | 45 (31.3%) | |
| Missing | 2 (100%) | 0 (0%) | 2 (1.4%) | |
| Coagulopathy | <.01 | |||
| No | 105 (89.7%) | 12 (10.3%) | 117 (81.3%) | |
| Yes | 11 (55%) | 9 (45%) | 20 (13.9%) | |
| Missing | 6 (85.7%) | 1 (14.3%) | 7 (4.9%) | |
| CRS | .02 | |||
| No | 54 (93.1%) | 4 (6.9%) | 58 (40.3%) | |
| Yes | 68 (80%) | 17 (20%) | 85 (59%) | |
| Missing | 0 (0%) | 1 (100%) | 1 (0.7%) | |
| ASTCT CRS | <.01 | |||
| 0 | 51 (92.7%) | 4 (7.3%) | 55 (38.2%) | |
| 1 | 34 (94.4%) | 2 (5.6%) | 36 (25%) | |
| 2 | 20 (83.3%) | 4 (16.7%) | 24 (16.7%) | |
| 3 | 12 (75%) | 4 (25%) | 16 (11.1%) | |
| 4 | 4 (33.3%) | 8 (66.7%) | 12 (8.3%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Severe CRS∗ | <.01 | |||
| No | 105 (91.3%) | 10 (8.7%) | 115 (79.9%) | |
| Yes | 16 (57.1%) | 12 (42.9%) | 28 (19.4%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| ICANS | .02 | |||
| No | 100 (88.5%) | 13 (11.5%) | 113 (78.5%) | |
| Yes | 21 (70.0%) | 9 (30.0%) | 30 (20.8%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Max grade of ICANS | .07 | |||
| 0 | 100 (88.5%) | 13 (11.5%) | 113 (78.5%) | |
| 1 | 12 (80%) | 3 (20%) | 15 (10.4%) | |
| 2 | 4 (80%) | 1 (20%) | 5 (3.5%) | |
| 3 | 3 (42.9%) | 4 (57.1%) | 7 (4.9%) | |
| 4 | 2 (66.7%) | 1 (33.3%) | 3 (2.1%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| CRS with HLH-like toxicities | <.01 | |||
| No | 117 (90.7%) | 12 (9.3%) | 129 (89.6%) | |
| Yes | 5 (33.3%) | 10 (66.7%) | 15 (10.4%) | |
| Intensive care required (days) | <.01 | |||
| Median (IQR) | 0 (0-0) | 6 (0-10.5) | 0 (0-2) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Peak ferritin (ng/mL) | <.01 | |||
| Median (IQR) | 1580 (637-3520) | 40 000 (11 800-157 000) | 2300 (900-9050) | |
| Missing | 17 (85%) | 3 (15%) | 20 (13.9%) | |
| Peak CRP (mg/dL) | <.01 | |||
| Median (IQR) | 2.35 (0.8-10) | 15.6 (7.63-32) | 3.27 (0.900-11.6) | |
| Missing | 20 (76.9%) | 6 (23.1%) | 26 (18.1%) | |
| No. of doses of tocilizumab | <.01 | |||
| Median (IQR) | 0 (0-0) | 1 (0-2) | 0 (0-0) | |
| Missing | 7 (87.5%) | 1 (12.5%) | 8 (5.6%) | |
| No. of days of steroid | .16 | |||
| Median (IQR) | 0 (0-0) | 0.50 (0-4.25) | 0 (0-0) | |
| Missing | 10 (83.3%) | 2 (16.7%) | 12 (8.3%) | |
| Infection within 30 days after CAR-Ts | .03 | |||
| No | 107 (87.7%) | 15 (12.3%) | 122 (84.7%) | |
| Yes | 15 (68.2%) | 7 (31.8%) | 22 (15.3%) | |
| Time from CAR-Ts to earliest infection (days)† | ‡ | |||
| Median (IQR) | 87 (10-211) | 17.5 (6.25-47.5) | 50 (10-192) | |
| Missing | 0 (0%) | 1 (100%) | 1 (1.9%) |
| Variable . | No PSN (n = 122) . | PSN (n = 22) . | Overall (N = 144) . | P value . |
|---|---|---|---|---|
| pRBC transfusions required | <.01 | |||
| No | 86 (97.7%) | 2 (2.3%) | 88 (61.1%) | |
| Yes | 33 (63.5%) | 19 (36.5%) | 52 (36.1%) | |
| Missing | 3 (75%) | 1 (25%) | 4 (2.8%) | |
| Platelet transfusions required | <.01 | |||
| No | 96 (99%) | 1 (1%) | 97 (67.4%) | |
| Yes | 24 (53.3%) | 21 (46.7%) | 45 (31.3%) | |
| Missing | 2 (100%) | 0 (0%) | 2 (1.4%) | |
| Coagulopathy | <.01 | |||
| No | 105 (89.7%) | 12 (10.3%) | 117 (81.3%) | |
| Yes | 11 (55%) | 9 (45%) | 20 (13.9%) | |
| Missing | 6 (85.7%) | 1 (14.3%) | 7 (4.9%) | |
| CRS | .02 | |||
| No | 54 (93.1%) | 4 (6.9%) | 58 (40.3%) | |
| Yes | 68 (80%) | 17 (20%) | 85 (59%) | |
| Missing | 0 (0%) | 1 (100%) | 1 (0.7%) | |
| ASTCT CRS | <.01 | |||
| 0 | 51 (92.7%) | 4 (7.3%) | 55 (38.2%) | |
| 1 | 34 (94.4%) | 2 (5.6%) | 36 (25%) | |
| 2 | 20 (83.3%) | 4 (16.7%) | 24 (16.7%) | |
| 3 | 12 (75%) | 4 (25%) | 16 (11.1%) | |
| 4 | 4 (33.3%) | 8 (66.7%) | 12 (8.3%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Severe CRS∗ | <.01 | |||
| No | 105 (91.3%) | 10 (8.7%) | 115 (79.9%) | |
| Yes | 16 (57.1%) | 12 (42.9%) | 28 (19.4%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| ICANS | .02 | |||
| No | 100 (88.5%) | 13 (11.5%) | 113 (78.5%) | |
| Yes | 21 (70.0%) | 9 (30.0%) | 30 (20.8%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Max grade of ICANS | .07 | |||
| 0 | 100 (88.5%) | 13 (11.5%) | 113 (78.5%) | |
| 1 | 12 (80%) | 3 (20%) | 15 (10.4%) | |
| 2 | 4 (80%) | 1 (20%) | 5 (3.5%) | |
| 3 | 3 (42.9%) | 4 (57.1%) | 7 (4.9%) | |
| 4 | 2 (66.7%) | 1 (33.3%) | 3 (2.1%) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| CRS with HLH-like toxicities | <.01 | |||
| No | 117 (90.7%) | 12 (9.3%) | 129 (89.6%) | |
| Yes | 5 (33.3%) | 10 (66.7%) | 15 (10.4%) | |
| Intensive care required (days) | <.01 | |||
| Median (IQR) | 0 (0-0) | 6 (0-10.5) | 0 (0-2) | |
| Missing | 1 (100%) | 0 (0%) | 1 (0.7%) | |
| Peak ferritin (ng/mL) | <.01 | |||
| Median (IQR) | 1580 (637-3520) | 40 000 (11 800-157 000) | 2300 (900-9050) | |
| Missing | 17 (85%) | 3 (15%) | 20 (13.9%) | |
| Peak CRP (mg/dL) | <.01 | |||
| Median (IQR) | 2.35 (0.8-10) | 15.6 (7.63-32) | 3.27 (0.900-11.6) | |
| Missing | 20 (76.9%) | 6 (23.1%) | 26 (18.1%) | |
| No. of doses of tocilizumab | <.01 | |||
| Median (IQR) | 0 (0-0) | 1 (0-2) | 0 (0-0) | |
| Missing | 7 (87.5%) | 1 (12.5%) | 8 (5.6%) | |
| No. of days of steroid | .16 | |||
| Median (IQR) | 0 (0-0) | 0.50 (0-4.25) | 0 (0-0) | |
| Missing | 10 (83.3%) | 2 (16.7%) | 12 (8.3%) | |
| Infection within 30 days after CAR-Ts | .03 | |||
| No | 107 (87.7%) | 15 (12.3%) | 122 (84.7%) | |
| Yes | 15 (68.2%) | 7 (31.8%) | 22 (15.3%) | |
| Time from CAR-Ts to earliest infection (days)† | ‡ | |||
| Median (IQR) | 87 (10-211) | 17.5 (6.25-47.5) | 50 (10-192) | |
| Missing | 0 (0%) | 1 (100%) | 1 (1.9%) |
ASTCT, American Society of Transplant and Cellular Therapy; IQR, interquartile range; pRBC, packed red blood cell.
Severe CRS: grade ≥3 CRS.
Among patients experiencing at least 1 infection.
P value not calculated because of unknown sequence of covariate and outcome.
Survival outcomes in patients with and without PSN
To evaluate the impact of PSN on long-term outcomes, we characterized survival in patients with and without PSN. Median time to death or censoring in this cohort was 381 days. Patients with PSN had inferior OS than those without PSN (1-year OS: 0.44 vs 0.92, respectively; P < .001; Figure 2E). Because high disease burden is a known prognostic factor for poor outcomes in patients receiving CAR-T therapy for B-ALL,2,23,25,28 we evaluated the impact of PSN in these patients. Development of PSN independently affected OS when analyzing a high disease burden–only cohort (P < .001; supplemental Figure 1), suggesting independent prognostic value of PSN in this high-risk cohort. Because only 3 patients with low disease burden experienced PSN, we did not have the power to evaluate the impact of PSN on OS in this group.
Patients with PSN had inferior RFS than those without PSN (1-year RFS: 0.35 vs 0.63, respectively; P = .01). This was despite excluding those with residual leukemia by day 35 (Figure 2F). Among patients with high-disease burden, there was a trend toward inferior RFS in patients with PSN compared with those without PSN (P = .08; supplemental Figure 1).
Cumulative incidence of NRM was higher among those with PSN than those without PSN (1-year NRM: 0.145 vs 0.000, respectively; P = .003; Figure 2G). There were no deaths from NRM within the first year in the patients who did not have PSN whereas 3 deaths occurred within the first year in patients with PSN. Causes of death included fungal infection at day +123 after infusion in 1 patient, and cardiopulmonary complications in 2 patients, 1 at day +207 without an intervening hematopoietic stem cell transplant (HSCT) and 1 at day +338 who had undergone HSCT at day +230 after tisagenlecleucel while in remission. The 1-year estimate of cumulative incidence of relapse was higher in the PSN group, but this difference was not statistically significant (0.51 vs 0.39, P = .2; Figure 2H).
We also analyzed outcomes using an alternative definition for PSN, categorizing patients into 0 to <14, 14 to <30, or ≥30 days of severe neutropenia. As shown in the supplemental Figure 2, we found that the group of patients with ≥30 days of severe neutropenia have significantly worse outcomes across OS, RFS, and NRM, compared with the groups with 14 to <30 days and <14 days for which outcomes were similar. For relapse, the estimates followed a similar pattern, but the difference was not significant.
Analysis of risk factors for development of PSN
We evaluated risk factors for development of PSN taking into account both pre–CAR-T infusion (Table 1) and post–CAR-T infusion variables (Table 2). The group with PSN had more lines of previous therapy (P = .04), higher baseline bone marrow disease burden (P < .01), ferritin (P = .007), lactate dehydrogenase (P = .01), and CRP (P < .01), and lower baseline platelet count (P < .01) and ANC (P < .01) than the cohort without PSN (Table 1). After infusion, the group with PSN more frequently had coagulopathy (P < .01), ICANS (P = .02), CRS (P = .02), CRS with HLH-like toxicities (P < .01), and higher CRS grade (P < .01). Those with PSN had higher peak CRP (P < .01) and ferritin (P < .01) after infusion, received a greater number of doses of tocilizumab (P < .01), and required a greater number of days of treatment in an intensive care unit (P < .01). Patients with PSN required more packed red blood cell and platelet transfusions after CAR-Ts (P < .01). Furthermore, the percentage of patients requiring ongoing transfusions beyond day 30 after infusion was greater in the PSN than the non-PSN cohort for both platelets (72.7% vs 7.5%, P < .01) and packed red blood cells (61.9% vs 5.9%, P < .01). Infections in the first 30 days after CAR-T infusion were more common in those who developed PSN compared with those who did not (P = .03). Among patients with infections, the median time to infection onset in patients with PSN was 17.5 days, compared with 87 days for those without PSN (Table 2). Details of infectious complications are listed in supplemental Table 2.
Developing a prediction model for PSN and evaluating performance in training data set
To investigate predictors of PSN, we performed least absolute shrinkage and selection operator-penalized logistic regression. Two preinfusion variables (disease burden and preinfusion CRP) and 2 postinfusion variables (severe CRS and peak ferritin) were identified as key predictors of PSN (see the supplemental Materials for details). Using these predictors, we developed preinfusion only, and preinfusion and postinfusion, point-based scoring systems that we termed the “CytoRisk score” (Figure 3A-B). We analyzed the performance of the CytoRisk score in the training data set. We report a 0.83 AUC in the training data for the preinfusion-only system and 0.91 AUC for the and preinfusion and postinfusion system. We identified that categorizing summative risk scores of ≥1 for the preinfusion score and ≥4 for the preinfusion and postinfusion score, have the largest Youden indices for predicting PSN (details in the supplemental Data).
Development of CytoRisk score and validation. (A) Initial 2-point preinfusion system. (B) Initial 5-point preinfusion and postinfusion system. (C) Validation receiver operating characteristic (ROC) curves for initial 2-point preinfusion, initial 5-point preinfusion and postinfusion, and CAR-HEMATOTOX systems. ∗For all risk scores, the AUC was significantly different from 0.50 (P < .001). (D) Optimized 4-point preinfusion system. (E) Optimized 7-point preinfusion and postinfusion system. (F) Validation ROC curves for optimized 4-point preinfusion, optimized 7-point preinfusion and postinfusion, and CAR-HEMATOTOX systems. ∗For all risk scores, the AUC was significantly different from 0.50 (P < .001). (G) Prediction performance for all candidate systems at the respective proposed threshold for high risk.
Development of CytoRisk score and validation. (A) Initial 2-point preinfusion system. (B) Initial 5-point preinfusion and postinfusion system. (C) Validation receiver operating characteristic (ROC) curves for initial 2-point preinfusion, initial 5-point preinfusion and postinfusion, and CAR-HEMATOTOX systems. ∗For all risk scores, the AUC was significantly different from 0.50 (P < .001). (D) Optimized 4-point preinfusion system. (E) Optimized 7-point preinfusion and postinfusion system. (F) Validation ROC curves for optimized 4-point preinfusion, optimized 7-point preinfusion and postinfusion, and CAR-HEMATOTOX systems. ∗For all risk scores, the AUC was significantly different from 0.50 (P < .001). (G) Prediction performance for all candidate systems at the respective proposed threshold for high risk.
Validation cohort characteristics
A CONSORT diagram for the validation cohort (n = 141 evaluable) is shown in supplemental Figure 4 and patient characteristics are shown in supplemental Table 1. Characteristics of the validation cohort were comparable with that of the training cohort including median age (12 vs 12.5 years), proportion female (41.8% vs 38.8%), proportion with previous HSCT (14.2% vs 22.2%), median baseline bone marrow disease burden (1% vs 1.8%), and proportion that developed PSN (21.3% vs 15.3%). Within the validation cohort there were no significant differences in age, sex, previous HSCT, or time from previous HSCT to CAR-Ts between patients with and without PSN. As in the training data set, higher preinfusion disease burden, CRP, and ferritin values, and lower preinfusion platelet and ANC values were observed in the PSN compared with the non-PSN group. After infusion, severe CRS was more common, and median peak ferritin was higher in the PSN than the non-PSN group.
Evaluating prediction model performance in validation data set
We compared the predictive performance of our proposed CytoRisk scoring systems and the previously established CAR-HEMATOTOX score in the validation cohort. Receiver operating characteristic curves and their respective AUCs with CAR-HEMATOTOX are reported in Figure 3C. In our validation data set, we computed an AUC of 0.81 for the proposed 2-point preinfusion-only scoring system. We calculated a higher AUC of 0.89 for the proposed 5-point and preinfusion and postinfusion scoring system compared with CAR-HEMATOTOX AUC of 0.85.
Model optimization and further validation analysis
Next, we sought to use the training data to further optimize our preinfusion model performance by evaluating 2 cut points each for disease burden and preinfusion CRP based on additional clinically relevant cut points (M3 bone marrow, >25% involvement, and CRP of >5.0 mg/dL). In Figure 3D-E we propose a 4-point preinfusion system, and a 7-point preinfusion and postinfusion system. Using the revised preinfusion 4-point model, the AUC for PSN was 0.83, and the revised 7-point preinfusion and postinfusion model AUC was 0.90, compared with the CAR-HEMATOTOX AUC of 0.85 (Figure 3F).
In Figure 3G, we report other performance metrics using the proposed high-risk thresholds established for each scoring system compared with CAR-HEMATOTOX.10 Our CytoRisk preinfusion scoring system had a similar pattern but higher operating characteristics including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) than CAR-HEMATOTOX. Our combined CytoRisk preinfusion and postinfusion systems had notably higher specificity and PPV, whereas retaining other comparable operating characteristics to CAR-HEMATOTOX.
To evaluate the clinical usefulness of a predictive score with embedded postinfusion variables, we studied timing of risk-defining events after CAR infusion. The median and maximum (max) days after CAR-T that patients were first identified with grade ≥3 CRS, peak ferritin of >3500 ng/mL, or peak ferritin of >10 000 ng/mL were 6 days (max, 12), 7 days (max, 14), and 8 days (max, 16), respectively. Using the combined preinfusion and postinfusion systems in the validation data, we could determine risk for PSN at a median of 8 days after infusion, and for all patients within 2 weeks after CAR-T infusion.
Evaluating model performance over time
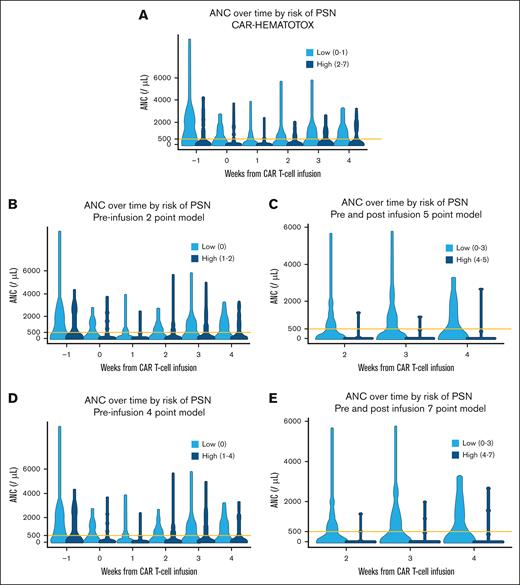
In Figure 4, the violin plots demonstrate neutrophil recovery over the first 4 weeks after CAR-Ts, stratifying ANC based on the various scoring systems. For all systems, patients identified as high risk for PSN had lower ANC across all time points tested than those not at risk of PSN. However, the preinfusion-only systems (including the 2-point and 4-point CytoRisk score and the CAR-HEMATOTOX) perform worse at stratifying ANC after week 2 compared with the combined preinfusion and postinfusion CytoRisk scores, which can effectively stratify ANC across all time points.
Violin plots of weekly ANC for each candidate system categorized by the respective high-risk grouping. Systems including postinfusion variables are restricted to weeks 2 to 4 when the highest risk grouping could be determined for all patients in the data set. Orange line overlaid at 500. (A) Violin plots of weekly ANC stratified by CAR-HEMATOTOX. (B) Violin plots of weekly ANC stratified by 2-point preinfusion variables. (C) Violin plots of weekly ANC stratified by 5-point preinfusion and postinfusion variables. (D) Violin plots of weekly ANC stratified by 4-point preinfusion variables. (E) Violin plots of weekly ANC stratified by 7-point preinfusion variables.
Violin plots of weekly ANC for each candidate system categorized by the respective high-risk grouping. Systems including postinfusion variables are restricted to weeks 2 to 4 when the highest risk grouping could be determined for all patients in the data set. Orange line overlaid at 500. (A) Violin plots of weekly ANC stratified by CAR-HEMATOTOX. (B) Violin plots of weekly ANC stratified by 2-point preinfusion variables. (C) Violin plots of weekly ANC stratified by 5-point preinfusion and postinfusion variables. (D) Violin plots of weekly ANC stratified by 4-point preinfusion variables. (E) Violin plots of weekly ANC stratified by 7-point preinfusion variables.
Discussion
The lack of a clinically relevant score predictive of post-CAR hematotoxicity in CAYAs with B-ALL is a fundamental gap in the field and efforts to investigate and validate a predictive hematotoxicity score designed for CAYAs with B-ALL are foundational. We describe the incidence, patterns of neutrophil recovery, risk factors, and outcomes for the development of PSN in CAYAs with B-ALL after infusion of tisagenlecleucel. We developed and validated, to our knowledge, the first risk score for PSN using data from CAYAs with B-ALL undergoing treatment with tisagenlecleucel, the CytoRisk score, and evaluated its performance along with that of the CAR-HEMATOTOX score in the CAYA B-ALL population.
Several definitions exist for thresholds of degree and duration of neutropenia.10,22,29 Although severe neutropenia occurs frequently after CAR-T infusion, it most often resolves by 2 weeks after onset.22,23 However, neutropenia has been reported to last months to years after CAR-T infusion, with some patients developing an “aplastic” phenotype that does not recover despite intervention with granulocyte colony-stimulating factor (G-CSF).10,29-31 We therefore chose to define PSN as duration of ≥30 days of ANC of <0.5 x 103/μL, to identify patients who remain with neutropenia beyond 2 weeks and are thereby at highest risk of complications. The infection risk in these patients is further compounded in context of CD19 CAR-Ts by expected B-cell aplasia and hypogammaglobulinemia.13,15,18 Using our definition of PSN, we found PSN occurred frequently in both our training and validation cohorts, and was associated with inferior OS and RFS compared with those without PSN, even when stratified by disease burden, a known risk factor for inferior outcome.2,23,25,28 Furthermore, patients with PSN experienced more frequent NRM, a greater number of early infections, greater need for transfusions, and longer intensive care unit admissions, highlighting the increased health care use in this population. We also conducted a supplementary analysis using an alternative definition of PSN (<14 days, 14-30 days, and ≥30 days) to evaluate our choice of definition, and this analysis provided further confirmation that our approach of PSN of ≥30 days identifies patients at highest risk.
Several studies of adult patients have evaluated risk factors for development of cytopenias after CAR-T treatment. Rejeski et al showed that in adults receiving CAR-T therapy for lymphoma, preinfusion platelet count, ANC, hemoglobin, CRP, and ferritin were predictive for development of cytopenias, and these were used to develop the CAR-HEMATOTOX scoring system.10 Notably, preinfusion disease burden was not included in this model although other studies have shown preinfusion bone marrow involvement to be predictive for development of cytopenias even in patients with lymphoma and multiple myeloma.11,32,33 A large, single-center study34 showed that patients with leukemia had higher incidence and longer duration of PSN compared with patients receiving CAR-Ts for lymphomas or solid tumors, and the authors hypothesized that this could be because of decreased infiltration of the bone marrow by lymphomas and solid tumors and thereby a superior hematopoietic reserve. Additionally, an association between the presence of inflammatory toxicities after CAR-T infusion and development of cytopenias has also been reported, with some studies suggesting cytopenias be considered in the definition of immune effector cell–associated HLH-like syndrome.31,33,35-37 Similarly, in our study, occurrence of inflammation (high-grade CRS and peak ferritin) after CAR-T infusion was predictive for development of cytopenias. In contrast, the study that developed the CAR-HEMATOTOX score in adults with lymphoma10 did not identify postinfusion inflammatory variables to be associated with prolonged cytopenias. Differences in disease biology, age, CAR-T products used, and characteristics of inflammatory toxicities in adults with lymphoma or myeloma compared with pediatric patients with ALL likely explain these findings. Therefore, because the variables associated with risk of PSN in our cohort were distinct compared with those used in the CAR-HEMATOX scoring system, we developed and validated an alternate scoring system, the CytoRisk score.
We compared performance of the CytoRisk score with the CAR-HEMATOTOX score. The CytoRisk 5-point and 7-point preinfusion and postinfusion scores had superior AUC than the CAR-HEMATOTOX score (AUC of 0.89 and 0.90, respectively, vs 0.85) and our revised 7-point score had the most optimal testing characteristics of all the systems tested. Although postinfusion variables were used in the score, all highest-risk patients were identified within 2 weeks after infusion, supporting the practical utility of this approach. Overall, the CAR-HEMATOTOX score has a lower PPV and specificity but higher sensitivity and NPV for PSN in B-ALL, which means that not all patients considered at high risk will develop severe hematotoxicity. Used in combination, our preinfusion CytoRisk score has high sensitivity and NPV, useful in identifying patients at low risk for PSN, and the combined preinfusion and postinfusion CytoRisk score, which is optimized for high specificity and PPV, is most useful for identifying patients at highest risk for PSN. We therefore recommend using the preinfusion CytoRisk score as an initial screen to identify patients at risk for PSN before CAR-T therapy, and the combined preinfusion and postinfusion CytoRisk scored applied within 2 weeks after infusion to identify patients at highest risk for PSN. Alternatively, the CAR-HEMATOTOX score could also be used to screen patients at risk of hematotoxicity and our CytoRisk score can be used after infusion to identify with high specificity those at highest risk of developing severe neutropenia of ≥30 days.
There are several clinical implications for this work. The identification of patients at high risk for PSN could allow for increased vigilance in these patients, in particular for infectious complications that remain a major challenge in pediatric patients receiving CAR-T therapies.38 Monitoring for, and applying the preinfusion and postinfusion CytoRisk scoring system in the first 2 weeks after CAR, also allows for a therapeutic window for early interventions. Because PSN and associated complications may be preventable through earlier implementation of antibiotics39 and/or mold-active fungal prophylaxis,40,41 or the earlier use of G-CSF,42-46 the CytoRisk score could identify patients at highest risk for PSN for whom study of these interventions would be warranted. However, in patients with this severe phenotype, it is possible that the use of G-CSF alone will not suffice, particularly if there is a significantly decreased hematopoietic reserve. In such cases, in which a previous transplant donor is available, a CD34+ stem cell boost could be anticipated and requested in advance, because the safety and efficacy of this approach has been demonstrated.47-50 In contrast, patients at low risk of developing PSN may be spared the use of intensified prophylactic antimicrobials and allowed a less-rigorous postinfusion laboratory monitoring schedule, because need for packed red blood cell and platelet transfusion requirements will be lower.
The mechanisms underlying the development of hematotoxicity are yet to be fully established, with studies demonstrating decreased bone marrow hematopoietic reserve, immune dysregulation at baseline, clonal T-cell expansion, and inflammatory cytokine production after CAR-T infusion31,51-53 as being possible contributors. A better understanding of these mechanisms could allow for use of targeted therapies to circumvent the deleterious impact of inflammation after CAR-T therapy.
A strength of our study is the size of our training and validation data sets in the context of pediatric patients receiving commercially available CD19 CAR-T therapy. The retrospective nature of our study with its inherent biases is a limitation along with lack of longer-term data. We were limited in our analysis of impact of G-CSF use because these data were not available. Our study excluded patients with refractory disease or death before day 35 to identify cytopenias not driven by leukemic progression. Prospective evaluation of our score in all tisagenlecleucel recipients will help to further validate its clinical utility.
In conclusion, our study is, to our knowledge, the first to report on the incidence, patterns of neutrophil recovery, and risk factors for the development of PSN in CAYAs with B-ALL after infusion of tisagenlecleucel. The CytoRisk score that we developed and validated allows for identification of patients at highest risk of PSN. Prospective studies using the CytoRisk score to stratify patients in need of prophylactic antimicrobials or to intervene with strategies such as use of preemptive G-CSF are warranted, as are mechanistic studies to ultimately identify therapies to alleviate this toxicity.
Acknowledgments
The authors acknowledge the following individuals for their major roles in supporting successful execution of this multi-institutional study: E.E. for regulatory support; Anika Dove and Daisy Torres for administrative support; Neil Morimoto for legal council and contracting; and Anne Marcy, Michelle Fujimoto, Jennifer Sheppard, Jean Sosna, Victoria Koch, Katie Doherty, Emily Bakinowski, Elizabeth Klein, Daritzya Baraja, Courtney Newbold, Glenn McWillians, Maggie Dyer, Kasey Abrahamnson, Angie Peltz, Ahmed Tahoun, Mary Suarez, Megan Hanby, Stacy Cooper, Simran Narula, and Brad Muller for data management.
The Stanford research electronic data capture (REDCap) platform (http://redcap.stanford.edu) has been developed and is operated by Stanford Medicine Research Technology team. The REDCap platform services at Stanford are subsidized by the Stanford School of Medicine Research Office, and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1 TR003142. Study data were collected and managed using REDCap electronic data capture tools.54,55 The details are posted here: https://medwiki.stanford.edu/pages/viewpage.action?pageId=120882342.
This study was supported, in part, by a grant from the St. Baldrick’s Foundation to the Empowering Immunotherapies for Children’s Cancers Team (C.L.M.). C.L.M. is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research. K.O.M. was supported by Siragusa Transplant Center Grant and NUCATS K12 award 1K12TR005104-01. Biostatistics support has been provided by the Biostatistics Shared Resource of the St. Jude Children’s Research Hospital and St. Jude Comprehensive Cancer Center (NIH P30CA021765).
Authorship
Contribution: S.N., S.S., A.C.T., S.D., L.M.S., and K.O.M. designed the research, performed the analysis, and wrote the paper; G.L.C., V.A.F., R.H.R., X.L.Z., A.V., J.R., H.L.P., S.J., C.L.P., J.-A.T., A.M., M.R.V., D.M., E.H., N.K., C.L.B., M.Q., E.B., A.K.K., S.H.C.B., E.T., M.L.H., P.S., C.K., V.C., H.E.S., E.E., K.J.C., T.W.L., and C.L.M. contributed patients to the study; S.P., K.P.N., and C.B. contributed to database design, data collection, and quality control; and all authors were involved in final manuscript approval and are accountable for all aspects of this work.
Conflict-of-interest disclosure: C.L.M. holds multiple patents related to chimeric antigen receptor (CAR) T-cell therapies; receives royalties for CD22-CAR from CARGO Therapeutics; holds equity in CARGO Therapeutics, Link Cell Therapies, GBM NewCo, and Ensoma; consults for CARGO Therapeutics, Link Cell Therapies, GBM NewCo, Ensoma, Immatics, and AstraZeneca; and receives research funding from Tune Therapeutics. R.H.R. has received honoraria from Novartis; and consulting fees from Pfizer. V.A.F. consults for Adaptimmune. T.W.L. holds equity in Advanced Microbubbles; consults for Bayer, Massive Bio, AI Therapeutics, Jazz Pharmaceuticals, GentiBio, ITM Oncologics, and GlaxoSmithKline; and receives research funding from Pfizer, Bayer, Turning Point Therapeutics, Lilly, Roche/Genentech, Taiho, Advanced Accelerator Applications/Novartis, BioAtla, Hoffman-La Roche, Exelixis, and Adaptimmune. The remaining authors declare no competing financial interests.
Correspondence: Swati Naik, Department of Bone Marrow Transplantation and Cellular Therapy, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; email: swati.naik@stjude.org; and Kevin O. McNerney, Department of Hematology, Oncology, and Stem Cell Transplant, Lurie Children’s Hospital, 875 N Michigan Ave, Room 14-113, Chicago, IL; email: kmcnerney@luriechildrens.org.
References
Author notes
L.M.S. and K.O.M. contributed equally to this study.
Publication-related data are available on request from the corresponding authors, Swati Naik (swati.naik@stjude.org) and Kevin O. McNerney (kmcnerney@luriechildrens.org).
The full-text version of this article contains a data supplement.