Key Points
Maintenance romidepsin after AHCT in an unselected population of patients with PTCL did not meet the primary efficacy end point.
Maintenance romidepsin is feasible after AHCT.
Visual Abstract
Patients with peripheral T-cell lymphoma (PTCL) have suboptimal outcomes. Autologous hematopoietic stem-cell transplantation (AHCT) is a therapeutic strategy for patients in first complete remission (CR1) or first partial remission (PR1), with median intent-to-treat progression-free survival (PFS) of 36% to 48%. Romidepsin is a histone deacetylase inhibitor used for treatment of relapsed/refractory PTCL. We present a multicenter study to evaluate the PFS of patients receiving maintenance therapy with romidepsin after AHCT. Twenty-six patients who underwent AHCT in CR1 or PR1 were evaluated for the primary end point of 2-year PFS. The exploratory cohort enrolled patients who underwent transplantation during or after CR/PR2 (n = 5) or high-risk histologies (n = 2). Patients underwent AHCT with carmustine, etoposide, cytarabine, and melphalan conditioning. Romidepsin 14 mg/m2 was started on days 42 to 80 after -AHCT every other week until 6 months of -AHCT, every 3 weeks between 6 months and 1-year of -AHCT, and every 4 weeks between 1 and 2 years of -AHCT. PFS was estimated using the Kaplan-Meier analysis. Forty-seven patients consented and 13 did not receive romidepsin. With a median progression-free follow-up of 32 months (range, 24-36), 15 of the first 25 patients in cohort 1 were progression-free after 2 years. The estimated 2-year PFS was 62% (95% confidence interval, 45-83). The most common toxicities were fatigue (n = 24, 73%), decreased platelets (n = 16, 48%), and anemia (n = 16, 48%). Although the study did not meet its desired primary efficacy end point, maintenance romidepsin was feasible, with a favorable estimated 2-year PFS of 62%. This trial was registered at www.ClinicalTrials.gov as #NCT01908777.
Introduction
Peripheral T-cell lymphomas (PTCL) represent a heterogeneous group of aggressive non-Hodgkin lymphomas. Treatment strategies have been extrapolated from aggressive B-cell lymphomas, with most patients receiving a CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–like regimen as up-front therapy. However, the efficacy of CHOP has been inadequate for most patients with PTCL, with historic and retrospective 5-year progression-free survival (PFS) rates of 13% to 36%.1-3
Additional agents have been studied in combination with frontline CHOP regimens as an up-front strategy to improve the outcomes. The most successful combination was evaluated in ECHELON-2, a prospective, randomized, phase 3 study of brentuximab vedotin with cyclophosphamide, doxorubicin, and prednisone (A + CHP) in subjects with CD30-expressing PTCL. This study demonstrated both improved PFS and overall survival (OS) when compared with CHOP, with a 3-year PFS of 57% in the A+CHP arm vs 44% in the CHOP arm.4 The addition of etoposide to up-front CHOP therapy in the cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone (CHOEP) regimen has also demonstrated improvement when compared with outcomes with CHOP alone, with a 3-year event-free survival of 75% in the CHOEP group vs 51% in the CHOP group (P = .003) for patients under 60 years of age who had normal lactate dehydrogenase.5 Notably, both studies predominantly enrolled patients with anaplastic large-cell lymphoma, which generally has a more favorable outcome than other subtypes of nodal PTCL.
Other agents have been studied in addition to CHOP-like regimens as part of frontline therapy but have not demonstrated improvement compared with historical PFS or OS. Romidepsin is a histone deacetylase inhibitor that was US Food and Drug Administration–approved for the treatment of relapsed/refractory T-cell lymphoma based on the results of a phase 2 trial demonstrating an objective response rate of 25%.6 Romidepsin was also studied in combination with CHOP in a phase 3 study of 421 patients undergoing up-front treatment for PTCL. Patients in the romidepsin + CHOP (Ro-CHOP) arm did not experience improved PFS or OS compared with those treated with CHOP alone. At a median follow-up of 27.5 months, the median PFS for Ro-CHOP vs CHOP was 12.0 months (95% confidence interval [CI], 9.0-25.8) vs 10.2 months (95% CI, 7.4-13.2), and the median OS for Ro-CHOP vs CHOP was 51.8 months (95% CI, 35.7-72.6) vs 42.9 months (95% CI, 29.9 to not evaluable). Treatment-related adverse events with combination therapy also led to more frequent dose interruption (36% vs 20%) and dose reduction (26% vs 15%) in patients receiving Ro-CHOP vs CHOP.7 As this study did not meet its primary end point of improved PFS, the PTCL indication for romidepsin was withdrawn. However, in an exploratory analysis, the median PFS of patients with PTCL-T follicular helper phenotype (Tfh) subtypes was significantly longer in Ro-CHOP vs CHOP (19.5 vs 10.6 months; hazard ratio, 0.703; 95% CI, 0.502-0.985), suggesting that this subset may derive additional benefit from romidepsin.8
Another therapeutic strategy to improve outcomes after up-front PTCL therapy for patients with chemosensitive disease in first complete remission (CR1) or first partial remission (PR1) is to consolidate the up-front response to autologous hematopoietic cell transplantation (AHCT). An unplanned analysis of ECHELON-2 suggested a PFS benefit to consolidation with AHCT,9 and an analysis of the Netherlands population–based data suggested an OS benefit with AHCT consolidation.10 However, relapses still occur, and PFS after AHCT remains suboptimal.11,12 In the first multicenter study of AHCT in PTCL (n = 83), 3-year PFS by intent to treat was 36%12 and in a phase 2 study of AHCT in untreated PTCL with 160 patients in the intent-to-treat population, the 5-year PFS was 44% (95% CI, 36-52).11 Retrospective single-center data of outcomes after AHCT have shown a 4-year event-free survival of 48%.13 A persistent, unmet therapeutic need exists for improved PFS and OS after AHCT in patients with PTCL.
Most patients with PTCL who experience posttransplantation relapse or progressive disease do so within the first 24 months of diagnosis. In a large retrospective cohort from the United States, Sweden, and Canada, 64% of the 775 patients progressed within the first 24 months.14 These relatively early relapses may be driven by the proliferation of residual tumor cells that remain after transplantation. As maintenance therapy has successfully improved PFS in other lymphomas, such as mantle cell (rituximab)15 and Hodgkin lymphoma (brentuximab vedotin),16 we hypothesized that maintenance therapy could extend the initial remission after induction therapy and AHCT for patients with PTCL. A maintenance strategy of programmed cell death protein-1 blockade after AHCT has shown positive results in PTCL.17 We present the results of the first multicenter prospective study to evaluate the PFS of patients with PTCL receiving maintenance romidepsin therapy after AHCT.
Methods
Patients
This was a multicenter, single-arm phase 2 study (ClinicalTrials.gov identifier: NCT01908777). We treated patients with standard-risk histologies, defined as PTCL, angioimmunoblastic T-cell lymphoma (AITL), anaplastic large-cell lymphoma, and enteropathy-type T-cell lymphoma. Twenty-six patients who underwent transplantation in the CR1 or PR1 were evaluated for the primary end point of PFS at 2 years posttransplantation (cohort 1). Patients who received romidepsin after transplantation were evaluated for the primary end point and were counted for the accrual total. Any patient who did not receive romidepsin after transplantation was replaced. A preplanned exploratory cohort enrolled 8 patients who either underwent transplantation in the second or later CR or PR (n = 5) or had high-risk histologies, defined as extranodal natural killer/T-cell lymphoma (n = 2). The last patient in this exploratory cohort was diagnosed with myeloid leukemia after enrolling and starting romidepsin. On repeat review of his initial pathology, he did not have PTCL and was therefore not eligible. The second exploratory cohort (cohort 2) was analyzed for secondary end points only.
Written informed consent was obtained from the patient or legally authorized representative. The study protocol and amendments were approved by the institutional review boards of each site and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices (supplemental Methods).
Treatment
The patients were enrolled in the study before receiving high-dose chemotherapy. Patients in cohort 1 received up-front treatment with anthracycline-containing regimens (Table 1). They underwent AHCT with carmustine, etoposide, cytarabine, and melphalan conditioning, and initiated treatment with romidepsin between days 42 and 80 after AHCT. To allow for recovery from AHCT-related toxicities and minimize ongoing therapy-related toxicity, patients received a single dose of romidepsin every other week until 6 months after transplantation, every 3 weeks between 6 months and 1 year of transplantation, and every 4 weeks between 1 and 2 years of transplantation. The potassium and magnesium levels were monitored for each infusion. Electrocardiograms were obtained at screening on the first day of treatment before the first dose of romidepsin and after the completion of the first dose of romidepsin. The planned dose of romidepsin was 14 mg/m2, with protocol-guided dose reduction for toxicity. Treatment was discontinued early because of drug intolerance, disease recurrence, or disease progression. Patients received antimicrobial prophylaxis, including antiviral, antibacterial, and antifungal prophylaxis, as per the standard-of-care guidelines at the treating institutions. The use of growth factors was not mandated in the protocol.
Clinical characteristics of patients enrolled in the study
| Characteristics . | Cohort 1 . | Cohort 2 . |
|---|---|---|
| (N = 26) . | (N = 7) . | |
| Histology, n(%) | ||
| Angioimmunoblastic T-cell lymphoma | 11 (42) | 1 (14) |
| PTCL, not otherwise specified | 7 (27) | 0 |
| Anaplastic large cell lymphoma, ALK– | 5 (19) | 1 (14) |
| Anaplastic large cell lymphoma, ALK+ | 1 (4) | 3 (43) |
| NK/T-cell lymphoma | 0 | 2 (29) |
| Enteropathy-associated T-cell lymphoma | 1 (4) | 0 |
| Monomorphic epitheliotropic intestinal T-cell lymphoma | 1 (4) | 0 |
| Prior regimen(s), n (%) | ||
| CHOEP | 13 (50) | |
| CHOP | 5 (19) | |
| EPOCH | 2 (8) | |
| CHOEP + lenalidomide | 2 (8) | |
| SMILE | 0 | 2 (29) |
| Others (CHOEP/ICE, CHOEP + HD-MTX, CHOP/CHOEP, CHOEP/CHEP, brentuximab vedotin, pralatrexate, R-EPOCH, R-CHOP, ICE) | 4 (15) | 5 (71) |
| Age at transplant, median (range) | 59 (37-73) | 59 (23-68) |
| Sex, n (%) | ||
| Male | 16 (62) | 2 (29) |
| Female | 10 (38) | 5 (71) |
| Disease status at transplant, n (%) | ||
| Complete response | 23 (88) | 6 (86) |
| Partial response | 3 (12) | 1 (14) |
| Median time from AHCT to first romidepsin, d (range) | 75 (42-102) | 49 (44-80) |
| Total doses of romidepsin, median (range) | 21 (1-33) | 8 (2-32) |
| Characteristics . | Cohort 1 . | Cohort 2 . |
|---|---|---|
| (N = 26) . | (N = 7) . | |
| Histology, n(%) | ||
| Angioimmunoblastic T-cell lymphoma | 11 (42) | 1 (14) |
| PTCL, not otherwise specified | 7 (27) | 0 |
| Anaplastic large cell lymphoma, ALK– | 5 (19) | 1 (14) |
| Anaplastic large cell lymphoma, ALK+ | 1 (4) | 3 (43) |
| NK/T-cell lymphoma | 0 | 2 (29) |
| Enteropathy-associated T-cell lymphoma | 1 (4) | 0 |
| Monomorphic epitheliotropic intestinal T-cell lymphoma | 1 (4) | 0 |
| Prior regimen(s), n (%) | ||
| CHOEP | 13 (50) | |
| CHOP | 5 (19) | |
| EPOCH | 2 (8) | |
| CHOEP + lenalidomide | 2 (8) | |
| SMILE | 0 | 2 (29) |
| Others (CHOEP/ICE, CHOEP + HD-MTX, CHOP/CHOEP, CHOEP/CHEP, brentuximab vedotin, pralatrexate, R-EPOCH, R-CHOP, ICE) | 4 (15) | 5 (71) |
| Age at transplant, median (range) | 59 (37-73) | 59 (23-68) |
| Sex, n (%) | ||
| Male | 16 (62) | 2 (29) |
| Female | 10 (38) | 5 (71) |
| Disease status at transplant, n (%) | ||
| Complete response | 23 (88) | 6 (86) |
| Partial response | 3 (12) | 1 (14) |
| Median time from AHCT to first romidepsin, d (range) | 75 (42-102) | 49 (44-80) |
| Total doses of romidepsin, median (range) | 21 (1-33) | 8 (2-32) |
EPOCH, etoposide, prednisone, doxorubicin, cyclophosphamide, and vincristine; HD-MTX, high-dose methotrexate; ICE, ifosfamide, carboplatin, and etoposide; NK, natural killer cell; SMILE, dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide.
Outcome measures
The primary objective of this study was to estimate the PFS at 2 years after AHCT for patients undergoing AHCT in CR1 or PR1 with standard-risk histologies. Secondary objectives included PFS at 2 years after transplantation for patients with high-risk histologies or transplant in ≥CR2/PR2, an estimate of OS at 2 years after transplantation, OS and PFS at 1 year after romidepsin completion, and tolerance to romidepsin after AHCT. OS was defined as the time to death or last follow-up from the AHCT date. CR and PR were defined in accordance with the Revised Response Criteria for Malignant Lymphoma,18 using positron emission tomography scans. Pretreatment bone marrow biopsies were required for patients with prior marrow involvement. Toxicities were scored using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
PFS and OS were estimated using the Kaplan-Meier analysis. The expected 2-year PFS after AHCT was 45%, and the study was designed for a desired 2-year PFS after transplantation of 70% with 90% power and 10% Type I error. Success was achieved if ≥15 of the first 25 patients enrolled in the study were progression-free 2 years post-AHCT. Patients who received romidepsin after transplantation were evaluable for the primary end point. Any patient who did not receive romidepsin after transplantation was replaced per protocol.
Results
Patients
Forty-seven patients consented to the trial and 13 patients did not receive romidepsin. Two patients did not undergo AHCT. Three patients relapsed before the first dose of romidepsin. One patient had progression of disease ∼1 month after transplantation, and 2 progressed between 2 and 3 months after transplantation. Three patients had cardiac issues precluding the administration of romidepsin (corrected QT interval prolongation in 1 patient, atrial fibrillation in a second, and abnormal ejection fraction in a third). Five patients declined treatment before the first dose of romidepsin. One patient received romidepsin but was later reclassified to a diagnosis of myeloid sarcoma and was removed from the analysis. Patient characteristics are summarized in Table 1. The most common histology represented in the trial was AITL.
Outcomes
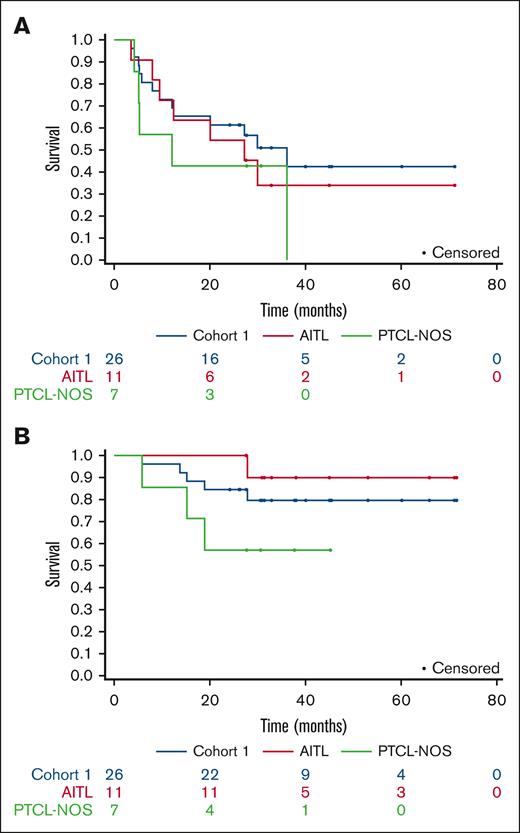
With a median follow-up of 32 months for patients remaining progression-free (range, 24-36), 15 out of the first 25 patients in cohort 1 were progression-free after 2 years. There were 12 total events among all 26 patients. The estimated 2-year PFS from diagnosis was 65% (95% CI, 49-86), and the estimated 2-year PFS from transplantation was 62% (95% CI, 45-83) (Figure 1). The median PFS was not reached (NR) (95% CI, 12 to NR). In cohort 2, with a median progression-free follow-up of 30 months (range, 24-37), there were 4 events among 7 patients. The estimated 2-year PFS rate was 43% (95% CI, 18-100). The median PFS was 14 months (95% CI, 5 to NR). For all patients, the estimated 2-year OS was 79% (95% CI, 66-94), and the median OS was NR (95% CI, 46 to NR). The overall follow-up time among the survivors was 38 months (range, 21-72 months). After romidepsin maintenance, the survivors were followed up for 22 months (range, 3-67 months). The estimated PFS at 1-year poststudy treatment was 52% (95% CI, 35-76) in cohort 1 and 43% (95% CI, 18-100) in cohort 2. In an exploratory subanalysis of 11 patients with AITL, the median PFS was 27 months (range, 12 to NR). Two-year PFS was 55% (95% CI, 32-94). One additional progression event was noted after the 36-month assessment period in cohort 1. With this additional follow-up, the median PFS in cohort 1 was 36 months (95% CI, 12.4 to NR) (Figure 1).
Survival curves. (A) PFS and (B) OS for cohort 1 patients, as assessed using Kaplan-Meier analysis. NOS, not otherwise specified.
Survival curves. (A) PFS and (B) OS for cohort 1 patients, as assessed using Kaplan-Meier analysis. NOS, not otherwise specified.
Toxicities
Thirty-four patients were evaluated for toxicity (Table 2). Across cohorts, 3 patients chose to withdraw from maintenance treatment (moved away from the study center = 1, infusion schedule too burdensome = 1, and poor quality of life [QOL] = 1). Three patients came off study due to toxicity (recurrent upper respiratory infections = 1, fatigue and anorexia = 1, weakness and pleural effusion = 1). Two patients required dose reduction from 14 mg/m2 to 10 mg/m2 (muscle pain/weakness = 1 and fatigue = 1), and 3 required further dose reduction to 8 mg/m2 (grade 3 neutropenia = 1, grade 3 neutropenia/thrombocytopenia = 1, and nausea/vomiting = 1). The most common toxicities (≥10% of patients, all grades) were nausea (n = 23, 68%), fatigue (n = 22, 65%), anemia (n = 16, 47%), decreased platelet count (n = 16, 48%), and white blood cells and neutrophils (all n = 15, 44%). The most common grade 3/4 toxicities were neutropenia (n = 8, 24%) and lymphopenia (n = 7, 21%). After starting romidepsin, 1 patient developed cytomegalovirus retinitis. One patient required hospitalization for febrile neutropenia, and 2 patients were hospitalized with fever. One patient was noted to have prolonged QTc on electrocardiogram; cardiac toxicities were otherwise not noted.
Romidepsin-related toxicities occurring in more than 15% of patients
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . |
|---|---|---|---|---|---|
| Nausea | 20 | 3 | 0 | 0 | 23 |
| Fatigue | 16 | 5 | 1 | 0 | 22 |
| Anemia | 8 | 4 | 4 | 0 | 16 |
| Neutrophil count decreased | 2 | 5 | 5 | 3 | 15 |
| Platelet count decreased | 10 | 1 | 2 | 2 | 15 |
| White blood cells decreased | 5 | 6 | 3 | 1 | 15 |
| Dysgeusia | 9 | 4 | 0 | 0 | 13 |
| Lymphocyte count decreased | 2 | 1 | 5 | 2 | 10 |
| Aspartate aminotransferase increased | 6 | 1 | 1 | 0 | 8 |
| Blood bilirubin increased | 3 | 3 | 1 | 0 | 7 |
| Hyperglycemia | 3 | 2 | 2 | 0 | 7 |
| Alanine aminotransferase increased | 4 | 1 | 1 | 0 | 6 |
| Vomiting | 5 | 1 | 0 | 0 | 6 |
| Anorexia | 2 | 3 | 0 | 0 | 5 |
| Constipation | 4 | 1 | 0 | 0 | 5 |
| Generalized muscle weakness | 4 | 0 | 1 | 0 | 5 |
| Hypoalbuminemia | 2 | 3 | 0 | 0 | 5 |
| Upper respiratory infection | 1 | 4 | 0 | 0 | 5 |
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . |
|---|---|---|---|---|---|
| Nausea | 20 | 3 | 0 | 0 | 23 |
| Fatigue | 16 | 5 | 1 | 0 | 22 |
| Anemia | 8 | 4 | 4 | 0 | 16 |
| Neutrophil count decreased | 2 | 5 | 5 | 3 | 15 |
| Platelet count decreased | 10 | 1 | 2 | 2 | 15 |
| White blood cells decreased | 5 | 6 | 3 | 1 | 15 |
| Dysgeusia | 9 | 4 | 0 | 0 | 13 |
| Lymphocyte count decreased | 2 | 1 | 5 | 2 | 10 |
| Aspartate aminotransferase increased | 6 | 1 | 1 | 0 | 8 |
| Blood bilirubin increased | 3 | 3 | 1 | 0 | 7 |
| Hyperglycemia | 3 | 2 | 2 | 0 | 7 |
| Alanine aminotransferase increased | 4 | 1 | 1 | 0 | 6 |
| Vomiting | 5 | 1 | 0 | 0 | 6 |
| Anorexia | 2 | 3 | 0 | 0 | 5 |
| Constipation | 4 | 1 | 0 | 0 | 5 |
| Generalized muscle weakness | 4 | 0 | 1 | 0 | 5 |
| Hypoalbuminemia | 2 | 3 | 0 | 0 | 5 |
| Upper respiratory infection | 1 | 4 | 0 | 0 | 5 |
Discussion
PTCL is a heterogenous and generally aggressive disease, and there is an unmet therapeutic need for improved up-front treatments. AHCT as consolidation in first remission is a therapeutic strategy that has been associated with improved PFS in phase 2 studies, registry studies, and retrospective analyses.1,11,19,20 In this article, we present the results of the maintenance of romidepsin after AHCT for PTCL. Although the study did not meet its desired primary efficacy end point, treatment with maintenance romidepsin resulted in an estimated 2-year PFS of 62%. By intention-to-treat, historical controls have shown PFS of 36% to 44%.11-13 A study powered to meet a smaller PFS improvement may demonstrate a benefit for maintenance romidepsin. Of the patients who received treatment, responses were durable, with a median PFS of 36 months in cohort 1. Regardless, there was no plateau on the Kaplan-Meier curve. Treatment with maintenance romidepsin was also well-tolerated in the posttransplantation setting. Most toxicities were hematologic in nature and most patients were able to continue treatment.
The limitations of the study include the small sample size and the single-arm design. This nonrandomized study would not be sufficient to demonstrate the superiority of a maintenance approach after AHCT. In addition, PTCL is an aggressive malignancy, and patients remain at risk of relapse during the time interval between transplantation and the initiation of maintenance. In our study, patients consented to maintenance therapy before transplantation. Thirteen of 47 (28%) patients who initially consented, did not receive maintenance. Two of the 13 (15%) patients did not receive transplantation, and 3 of the 13 (23%) progressed before the first dose of romidepsin. These patients, who were at higher risk for progression, would need alternative consolidative strategies to prolong remission. Notably, we saw evidence of later relapses, with 1 progression event occurring after the 36-month follow-up period in cohort 1. A phase 2 study of 8 cycles of pembrolizumab maintenance after AHCT for PTCL demonstrated a promising 18-month PFS of 83.6% but did not extend follow-up beyond 18 months.17 Maintenance treatments may prolong PFS in these aggressive lymphomas, instead of leading to a durable remission or cure. Future studies evaluating the superiority of a maintenance approach in PTCL would benefit from an extended follow-up. Finally, our study identified nausea, fatigue, and dysgeusia as common toxicities (Table 2) that can have a significant impact on QOL. As QOL is a major consideration in a maintenance strategy, future studies should include patient-reported outcomes to better characterize the tolerability of treatments.
Over the past several years, our understanding of the molecular heterogeneity and oncogenesis of histologies within PTCL has improved. Instead of treating all subtypes using the same approach, specific histologies may benefit from targeted treatments. Retrospective data indicate that histone deacetylase inhibitors, such as romidepsin, may have superior efficacy in AITL or PTCL-Tfh when compared with other subtypes of PTCL.21 Prospective data with romidepsin in addition to CHOP also suggest superior efficacy for PTCL-Tfh.8 In our population, we did not note improved PFS or OS in the AITL subgroup; however, definitive conclusions are limited by the small sample size of patients. In pembrolizumab maintenance after AHCT, no relapses were seen among 3 patients with extranodal natural killer T-cell lymphoma, a disease subtype with known sensitivity to programmed cell death protein-1 inhibition.17 A clinical trial to evaluate the efficacy of brentuximab vedotin maintenance after ASCT for CD30+ PTCL is currently underway (ClinicalTrials.gov identifier: NCT04334174). Future exploration of maintenance therapy in PTCL should be informed by the rational selection of appropriate targets based on cell markers or mutations that may drive specific lymphomas.
Acknowledgments
This work was supported by the Lymphoma Research Foundation, the Leukemia and Lymphoma Society (number 7026-21 and number 2332-70), and Nonna's Garden Foundation. The research was also supported by National Cancer Institute grant P30CA008748.
Authorship
Contribution: S.G. and S.M.H. designed the study; N.K., A.R.S., F.K., M.S., J.R., A.J.M., A.D.Z., A.N., D.J.S., P.D., A.H., A.K., C.S.S., G.L.S., M.J.M., T.D., H.H., N. Galasso, N. Ganesan, K.v.B., S.G., S.M.H., and P.B.D. collected the data; N.K., N. Ganesan, E.D., P.B.D., and S.M.H. analyzed the data; N.K., P.B.D., and S.M.H. wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.K. reports research support from Genentech; consulting from ADC Therapeutics; and honoraria from Kyowa Kirin. S.M.H. reports research support from ADC Therapeutics, Affimed, Aileron, Celgene, CRISPR Therapeutics, Daiichi Sankyo, Forty-Seven, Inc, Kyowa Hakko Kirin, Millennium/Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio; and consulting from Acrotech Biopharma, ADC Therapeutics, Astex, Auxilus Pharma, Merck, C4 Therapeutics, Celgene, Cimieo Therapeutics, Daiichi Sankyo, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, SecuraBio, Shoreline Biosciences, Inc, Takeda, Trillium Therapeutics, Tubulis, Verastem/SecuraBio, Vividion Therapeutics, and Yingli Pharma Ltd. A.J.M. reports research support from ADC Therapeutics, BeiGene, Miragen, Seattle Genetics, Merck, Bristol Myers Squibb (BMS), Incyte, and SecuraBio; and honoraria from Affimed, Imbrium Therapeutics L.P./Purdue, Janpix Ltd, Merck, Seattle Genetics, and Takeda. A.D.Z. reports financial compensation for consulting from Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, MorphoSys, AbbVie, AstraZeneca, and MEI Pharma; research funding from MEI Pharmaceuticals, Genentech/Roche, BeiGene, National Institutes of Health Specialized Programs of Research Excellence principal investigator (receives salary support and funds awarded to M.S.K.); and has served on the data monitoring committee for BeiGene (Chair) and BMS/Celgene/Juno. A.N. reports research funding from Pharmacyclics/AbbVie, Kite/Gilead, and Cornerstone; consultancy for Janssen, Morphosys, Cornerstone, Epizyme, EUSA Pharma, TG Therapeutics, ADC Therapeutics, and AstraZeneca; and honoraria from Pharmacyclics/AbbVie. M.S. reports research funding from Mustang Bio, BMS, Pharmacyclics, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; and consultancy for AbbVie, Genentech, AstraZeneca, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC Therapeutics, Fate Therapeutics, Janssen, and MEI Pharma. The remaining authors declare no competing financial interests.
Correspondence: Niloufer Khan, Division of Lymphoma, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; email: niloufer.khan@gmail.com.
References
Author notes
N.K. and P.B.D. contributed equally to this study.
Deidentified data are available upon request from the corresponding author, Niloufer Khan (niloufer.khan@gmail.com).
The full-text version of this article contains a data supplement.