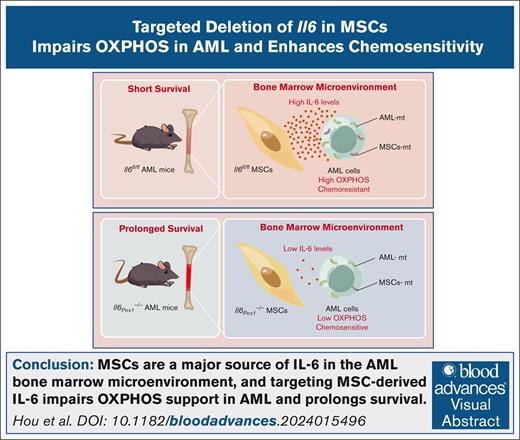
Key Points
IL-6 levels in the BM supernatant predict chemotherapy response in adult patients with AML.
Targeted deletion of Il6 in MSCs intensifies the cytotoxicity of Ara-C against AML and prolongs overall survival.
Visual Abstract
In acute myeloid leukemia (AML), elevated interleukin 6 (IL-6) levels in the bone marrow (BM) are linked to poor prognosis. However, the mechanisms driving this elevation and its role in chemoresistance remain unclear. Using the Prrx1-Cre system, we selectively deleted Il6 in BM mesenchymal stromal cells (MSCs) and established an AML mouse model. Our results show that MSCs are a major source of IL-6 in AML BM. Importantly, Il6 deletion in MSCs reduced oxidative phosphorylation (OXPHOS) activity in AML cells, slowed disease progression, and enhanced chemosensitivity to cytarabine (Ara-C). Similarly, the OXPHOS inhibitor IACS-010759 improved chemosensitivity in AML mice. Exogenous recombinant IL-6 reversed the chemosensitivity gains from Il6 deletion, confirming its role in chemoresistance. We further demonstrated that Il6 absence in MSCs inhibits mitochondria transfer to AML cells, dampening OXPHOS and enhancing Ara-C efficacy. In summary, our study underscores the critical role of Il6 from MSCs in AML progression and chemoresistance. Targeting IL-6 in MSCs may offer a promising therapeutic strategy for AML. This trial was registered at www.clinicaltrials.gov as #NCT06486350.
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous malignant clonal disease characterized by complex mutations and cytogenetic abnormalities. According to Surveillance, Epidemiology, and End Results cancer statistics, the 5-year survival rate for patients with AML remains disappointingly low at ∼30%.1 Despite substantial advancements in AML therapies, chemoresistance and relapse remain significant barriers to successful treatment.2 Mesenchymal stromal cells (MSCs) play a crucial role in the pathogenesis of AML. MSCs are “co-opted” by AML cells, which reprogram them to create a supportive microenvironment that promotes AML cell survival and proliferation. This interaction is not only pivotal in the initiation and progression of AML but is also a key factor in chemoresistance.3-6 Understanding the intricate interplay between AML cells and MSCs is essential for developing more effective therapies that target the microenvironment critical for AML cell survival and proliferation.
Interleukin 6 (IL-6), a pleiotropic cytokine, plays a significant role in the pathogenesis of various diseases.7 Elevated IL-6 levels in the plasma8 and BM aspirate9 of patients with AML are associated with poorer event-free survival and overall survival. However, the mechanisms driving the elevated levels of IL-6 in the BM microenvironment of patients with AML and its contribution to chemoresistance remain unclear. Although studies have confirmed the presence of IL-6 autocrine secretion in primary AML cells,10 there is no correlation between BM IL-6 levels and BM blast percentage at initial biopsy,9 suggesting that autocrine secretion by leukemia cells is not the primary determinant of IL-6 levels in the BM of patients with AML. Other research has shown that IL-6 secretion by AML-associated MSCs is significantly higher than that by MSCs from healthy individuals.11 This finding implicates AML-MSCs as a potential source of elevated IL-6 levels in the BM microenvironment, which may contribute to the chemoresistance observed in AML. However, the precise mechanisms underlying this relationship require further investigation to fully understand its implications for AML pathogenesis and treatment strategies.
Metabolic reprogramming in AML cells has emerged as a key factor in their response to chemotherapy stress.12,13 Unlike normal hematopoietic cells, AML cells exhibit a high oxidative phosphorylation (OXPHOS) status, characterized by increased oxygen consumption rate and mitochondrial adenosine triphosphate (ATP) production, elevated mitochondrial membrane potential and mass, and higher mitochondrial reactive oxygen species (mtROS) content.14,15 MSCs support these metabolic traits in AML cells via the CXCL12-CXCR4 signaling axis16 or by transferring protective mitochondria,17 thereby promoting chemoresistance. Additionally, recent studies have highlighted the crucial role of IL-6 in the metabolic reprogramming of tumor cells, including AML cells.18-20 Our previous research has shown that exogenous IL-6 can upregulate mitochondrial fusion and OXPHOS levels in AML cells, thereby inducing chemoresistance.21 However, the potential of targeting IL-6 in MSCs to mitigate chemoresistance in AML warrants further investigation.
In this study, we used a genetically engineered conditional knockout mouse model of AML, in which the Il6 gene was specifically deleted in MSCs, to demonstrate that IL-6 secreted by MSCs is a major contributor to the elevated IL-6 levels in the AML BM microenvironment. Our findings underscore the critical role of MSC-derived IL-6 in promoting AML progression and chemoresistance by facilitating the transfer of functional mitochondria to AML cells, thereby augmenting OXPHOS metabolic pathway. This discovery identifies the primary source of IL-6 within the AML BM microenvironment and elucidates the mechanism by which chemoresistance is induced in AML cells. Consequently, our study provides valuable insights that may inform the development of targeted therapeutic strategies aimed at modulating the AML BM microenvironment.
Methods
Patient samples
This single-arm study was conducted at Fujian Medical University Union Hospital from September 2023 to March 2025. A total of 66 BM samples were collected from newly diagnosed patients with AML (excluding acute promyelocytic leukemia [APL]), following the “Chinese guidelines for diagnosis and treatment of adult AML (not APL; 2023).”
Animal experiments
Mice (male and female; aged 8-10 weeks; weighing 20-28 g) were randomly assigned to groups. MLL::AF9 AML cells were generated as previously described.21MLL::AF9 retroviruses were produced by transfecting 293T cells with MSCV-MLL-AF9-IRES-YFP and the packaging plasmid pCL-ECO using Lipofectamine 3000 (Thermo Fisher Scientific). Lineage-negative fetal liver cells from C57BL/6J embryos were transduced with MLL::AF9 retroviruses and subsequently transplanted (3 × 105 cells per mouse) via tail vein injection into sublethally irradiated (6.5 Gy) C57BL/6J recipient mice. After leukemia development, MLL::AF9 yellow fluorescent protein positive (YFP+) AML cells were isolated by fluorescence-activated cell sorting, and 2 × 105 of these cells were IV injected into Il6Prrx1–/– mice and Il6fl/fl mice to obtain Il6Prrx1–/– AML mice and Il6fl/fl AML mice, respectively.
For the AML mouse chemosensitivity experiment, Il6fl/fl AML mice received injections of phosphate-buffered saline (PBS; 200 μL; intraperitoneal [i.p.]), cytarabine, (Ara-C; 100 mg/kg; i.p.; Cytosar), or Ara-C (100 mg/kg; i.p.) + IACS-010759 (OXPHOS inhibitor; 2.5 mg/kg; intragastric; MedChemExpress) for 5 consecutive days starting on day 18 after AML model induction. Il6Prrx1–/– AML mice received injections of PBS (200 μL; i.p.), Ara-C (100 mg/kg; i.p.), or Ara-C (100 mg/kg; i.p.) + IL-6 (3 ng/g; i.p.; MedChemExpress) for the same duration. AML burden was assessed at the disease end point, and overall survival was recorded.
The study was conducted in accordance with the Declaration of Helsinki guidelines and was approved by the Ethics Committee of Fujian Medical University Union Hospital (number 2019KJCX029), with informed consent obtained from all patients.
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Fujian Medical University, China (FJMUIACUC2021-0322).
Results
Elevated IL-6 levels in BM supernatant predict poor chemotherapy outcomes for patients with AML
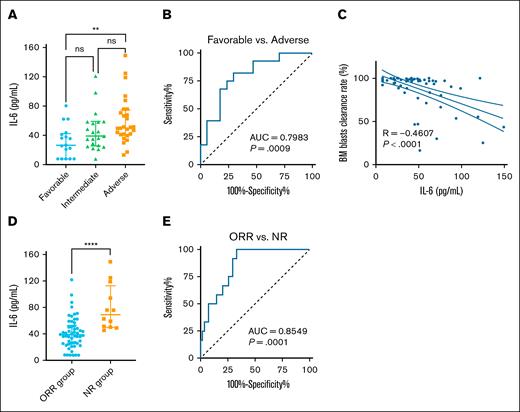
Current studies have shown that elevated IL-6 levels in the peripheral blood (PB)8 and BM9 supernatant of patients with AML are associated with inferior survival. To further investigate the relationship between BM IL-6 levels and chemotherapy outcomes in AML, we included 66 newly diagnosed adult patients with AML, of whom 59.09% received induction therapy with the “7+3” regimen (supplemental Table 1). Patients were stratified into high and low IL-6 groups based on the median BM IL-6 level. No significant differences were found between the 2 groups in terms of sex, age, white blood cell count, PB blast percentage, BM blast percentage, French-American-British classification, cytogenetics, or gene mutation status. However, according to European LeukemiaNet 2022 risk stratification, a significant difference in prognostic distribution was observed: the proportion of patients classified as adverse risk was notably higher in the high IL-6 group than the low IL-6 group (28.79% vs 13.64%), whereas the proportion of favorable-risk patients was markedly lower (4.55% vs 21.21%; Table 1). Furthermore, IL-6 levels showed a trend of increasing with worsening prognostic category, with a statistically significant difference observed between the adverse- and favorable-risk groups (Figure 1A). Receiver operating characteristic analysis further confirmed that IL-6 levels could distinguish between these 2 prognostic groups with reasonable accuracy (Figure 1B). These findings suggest that elevated BM IL-6 may be associated with poor-risk disease features and unfavorable clinical outcomes.
Clinical characteristics of patients with AML in the high and low IL-6 groups
| Patient parameter . | Low IL-6, <42.70 pg/mL (n = 34) . | High IL-6, ≥42.70 pg/mL (n = 32) . | P value . |
|---|---|---|---|
| Sex, male/female | 13/21 | 17/15 | .225 |
| Age, median ± SD, y | 47.26 ± 15.83 | 48.88 ± 17.63 | .697 |
| Median WBC (range), ×109/L | 17.59 (0.75-277.14) | 27.37 (1.03-291.44) | .202 |
| Median PB blasts (range), % | 41 (0-98) | 28 (0-98) | .690 |
| Median BM blasts (range), % | 35.5 (3.0-96.5) | 36.0 (5.0-95.5) | .705 |
| FAB | .643 | ||
| M0 | 1 | 0 | 1.000 |
| M1 | 2 | 4 | .420 |
| M2 | 12 | 9 | .532 |
| M4 | 0 | 1 | .485 |
| M5 | 19 | 18 | .976 |
| Karyotypes | .307 | ||
| Normal | 24 | 20 | .486 |
| t(8;21) | 4 | 1 | .357 |
| +8 | 2 | 1 | 1.000 |
| 11q23 | 1 | 5 | .100 |
| inv(16) | 1 | 1 | 1.000 |
| inv(3) | 0 | 1 | .485 |
| Others | 1 | 0 | 1.000 |
| Complex | 1 | 3 | .348 |
| ELN 2022 risk | .005 | ||
| Good | 14 | 3 | .003 |
| Intermediate | 11 | 10 | .923 |
| Poor | 9 | 19 | .007 |
| Gene mutations, +/− | .469 | ||
| FLT3 | 7/27 | 10/22 | .403 |
| NPM1 | 9/25 | 6/26 | .454 |
| DNMT3A | 4/30 | 4/28 | 1.000 |
| IDH2 | 6/28 | 1/31 | .106 |
| IDH1 | 2/32 | 3/29 | .668 |
| TET2 | 3/31 | 3/29 | 1.000 |
| RUNX1 | 2/32 | 5/27 | .251 |
| NRAS | 5/29 | 1/31 | .198 |
| CEBPA | 8/26 | 5/27 | .420 |
| WT1 | 3/31 | 2/30 | 1.000 |
| KIT | 4/30 | 4/28 | 1.000 |
| GATA2 | 1/33 | 5/27 | .100 |
| STAG2 | 1/33 | 2/30 | .608 |
| ROBO2 | 1/33 | 2/30 | .608 |
| RELN | 1/33 | 2/30 | .608 |
| ARID2 | 1/33 | 2/30 | .608 |
| ASXL1 | 3/31 | 4/28 | .705 |
| BCOR | 2/32 | 0/32 | .493 |
| EZH2 | 0/34 | 2/30 | .231 |
| SF3B1 | 1/33 | 0/32 | 1.000 |
| SRSF2 | 1/33 | 2/30 | .608 |
| ZRSR2 | 1/33 | 0/32 | 1.000 |
| TP53 | 1/33 | 1/31 | 1.000 |
| Patient parameter . | Low IL-6, <42.70 pg/mL (n = 34) . | High IL-6, ≥42.70 pg/mL (n = 32) . | P value . |
|---|---|---|---|
| Sex, male/female | 13/21 | 17/15 | .225 |
| Age, median ± SD, y | 47.26 ± 15.83 | 48.88 ± 17.63 | .697 |
| Median WBC (range), ×109/L | 17.59 (0.75-277.14) | 27.37 (1.03-291.44) | .202 |
| Median PB blasts (range), % | 41 (0-98) | 28 (0-98) | .690 |
| Median BM blasts (range), % | 35.5 (3.0-96.5) | 36.0 (5.0-95.5) | .705 |
| FAB | .643 | ||
| M0 | 1 | 0 | 1.000 |
| M1 | 2 | 4 | .420 |
| M2 | 12 | 9 | .532 |
| M4 | 0 | 1 | .485 |
| M5 | 19 | 18 | .976 |
| Karyotypes | .307 | ||
| Normal | 24 | 20 | .486 |
| t(8;21) | 4 | 1 | .357 |
| +8 | 2 | 1 | 1.000 |
| 11q23 | 1 | 5 | .100 |
| inv(16) | 1 | 1 | 1.000 |
| inv(3) | 0 | 1 | .485 |
| Others | 1 | 0 | 1.000 |
| Complex | 1 | 3 | .348 |
| ELN 2022 risk | .005 | ||
| Good | 14 | 3 | .003 |
| Intermediate | 11 | 10 | .923 |
| Poor | 9 | 19 | .007 |
| Gene mutations, +/− | .469 | ||
| FLT3 | 7/27 | 10/22 | .403 |
| NPM1 | 9/25 | 6/26 | .454 |
| DNMT3A | 4/30 | 4/28 | 1.000 |
| IDH2 | 6/28 | 1/31 | .106 |
| IDH1 | 2/32 | 3/29 | .668 |
| TET2 | 3/31 | 3/29 | 1.000 |
| RUNX1 | 2/32 | 5/27 | .251 |
| NRAS | 5/29 | 1/31 | .198 |
| CEBPA | 8/26 | 5/27 | .420 |
| WT1 | 3/31 | 2/30 | 1.000 |
| KIT | 4/30 | 4/28 | 1.000 |
| GATA2 | 1/33 | 5/27 | .100 |
| STAG2 | 1/33 | 2/30 | .608 |
| ROBO2 | 1/33 | 2/30 | .608 |
| RELN | 1/33 | 2/30 | .608 |
| ARID2 | 1/33 | 2/30 | .608 |
| ASXL1 | 3/31 | 4/28 | .705 |
| BCOR | 2/32 | 0/32 | .493 |
| EZH2 | 0/34 | 2/30 | .231 |
| SF3B1 | 1/33 | 0/32 | 1.000 |
| SRSF2 | 1/33 | 2/30 | .608 |
| ZRSR2 | 1/33 | 0/32 | 1.000 |
| TP53 | 1/33 | 1/31 | 1.000 |
ELN 2022, European LeukemiaNet 2022; FAB, French-American-British classification; SD, standard deviation; WBC, white blood cell.
IL-6 in BM supernatant as a predictor of poor chemotherapy outcomes in AML. (A) IL-6 levels in the BM supernatant of patients with AML stratified by ELN 2022 risk classification. (B) Receiver operating characteristic (ROC) curve of IL-6 in the BM supernatant for distinguishing between favorable- and adverse-risk AML groups. (C) Correlation between IL-6 levels in the BM supernatant and the BM blast clearance rate. (D) IL-6 levels in the BM supernatant of patients with AML with different chemotherapy efficacy. (E) ROC curve of IL-6 in the BM supernatant for distinguishing between ORR and NR groups. Data are presented as median with interquartile range. Differences analyzed using Kruskal-Wallis test with Dunn multiple comparisons test (A), area under the ROC curve (B,E), Spearman correlation (C), or Mann-Whitney test (D). ∗∗P < .01; ∗∗∗∗P < .0001. AUC, area under the curve; ns, not significant.
IL-6 in BM supernatant as a predictor of poor chemotherapy outcomes in AML. (A) IL-6 levels in the BM supernatant of patients with AML stratified by ELN 2022 risk classification. (B) Receiver operating characteristic (ROC) curve of IL-6 in the BM supernatant for distinguishing between favorable- and adverse-risk AML groups. (C) Correlation between IL-6 levels in the BM supernatant and the BM blast clearance rate. (D) IL-6 levels in the BM supernatant of patients with AML with different chemotherapy efficacy. (E) ROC curve of IL-6 in the BM supernatant for distinguishing between ORR and NR groups. Data are presented as median with interquartile range. Differences analyzed using Kruskal-Wallis test with Dunn multiple comparisons test (A), area under the ROC curve (B,E), Spearman correlation (C), or Mann-Whitney test (D). ∗∗P < .01; ∗∗∗∗P < .0001. AUC, area under the curve; ns, not significant.
The clearance of BM blasts is an important indicator for assessing chemotherapy efficacy. Chemotherapy efficacy was assessed based on standard clinical response criteria, including complete remission (CR), CR with incomplete hematologic recovery, partial remission, and nonresponse (NR), as defined by BM blast percentages and hematologic recovery after induction chemotherapy. Spearman analysis revealed a negative correlation between IL-6 levels and the BM blast clearance rate, which reflects the speed at which blast cells are eliminated from the BM after chemotherapy initiation (Figure 1C). Stratification by initial chemotherapy efficacy showed that the median IL-6 levels in the NR group (69.22 pg/mL; interquartile range, 49.77-112.81) was significantly higher than that in the objective response rate (ORR) group (33.38 pg/mL; interquartile range, 24.95-53.06; Figure 1D). ORR is defined as the proportion of patients who achieved a CR, CR with incomplete hematologic recovery, or partial remission, based on standard clinical criteria. Receiver operating characteristic analysis further confirmed the efficient discriminatory power of IL-6 levels in distinguishing NR from ORR groups (Figure 1E). Therefore, the elevated IL-6 levels in the BM supernatant of newly diagnosed patients with AML may serve as a predictor of poor chemotherapy outcomes.
MSCs are the primary source of IL-6 in the BM microenvironment of AML
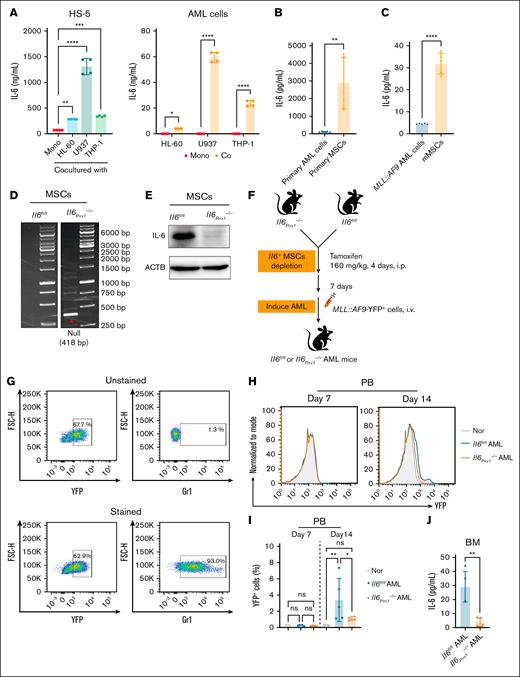
To elucidate the primary source of IL-6 in the AML BM microenvironment, we cocultured human AML cell lines with the human BM stromal cell line HS-5 in vitro to simulate the in vivo BM microenvironment.22 Our findings indicated that IL-6 secretion levels were elevated in both human AML cells and HS-5 cells after coculture (Figure 2A). Notably, HS-5 cells exhibited substantially higher IL-6 secretion than AML cells. A similar phenomenon was observed in primary MSCs derived from both patients with AML and MLL::AF9-induced AML mice, which exhibited markedly higher IL-6 secretion than matched AML cells (Figure 2B-C). These findings further support the notion that MSCs are a major contributor to the elevated IL-6 levels in the AML BM microenvironment. To substantiate this hypothesis, we generated Prrx1-CreERT2; Il6fl/fl(referred to as Il6Prrx1–/–) mice by crossbreeding Il6fl/fl mice with Prrx1-CreERT2 mice (supplemental Figure 1A) and performed genotyping by polymerase chain reaction (supplemental Figure 1B). Prrx1, a marker of MSCs,23,24 enables the conditional deletion of the Il6 gene in MSCs upon tamoxifen (TAM) induction. After TAM induction, we isolated genomic DNA from various tissues and BM-derived MSCs of Il6Prrx1–/– mice (supplemental Figure 1C), revealing efficient Il6 gene deletion specifically in MSCs (Figure 2D). This targeted gene deletion was further validated by western blot (Figure 2E). Moreover, we evaluated whether Il6 knockout affects MSC function. No significant differences were observed in the proportion of MSCs (supplemental Figure 1D) or their viability (supplemental Figure 1E) between Il6Prrx1–/– and Il6fl/fl mice, indicating that MSC survival and maintenance within the AML-supportive niche remain intact. Specifically, histological analyses using Von Kossa staining revealed that Il6 deletion led to reduced mineralized bone area, whereas fatty acid-binding protein 4 staining showed that adipocyte numbers remained unchanged (supplemental Figure 1F-G), suggesting Il6 deletion partially impaired MSC osteogenic differentiation without altering their adipogenic potential.
MSCs are the primary source of IL-6 in the AML BM microenvironment. (A) Coculture of human AML cell lines (HL-60/U937/THP-1 cells) with the human BM stromal cell HS-5 to simulate the BM microenvironment. IL-6 secretion levels of AML and HS-5 cells were measured by enzyme-linked immunosorbent assay (ELISA) after 24 hours of culture (n = 4). (B-C) IL-6 levels in the 24-hour cell culture supernatants measured by ELISA: primary AML cells and MSCs (B) isolated from patients with AML (n = 5); and MLL::AF9 AML cells and mMSCs (C) from MLL::AF9 AML mice (n = 5). (D-E) Validation of Il6 knockout in mouse BM MSCs by polymerase chain reaction (D) and western blot (E). (F) Flowchart for the construction of the Il6Prrx1–/– and Il6fl/fl AML mouse model. (G) FCM analysis of Gr-1 expression in YFP+MLL::AF9 cells. (H-I) FCM analysis of the proportion of YFP+ AML cells in the PB of mice; representative FCM plots (H); bar chart (I) of YFP+ AML cell proportion (n = 5). (J) ELISA analysis of the effect of Il6 deletion in MSCs on IL-6 levels in the BM supernatant of AML mice (n = 5). Data are presented as mean ± standard error of the mean (SEM; panel A) or standard deviation (SD; panels B-C,I-J). Differences were analyzed using ordinary 1-way analysis of variance (ANOVA) with Dunnett multiple comparisons test (panel A; for HS-5 cells), ordinary 1-way ANOVA with Tukey multiple comparisons test (panel I), 2-way ANOVA with Sidak multiple comparisons test (panel A; for AML cells), or 2-tailed unpaired t test (panels B-C,J). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Co, cocultured; Mono, monocultured; mMSCs, mouse MSCs; Nor, normal healthy mice; ns, not significant.
MSCs are the primary source of IL-6 in the AML BM microenvironment. (A) Coculture of human AML cell lines (HL-60/U937/THP-1 cells) with the human BM stromal cell HS-5 to simulate the BM microenvironment. IL-6 secretion levels of AML and HS-5 cells were measured by enzyme-linked immunosorbent assay (ELISA) after 24 hours of culture (n = 4). (B-C) IL-6 levels in the 24-hour cell culture supernatants measured by ELISA: primary AML cells and MSCs (B) isolated from patients with AML (n = 5); and MLL::AF9 AML cells and mMSCs (C) from MLL::AF9 AML mice (n = 5). (D-E) Validation of Il6 knockout in mouse BM MSCs by polymerase chain reaction (D) and western blot (E). (F) Flowchart for the construction of the Il6Prrx1–/– and Il6fl/fl AML mouse model. (G) FCM analysis of Gr-1 expression in YFP+MLL::AF9 cells. (H-I) FCM analysis of the proportion of YFP+ AML cells in the PB of mice; representative FCM plots (H); bar chart (I) of YFP+ AML cell proportion (n = 5). (J) ELISA analysis of the effect of Il6 deletion in MSCs on IL-6 levels in the BM supernatant of AML mice (n = 5). Data are presented as mean ± standard error of the mean (SEM; panel A) or standard deviation (SD; panels B-C,I-J). Differences were analyzed using ordinary 1-way analysis of variance (ANOVA) with Dunnett multiple comparisons test (panel A; for HS-5 cells), ordinary 1-way ANOVA with Tukey multiple comparisons test (panel I), 2-way ANOVA with Sidak multiple comparisons test (panel A; for AML cells), or 2-tailed unpaired t test (panels B-C,J). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Co, cocultured; Mono, monocultured; mMSCs, mouse MSCs; Nor, normal healthy mice; ns, not significant.
On day 7 after TAM induction, Il6fl/fl and Il6Prrx1–/– mice were IV injected with YFP+MLL::AF9 AML cells to establish respective AML mouse models (Figure 2F). To further validate the AML identity of YFP+MLL::AF9 cells, flow cytometry (FCM) analysis showed that >90% of YFP+ cells expressed Gr-1, confirming their myeloid lineage origin (Figure 2G). By day 14 after transplantation, PB smears confirmed the presence of blasts (supplemental Figure 1H), and FCM analysis revealed a significantly reduced proportion of YFP+MLL::AF9 AML cells in the PB of Il6Prrx1–/– recipient mice, compared with Il6fl/fl controls (Figure 2H-I). Enzyme-linked immunosorbent assay analysis demonstrated a substantial reduction in IL-6 levels in the BM supernatant of Il6Prrx1–/– AML mice compared with Il6fl/fl AML mice (Figure 2J). Additionally, Il6 knockout in MSCs did not significantly alter the proportions of major immune cell populations, including T cells, B cells, and natural killer cells, in the AML BM microenvironment (supplemental Figure 1I), suggesting that the effects of MSC-derived IL-6 are likely exerted directly on AML cells rather than through modulation of immune cell composition. Collectively, these data underscore the pivotal role of MSC-derived IL-6 in the AML BM microenvironment.
Il6 deletion in MSCs slows down the progression of AML mice and impairs the high OXPHOS status in AML cells
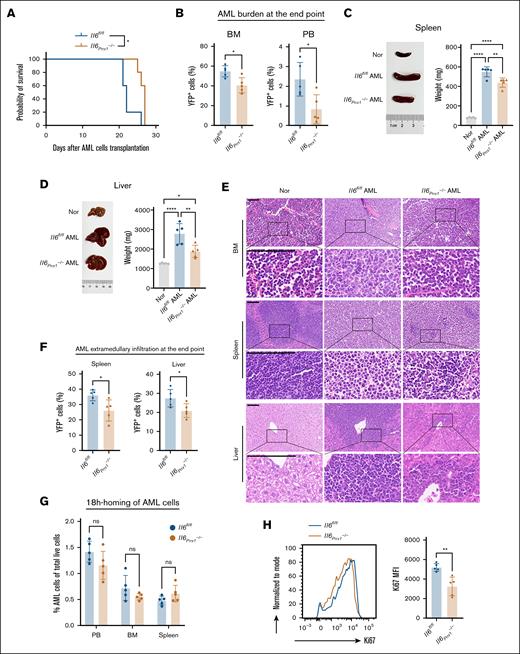
We conducted an investigation into the impact of Il6 gene ablation in MSCs on disease progression in AML mice. Mice with MSC-specific knockout of the Il6 demonstrated attenuated disease progression relative to Il6fl/fl AML mice, as evidenced by a modest extension of overall survival (Figure 3A), reduced AML cell burden in the BM and PB (Figure 3B), and diminished organomegaly in the spleen and liver (Figure 3C-D). Furthermore, histological and FCM analyses revealed decreased infiltration of AML cells in the spleen and liver (Figure 3E-F). To elucidate the temporal dynamics of Il6 gene deletion, we induced Il6 ablation in MSCs at 3 days after AML cell transplantation (supplemental Figure 2A). This intervention similarly delayed AML progression, as demonstrated by the data (supplemental Figure 2B-F). Notably, there was no significant difference in the homing of AML cells to the BM and spleen between Il6fl/fl and Il6Prrx1–/– recipient mice (Figure 3G; supplemental Figure 2G). In contrast, Ki67 staining revealed a marked reduction in the proliferative capacity of AML cells in Il6Prrx1–/– mice (Figure 3H), suggesting that stromal IL-6 primarily influences AML progression through effects on cell proliferation rather than homing. Collectively, these findings highlight MSC-derived IL-6 as a key facilitator of AML pathogenesis, with Il6 gene deletion in MSCs slowing disease progression regardless of the timing relative to AML cell transplantation.
Deletion of Il6 in MSCs inhibits the progression of AML. (A) Kaplan-Meier survival curves of Il6fl/fl and Il6Prrx1–/– AML mice. (B) The proportion of AML cells in the BM and PB of Il6fl/fl and Il6Prrx1–/– AML mice. (C) Spleen size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (D) Liver size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (E) Hematoxylin and eosin (H&E) staining was used to assess the infiltration of AML cells in BM, splenic, and hepatic tissues (scale bar, 100 μm). (F) FCM was used to quantify the infiltration ratio of AML cells in the splenic and hepatic tissues. (G) FCM analysis of YFP+MLL:: AF9 AML cell homing to PB, BM, and spleen at 18 hours after transplantation into Il6fl/fl and Il6Prrx1–/– recipient mice. (H) Ki67 staining of AML cell isolated from Il6fl/fl and Il6Prrx1–/– AML mice to assess proliferative activity (n = 5 mice per group). Data are presented as mean ± SD. Log-rank (Mantel-Cox) test was used for survival study comparisons (panel A). Differences analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-D), 2-way ANOVA with Sidak multiple comparisons test (panel G), or 2-tailed unpaired t test (panels B,F,H). ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. Nor, normal healthy mice; ns, not significant.
Deletion of Il6 in MSCs inhibits the progression of AML. (A) Kaplan-Meier survival curves of Il6fl/fl and Il6Prrx1–/– AML mice. (B) The proportion of AML cells in the BM and PB of Il6fl/fl and Il6Prrx1–/– AML mice. (C) Spleen size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (D) Liver size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (E) Hematoxylin and eosin (H&E) staining was used to assess the infiltration of AML cells in BM, splenic, and hepatic tissues (scale bar, 100 μm). (F) FCM was used to quantify the infiltration ratio of AML cells in the splenic and hepatic tissues. (G) FCM analysis of YFP+MLL:: AF9 AML cell homing to PB, BM, and spleen at 18 hours after transplantation into Il6fl/fl and Il6Prrx1–/– recipient mice. (H) Ki67 staining of AML cell isolated from Il6fl/fl and Il6Prrx1–/– AML mice to assess proliferative activity (n = 5 mice per group). Data are presented as mean ± SD. Log-rank (Mantel-Cox) test was used for survival study comparisons (panel A). Differences analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-D), 2-way ANOVA with Sidak multiple comparisons test (panel G), or 2-tailed unpaired t test (panels B,F,H). ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. Nor, normal healthy mice; ns, not significant.
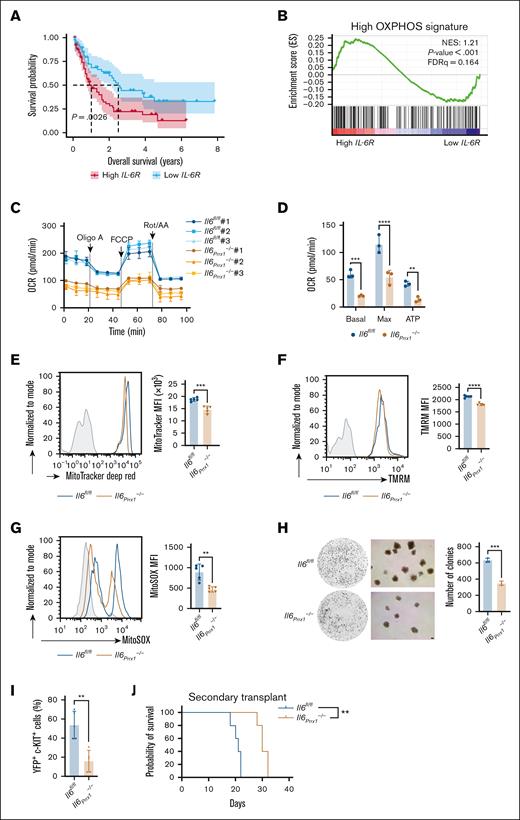
The IL-6 receptor (IL-6R) is critical cellular receptor for mediating exogenous IL-6 signaling, with its upregulation suggesting enhanced signal transduction.7 We observed a significant increase in IL-6R messenger RNA expression in AML cells compared with normal hematopoietic cells using the Gene Expression Omnibus and BloodSpot databases (supplemental Figure 3A-B). Further analysis using the Cancer Genome Atlas database revealed that high IL-6R expression in patients with AML was associated with significantly shorter overall survival (Figure 4A). Gene ontology analysis revealed that differentially expressed genes were significantly enriched in biological processes related to the “positive regulation of metabolic processes” (supplemental Figure 3C). To further characterize the metabolic alterations, we performed gene set enrichment analysis, which identified significant enrichment of multiple metabolic pathways (supplemental Figure 3D). Strikingly, the high OXPHOS gene signature was significantly enriched in the high IL-6R expression group (Figure 4B), suggesting that enhanced IL-6 signaling may promote OXPHOS levels in AML cells. Seahorse metabolic analysis of mitochondrial respiration revealed reduced oxygen consumption rate in AML cells from the BM of Il6Prrx1–/– AML mice compared to Il6fl/fl AML mice (Figure 4C-D). FCM analysis further confirmed this reduction in mitochondrial activity, as evidenced by decreased mitochondrial mass (Figure 4E), mitochondrial membrane potential (Figure 4F), and mtROS levels (Figure 4G). Together, these findings indicate that Il6 knockout in MSCs attenuates mitochondrial function and diminishes the high OXPHOS status in AML cells. The reduced colony-forming capacity (Figure 4H), lower frequency of leukemia stem cells (LSCs; YFP+ c-Kit+; Figure 4I), and prolonged survival in secondary transplant recipients receiving Il6Prrx1–/– AML cells (Figure 4J) collectively indicate that MSC-derived IL-6 is essential for maintaining AML stemness and self-renewal. These findings also suggest that the impaired OXPHOS status observed in Il6Prrx1–/– AML cells may contribute to the diminished stem-like properties.
The absence of Il6 in MSCs impairs the OXPHOS status in AML cells. (A) Overall survival analysis of patients with AML with high (n = 65) and low expression (n = 65) of IL-6R from the TCGA database. (B) Gene set enrichment analysis of the high OXPHOS gene signature in patients with AML with high and low IL-6R expression from the TCGA database. (C) Measurement of mitochondrial respiration in AML cells isolated from Il6fl/fl and Il6Prrx1–/– AML mice using a Seahorse Bioscience XF analyzer (n = 3). (D) Calculation of basal respiration, maximal respiration, and mitochondrial ATP production based on measurements shown in panel C (n = 3). (E-G) FCM analysis of mitochondrial mass (E), membrane potential (F), and mtROS levels (G) in AML cells isolated from Il6fl/fl and Il6Prrx1–/– AML mice (n = 5). (H) Colony formation assay of AML cells isolated from Il6fl/fl and Il6Prrx1–/– AML mice (n = 3; scale bar, 100 μm). (I) FCM analysis of LSC frequency, defined as YFP+ c-KIT+ cells, in the BM of Il6fl/fl and Il6Prrx1–/– mice (n = 5). (J) Kaplan-Meier survival curves of secondary recipient mice transplanted with 2 × 105 AML cells derived from primary Il6fl/fl or Il6Prrx1–/– AML mice (n = 5). Data are presented as mean ± SD. Log-rank (Mantel-Cox) test was used for survival study comparisons (panels A,J). Differences were analyzed using 2-way ANOVA with Sidak multiple comparisons test (panel D) or 2-tailed unpaired t test (panels E-I). ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ATP, adenosine triphosphate; Basal, basal respiration; FDRq, false discovery rate q-value; Max, maximal respiration; MFI, mean fluorescence intensity; NES, normalized enrichment score; OCR, oxygen consumption rate.
The absence of Il6 in MSCs impairs the OXPHOS status in AML cells. (A) Overall survival analysis of patients with AML with high (n = 65) and low expression (n = 65) of IL-6R from the TCGA database. (B) Gene set enrichment analysis of the high OXPHOS gene signature in patients with AML with high and low IL-6R expression from the TCGA database. (C) Measurement of mitochondrial respiration in AML cells isolated from Il6fl/fl and Il6Prrx1–/– AML mice using a Seahorse Bioscience XF analyzer (n = 3). (D) Calculation of basal respiration, maximal respiration, and mitochondrial ATP production based on measurements shown in panel C (n = 3). (E-G) FCM analysis of mitochondrial mass (E), membrane potential (F), and mtROS levels (G) in AML cells isolated from Il6fl/fl and Il6Prrx1–/– AML mice (n = 5). (H) Colony formation assay of AML cells isolated from Il6fl/fl and Il6Prrx1–/– AML mice (n = 3; scale bar, 100 μm). (I) FCM analysis of LSC frequency, defined as YFP+ c-KIT+ cells, in the BM of Il6fl/fl and Il6Prrx1–/– mice (n = 5). (J) Kaplan-Meier survival curves of secondary recipient mice transplanted with 2 × 105 AML cells derived from primary Il6fl/fl or Il6Prrx1–/– AML mice (n = 5). Data are presented as mean ± SD. Log-rank (Mantel-Cox) test was used for survival study comparisons (panels A,J). Differences were analyzed using 2-way ANOVA with Sidak multiple comparisons test (panel D) or 2-tailed unpaired t test (panels E-I). ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ATP, adenosine triphosphate; Basal, basal respiration; FDRq, false discovery rate q-value; Max, maximal respiration; MFI, mean fluorescence intensity; NES, normalized enrichment score; OCR, oxygen consumption rate.
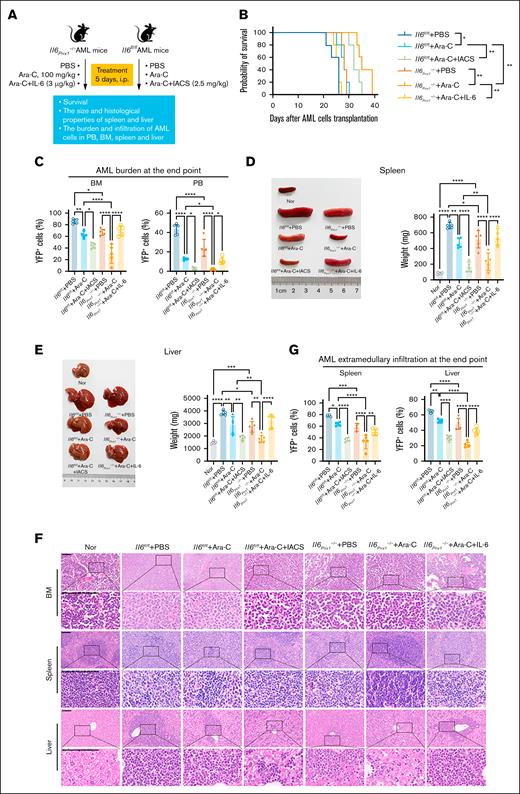
Il6 deletion in MSCs enhances Ara-C chemotherapy efficacy in AML mice by targeting high OXPHOS status in AML cells
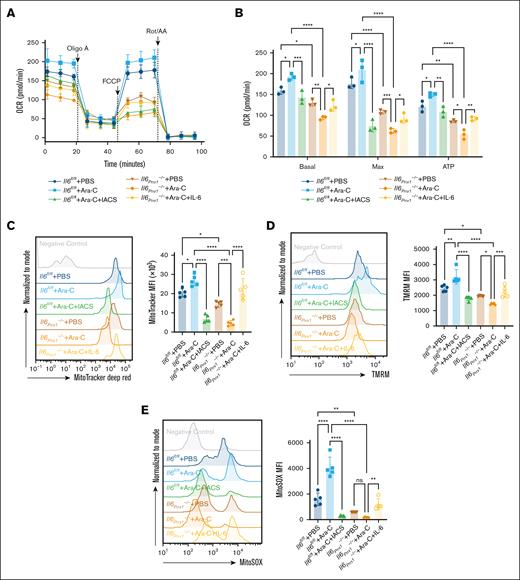
To investigate the impact of Il6 deletion in MSCs on chemotherapeutic response, we treated Il6fl/fl and Il6Prrx1–/– AML mice with Ara-C (Figure 5A). Il6Prrx1–/– AML mice showed increased sensitivity to Ara-C, demonstrated by significantly prolonged overall survival (Figure 5B), reduced AML burden in both the BM and PB (Figure 5C), attenuated splenomegaly and hepatomegaly (Figure 5D-E), and decreased infiltration of AML cells in the spleen and liver (Figure 5F-G). Moreover, recombinant mouse IL-6 protein administration restored Ara-C chemoresistance in Il6Prrx1–/– AML mice (Figure 5B-G). Conversely, OXPHOS inhibition with IACS-010759 enhanced Ara-C chemosensitivity in Il6fl/fl AML mice (Figure 5B-G). Subsequently, we assessed the OXPHOS status of residual AML cells in the BM after Ara-C treatment. Residual AML cells from Il6fl/fl AML mice after Ara-C treatment displayed a high OXPHOS status compared to the PBS controls (Figure 6). Il6 deletion in MSCs significantly reduced this heightened OXPHOS status of residual AML cells after Ara-C treatment, which was reversed by IL-6 supplementation (Figure 6). Additionally, OXPHOS inhibition with IACS-010759 markedly reduced the high OXPHOS status of residual AML cells in the BM of Il6fl/fl AML mice, phenocopying the effects observed with Il6 deletion in MSCs. These results suggest that deletion of Il6 in MSCs can attenuate the high OXPHOS status of AML cells, thereby diminishing their chemoresistance to Ara-C.
Deletion of Il6 in MSCs enhances chemosensitivity of AML mice to Ara-C. (A) Schematic diagram of the experimental process in the mouse model. (B) Kaplan-Meier survival curves of AML mice. (C) AML burden in the BM and PB of AML mice. (D) Spleen size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (E) Liver size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (F) H&E staining was used to assess the infiltration of AML cells in the BM, splenic, and hepatic tissues (scale bar, 100 μm). (G) FCM was used to quantify the infiltration ratio of AML cells in the splenic and hepatic tissues (n = 5 mice per group). Data are presented as mean ± SD. Log-rank (Mantel-Cox) test was used for survival study comparisons (panel B). Differences were analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-E,G). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. IACS, IACS-010759 (OXPHOS inhibitor); Nor, normal healthy mice.
Deletion of Il6 in MSCs enhances chemosensitivity of AML mice to Ara-C. (A) Schematic diagram of the experimental process in the mouse model. (B) Kaplan-Meier survival curves of AML mice. (C) AML burden in the BM and PB of AML mice. (D) Spleen size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (E) Liver size and weight in Il6fl/fl and Il6Prrx1–/– AML mice. (F) H&E staining was used to assess the infiltration of AML cells in the BM, splenic, and hepatic tissues (scale bar, 100 μm). (G) FCM was used to quantify the infiltration ratio of AML cells in the splenic and hepatic tissues (n = 5 mice per group). Data are presented as mean ± SD. Log-rank (Mantel-Cox) test was used for survival study comparisons (panel B). Differences were analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-E,G). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. IACS, IACS-010759 (OXPHOS inhibitor); Nor, normal healthy mice.
Deletion of Il6 in MSCs attenuates the OXPHOS status of residual AML cells after Ara-C treatment. (A) Measurement of mitochondrial respiration in AML cells sorted from AML mice using the Seahorse Bioscience XF Analyzer (n = 3). (B) Calculation of basal respiration, maximum respiration, and mitochondrial ATP production based on the measurements in panel A (n = 3). (C-E) FCM analysis of mitochondrial mass (C), membrane potential (D), and mtROS levels (E) in AML cells sorted from AML mice (n = 5). Data are presented as mean ± SD. Differences analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-E) or 2-way ANOVA with Sidak multiple comparisons test (panel B). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ATP, adenosine triphosphate; Basal, basal respiration; IACS, IACS-010759 (OXPHOS inhibitor); Max, maximal respiration; MFI, mean fluorescence intensity; ns, not significant; OCR, oxygen consumption rate; TMRM, tetramethylrhodamine methyl ester.
Deletion of Il6 in MSCs attenuates the OXPHOS status of residual AML cells after Ara-C treatment. (A) Measurement of mitochondrial respiration in AML cells sorted from AML mice using the Seahorse Bioscience XF Analyzer (n = 3). (B) Calculation of basal respiration, maximum respiration, and mitochondrial ATP production based on the measurements in panel A (n = 3). (C-E) FCM analysis of mitochondrial mass (C), membrane potential (D), and mtROS levels (E) in AML cells sorted from AML mice (n = 5). Data are presented as mean ± SD. Differences analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-E) or 2-way ANOVA with Sidak multiple comparisons test (panel B). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ATP, adenosine triphosphate; Basal, basal respiration; IACS, IACS-010759 (OXPHOS inhibitor); Max, maximal respiration; MFI, mean fluorescence intensity; ns, not significant; OCR, oxygen consumption rate; TMRM, tetramethylrhodamine methyl ester.
To investigate the impact of Il6 knockout in MSCs on AML relapse, we transplanted normal BM mononuclear cells into lethally irradiated Il6fl/fl and Il6Prrx1–/– AML mice to mimic clinical therapeutic transplantation (supplemental Figure 4A). AML relapse was delayed in Il6Prrx1–/– mice, as indicated by a later onset of disease-related symptoms and prolonged survival compared to controls (supplemental Figure 4B-F).
Decreased active mitochondrial transfer from Il6Prrx1–/– MSCs to AML cells
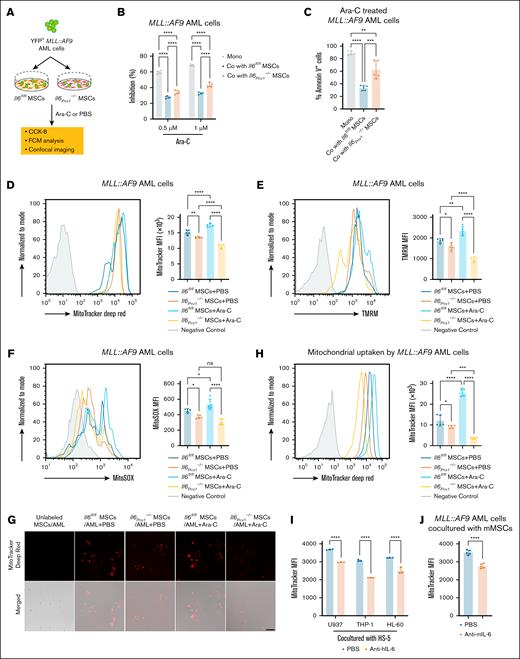
To elucidate whether enhanced mitochondria function in AML cells is a critical mechanism by which MSC-derived IL-6 mediates chemoresistance, we established an in vitro adhesion coculture system. MSCs isolated from the BM of Il6Prrx1–/– and Il6fl/fl mice were directly cocultured with MLL::AF9 AML cells (Figure 7A). Consistent with in vivo observations, MLL::AF9 AML cells cocultured with Il6Prrx1–/– MSCs showed increased sensitivity to Ara-C compared to those cocultured with Il6fl/fl MSCs (Figure 7B-C; supplemental Figure 5A). Mitochondrial mass, mitochondrial membrane potential, and mtROS levels in MLL::AF9 AML cells were significantly reduced when cocultured with Il6Prrx1–/– MSCs compared to those cocultured with Il6fl/fl MSCs, irrespective of Ara-C treatment (Figure 7D-F). Notably, Ara-C exposure significantly increased mitochondrial mass, membrane potential, and mtROS levels in surviving MLL::AF9 AML cells cocultured with Il6fl/fl MSCs compared to PBS treatment (Figure 7D-F). Our group25 and others17 have previously demonstrated that MSCs can transfer mitochondria to AML cells, thereby enhancing their OXPHOS levels to resist Ara-C. Furthermore, we examined mitochondrial transfer using MitoTracker Deep Red to label MSC mitochondria. Consistent with our expectations, Ara-C treatment resulted in an increased presence of mitochondria from Il6fl/fl MSCs in surviving MLL::AF9 AML cells (Figure 7G-H). However, the number of mitochondria transferred from Il6Prrx1–/– MSCs to MLL::AF9 AML cells was significantly lower than that from Il6fl/fl MSCs, regardless of Ara-C treatment (Figure 7G-H). These results suggest that MSC-derived IL-6 promotes mitochondrial transfer to AML cells, thereby enhancing their chemoresistance to Ara-C. Furthermore, pretreatment with IL-6–neutralizing antibodies significantly reduced mitochondrial transfer from MSCs to AML cells (Figure 7I-J), underscoring the therapeutic potential of targeting the IL-6/IL-6R signaling axis in AML.
MSC-derived IL-6 increases the active mitochondria in AML cells to resist Ara-C treatment. (A) Experimental layout for assessing mitochondrial function of MLL::AF9 AML cells cocultured with MSCs isolated and purified from mouse BM, followed by treatment with Ara-C or PBS. (B) CCK-8 assay to measure the inhibitory effect of Ara-C on MLL::AF9 AML cells after in vitro coculture (n = 5). (C) Apoptotic cells were determined by FCM analysis of allophycocyanin-annexin V and 7-aminoactinomycin D staining (n = 5). (D-F) FCM analysis of mitochondrial mass (D), mitochondrial membrane potential (E), and mtROS levels (F) in MLL::AF9 AML cells after in vitro coculture (n = 5). (G-H) Laser scanning confocal microscopy (G) and FCM (H) to observe mitochondrial transfer from MSCs to MLL::AF9 AML cells (n = 5; scale bar, 50 μm). (I-J) FCM analysis of mitochondrial transfer from HS-5 (n = 3) (I) or mouse MSCs (n = 5) (J) to AML cells, after pretreatment with anti-human IL-6 (anti–hIL-6) or anti-mouse IL-6 (anti–mIL-6) neutralizing antibodies. Data are presented as mean ± SD (panels B-F,H,J) or SEM (panel I). Differences were analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-F,H), 2-way ANOVA with Sidak multiple comparisons test (panels B,I), or 2-tailed unpaired t test (panel J). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Co, cocultured; mMSCs, mouse MSCs; Mono, monocultured; ns, not significant.
MSC-derived IL-6 increases the active mitochondria in AML cells to resist Ara-C treatment. (A) Experimental layout for assessing mitochondrial function of MLL::AF9 AML cells cocultured with MSCs isolated and purified from mouse BM, followed by treatment with Ara-C or PBS. (B) CCK-8 assay to measure the inhibitory effect of Ara-C on MLL::AF9 AML cells after in vitro coculture (n = 5). (C) Apoptotic cells were determined by FCM analysis of allophycocyanin-annexin V and 7-aminoactinomycin D staining (n = 5). (D-F) FCM analysis of mitochondrial mass (D), mitochondrial membrane potential (E), and mtROS levels (F) in MLL::AF9 AML cells after in vitro coculture (n = 5). (G-H) Laser scanning confocal microscopy (G) and FCM (H) to observe mitochondrial transfer from MSCs to MLL::AF9 AML cells (n = 5; scale bar, 50 μm). (I-J) FCM analysis of mitochondrial transfer from HS-5 (n = 3) (I) or mouse MSCs (n = 5) (J) to AML cells, after pretreatment with anti-human IL-6 (anti–hIL-6) or anti-mouse IL-6 (anti–mIL-6) neutralizing antibodies. Data are presented as mean ± SD (panels B-F,H,J) or SEM (panel I). Differences were analyzed using ordinary 1-way ANOVA with Tukey multiple comparisons test (panels C-F,H), 2-way ANOVA with Sidak multiple comparisons test (panels B,I), or 2-tailed unpaired t test (panel J). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Co, cocultured; mMSCs, mouse MSCs; Mono, monocultured; ns, not significant.
Additionally, FCM analysis demonstrated a reduced proportion of cells expressing the LSC marker YFP+ c-KIT+ in MLL::AF9 AML cells cocultured with Il6Prrx1–/– MSCs (supplemental Figure 5B). This result is consistent with our in vivo findings and further supports the potential role of MSC-derived IL-6 in maintaining or enriching LSCs.
Discussion
In patients with AML, elevated IL-6 levels in the plasma8 and BM supernatant9 have been shown to correlate negatively with prognosis. Our study further reveals that elevated IL-6 levels in the BM at diagnosis are predictive of poor chemotherapy outcomes in patients with AML. This association prompted an in-depth investigation into the origins of elevated IL-6 within the BM microenvironment. Using a direct-contact coculture system, we found that IL-6 secretion by MSCs far exceeded that by AML cells themselves. To further elucidate the role of MSCs in IL-6 production in vivo, we generated an MSC-specific Il6 knockout AML mouse model using the Cre-LoxP system, with induction by TAM. Our results demonstrate that the targeted deletion of Il6 in MSCs significantly reduced IL-6 levels in the BM supernatant of these AML mice, thereby establishing MSCs as the primary source of elevated IL-6 in the AML BM environment.
In our previous study,21,26 we established that IL-6 secreted by MSCs enhances AML chemoresistance by activating mitochondrial function through 2 key mechanisms: the serine-phosphorylated STAT3 signaling axis and mitofusin 1–mediated mitochondrial fusion. These processes collectively boost OXPHOS, which is associated with resistance to Ara-C. We also showed that AML cells can stimulate IL-6 production in MSCs, reinforcing this prosurvival signaling loop. Importantly, CRISPR/Cas9-mediated IL-6 knockout in HS-5 cells disrupted this axis, reducing OXPHOS and sensitizing AML cells to chemotherapy. Building on this mechanistic framework, our study uses an in vivo conditional Il6 knockout model to validate and extend these findings. We demonstrate that MSC Il6 deletion reduces AML OXPHOS, impairs leukemia stemness, and enhances chemotherapy efficacy, thereby strengthening the clinical relevance of targeting MSC-derived IL-6 in the AML microenvironment.
Recent phase 1 clinical trial findings have underscored the therapeutic potential of combining the anti–IL-6R monoclonal antibody tocilizumab with standard intensive induction chemotherapy for AML, demonstrating promising efficacy.27 Furthermore, studies have indicated that IL-6 blockade using antibodies can reverse BM failure associated with AML and enhance overall survival.28 In our study, we present evidence that deletion of Il6 in MSCs impedes AML progression, as demonstrated in AML mouse models both before and after the induction of Il6 gene deletion in MSCs. These results provide compelling experimental support for the clinical application of MSC-derived IL-6 as a therapeutic target to ameliorate the outcomes in patients with AML.
Recent studies demonstrate that MSCs promote AML chemoresistance via 2 distinct mechanisms: mitochondrial transfer–mediated OXPHOS enhancement and redox balance modulation–induced antioxidant defense augmentation.3,16,17 A key finding of our study is that targeted deletion of Il6 in MSCs increases the cytotoxicity of Ara-C against AML and prolongs overall survival. Mechanistically, the heightened chemosensitivity of AML cells after Il6 deletion in MSCs is attributed to the impeded transfer of mitochondria from MSCs to AML cells. This reduction in active mitochondrial content within AML cells subsequently lowers OXPHOS levels, disrupting the energy supply and survival mechanisms14,29 of the AML cells and increasing their vulnerability to chemotherapeutic agents. Both genetic deletion of Il6 in MSCs and pharmacological inhibition of OXPHOS using the inhibitor IACS-010759 significantly reduce chemoresistance in AML.
Furthermore, Il6 knockout in MSCs not only significantly reduces OXPHOS levels but also impairs AML cell stemness, aligning with findings by Shao et al,30 which demonstrated that elevated high OXPHOS supports leukemic stemness. This loss of stemness may, in part, explain the delayed relapse observed in Il6Prrx1–/– AML mice. Notably, although prior studies11,31 have demonstrated IL-6 production by MSCs, our work provides additional mechanistic insights into how MSC IL-6 promotes AML maintenance by supporting OXPHOS activity, facilitating mitochondrial transfer, and enhancing leukemia stemness and relapse potential. Moreover, the use of a conditional Il6 knockout model in MSCs allowed us to dissect the specific contribution of MSC IL-6 in vivo in a way that complements and extends existing findings.
Mitochondrial energy metabolism is increasingly recognized as being driven by oxidative stress, a pivotal mechanism enhancing OXPHOS metabolism.32,33 Marlein et al34 demonstrated that superoxide, generated by the NADPH oxidase 2 in AML cells, stimulates the transfer of mitochondria from MSCs to AML cells, thereby enhancing OXPHOS. Additionally, the elevated levels of ROS, a byproduct of OXPHOS metabolism induced by IL-6 in AML cells, may also facilitate this transfer process. Our in vitro coculture experiments, using mouse BM MSCs and MLL::AF9 AML cells, revealed a notable reduction in ROS levels within AML cells cocultured with Il6Prrx1–/– MSCs. Moreover, the transfer of mitochondria to AML cells was substantially diminished with Il6Prrx1–/– MSCs compared to their Il6fl/fl counterparts. Nevertheless, the precise molecular mechanisms underlying IL-6–induced mitochondrial transfer and the clinical implications of this phenomenon in AML warrant further investigation.
Although our study underscores the critical role of MSC-derived IL-6 in promoting OXPHOS activity, stemness, and chemoresistance in AML cells, we recognize that it does not fully address the potential contributions of IL-6 from other cellular sources in the BM microenvironment. Previous studies have shown that myeloid cells, endothelial cells, and other stromal components are also capable of producing IL-6,4,35,36 which may influence leukemic cell behavior either independently or in cooperation with MSCs. Although our functional and comparative analyses support MSCs as a predominant source of IL-6 in our model, a comprehensive understanding of IL-6 signaling in AML will require systematic mapping of IL-6–producing cell types and their contextual roles. In addition, although our study establishes a causal link between MSC-derived IL-6 and mitochondrial remodeling in AML, we focused primarily on stromal-AML interactions. The role of IL-6 signaling, in conjunction with immune cell modulation and the differentiation potential of MSCs, as well as its impact on the broader BM ecosystem, remains to be fully elucidated. Furthermore, our findings are largely based on murine models and in vitro systems, and future validation using human patient–derived xenografts and clinical samples will be important to confirm translational relevance.
In summary, our study identifies MSCs as the primary source of IL-6 in the AML BM microenvironment and elucidates how MSC-derived IL-6 modulates mitochondrial energy metabolism in vivo, thereby enhancing AML cell survival and chemoresistance. Targeting MSC-derived IL-6 may thus represent an effective strategy to improve the efficacy of AML treatment outcomes.
Acknowledgments
The authors thank the Cell Bank of Chinese Academy of Sciences and the Center for Excellence in Molecular Cell Science of Chinese Academy of Sciences, for providing the cell lines and related technical services.
This work was supported by Joint Funds for the Innovation of Science and Technology, Fujian Province of China (2023Y9122 [H.H.]; 2024Y9231 [D.H.]), Fujian Provincial Natural Science Foundation of China (2024J01594; D.H.), and Fujian Provincial Government-Funded Project of the Construction of High-Level Laboratory of China (Min201704; H.H.).
Authorship
Contribution: H.H., D.H., and D.C. conceived of the study, designed experiments, interpreted data, and wrote the manuscript; D.H., D.C., W.D., X.L., M.T., X.Z., L.W., J.L., J.W., and X.W. conducted experiments and acquired data; W.D. and M.T. performed bioinformatic analysis; and D.C., L.W., and Q.F. obtained and interpreted clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Huifang Huang, Central Laboratory, Fujian Medical University Union Hospital, 29 Xinquan Rd, Fuzhou 350001, China; email: huanghuif@fjmu.edu.cn.
References
Author notes
D.H. and D.C. contributed equally to this study.
Original data are available on request from the corresponding author, Huifang Huang (huanghuif@fjmu.edu.cn).
The full-text version of this article contains a data supplement.