Key Points
Persistence of IDH1 mutations in complete remission is not associated with increased risk of relapse in AML.
Mutant IDH2 MRD in complete remission is associated with higher relapse risk in mutant IDH2 AML without concomitant mutant NPM1 or FLT3-ITD.
Visual Abstract
Molecular measurable residual disease (MRD) assessment in patients with acute myeloid leukemia (AML) has been established for only a few specific markers: mutant NPM1 and FLT3 internal tandem duplication (ITD). Mutations in isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) are present in ∼20% of patients with AML. However, validation of mutant IDH1/2 MRD has been hampered by cohort size as well as the availability of highly sensitive and specific MRD detection assays. Here, we comprehensively investigate the impact of persistent IDH1/2 mutations in complete remission after intensive chemotherapy in a cohort of 163 newly diagnosed patients with IDH-mutant AML, enrolled in Dutch-Belgian Cooperative Trial Group for Hematology-Oncology and Swiss Group for Clinical Cancer Research clinical trials, using a next-generation sequencing (NGS)–based approach, targeting all hot spot mutations in IDH1 (R132) and IDH2 (R140 and R172). The high sensitivity (10–4) as well as the levels of persistent IDH1/2 mutations detected by the NGS-based approach were confirmed by an independent super rolling circle amplification assay. We demonstrate that the risk of relapse was significantly increased in patients with AML with measurable persistent IDH2 mutations (P = .027; subdistribution hazard ratio (SHR), 2.34) but not in patients with persistent mutant IDH1 (P = .591; SHR, 0.80). Moreover, the association of persistence of mutant IDH2 and increased risk of relapse was most pronounced in patients with mutant IDH2 AML without concomitant NPM1 mutations or FLT3-ITD (P = .011; SHR, 5.29). Thus, mutant IDH2 appears as a potentially useful novel molecular MRD marker with prognostic significance in AML.
Introduction
Mutations in the isocitrate dehydrogenase 1 (IDH1) or the isocitrate dehydrogenase 2 (IDH2) genes are among the most common in acute myeloid leukemia (AML), occurring in ∼20% of all cases (IDH1 in 8%-19% and IDH2 in 7%-14%).1-7 Both IDH genes encode an enzyme that converts isocitrate to α-ketoglutarate to produce NADPH from NADP+.8 Mutations nearly always occur in hot spot regions in the enzymatic active site of IDH1 (R132) and IDH2 (R140 and R172) and result in a neomorphic enzyme that produces the oncometabolite 2-hydroxyglutarate (2-HG). Increased levels of 2-HG alter DNA methylation and histone modification, thereby impairing myeloid differentiation and promoting leukemogenesis.9-11IDH mutations are associated with cytogenetically normal AML and often co-occur with mutations in DNMT3A, NPM1, and FLT3.12-15
The prognostic impact of IDH1/2 mutations at diagnosis either alone or in combination with frequently co-occurring mutations is still unclear. Although it was demonstrated that variations in treatment outcome might correlate with specific types of IDH mutation and concurrent mutations, the overall associations with treatment response in mutant IDH AML have been controversial.7,13,16-20 The emergence of small-molecule inhibitors targeting mutant IDH in recent years (ie, ivosidenib for IDH1 and enasidenib for IDH2) has expanded therapeutic options for patients with IDH-mutated AML, and their effectiveness either as monotherapy or combination therapy has been recently confirmed. In particular, for patients with AML ineligible for intensive chemotherapy, addition of either of the IDH inhibitors to azacitidine treatment has shown significant clinical benefits compared to azacitidine as a single agent.21,22
Molecular measurable residual disease (MRD) detection in guiding treatment decisions in AML has been established in various settings as an informative approach, because it has demonstrated independent prognostic value for patient outcomes.23-25 However, routine molecular MRD assessment in AML is still limited to a few specific markers: RUNX1::RUNX1T1 and CBFB::MYH11 in core-binding factor leukemia and mutated NPM1 by reverse transcriptase quantitative polymerase chain reaction, whereas next-generation sequencing (NGS)–based assays for mutant NPM1 and FLT3 internal tandem duplication (ITD) MRD have recently shown promise as well.24,26-31IDH mutations are attractive targets for MRD detection in AML, given that these mutations occur relatively frequently, involve mutational hotspots, and are considered to be stable during the course of disease. However, the prognostic value of persistent IDH1/2 mutations has yet to be firmly established, as previous results are conflicting.15,32-36 In addition, in individuals with clonal hematopoiesis (CH), a condition characterized by clonal expansion of mutant hematopoietic stem cells without evidence of hematologic disease, the presence of IDH mutations might confer an increased risk of progression to AML. Similarly, the persistence of certain IDH mutations after treatment might indeed be indicative of inferior outcomes.37-39
We explored the prognostic impact of persistent IDH1 and IDH2 mutations in a cohort of 163 newly diagnosed patients with IDH-mutant AML who achieved complete remission (CR) after intensive chemotherapy. We performed NGS to detect persistent IDH1 and IDH2 mutations with high sensitivity and compared this approach to a super rolling circle amplification (superRCA)–based technology. Associations between persistence of IDH mutations in CR and treatment outcome were investigated.
Methods
Patients and samples
A total of 163 patients newly diagnosed with AML carrying IDH1 or IDH2 mutations at diagnosis, enrolled in the Dutch-Belgian Cooperative Trial Group for Hematology-Oncology (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) clinical trials HO42A (NL193/NTR230; n = 25), HO92 (n = 10), HO102 (NL2070/NTR2187; n = 37), and HO132 (NL2070/NTR2187; n = 91) were included for MRD assessment. All patients received 2 cycles of “7+3”-based intensive chemotherapy according to their respective treatment protocol.40-44 Treatment protocols and patient eligibility criteria are available on the HOVON website (http://www.hovon.nl). All participants provided written informed consent in accordance with the Declaration of Helsinki.
Patients attaining CR or CR with incomplete hematologic recovery (<5% blast cells; hereafter collectively referred to as CR) after 2 cycles of induction chemotherapy were included in the study. Samples were taken within a defined interval in remission between 21 days and 4 months after the start of cycle 2 and before consolidation therapy.
Mutation detection with targeted NGS
At the time of diagnosis, NGS for gene mutations was performed on diagnostic bone marrow or peripheral blood samples using the TruSight Myeloid Sequencing Panel (Illumina, San Diego, CA) targeting 54 frequently mutated genes in hematologic malignancies.45 For MRD assessment, deep sequencing targeting exon 4 of IDH1 and IDH2 was performed on 250 ng of DNA at time of CR. A detailed library preparation protocol is described in the supplemental Methods. Final NGS libraries were paired-end sequenced (2 × 151 bp) using a NovaSeq 6000 SP Reagent Kit v1.5 (Illumina, San Diego, CA). The average read coverage was 69 600. The limit of detection (LoD) for each mutation was determined using 32 buffy coat DNA samples of healthy individuals. The resulting variant allele frequencies (VAFs) were used to calculate an MRD threshold, defined as the mean VAF plus 3 standard deviations. MRD was defined as VAF >0.0166% for IDH1(p.R132C), VAF >0.0222% for IDH2(p.R140Q), and VAF >0.01% for IDH2(p.R172K). MRD assessment for mutant NPM1 and FLT3-ITD in CR was performed as described before.27
Mutation detection with superRCA
SuperRCA is a highly sensitive and selective molecule amplification technology to detect mutations that uses RCA and PadLock probes to convert nucleic acid into large fluorescent particles that are enumerated using flow cytometry.46 Genotyping PadLock probes are used to distinguish between wild-type and mutant sequences, generating the VAF.47
A total of 128 out of 163 patients with AML carrying an IDH1 or IDH2 mutation at diagnosis were included for validation of NGS deep sequencing. SuperRCA was performed according to the manufacturer’s protocol with 250 ng of DNA per reaction using 12 cycles of polymerase chain reaction amplification.46 Samples were analyzed using a multiplex superRCA Mutation Kit covering IDH1 and IDH2 (Rarity Bioscience, Uppsala, Sweden). Mutant IDH1/2 and wild-type IDH1/2 controls were consistently incorporated during all assay procedures. The LoD was set to 0.01% VAF for mutant IDH1/2. The final readout of the superRCA products was performed using a FACSLyric flow cytometer with a 96-well plate loader adapter (Becton Dickinson, Franklin Lakes, NJ). VAFs were determined for each sample by calculating the ratio of IDH1/2 mutant events to the total of IDH1/2 wild-type and mutant events. MRD was defined as a VAF above the LoD. MRD negativity was defined as a VAF below the LoD or below the limit of blank (LoB). If the VAF was below the LoD, but above the LoB, samples were defined as MRD unclassified. A detailed protocol can be found in the supplemental Methods.
Statistical analyses
Associations between variables were tested by the Fisher exact test for categorical variables and by the Mann-Whitney U test for continuous variables. The primary end point of interest was the cumulative incidence of relapse (CIR). Secondary end point was overall survival (OS). Kaplan-Meier estimates were used to compare OS between subgroups. The reverse Kaplan-Meier estimate was used to determine the median follow-up. Differences in relapse incidence were evaluated using the Gray method and the Fine and Gray model for competing risks, whereas the log-rank test and the Cox proportional hazards model were used for survival analyses. Death was considered a competing event for relapse. OS and CIR were calculated from the date of sampling in CR to the date of an event. All P values were 2-sided, and a P value < .05 was considered statistically significant. Statistical analyses were performed with Stata Statistical Software, Release 18.0 (Stata, College Station, TX).
Results
Characteristics of the cohort of patients with mutant IDH1/IDH2 AML
Among the 163 patients with AML, 73 harbored mutations in IDH1 and 92 patients in IDH2. Two patients carried mutations in both IDH genes, with mutant IDH1 being the major clone in both patients. The median age of the total cohort was 53 years (range, 19-65). Most patients had achieved CR after the first cycle of induction chemotherapy (82.2%; supplemental Table 1). No significant differences were observed in the clinical characteristics between patients with IDH1- and IDH2-mutated AML (supplemental Table 2). Event-free survival (EFS) and OS were comparable between patients with IDH1- and IDH2-mutated AML (median follow-up, 78.2 months [interquartile range, 63.1-118.3]; supplemental Figure 1). According to the European LeukemiaNet 2022 risk classification (ELN2022), 62 patients with AML (38.0%) were classified as favorable risk, 55 (33.7%) as intermediate risk, and 46 (28.2%) as adverse risk. Chromosomal abnormalities were found in 35 patients with IDH-mutated AML (20.9%), of whom 5 had complex karyotypes according to ELN2022. NPM1 was the most frequently comutated gene (55.2%), followed by DNMT3A (42.3%) and FLT3 (36.9%; including ITD in 25.2% and tyrosine kinase domain mutations in 11.7%; Figure 1). In addition, 54 patients (33.1%) carried ≥1 myelodysplasia-related gene mutation. Concurrent mutations in PTPN11 (19.7% vs 7.8%; P = .034) and NRAS (25.4% vs 11.1%; P = .022) were significantly more often observed in IDH1-mutated patients than IDH2-mutated patients (Figure 1).
Co-occurring mutations at diagnosis in patients with AML carrying mutant IDH1 and IDH2. A total of 163 patients were included. Blue bars indicate whether a mutation is present at diagnosis. Red indicates (top rows) whether a mutation is present in CR.
Co-occurring mutations at diagnosis in patients with AML carrying mutant IDH1 and IDH2. A total of 163 patients were included. Blue bars indicate whether a mutation is present at diagnosis. Red indicates (top rows) whether a mutation is present in CR.
Mutant IDH MRD detection by NGS deep sequencing and superRCA
Among the 163 patients with IDH-mutated AML, mutant IDH1 or IDH2 was detectable after the second cycle of intensive chemotherapy in 72 patients (44%). The median VAF of persistent IDH mutations was 0.43% (range, 0.17%-41.2%), with 43 patients (60%) having a mutant IDH VAF <1.0% (supplemental Figure 2). A superRCA approach was used as validation in 128 of 163 available patients with AML enrolled in the mutant IDH AML cohort. Of these patients, 114 (89%) had concordant MRD results between superRCA and NGS (supplemental Table 3). Discordant cases included 1 patient without detectable MRD using superRCA but detectable by NGS, whereas 5 patients had detectable MRD by superRCA but not by NGS. Eight patients could not be classified by superRCA because the results were above the LoB but below the LoD. VAFs by NGS and superRCA were highly similar (r = 0.9952; supplemental Figure 3A). Moreover, mutations with low VAFs of <1.0% were also highly similar (r = 0.9893; supplemental Figure 3B), indicating that deep sequencing approach can accurately detect persistent mutant IDH with high sensitivity. Further analyses were performed using the MRD data following the NGS approach.
Prognostic relevance of molecular MRD in mutant IDH1 and IDH2 AML
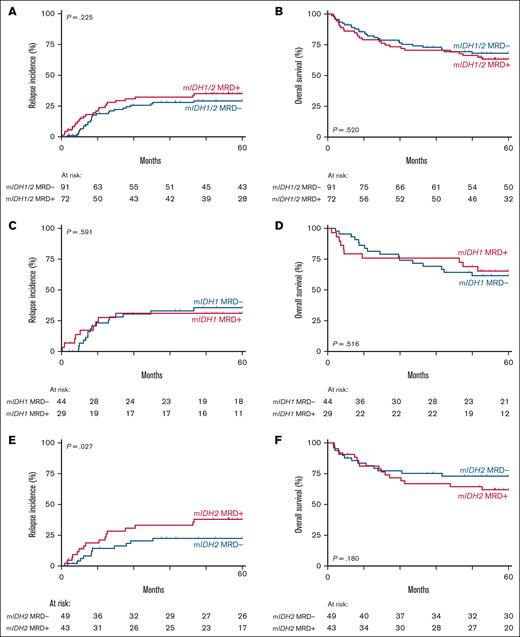
Next, we examined the differences in clinical characteristics and outcome between patients with and without persistent IDH mutations in CR by NGS. Patients with detectable mutant IDH1/2 MRD were significantly older (median age, 57 years [MRD] vs 50 years [no MRD]; P = .002), more often classified as ELN2022 adverse risk (37.5% vs 20.9%; P = .023), and more frequently did not receive consolidation therapy (18.1% vs 2.2%; P = .001; supplemental Table 4). Myelodysplasia-related (MR) gene mutations were more often present in patients with detectable mutant IDH1/2 MRD (MRD, 41.7%; no MRD, 26.4%; P = .045), whereas mutations in PTPN11 were less frequent (MRD, 5.6%; no MRD, 18.7%; P = .017; supplemental Table 5). CIR and OS were similar in patients with and without detectable mutant IDH MRD (Figure 2A-B), and no significant difference was observed in CIR and OS between those with a VAF <1% and those with a VAF >1% (supplemental Figure 4).
Survival analyses of patients with AML by mutant IDH MRD status. (A-B) CIR (A) and OS (B) of patients with IDH-mutated AML with detectable (red) and undetectable mutant IDH in CR (blue). (C-D) CIR (C) and OS (D) of patients with IDH1-mutated AML with detectable (red) and undetectable mutant IDH1 in CR (blue). (E-F) CIR (E) and OS (F) of IDH2-mutated patients with IDH2-mutated AML with detectable (red) and undetectable mutant IDH2 in CR (blue).
Survival analyses of patients with AML by mutant IDH MRD status. (A-B) CIR (A) and OS (B) of patients with IDH-mutated AML with detectable (red) and undetectable mutant IDH in CR (blue). (C-D) CIR (C) and OS (D) of patients with IDH1-mutated AML with detectable (red) and undetectable mutant IDH1 in CR (blue). (E-F) CIR (E) and OS (F) of IDH2-mutated patients with IDH2-mutated AML with detectable (red) and undetectable mutant IDH2 in CR (blue).
Subsequently, we examined the association of clinical outcomes with detectable mutant IDH1 and IDH2 MRD separately. Persistence of IDH1 mutations in CR was found in 29 of 73 patients (40%). Patients with AML with detectable mutant IDH1 MRD received consolidation therapy less frequently (17.2% vs 2.3%). No other significant clinical and molecular differences were apparent between patients with and without mutant IDH1 MRD (Table 1; supplemental Table 6). Importantly, no association between persistence of IDH1 mutations in CR, regardless of VAF, was apparent in terms of CIR and OS (Figure 2C-D; supplemental Figure 5A-B). In patients with IDH2-mutated AML, 43 of 92 had persistent IDH2 mutations in CR (47%). Patients with detectable mutant IDH2 MRD presented significantly more often with MR gene mutations (46.5% vs 22.4%; P = .017) and received consolidation therapy less often (18.6% vs 2.0%; P = .041; Table 1; supplemental Table 6). Although the presence of mutant IDH2 in CR was not significantly associated with inferior OS (P = .180), a significantly increased risk of relapse was observed compared to those without detectable mutant IDH2 MRD (P = .027; SHR, 2.34; Figure 2E-F). Moreover, this was most pronounced in patients with a persistent IDH2 mutation with a VAF >1% (supplemental Figure 5C-D). The increased risk of relapse was not specifically associated with the R140 or R172 IDH2 hotspot mutation (supplemental Figure 6).
Patient characteristics by type of IDH mutation and MRD status
| Patient characteristic . | Mutant IDH1 MRD absent (N = 44) . | Mutant IDH1 MRD present (N = 29) . | P value IDH1 . | Mutant IDH2 MRD absent (N = 49) . | Mutant IDH2 MRD present (N = 43) . | P value IDH2 . |
|---|---|---|---|---|---|---|
| HOVON trial, n (%) | .548 | .231 | ||||
| HO42A | 4 (9.1) | 5 (17.2) | 12 (24.5) | 4 (9.3) | ||
| HO92 | 4 (9.1) | 1 (3.4) | 3 (6.1) | 2 (4.7) | ||
| HO102 | 11 (25.0) | 5 (17.2) | 11 (22.4) | 10 (23.3) | ||
| HO132 | 25 (56.8) | 18 (62.1) | 23 (46.9) | 27 (62.8) | ||
| Age, median (range), y | 50 (27-65) | 57 (20-65) | .122 | 51 (19-64) | 58 (26-65) | .003 |
| Sex, n (%) | .340 | .528 | ||||
| Male | 28 (63.6) | 15 (51.7) | 30 (61.2) | 23 (53.5) | ||
| Female | 16 (36.4) | 14 (48.3) | 19 (38.8) | 20 (46.5) | ||
| White blood cell count at entry, n (%) | .392 | .336 | ||||
| ≤100 × 109/L | 39 (88.6) | 28 (96.6) | 48 (98.0) | 40 (93.0) | ||
| >100 × 109/L | 5 (11.4) | 1 (3.4) | 1 (2.0) | 3 (7.0) | ||
| Last treatment before CR, n (%) | .181 | .311 | ||||
| Cycle 1 | 40 (90.9) | 23 (79.3) | 41 (83.7) | 32 (74.4) | ||
| Cycle 2 | 4 (9.1) | 6 (20.7) | 8 (16.3) | 11 (25.6) | ||
| Consolidation therapy, n (%) | .014 | .041 | ||||
| No consolidation | 1 (2.3) | 5 (17.2) | 1 (2.0) | 8 (18.6) | ||
| Third cycle chemotherapy | 6 (13.6) | 4 (13.8) | 12 (24.5) | 6 (14.0) | ||
| Autologous HSCT | 23 (52.3) | 6 (20.7) | 15 (30.6) | 14 (32.6) | ||
| Allogeneic HSCT | 14 (31.8) | 14 (48.3) | 21 (42.9) | 15 (34.9) | ||
| ELN risk classification 2022, n (%) | .274 | .043 | ||||
| Favorable | 22 (50.0) | 9 (31.0) | 21 (42.9) | 10 (23.3) | ||
| Intermediate | 11 (25.0) | 10 (34.5) | 19 (38.8) | 16 (37.2) | ||
| Adverse | 11 (25.0) | 10 (34.5) | 9 (18.4) | 17 (39.5) | ||
| Mutations, n (%) | ||||||
| Mutant NPM1 | 28 (63.6) | 13 (44.8) | .150 | 27 (55.1) | 22 (51.2) | .834 |
| Mutant FLT3-ITD | 9 (20.5) | 5 (17.2) | 1.000 | 10 (20.4) | 17 (39.5) | .066 |
| Mutant TP53 | 1 (2.3) | 2 (6.9) | .559 | 1 (2.0) | 0 (0) | 1.000 |
| Myelodysplasia-related gene mutations | 14 (31.8) | 10 (34.5) | 1.000 | 11 (22.4) | 20 (46.5) | .017 |
| Cytogenetics, n (%)∗ | ||||||
| Normal karyotype | 33 (75.0) | 22 (75.9) | .782 | 37 (75.5) | 34 (79.1) | 1.000 |
| Complex karyotype ELN2022 | 1 (2.3) | 2 (6.9) | .563 | 1 (2.0) | 1 (2.3) | 1.000 |
| Patient characteristic . | Mutant IDH1 MRD absent (N = 44) . | Mutant IDH1 MRD present (N = 29) . | P value IDH1 . | Mutant IDH2 MRD absent (N = 49) . | Mutant IDH2 MRD present (N = 43) . | P value IDH2 . |
|---|---|---|---|---|---|---|
| HOVON trial, n (%) | .548 | .231 | ||||
| HO42A | 4 (9.1) | 5 (17.2) | 12 (24.5) | 4 (9.3) | ||
| HO92 | 4 (9.1) | 1 (3.4) | 3 (6.1) | 2 (4.7) | ||
| HO102 | 11 (25.0) | 5 (17.2) | 11 (22.4) | 10 (23.3) | ||
| HO132 | 25 (56.8) | 18 (62.1) | 23 (46.9) | 27 (62.8) | ||
| Age, median (range), y | 50 (27-65) | 57 (20-65) | .122 | 51 (19-64) | 58 (26-65) | .003 |
| Sex, n (%) | .340 | .528 | ||||
| Male | 28 (63.6) | 15 (51.7) | 30 (61.2) | 23 (53.5) | ||
| Female | 16 (36.4) | 14 (48.3) | 19 (38.8) | 20 (46.5) | ||
| White blood cell count at entry, n (%) | .392 | .336 | ||||
| ≤100 × 109/L | 39 (88.6) | 28 (96.6) | 48 (98.0) | 40 (93.0) | ||
| >100 × 109/L | 5 (11.4) | 1 (3.4) | 1 (2.0) | 3 (7.0) | ||
| Last treatment before CR, n (%) | .181 | .311 | ||||
| Cycle 1 | 40 (90.9) | 23 (79.3) | 41 (83.7) | 32 (74.4) | ||
| Cycle 2 | 4 (9.1) | 6 (20.7) | 8 (16.3) | 11 (25.6) | ||
| Consolidation therapy, n (%) | .014 | .041 | ||||
| No consolidation | 1 (2.3) | 5 (17.2) | 1 (2.0) | 8 (18.6) | ||
| Third cycle chemotherapy | 6 (13.6) | 4 (13.8) | 12 (24.5) | 6 (14.0) | ||
| Autologous HSCT | 23 (52.3) | 6 (20.7) | 15 (30.6) | 14 (32.6) | ||
| Allogeneic HSCT | 14 (31.8) | 14 (48.3) | 21 (42.9) | 15 (34.9) | ||
| ELN risk classification 2022, n (%) | .274 | .043 | ||||
| Favorable | 22 (50.0) | 9 (31.0) | 21 (42.9) | 10 (23.3) | ||
| Intermediate | 11 (25.0) | 10 (34.5) | 19 (38.8) | 16 (37.2) | ||
| Adverse | 11 (25.0) | 10 (34.5) | 9 (18.4) | 17 (39.5) | ||
| Mutations, n (%) | ||||||
| Mutant NPM1 | 28 (63.6) | 13 (44.8) | .150 | 27 (55.1) | 22 (51.2) | .834 |
| Mutant FLT3-ITD | 9 (20.5) | 5 (17.2) | 1.000 | 10 (20.4) | 17 (39.5) | .066 |
| Mutant TP53 | 1 (2.3) | 2 (6.9) | .559 | 1 (2.0) | 0 (0) | 1.000 |
| Myelodysplasia-related gene mutations | 14 (31.8) | 10 (34.5) | 1.000 | 11 (22.4) | 20 (46.5) | .017 |
| Cytogenetics, n (%)∗ | ||||||
| Normal karyotype | 33 (75.0) | 22 (75.9) | .782 | 37 (75.5) | 34 (79.1) | 1.000 |
| Complex karyotype ELN2022 | 1 (2.3) | 2 (6.9) | .563 | 1 (2.0) | 1 (2.3) | 1.000 |
HSCT, hematopoietic stem cell transplantation.
Missing data for 3 patients.
Prognostic relevance of MRD in mutant IDH1 or IDH2 AML stratified by NPM1 mutational and FLT3-ITD status
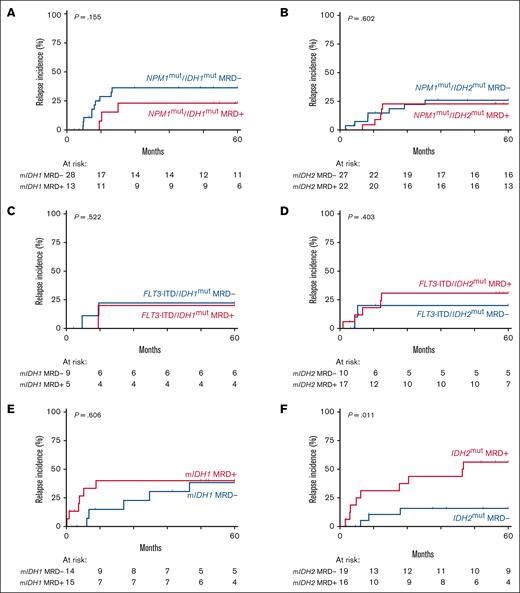
Within our mutant IDH AML cohort, 55% of patients had a concomitant NPM1 mutation at diagnosis (Figure 1). To determine whether mutant IDH1/2 MRD carries prognostic value in patients with AML with (n = 90) or without mutant NPM1 (n = 73), we divided our cohort based on mutant NPM1 status. No prognostic value for CIR and OS was observed for IDH1 or IDH2 mutations as MRD marker in patients with NPM1-mutated AML (Figure 3A-B; supplemental Figure 7A-B). At diagnosis, 41 patients (25%) of all IDH-mutant cases had a co-occurring FLT3-ITD (Figure 1). No added prognostic value for relapse was found for mutant IDH1 or IDH2 MRD with concurrent FLT3-ITD at diagnosis (Figure 3C-D; supplemental Figure 7C-D).
Relapse incidence of patients with AML with mutant IDH MRD stratified by NPM1 mutational and FLT3-ITD status at diagnosis. (A) CIR of patients with IDH1-mutated AML with detectable (red) and undetectable (blue) mutant IDH1 MRD among those with a concomitant NPM1 mutation at diagnosis. (B) CIR of patients with IDH2-mutated AML with detectable (red) and undetectable (blue) mutant IDH2 MRD among those with a concomitant NPM1 mutation at diagnosis. (C) CIR of patients with IDH1-mutated AML with detectable (red) and undetectable (blue) mutant IDH1 MRD in those with a concomitant FLT3-ITD at diagnosis. (D) CIR of patients with IDH2-mutated AML with detectable (red) and undetectable (blue) mutant IDH2 MRD among those with a concomitant FLT3-ITD at diagnosis. (E) CIR of patients with IDH1-mutated AML with detectable (red) and undetectable (blue) mutant IDH1 MRD in those without concomitant mutant NPM1 or FLT3-ITD at diagnosis. (F) CIR of patients with IDH2-mutated AML with detectable (red) and undetectable (blue) mutant IDH2 MRD in those without concomitant mutant NPM1 or FLT3-ITD at diagnosis.
Relapse incidence of patients with AML with mutant IDH MRD stratified by NPM1 mutational and FLT3-ITD status at diagnosis. (A) CIR of patients with IDH1-mutated AML with detectable (red) and undetectable (blue) mutant IDH1 MRD among those with a concomitant NPM1 mutation at diagnosis. (B) CIR of patients with IDH2-mutated AML with detectable (red) and undetectable (blue) mutant IDH2 MRD among those with a concomitant NPM1 mutation at diagnosis. (C) CIR of patients with IDH1-mutated AML with detectable (red) and undetectable (blue) mutant IDH1 MRD in those with a concomitant FLT3-ITD at diagnosis. (D) CIR of patients with IDH2-mutated AML with detectable (red) and undetectable (blue) mutant IDH2 MRD among those with a concomitant FLT3-ITD at diagnosis. (E) CIR of patients with IDH1-mutated AML with detectable (red) and undetectable (blue) mutant IDH1 MRD in those without concomitant mutant NPM1 or FLT3-ITD at diagnosis. (F) CIR of patients with IDH2-mutated AML with detectable (red) and undetectable (blue) mutant IDH2 MRD in those without concomitant mutant NPM1 or FLT3-ITD at diagnosis.
In patients with IDH1-mutated AML without mutant NPM1/FLT3-ITD, the CIR was not significantly increased in patients with persistent mutant IDH1 (P = .606; Figure 3E). In contrast, detectable mutant IDH2 MRD was associated with a significantly increased risk of relapse in mutant IDH2 patients without mutant NPM1/FLT3-ITD (P = .011; SHR, 5.29; Figure 3F). The OS of mutant IDH2 patients without mutant NPM1/FLT3-ITD with mutant IDH2 MRD did not significantly differ from those without mutant IDH2 MRD (supplemental Figure 8B). The increased risk of relapse was most apparent in mutant IDH2 patients without mutant NPM1/FLT3-ITD with detectable mutant IDH2 with a VAF >1.0% (supplemental Figure 9C). In mutant IDH1 patients without mutant NPM1/FLT3-ITD, no significant difference in relapse risk was observed at the VAF cutoff.
In multivariable analysis, detectable mutant IDH2 MRD remained an independent risk factor for relapse in patients with IDH2-mutated AML without mutant NPM1/FLT3-ITD (supplemental Table 7).
Discussion
Molecular MRD detection in AML can be used to identify patients at high risk of relapse and inferior survival, yet molecular MRD in clinical care is currently restricted to the availability of a few validated molecular biomarkers in AML.24 In this study, we demonstrate that mutant IDH2 NGS-MRD status in patients with IDH2mut/NPM1wt AML holds significant prognostic value and therefore can be considered for use as a MRD molecular biomarker for prospective assessment of depth of remission and for monitoring the emergence of leukemic clones.
Mutations in IDH1 and IDH2 are among the most common abnormalities, occurring in up to 20% of all patients, and generally represent early events in AML evolution.5-7 They are also commonly described as mutations occurring in CH, in which hematopoietic stem cells acquire somatic mutations without any underlying malignancy. Although the presence of IDH mutations has been described as a high-risk factor for progression toward AML,39,48-50 it remains unsettled whether IDH mutations can be predictive of impending relapse. We have previously reported that frequently mutated genes associated with CH, including DNMT3A, TET2, and ASXL1, are unsuitable MRD markers.25,45 At that time, the small numbers of mutant IDH cases did not enable a critical analysis of whether the persistence of measurable IDH-mutated cells in remission indicated a CH state rather than evidence of emerging recurrent disease.
In this study, in which we evaluated the impact of residual IDH mutations in a large cohort of patients with AML, we identified residual IDH2-mutant cells but not persistent IDH1 mutations as significantly prognostic for relapse, in line with recently published studies addressing the utility of mutant IDH1 and IDH2 MRD in AML.51,52 This increased risk of relapse was found only in patients with AML with persistent IDH2 mutations and NPM1 wild type without FLT3-ITD at diagnosis. Of note, stratifying the mutant IDH cohort based on FLT3-ITD and NPM1 mutation status leads to a smaller subset of patients, and validation in external or larger cohorts is therefore warranted. Because mutant IDH1 and IDH2 exert the same biological effect, that is the production of the oncometabolite 2-HG, and only differ in their localization, it is difficult to explain the prognostic difference based on these data. Nonetheless, the lack of prognostic value of persistent IDH1, irrespective of clone size and NPM1 mutant status, suggests that persistence of only IDH1 mutations indicate a state of CH rather than residual disease. This is consistent with a previous study that demonstrated that mutant IDH1/2 is more representative of CH rather than leukemia in patients with NPM1mut AML who achieved CR, defined as the absence of mutant NPM1 transcripts.53
The observed increased risk of relapse in patients without FLT3-ITD/NPM1wt with persistent mutant IDH2 was highest among those with a VAF >1%, which might imply that the clone size of IDH2 mutations holds prognostic value. Because IDH mutations are considered to be acquired early in AML evolution,54 the relatively high level of VAF might suggest a clone that is already expanding, thereby possibly explaining the increased relapse rate in these patients. Interestingly, patients with detectable IDH MRD were less likely to receive consolidation therapy regardless of the type of IDH mutation, which could potentially bias the prognostic value of persistent IDH mutations. However, in a multivariable analysis in which allogeneic stem cell transplantation is considered as a time-dependent covariate, persistence of mutant IDH2 in patients without baseline FLT3-ITD and wild-type NPM1 leads to an almost fivefold increased risk of relapse, in line with recent data from the Pre-MEASURE project evaluating mutant IDH2 MRD in patients before allogeneic transplant.52 This indicates that mutant IDH2 MRD testing in this patient subgroup is beneficial to predict which patients have a high risk of relapse. In addition, due to the small number of coexisting mutations, the influence of persistence of other mutations could not be addressed. In particular, mutations found in MR genes have a high frequency in patients with IDH2mut/NPM1wt AML without FLT3-ITD. Unfortunately, the low number of patients with IDH2-mutated AML with concurrent MR mutations did not allow further analyses in this current study. Whether IDH2 MRD provides any additional predictive value for relapse risk in patients with MR-AML merits further investigation in larger cohorts.
In recent years, the IDH inhibitors ivosidenib (IDH1) and enasidenib (IDH2) have been approved by the US Food and Drug Administration and the European Medicine Agency (ivosidenib only) for the treatment of mutant IDH AML.55,56 Treatment with ivosidenib led to more favorable clinical outcomes when given as monotherapy in patients with relapsed or refractory IDH-mutated AML57,58 or in combination with azacitidine in newly diagnosed patients ineligible for intensive chemotherapy, in which clearance of IDH1 mutations was more frequently observed.21 Similarly, patients with IDH2-mutated AML treated with enasidenib had greater response rates than patients treated with other treatment regimes,59,60 and combinational therapy with azacitidine showed promising results especially for patients who previously received enasidenib alone.22,61 However, none of these studies report on the effect of mutant IDH2 MRD. Although MRD assessment has the potential to improve outcomes for patients with AML, it is primarily performed in patients receiving intensive chemotherapy.30 Given the promising results of azacitidine in combination with ivosidenib or enasidenib, it certainly is of added value to investigate whether mutant IDH MRD holds prognostic value for patients with AML receiving IDH inhibitors as part of less-intensive treatment regimes. Particularly, for patients with IDH1-mutated AML, in whom ivosidenib treatment has shown deep and durable remissions in difficult-to-treat patient populations, the prognostic relevance of mutant IDH1 MRD should be reevaluated. Importantly, the added value of enasidenib and ivosidenib to intensive induction chemotherapy for patients with newly diagnosed IDH-mutated AML is currently investigated in a phase 3, multicenter, double-blind randomized clinical trial (ClinicalTrials.gov identifier: NCT03839771), in which the association of relapse risk with persistence of mutant IDH in CR in patients with AML treated with IDH inhibitors will be addressed.
In conclusion, MRD assessment for mutant IDH2 identifies patients with an increased risk of relapse and expands the limited number of molecular targets currently available for NGS-based MRD detection in AML.
Acknowledgments
The authors are grateful to all Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) centers that participated in the clinical trials that formed the basis for this study. The authors also thank Eric Bindels for performing next-generation sequencing.
This work was made possible through support received from the Dutch Cancer Society “Koningin Wilhemina Fonds” (Queen Wilhelmina Fund; EMCR 2019-12507 and infra 2021-13650).
Authorship
Contribution: C.M.V., E.L.B., and P.J.M.V. designed the study; C.M.V., L.B., F.G.K., M.R., M.E., and S.N. performed experiments; E.L.B., C.M.V., F.G.K., and J.M.L.K. analyzed data; E.L.B. and C.M.V. prepared the figures; E.L.B., C.M.V., B.L., and P.J.M.V. drafted the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: M.E. and S.N. are employed by Rarity Bioscience. The remaining authors declare no competing financial interests.
Correspondence: Peter J. M. Valk, Department of Hematology, Erasmus University Medical Center Rotterdam, Nc-806, Wytemaweg 80, 3015 CN Rotterdam, The Netherlands; email: p.valk@erasmusmc.nl.
References
Author notes
E.L.B. and C.M.V. contributed equally to this work.
The data sets generated during and/or analyzed during this study are available on reasonable request from the corresponding author, Peter J. M. Valk (p.valk@erasmusmc.nl).
The full-text version of this article contains a data supplement.