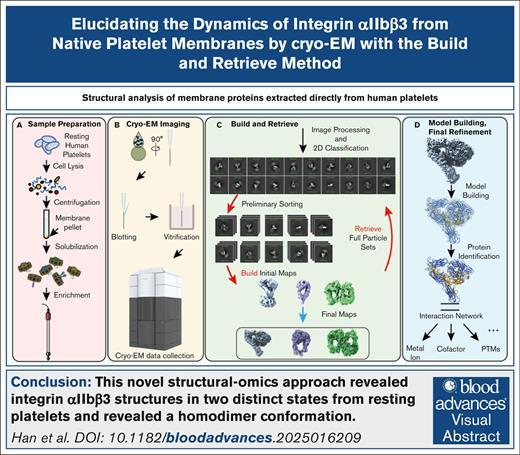
Key Points
We report the first structural analysis of platelet membrane proteins extracted directly from human platelet membranes.
Our novel structural-omics approach allowed us to solve integrin αIIbβ3 structures in 2 distinct states from resting human platelets.
Visual Abstract
Platelets fulfill their essential physiological roles sensing the extracellular environment through their membrane proteins. The native membrane environment provides essential regulatory cues that affect the protein structure and mechanism of action. Single-particle cryogenic electron microscopy (cryo-EM) has transformed structural biology by allowing high-resolution structures of membrane proteins to be solved from homogeneous samples. Our recent breakthroughs in data processing now make it feasible to obtain atomic-level-resolution protein structures from crude preparations in their native environments by integrating cryo-EM with the “build-and-retrieve” (BaR) data processing methodology. We applied this iterative bottom-up methodology on resting human platelet membranes for an in-depth systems biology approach to uncover how lipids, metal binding, post-translational modifications, and cofactor associations in the native environment regulate platelet function at the molecular level. Here, we report using cryo-EM followed by the BaR method to solve the unmodified integrin αIIbβ3 structure directly from resting human platelet membranes in its inactivated and intermediate states at 2.75 and 2.67 Å, respectively. Furthermore, we also solved a novel dimer conformation of αIIbβ3 at 2.85 Å formed by 2 intermediate states of αIIbβ3. This may indicate a previously unknown self-regulatory mechanism of αIIbβ3 in its native environment. In conclusion, our data show the power of using cryo-EM with the BaR method to determine 3 distinct structures including a novel dimer directly from natural sources. This approach allows us to identify unrecognized regulation mechanisms for proteins without artifacts owing to purification processes. These data have the potential to enrich our understanding of platelet signaling circuitry.
Introduction
Platelets are central players in primary hemostasis, thrombosis, inflammation, and vascular biology.1,2 They also play critical roles in diverse physiological and pathological processes, including inflammation, host defense, cancer, neurodegenerative, and cardiovascular diseases.3-5 Platelet membrane proteins respond to environmental changes by relaying the extracellular information across the membrane to mediate the platelet response.5-7 Although platelets patrol the vascular system’s integrity, membrane proteins sense and are regulated by their microenvironment. Understanding the interaction network of platelet membrane proteins will enhance our understanding of platelet biology.
Structural biology approaches such as crystallography and cryogenic electron microscopy (cryo-EM) have uncovered the functions of platelet membrane proteins by obtaining high-resolution structures of G-protein coupled receptors, adhesion molecules, and other surface receptors.8-10 They have also uncovered the binding sites and mechanisms of antiplatelet therapies.11,12 However, to understand the impact of the native environment on platelet physiology, we need approaches beyond traditional structural biology. Combining proteomics with high-resolution unmodified protein complex structures is essential for advancing our knowledge of platelet membrane protein function in situ. Although traditional proteomics offers valuable network insights, it lacks high-resolution structural information.13,14 Similarly, traditional structural approaches require highly purified protein samples, limiting their application in studying integrated systems within cells, tissues, or organs.15 Moreover, the dynamics of membrane proteins pose further challenges to obtaining structural information without introducing stabilizing modifications in overexpression systems.16-18 Finally, extensive purification of exogenously expressed proteins can lead to the loss of native regulatory cues and rare protein conformations.
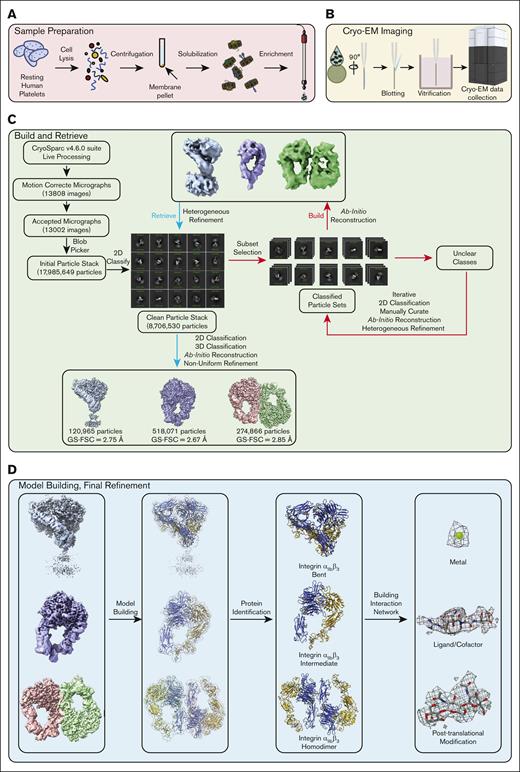
Recent advancements in data processing enable unprecedented insights into platelet membrane proteins in their native environment. These cutting-edge developments in the data processing algorithm make using cryo-EM for studying whole-cell, tissue, or organ samples feasible.19-23 A novel iterative methodology, build-and-retrieve (BaR), was developed to process heterogeneous cryo-EM sample data sets (Figure 1).21 This method allows us to analyze crude samples through in silico purification during data processing from a large heterogeneous data set.19-23
Build-and-Retrieve (BaR) Pipeline. Integrating cryo-EM with the BaR data processing methodology allows us to obtain atomic-level-resolution protein structures directly from crude preparations straight from their native environment. After sample extraction (A), a total of 13808 raw micrographs were collected using cryo-EM (B). Following the BaR protocol (C), an unbiased selection was applied on 13,002 motion-corrected micrographs to generate 17,985,649 initial particles. The 2D classification was used to generate a subset of a clean particle stack that contains 8,706,530 particles. Classes of particles that contain clear 2D features were used to “build” the low-resolution initial models, which were used to “retrieve” more particles from the clean particle stack. The improved maps at ∼3 Å resolution were used for protein identification (D), and final maps were used for model building and refinement. 2D, 2-dimensional; 3D, 3-dimensional; FSC, Fourier shell correlation; GS-FSC, gold-standard Fourier shell correlation.
Build-and-Retrieve (BaR) Pipeline. Integrating cryo-EM with the BaR data processing methodology allows us to obtain atomic-level-resolution protein structures directly from crude preparations straight from their native environment. After sample extraction (A), a total of 13808 raw micrographs were collected using cryo-EM (B). Following the BaR protocol (C), an unbiased selection was applied on 13,002 motion-corrected micrographs to generate 17,985,649 initial particles. The 2D classification was used to generate a subset of a clean particle stack that contains 8,706,530 particles. Classes of particles that contain clear 2D features were used to “build” the low-resolution initial models, which were used to “retrieve” more particles from the clean particle stack. The improved maps at ∼3 Å resolution were used for protein identification (D), and final maps were used for model building and refinement. 2D, 2-dimensional; 3D, 3-dimensional; FSC, Fourier shell correlation; GS-FSC, gold-standard Fourier shell correlation.
In this study, we applied cryo-EM coupled with BaR to resting platelet membrane proteins at ∼200 kDa from healthy individuals. We solved the structures of αIIbβ3 directly from native human platelet membranes. We identified αIIbβ3 structures in 2 distinct states, the inactivated and intermediate states, at 2.75 and 2.67 Å, respectively. We also observed a novel homodimer conformation at 2.85 Å in which the head regions of 2 αIIbβ3 molecules in intermediate states were facing each other.
Materials and methods
Cryo-EM sample preparation
Data collection and processing
Data were collected on a Titan Krios with a K3 detector in super-resolution mode at 81 000 magnifications and processed in the CryoSPARC v4.6.0 suite using the BaR protocol as described previously.19-23 The strategies are presented in Figure 1. Protein identification, model building, and refinement were performed as described.19-23 The detailed protocols are described in the supplemental Methods.
Quantification and statistical analysis
Gold-standard Fourier shell correlation curves at a threshold of 0.143 were computed using CryoSPARC v4.6.0 to obtain the final resolutions of protein models. The final atomic models were evaluated using MolProbity.
Proteomic analysis of platelet membrane fractions
Sample preparation for proteomic analysis was performed as described previously.20,21 The detailed protocols for protein digestion and identification are described in the supplemental Methods.
Blood was collected from healthy volunteers after consent was obtained in accordance with the Case Western Reserve University institutional review board–approved protocol.
Results
Using cryo-EM coupled with the BaR data processing methodology (Figure 1), we solved 3 distinct cryo-EM maps at resolutions of 2.75, 2.67, and 2.85 Å, respectively (Table 1; Figure 1). After model building and protein identification, all 3 maps were integrin αIIbβ3 in 3 different states: inactivated bent state, intermediate extended state, and a novel homodimer state that was formed by 2 integrin molecules in the extended state. Although we did not target integrin αIIbβ3 specifically or intentionally, it was not surprising that integrin αIIbβ3 was the first identified protein given that it is one of the most abundant membrane proteins on human platelets at ∼200 kDa.24
Cryo-EM data collection, processing, and refinement statistics
| Data set . | Bent conformation . | Intermediate conformation . | Homodimer conformation . |
|---|---|---|---|
| Data collection and processing | |||
| Magnification | 81 000 | ||
| Voltage (kV) | 300 | ||
| Electron microscope | Krios-GIF-K3 | ||
| Defocus range | −0.8 to 1.5 | ||
| Total exposure time (s) | 4 | ||
| Pixel size (Å) | 1.07 | ||
| Total dose (e−/A2) | 37.3 | ||
| No. of frames | 40 | ||
| Dose rate (e−/phys. pixel/s) | 11.71 | ||
| No. of initial micrographs | 13 807 | ||
| No. of initial particles | 17 985 649 | ||
| No. of final particles | 120 965 | 518 071 | 274 866 |
| Symmetry | C1 | C1 | C1 |
| GS-FSC resolution (Å) | 2.75 | 2.67 | 2.85 |
| FSC threshold | 0.143 | ||
| Refinement | |||
| Initial model | AlphaFold | ||
| Model resolution cutoff (Å) | 0.1 | 0.1 | 0.1 |
| No. of protein residues | 1602 | 1106 | 2196 |
| No. of ligands | Ca = 7 | Ca = 6 | Ca = 12 |
| Mg = 1 | Mg = 1 | Mg = 2 | |
| NAG = 18 | NAG =, 10 | NAG = 18 | |
| BMA = 4 | BMA = 3 | BMA = 3 | |
| RMSD | |||
| Bond lengths (Å) | 0.005 | 0.007 | 0.004 |
| Bond angles (°) | 0.704 | 0.861 | 0.650 |
| Validation | |||
| MolProbity score | 2.56 | 2.13 | 2.66 |
| Clash score | 25.16 | 10.81 | 15.26 |
| Rotamer outliers (%) | 2.49 | 1.73 | 4.59 |
| Ramachandran plot (%) | |||
| Favored (%) | 94.59 | 94.19 | 92.49 |
| Allowed (%) | 5.22 | 5.63 | 7.19 |
| Disallowed (%) | 0.19 | 0.18 | 0.32 |
| Data set . | Bent conformation . | Intermediate conformation . | Homodimer conformation . |
|---|---|---|---|
| Data collection and processing | |||
| Magnification | 81 000 | ||
| Voltage (kV) | 300 | ||
| Electron microscope | Krios-GIF-K3 | ||
| Defocus range | −0.8 to 1.5 | ||
| Total exposure time (s) | 4 | ||
| Pixel size (Å) | 1.07 | ||
| Total dose (e−/A2) | 37.3 | ||
| No. of frames | 40 | ||
| Dose rate (e−/phys. pixel/s) | 11.71 | ||
| No. of initial micrographs | 13 807 | ||
| No. of initial particles | 17 985 649 | ||
| No. of final particles | 120 965 | 518 071 | 274 866 |
| Symmetry | C1 | C1 | C1 |
| GS-FSC resolution (Å) | 2.75 | 2.67 | 2.85 |
| FSC threshold | 0.143 | ||
| Refinement | |||
| Initial model | AlphaFold | ||
| Model resolution cutoff (Å) | 0.1 | 0.1 | 0.1 |
| No. of protein residues | 1602 | 1106 | 2196 |
| No. of ligands | Ca = 7 | Ca = 6 | Ca = 12 |
| Mg = 1 | Mg = 1 | Mg = 2 | |
| NAG = 18 | NAG =, 10 | NAG = 18 | |
| BMA = 4 | BMA = 3 | BMA = 3 | |
| RMSD | |||
| Bond lengths (Å) | 0.005 | 0.007 | 0.004 |
| Bond angles (°) | 0.704 | 0.861 | 0.650 |
| Validation | |||
| MolProbity score | 2.56 | 2.13 | 2.66 |
| Clash score | 25.16 | 10.81 | 15.26 |
| Rotamer outliers (%) | 2.49 | 1.73 | 4.59 |
| Ramachandran plot (%) | |||
| Favored (%) | 94.59 | 94.19 | 92.49 |
| Allowed (%) | 5.22 | 5.63 | 7.19 |
| Disallowed (%) | 0.19 | 0.18 | 0.32 |
BMA, beta-D-mannose; Ca, calcium; Mg, magnesium; NAG, N-acetylglucosamine.
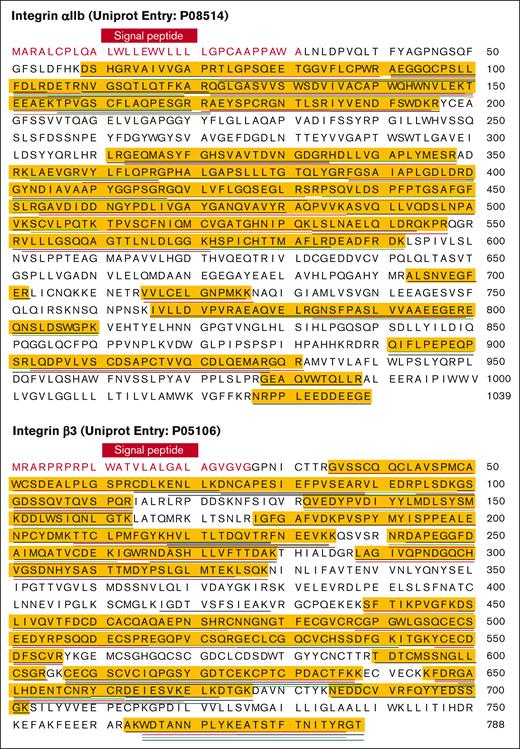
We used proteomics to investigate the composition of the peak at ∼200 kDa and to verify the existence of integrin αIIbβ3 in the sample. We identified ∼800 different proteins. The 35 most abundant proteins in the peak, ranked by intensity-based absolute quantification values, are presented in Table 2. This is calculated by summing the intensities of all detected peptides of a protein and dividing them by the number of theoretically observable peptides for that protein. We confirmed the presence of integrin αIIbβ3 (Figure 2; supplemental Table 3). Unsurprisingly, the integrin β3 subunit was the second most abundant protein, followed by integrin αIIb as the third (Table 2).
Top 35 proteins in the MS study
| Protein IDs (UniProt) . | Protein names . | Gene names . | Molecular weight (kDa) . | MS/MS count . | iBAQ (high to low) . | Peptides . | Sequence coverage (%) . |
|---|---|---|---|---|---|---|---|
| P02647 | Apolipoprotein A-I | APOA1 | 30.777 | 324 | 28.0858287811279 | 42 | 83.1 |
| P05106 | Integrin β3 | ITGB3 | 87.057 | 1076 | 28.0384712219238 | 59 | 63.8 |
| P08514 | Integrin αIIb | ITGA2B | 113.38 | 1856 | 26.633394241333 | 64 | 52.6 |
| P27105 | Erythrocyte band 7 integral membrane protein | STOM | 31.73 | 172 | 26.4323234558105 | 18 | 72.9 |
| P02776 | Platelet factor 4 | PF4 | 10.845 | 32 | 26.428861618042 | 6 | 36.6 |
| Q93084 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 | ATP2A3 | 109.25 | 349 | 26.3005466461182 | 56 | 48.9 |
| P16671 | Platelet glycoprotein 4 | CD36 | 53.053 | 111 | 26.1360607147217 | 20 | 37.1 |
| P13224 | Platelet glycoprotein Ib beta chain | GP1BB | 21.717 | 21 | 26.1172771453857 | 6 | 23.8 |
| P21926 | CD9 antigen | CD9 | 25.416 | 28 | 26.076961517334 | 4 | 15.4 |
| P14406 | Cytochrome c oxidase subunit 7A2, mitochondrial | COX7A2 | 9.3959 | 11 | 25.9447402954102 | 3 | 27.7 |
| P09669 | Cytochrome c oxidase subunit 6C | COX6C | 8.7813 | 10 | 25.8964748382568 | 5 | 38.7 |
| P02675;CON__P02676 | Fibrinogen beta chain | FGB | 55.928 | 347 | 25.8412456512451 | 48 | 76.8 |
| P06576 | ATP synthase subunit beta, mitochondrial | ATP5B | 56.559 | 314 | 25.5996017456055 | 29 | 73.7 |
| P25705 | ATP synthase subunit alpha, mitochondrial | ATP5A1 | 59.75 | 238 | 25.5468673706055 | 49 | 66.9 |
| P61224;A6NIZ1 | Ras-related protein Rap-1b | RAP1B | 20.825 | 60 | 25.5407028198242 | 13 | 67.4 |
| P14770 | Platelet glycoprotein IX | GP9 | 19.046 | 41 | 25.5176124572754 | 6 | 30.5 |
| P02679 | Fibrinogen gamma chain | FGG | 51.511 | 215 | 25.493444442749 | 38 | 72 |
| P05556 | Integrin β1 | ITGB1 | 88.414 | 223 | 25.4156093597412 | 43 | 47.6 |
| P62873;P16520 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | GNB1 | 37.377 | 80 | 25.2648124694824 | 19 | 73.2 |
| P56385 | ATP synthase subunit e, mitochondrial | ATP5I | 7.9331 | 27 | 25.2109661102295 | 7 | 71 |
| P48047 | ATP synthase subunit O, mitochondrial | ATP5O | 23.277 | 63 | 25.2020225524902 | 15 | 75.1 |
| P20674 | Cytochrome c oxidase subunit 5A, mitochondrial | COX5A | 16.762 | 26 | 25.1677932739258 | 7 | 38 |
| P07359 | Platelet glycoprotein Ib alpha chain | GP1BA | 71.539 | 76 | 25.161771774292 | 15 | 21.3 |
| O75947 | ATP synthase subunit d, mitochondrial | ATP5H | 18.491 | 67 | 25.0077419281006 | 14 | 84.5 |
| P30273 | High affinity immunoglobulin epsilon receptor subunit gamma | FCER1G | 9.6674 | 8 | 24.9811992645264 | 3 | 30.2 |
| P04899;P11488;P19087;A8MTJ3;P09471 | Guanine nucleotide-binding protein G(i) subunit α2 | GNAI2 | 40.45 | 148 | 24.9714965820313 | 29 | 72.4 |
| P16284 | Platelet endothelial cell adhesion molecule | PECAM1 | 82.521 | 248 | 24.9365634918213 | 40 | 53.5 |
| Q9Y490;Q9Y4G6 | Talin-1 | TLN1 | 269.76 | 820 | 24.7005462646484 | 156 | 73.4 |
| P02489 | Alpha-crystallin A chain | CRYAA | 19.909 | 54 | 24.5251007080078 | 7 | 36.4 |
| P14625;Q58FF3 | Endoplasmin | HSP90B1 | 92.468 | 172 | 24.4852981567383 | 43 | 53.1 |
| O75964 | ATP synthase subunit g, mitochondrial | ATP5L | 11.428 | 23 | 24.4625129699707 | 6 | 52.4 |
| P23229 | Integrin α6 | ITGA6 | 126.6 | 233 | 24.3582363128662 | 56 | 50 |
| Q9Y277 | Voltage-dependent anion-selective channel protein 3 | VDAC3 | 30.658 | 53 | 24.3242301940918 | 14 | 62.2 |
| P40197 | Platelet glycoprotein V | GP5 | 60.958 | 134 | 24.2378311157227 | 24 | 50.5 |
| P21333;Q14315;O75369 | Filamin A | FLNA | 280.74 | 658 | 24.2292003631592 | 155 | 61.5 |
| Protein IDs (UniProt) . | Protein names . | Gene names . | Molecular weight (kDa) . | MS/MS count . | iBAQ (high to low) . | Peptides . | Sequence coverage (%) . |
|---|---|---|---|---|---|---|---|
| P02647 | Apolipoprotein A-I | APOA1 | 30.777 | 324 | 28.0858287811279 | 42 | 83.1 |
| P05106 | Integrin β3 | ITGB3 | 87.057 | 1076 | 28.0384712219238 | 59 | 63.8 |
| P08514 | Integrin αIIb | ITGA2B | 113.38 | 1856 | 26.633394241333 | 64 | 52.6 |
| P27105 | Erythrocyte band 7 integral membrane protein | STOM | 31.73 | 172 | 26.4323234558105 | 18 | 72.9 |
| P02776 | Platelet factor 4 | PF4 | 10.845 | 32 | 26.428861618042 | 6 | 36.6 |
| Q93084 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 | ATP2A3 | 109.25 | 349 | 26.3005466461182 | 56 | 48.9 |
| P16671 | Platelet glycoprotein 4 | CD36 | 53.053 | 111 | 26.1360607147217 | 20 | 37.1 |
| P13224 | Platelet glycoprotein Ib beta chain | GP1BB | 21.717 | 21 | 26.1172771453857 | 6 | 23.8 |
| P21926 | CD9 antigen | CD9 | 25.416 | 28 | 26.076961517334 | 4 | 15.4 |
| P14406 | Cytochrome c oxidase subunit 7A2, mitochondrial | COX7A2 | 9.3959 | 11 | 25.9447402954102 | 3 | 27.7 |
| P09669 | Cytochrome c oxidase subunit 6C | COX6C | 8.7813 | 10 | 25.8964748382568 | 5 | 38.7 |
| P02675;CON__P02676 | Fibrinogen beta chain | FGB | 55.928 | 347 | 25.8412456512451 | 48 | 76.8 |
| P06576 | ATP synthase subunit beta, mitochondrial | ATP5B | 56.559 | 314 | 25.5996017456055 | 29 | 73.7 |
| P25705 | ATP synthase subunit alpha, mitochondrial | ATP5A1 | 59.75 | 238 | 25.5468673706055 | 49 | 66.9 |
| P61224;A6NIZ1 | Ras-related protein Rap-1b | RAP1B | 20.825 | 60 | 25.5407028198242 | 13 | 67.4 |
| P14770 | Platelet glycoprotein IX | GP9 | 19.046 | 41 | 25.5176124572754 | 6 | 30.5 |
| P02679 | Fibrinogen gamma chain | FGG | 51.511 | 215 | 25.493444442749 | 38 | 72 |
| P05556 | Integrin β1 | ITGB1 | 88.414 | 223 | 25.4156093597412 | 43 | 47.6 |
| P62873;P16520 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | GNB1 | 37.377 | 80 | 25.2648124694824 | 19 | 73.2 |
| P56385 | ATP synthase subunit e, mitochondrial | ATP5I | 7.9331 | 27 | 25.2109661102295 | 7 | 71 |
| P48047 | ATP synthase subunit O, mitochondrial | ATP5O | 23.277 | 63 | 25.2020225524902 | 15 | 75.1 |
| P20674 | Cytochrome c oxidase subunit 5A, mitochondrial | COX5A | 16.762 | 26 | 25.1677932739258 | 7 | 38 |
| P07359 | Platelet glycoprotein Ib alpha chain | GP1BA | 71.539 | 76 | 25.161771774292 | 15 | 21.3 |
| O75947 | ATP synthase subunit d, mitochondrial | ATP5H | 18.491 | 67 | 25.0077419281006 | 14 | 84.5 |
| P30273 | High affinity immunoglobulin epsilon receptor subunit gamma | FCER1G | 9.6674 | 8 | 24.9811992645264 | 3 | 30.2 |
| P04899;P11488;P19087;A8MTJ3;P09471 | Guanine nucleotide-binding protein G(i) subunit α2 | GNAI2 | 40.45 | 148 | 24.9714965820313 | 29 | 72.4 |
| P16284 | Platelet endothelial cell adhesion molecule | PECAM1 | 82.521 | 248 | 24.9365634918213 | 40 | 53.5 |
| Q9Y490;Q9Y4G6 | Talin-1 | TLN1 | 269.76 | 820 | 24.7005462646484 | 156 | 73.4 |
| P02489 | Alpha-crystallin A chain | CRYAA | 19.909 | 54 | 24.5251007080078 | 7 | 36.4 |
| P14625;Q58FF3 | Endoplasmin | HSP90B1 | 92.468 | 172 | 24.4852981567383 | 43 | 53.1 |
| O75964 | ATP synthase subunit g, mitochondrial | ATP5L | 11.428 | 23 | 24.4625129699707 | 6 | 52.4 |
| P23229 | Integrin α6 | ITGA6 | 126.6 | 233 | 24.3582363128662 | 56 | 50 |
| Q9Y277 | Voltage-dependent anion-selective channel protein 3 | VDAC3 | 30.658 | 53 | 24.3242301940918 | 14 | 62.2 |
| P40197 | Platelet glycoprotein V | GP5 | 60.958 | 134 | 24.2378311157227 | 24 | 50.5 |
| P21333;Q14315;O75369 | Filamin A | FLNA | 280.74 | 658 | 24.2292003631592 | 155 | 61.5 |
ATP, adenosine triphosphate; FSC, Fourier shell correlation; GS-FSC, gold-standard Fourier shell correlation; iBAQ, intensity-based absolute quantification; ID, identification; MS, mass spectrometry.
Peptide coverage of the human αIIbβ3 by mass spectrometry. Full sequence of integrin αIIb (top) and β3 (bottom) were obtained from the UniProt database. The signal peptides were colored in red, and the mature proteins were colored in black. Each underline indicates a peptide of integrin αIIb (top) or β3 (bottom) identified from the mass spectrometry study. Peptide coverage of integrin αIIb (top) and β3 (bottom) was highlighted in yellow.
Peptide coverage of the human αIIbβ3 by mass spectrometry. Full sequence of integrin αIIb (top) and β3 (bottom) were obtained from the UniProt database. The signal peptides were colored in red, and the mature proteins were colored in black. Each underline indicates a peptide of integrin αIIb (top) or β3 (bottom) identified from the mass spectrometry study. Peptide coverage of integrin αIIb (top) and β3 (bottom) was highlighted in yellow.
Inactivated bent state of αIIbβ3: the overall architecture
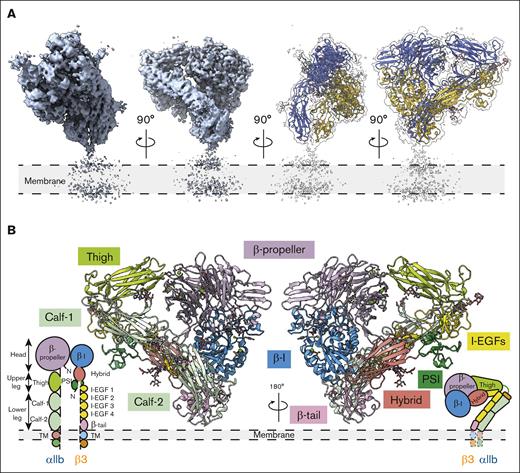
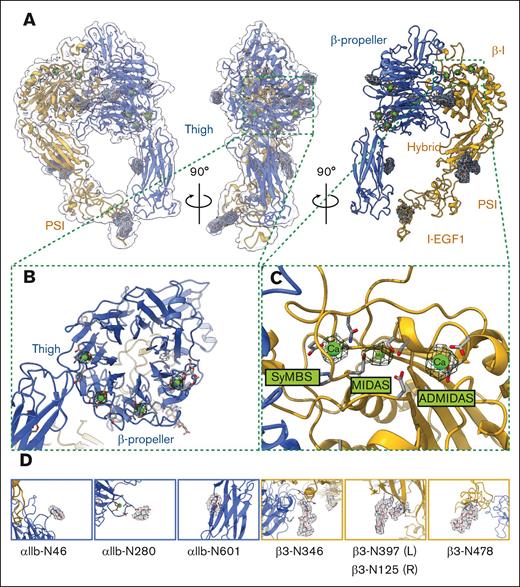
Our cryo-EM structure of native inactivated αIIbβ3 adopts the bent conformation seen previously in the crystal structures of the ectodomain and the 2 most recent cryo-EM models of full-length integrin in native lipids and detergent, respectively8,10,25 (Figure 3; supplemental Figure 1). Our inactivated αIIbβ3 cryo-EM structure is a heterodimer with overall dimensions of 114 Å × 82 Å × 110 Å, excluding the density of the nanodisc. The full topology of the heterodimer with all 12 extracellular subdomains and the nanodisc density is clearly visible in the cryo-EM map, which makes assigning the molecule’s orientation to the membrane surface feasible. However, no density is detected for the transmembrane α-helices and αIIb or β3 short cytoplasmic tails. Our αIIbβ3 structure is superimposable onto the X-ray structure of the complete ectodomain of integrin αIIbβ3 (3FCS.pdb25) with a root mean square deviation (RMSD) between the respective 1521 Cαs of 2.033 Å and to the 2 recent inactive αIIbβ3 cryo-EM structures (8T2V.pdb8 and 8GCD.pdb10) with RMSDs between the respective 1393 Cαs of 1.570 Å (8T2V.pdb) and 1308 Cαs of 1.141 Å (8GCD.pdb).8,10 The overall architecture of our integrin structure agrees with the previous structural observations,8,10,25,26 indicating that solving high-resolution membrane protein structure directly from platelet membranes without purification using BaR data processing is feasible and valid.
The overall architecture of integrin αIIbβ3 in the bent conformation. (A) The overall cryo-EM map of integrin αIIbβ3 in bent conformation at 2.75 Å resolution is shown. The integrin αIIbβ3 model was shown in ribbon diagrams with the αIIb subunit colored in royal blue and the β3 subunit colored in goldenrod here and in subsequent figures. (B) A total of 12 subdomains of the ectodomain are resolved. The integrin αIIbβ3 model was shown in ribbon diagrams with each subdomain colored as follows: β-propeller in purple, thigh in lime green, calf in light green, β-I in blue, hybrid in salmon, PSI in green, I-EGFs in yellow, and β-tail in pink. I-EGF, epidermal growth factor.
The overall architecture of integrin αIIbβ3 in the bent conformation. (A) The overall cryo-EM map of integrin αIIbβ3 in bent conformation at 2.75 Å resolution is shown. The integrin αIIbβ3 model was shown in ribbon diagrams with the αIIb subunit colored in royal blue and the β3 subunit colored in goldenrod here and in subsequent figures. (B) A total of 12 subdomains of the ectodomain are resolved. The integrin αIIbβ3 model was shown in ribbon diagrams with each subdomain colored as follows: β-propeller in purple, thigh in lime green, calf in light green, β-I in blue, hybrid in salmon, PSI in green, I-EGFs in yellow, and β-tail in pink. I-EGF, epidermal growth factor.
The head and upper leg regions are 114 Å long measured from Thr150 (UniProt entry: P08514) from the β-propeller domain to Thr501 at the end of the thigh domain; the lower leg region is 98.5 Å from the calcium at the αIIb genu point to Glu991 at the end of the calf-2 domain. Although the TM α-helixes in the bent αIIbβ3 are unsolved, we can assign the ectodomains’ orientation in relation to the membrane, given that the nanodisc density is visible (Figure 3). The molecule forms a “squatting” conformation (Figure 3). The β-propeller domain of the αIIb subunit forms a 36.4° angle with the thigh domain, and a 48.1° angle is formed between the thigh and calf-1 domains. The calf-2 domain in the lower leg region forms a 62.5° angle to the membrane (Figure 3; supplemental Figure 1). The head and upper leg regions of the β3 subunit (βI, hybrid, and plexin-semaphorin-integrin [PSI] domains) form at a sharper 16.2° angle with the lower leg region (epidermal growth factors [EGFs] and β-tail domains), and the lower leg region forms a 36.1° angle with the cell membrane (Figure 3; supplemental Figure 1). The head and upper leg regions of αIIbβ3 create a plane tilted at 49.2° to the cell surface, resulting in a more “relaxed” bending of the αIIb subunit and a tighter bending of the β3 subunit (Figure 3; supplemental Figure 1). The molecule is 110 Å tall, measured from the head region’s highest point (His61 from the β-propeller domain) to the membrane surface (Figure 3; supplemental Figure 1).
Using the 3-dimensional Flexible Refinement function embedded in the CryoSPARC v4.6.0 suite,27,28 we determined the local motion and dynamics of the flexible αIIbβ3 from the cryo-EM data set in the bent conformation (supplemental Figure 1E; supplemental Movie 1). Even in the single high-resolution “canonical” 3-dimensional density map of αIIbβ3, the molecule displays conformational variability and flexible (nonrigid) motion. The lower leg region demonstrated the highest dynamics followed by the upper leg region (supplemental Figure 1E; supplemental Movie 1). This flexibility leads to the molecule continuously oscillating on the cell membrane. In contrast, the entire head region is quite stable with minimal dynamics. This is in agreement with our local resolution map (supplemental Figure 1F): the head region has the highest resolution (∼2.5 Å), followed by the upper leg region (∼3 Å). Owing to the molecular flexibility, the resolution of the lower leg region ranges from 3.5 Å to +5 Å, where the β-tail has the lowest resolution.
Inactivated bent state of αIIbβ3: glycosylation and ion-binding sites
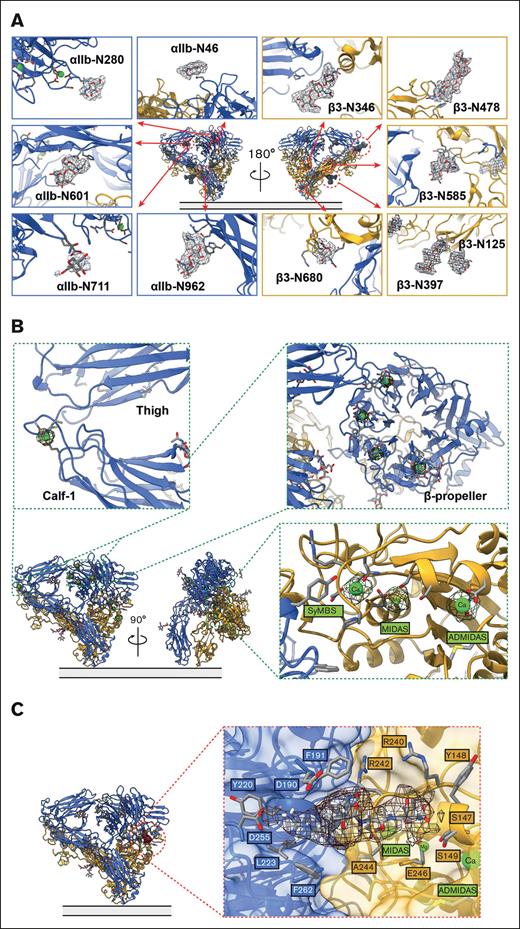
As a glycoprotein, integrin αIIbβ3 has 11 predicted N-linked glycans: 5 on the αIIb subunit (αIIb-N46 and αIIb-N280 in the β-propeller domain, αIIb-N601 in the thigh domain, αIIb-N711 in the calf-1 domain, and αIIb-N962 in the calf-2 domain) (UniProt entry: P08514) and 6 on the β3 subunit (β3-N346 in the βI domain, β3-N125 and β3-N397 in the hybrid domain, β3-N478 in the I-EGF1 domain, β3-N585 in the I-EGF3 domain, and β3-N680 in the β-tail domain) (UniProt entry: P05106).29 Clear densities of all 11 N-glycans are visible in our cryo-EM map of integrin αIIbβ3 directly extracted from resting human platelet membranes (Figure 4A). In agreement with previous structural data,8 we observed all ion-binding sites on the molecule (Figure 4B). Unlike previous studies that added Mg2+, Ca2+, or both during the purification of αIIbβ3, we did not provide exogenous divalent ions to our heterogeneous cryo-EM sample during our sample preparation except at the initial washing step of the intact platelet membrane with high salt buffer to remove soluble proteins. Therefore, all ion-binding was from the original whole blood sample from the donors. There are 4 Ca2+ binding sites at the β-propeller domain, 1 Ca2+ at the αIIb genu point, and 3 divalent ions at the βI domain: a Mg2+ at the metal ion–dependent adhesion site (MIDAS), a Ca2+ at the adjacent to MIDAS (ADMIDAS), and a Ca2+ at the synergistic metal ion–binding site (SyMBS) (Figure 4B).
The interaction network of integrin αIIbβ3 in the bent conformation. (A) 11-N-linked glycan sites were preserved in the bent integrin αIIbβ3 obtained from the native environment. (B) Closeup of the cryo-EM density of the metal ions, 1 at the α-genu (top left insert), 4 at the β-propeller (top right insert), and 3 at SyMBS (occupied by a Ca2+), MIDAS (occupied by a Mg2+), and ADMIDAS (occupied by a Ca2+) of the β-I domain (bottom right insert). (C) A clear density of an RGD motif–containing peptide was observed in the ligand-binding site.
The interaction network of integrin αIIbβ3 in the bent conformation. (A) 11-N-linked glycan sites were preserved in the bent integrin αIIbβ3 obtained from the native environment. (B) Closeup of the cryo-EM density of the metal ions, 1 at the α-genu (top left insert), 4 at the β-propeller (top right insert), and 3 at SyMBS (occupied by a Ca2+), MIDAS (occupied by a Mg2+), and ADMIDAS (occupied by a Ca2+) of the β-I domain (bottom right insert). (C) A clear density of an RGD motif–containing peptide was observed in the ligand-binding site.
Inactivated bent state of αIIbβ3: liganded by an RGD motif–containing peptide
Interestingly, the bent inactivated αIIbβ3 structure is liganded by a long peptide with an Arg-Gly-Asp (RGD) motif (Figure 4C). In this “squatting” conformation, the accessible ligand-binding site at the heterodimer interface of αIIbβ3 faces forward, 62 Å from the membrane surface. The accessibility of the ligand-binding site of αIIbβ3 in its bent conformation agrees with the 2 most recent αIIbβ3 cryo-EM structures.8,10
In our cryo-EM density map at 2.75 Å resolution, the ligand-binding site of bent inactivated αIIbβ3 is occupied by a clear density of an RGD motif–containing peptide. Our αIIbβ3 structure is superimposable onto the X-ray structure of integrin αIIbβ3 headpiece bound to fibrinogen γ chain peptide by Springer et al26 (2VDO.pdb) with an RMSD between the respective βI domain 168 Cαs of 0.366 Å, an RMSD between the β-propeller domain 390 Cαs of 0.258 Å, and an RMSD between the entire headpiece 658 Cαs of 0.729 Å. In agreement with the previous work, the surface representation shows that the RGD peptide sits in the groove formed by the heterodimer interface of αIIbβ3. Our BaR sample of resting human platelet membrane is directly extracted from the endogenous resource, and the RGD motif is a conserved sequence recognized by the integrin binding site, which suggests that this peptide should belong to a natural ligand of αIIbβ3. We observed some clear ligand density extended from the RGD motif (supplemental Figure 1H). A long density protruding from the binding pocket almost acts as a staple to tape the molecule in its bent conformation (supplemental Figure 1H). However, without knowing the precise amino acid sequence, we could not identify or track the peptide’s origin owing to the weak electron density.
Intermediate state of αIIbβ3: the overall architecture
The dynamic nature of αIIbβ3 is required for its inside-out and outside-in signaling. We observed a second conformation of αIIbβ3 in an intermediate state in our BaR data set from the resting human platelet membrane, indicating flexible structural rearrangement of αIIbβ3 in the inactivated platelet. Although the lower leg region was visible in the 2-dimensional classification (supplemental Figure 2B), its electron density was lost during the following data processing owing to the protein’s dynamics. Only the closed headpiece, containing the full head region and the upper leg region (β-propeller, thigh, and partial calf-1 domains on the αIIb subunit and βI, hybrid, PSI, and partial I-EGF1 domains on the β3 subunit), was solved in the αIIbβ3 intermediate state at 2.67 Å (Figure 5A; supplemental Figure 2).
The architecture of integrin αIIbβ3 in the intermediate conformation and its interaction network. (A) The overall cryo-EM map of integrin αIIbβ3 in intermediate conformation at 2.67 Å resolution is shown. The right panel is the side of the headpiece facing the cell membrane. (B) Closeup of the cryo-EM density of the 4 metal ions at the β-propeller domain. (C) Closeup of the cryo-EM density of the 3 metal ions at SyMBS (Ca2+), MIDAS (Mg2+), and ADMIDAS (Ca2+) of the β-I domain. (D) 7-N-linked glycan sites within the solved region of the intermediate integrin αIIbβ3 were preserved.
The architecture of integrin αIIbβ3 in the intermediate conformation and its interaction network. (A) The overall cryo-EM map of integrin αIIbβ3 in intermediate conformation at 2.67 Å resolution is shown. The right panel is the side of the headpiece facing the cell membrane. (B) Closeup of the cryo-EM density of the 4 metal ions at the β-propeller domain. (C) Closeup of the cryo-EM density of the 3 metal ions at SyMBS (Ca2+), MIDAS (Mg2+), and ADMIDAS (Ca2+) of the β-I domain. (D) 7-N-linked glycan sites within the solved region of the intermediate integrin αIIbβ3 were preserved.
Comparison between intermediate and bent states of αIIbβ3
There is a distinct difference between the top views of the bent and intermediate states, which validates that there were indeed 2 different conformations of integrin αIIbβ3 in our data set. The resolution of the density maps of both bent and intermediate states at the ligand-binding site was ∼2.5 Å. At this resolution, we can confidently assign all side chains of each amino acid. In contrast to a clear electron density of an RGD motif–containing peptide in the bent conformation, the ligand-binding site of the intermediate state was unoccupied, which convinced us to separate those particles into 2 different states. In addition, the densities of 3 divalent ions at the MIDAS, ADMIDAS, and SyMBS were equally strong in the bent conformation (Figure 4B). However, the electron density of the Mg2+ at the MIDAS was significantly weaker than the other 2 Ca2+ in the intermediate state (Figure 5C). The Mg2+ at the MIDAS is critical for αIIbβ3 ligand binding, which further supports that the closed headpiece in the intermediate state is unliganded. Furthermore, an additional density can be observed in the 2-dimensional projection of the top view of the bent conformation but not in the intermediate state. This density came from the lower leg region, which can be seen through the center of the headpiece from the top of the molecule (supplemental Figure 3A). In contrast, the lower leg region extends outward and is no longer visible through the center of the headpiece in the intermediate state (supplemental Figure 3A). The RMSD between the respective 834 Cαs of the intermediate state and the same headpiece region in our inactivated bent state is 1.662 Å, which indicates that these 2 conformations are similar but not the same. Compared with the inactivated state, the most noticeable overall structural shift of the headpiece came from the thigh domain in the αIIb subunit (shifted 9 Å) and the hybrid (shifted 6 Å), PSI, and I-EGF1 domains (shifted 9 Å) in the β3 subunit (supplemental Figure 3B).
Seven glycosylation sites were elucidated within the solved region of the intermediate state: 3 on the αIIb subunit (αIIb-N46 and αIIb-N280 in the β-propeller domain and αIIb-N601 in the thigh domain) and 4 on the β3 subunit (β3-N346 in the βI domain, β3-N125 and β3-N397 in the hybrid domain, and β3-N478 in the I-EGF1 domain). This is similar to the inactivated state (Figure 5D).
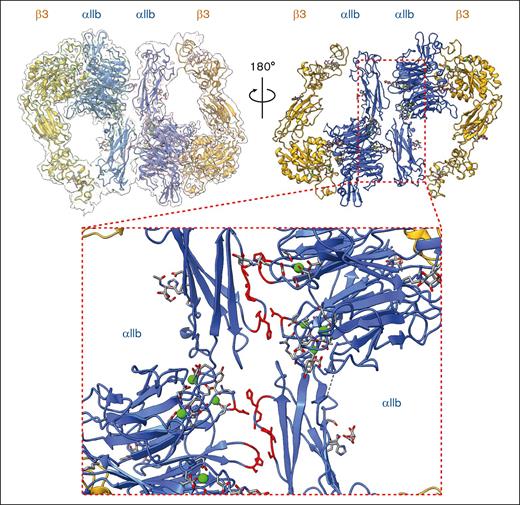
Novel homodimer of αIIbβ3: the overall architecture and dimer interface
A novel homodimer conformation of αIIbβ3 at 2.85 Å was also observed in which the head regions of 2 αIIbβ3 molecules in intermediate states were facing each other (Figure 6, top; supplemental Figure 4). This additional homodimer interface was formed between 2 αIIb subunits and locked 2 molecules in a “face-to-face” and “head-to-toe” conformation (Figure 6, bottom). In particular, there are 2 clusters of interaction formed between Ser60-Gly62 on the β-propeller domain and Pro574-Cys576 on the thigh domain, and another 2 clusters of interaction formed between Gly570-His572 on the thigh domain and Asp460 on the β-propeller domain.
A novel homodimer conformation formed by 2 intermediate integrin αIIbβ3 molecules was revealed. The overall cryo-EM map of integrin αIIbβ3 in homodimer conformation at 2.85 Å resolution is shown. The top right panel is the side of the headpiece facing the cell membrane. The bottom insert shows the closeup of the dimer interface formed by 2 αIIb subunits from 2 individual integrin αIIbβ3 molecules. The residues that are involved in the dimer interface were colored in red.
A novel homodimer conformation formed by 2 intermediate integrin αIIbβ3 molecules was revealed. The overall cryo-EM map of integrin αIIbβ3 in homodimer conformation at 2.85 Å resolution is shown. The top right panel is the side of the headpiece facing the cell membrane. The bottom insert shows the closeup of the dimer interface formed by 2 αIIb subunits from 2 individual integrin αIIbβ3 molecules. The residues that are involved in the dimer interface were colored in red.
The homodimer showed similar glycosylation and ion-binding profiles compared with the previous 2 structures. Like the intermediate conformation, we did not observe any ligand density in either αIIbβ3 molecule of the homodimer (Figure 6, top). Together with our observations in the first 2 structures, this unique integrin αIIbβ3 architecture identified directly from the native platelet membrane may indicate a previously unknown self-regulatory effect of αIIbβ3 to temporally regulate its ligand accessibility in the intermediate state.
Discussion
Platelets are essential for the vascular system and hemostasis. They are in a balance between the quiescent inactivated state in normal conditions and quick activation upon injury, maintained by interactions between proteins and biomacromolecules on the platelet membrane to sense and relay extracellular stimuli.1,2,7 Mutations, structural changes, or altered expression levels of these proteins profoundly affect platelet physiology and are associated with numerous disorders.3,30 In addition, the native membrane provides crucial regulatory cues, including ions, post-translational modifications, and ligands that coordinate protein structure and function. Defining the physical interactions of this network of membrane proteins will shed light on understanding basic platelet biology and physiology.
Advancements in single-particle cryo-EM enable solving near-atomic-resolution structures of macromolecules without high-quality crystals. This is critical for studying membrane proteins and larger complexes, whose sample preparation and crystallization are difficult. The rapid developments of cryo-EM expand the molecular weight range and enable structure determination directly from native cell extracts. However, solving multiple proteins or multiple states of a single protein from a raw heterogeneous sample remains a challenge for single-particle cryo-EM.
In this study, we combined cryo-EM with the BaR data processing method to analyze the platelet membrane proteins to bridge structural biology with omics. BaR is an iterative bottom-up approach that uses in silico purification to obtain high-resolution cryo-EM maps to solve structures of proteins in different states in the native environment.19-23 We solved integrin αIIbβ3 in 3 different states: bent (2.75 Å), intermediate (2.67 Å), and a homodimer conformation formed by 2 integrin molecules in their intermediate state (2.85 Å).
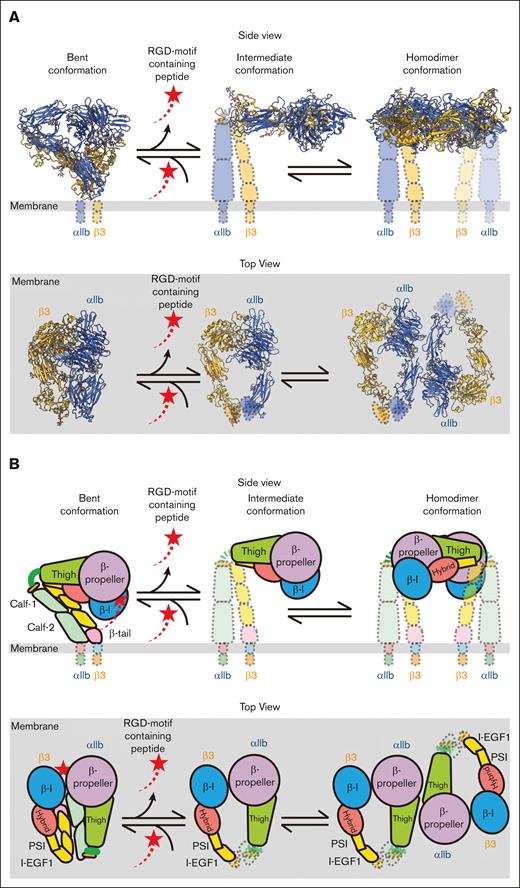
Integrin αIIbβ3, also known as GPIIb/IIIa, is a cell surface receptor that plays a crucial role in platelet function. When activated, αIIbβ3 undergoes conformational changes that enable it to bind to fibrinogen and other ligands, which further facilitates platelet aggregation. The previous structural studies with αIIbβ3 in different states have provided key insights into its mechanism of action. Our data with αIIbβ3 directly from resting human platelets add new information on the highly dynamic nature of this protein before activation. We also identified the structural rearrangement among the 3 states (Figure 7) and the local dynamics and flexibility of αIIbβ3 in the bent conformation.
Proposed model for the dynamics of integrin αIIbβ3 on the resting human platelet membrane. On the surface of the resting human platelets, integrin αIIbβ3 has 3 major conformations: inactivated bent, intermediate, and homodimer. An RGD motif–containing ligand (red shooting star) binds to the bent conformation and keeps the ligand-binding site occupied when the molecule is inactive. The ligand releases whereas αIIbβ3 extends to the intermediate state. Two adjacent αIIbβ3 molecules in the intermediate state can form a homodimer on the surface. There is a dynamical conformational equilibrium between the intermediate state and the homodimer state of αIIbβ3 on the human platelets. (A) Diagram with solved integrin αIIbβ3 structures in their corresponding location. (B) Cartoon models of the molecule. The top panels of both panels A and B show the side view of the molecules in which the extracellular side is on the top and the intracellular side is on the bottom. The bottom panels of both panels A and B show the top view of the molecules where the platelet membrane (gray) lies on the bottom.
Proposed model for the dynamics of integrin αIIbβ3 on the resting human platelet membrane. On the surface of the resting human platelets, integrin αIIbβ3 has 3 major conformations: inactivated bent, intermediate, and homodimer. An RGD motif–containing ligand (red shooting star) binds to the bent conformation and keeps the ligand-binding site occupied when the molecule is inactive. The ligand releases whereas αIIbβ3 extends to the intermediate state. Two adjacent αIIbβ3 molecules in the intermediate state can form a homodimer on the surface. There is a dynamical conformational equilibrium between the intermediate state and the homodimer state of αIIbβ3 on the human platelets. (A) Diagram with solved integrin αIIbβ3 structures in their corresponding location. (B) Cartoon models of the molecule. The top panels of both panels A and B show the side view of the molecules in which the extracellular side is on the top and the intracellular side is on the bottom. The bottom panels of both panels A and B show the top view of the molecules where the platelet membrane (gray) lies on the bottom.
Our αIIbβ3 structures in bent and intermediate conformations are in agreement with their corresponding crystal and cryo-EM structures.8,10,25 Our cryo-EM sample was prepared directly from its native environment without additional affinity enrichment. This preserved the natural regulatory cues, such as glycosylation, ion binding, and cofactors, and provided new insights into how the environment regulates αIIbβ3 structure and function.
First, N-linked glycosylation is critical to regulating the adhesion function of integrins.29 The role of each N-linked glycan on αIIbβ3 integrin structure and function was thoroughly studied by mutagenesis in HEK293FT cells.29 Our structural data provided additional clear visual support for the presence of all 11 N-linked glycans (Figure 4A). Micro-, macro-, and meta-heterogeneity of glycosylation can affect protein structure and function.31 We observed micro- and macroheterogeneity of N-linked glycosylation among the bent, intermediate, and dimer conformations. The structural and functional impact of heterogeneous glycosylation of integrin αIIbβ3 is an area for future exploration. The electron density was weaker in the I-EGF1 domain in both the intermediate and the dimer conformations. Thus, although we did observe a clear N-linked glycan around the N478 site in the β3 subunit, assigning the precise location of the NAG was challenging given that 2 additional asparagine residues (N475 and N509) were in close proximity. Future studies will determine the functional significance of the interplay of these 3 asparagines on N-linked glycosylation in the I-EGF1 domain.
Second, we observed all ion-binding sites on the αIIbβ3 molecule (Figures 4B and 5B-C). We observed clear electron densities of 4 Ca2+ binding sites at the β-propeller domain, 1 Ca2+ at the αIIb genu point, and 3 divalent ions binding at the MIDAS, ADMIDAS, and SyMBS at the βI domain, which indicates that all ion binding from the original whole blood from the donor is preserved. This demonstrates the power of our approach to preserve the interactions from the original native environment.
Third, the αIIbβ3 cryo-EM map of the bent conformation showed a clear density from a peptide that contains an RGD motif at the ligand-binding site (Figure 4C). This agrees with the overall architecture of the bent conformation and the most recent cryo-EM structure of the full-length αIIbβ3,8 in which the whole molecule was “squatting” on the lipidic nanoparticles and the ligand-binding site was fully accessible. This challenges the conventional switchblade model of αIIbβ3 activation, in which the headpiece of the molecule points to the membrane to maintain its low affinity for the ligand.8 We cannot rule out that this observation is caused by our extraction strategy. Coller32 suggested that extraction conditions affect the overall structure of αIIbβ3. Therefore, further studies are needed to carefully evaluate the impact of the lipidic mimetic environment and extraction conditions on αIIbβ3 structure.
Talin, an integrin-actin adapter, connects integrins and the cytoskeleton.33-36 In platelets, binding between talin and β3 subunit is crucial for αIIbβ3 “inside-out” signaling. Filamin A is also associated with both inactive and active αIIbβ3 to regulate bidirectional signaling in platelets.37 Both proteins were identified in our mass spectrometry data, but neither was associated with αIIbβ3 in cryo-EM structures. Our high salt buffer wash (1 M NaCl) likely dissociated the interaction among αIIbβ3, talin, and filamin A. Future studies using different enrichment methods may preserve αIIbβ3 association with accessory proteins.
We also identified a third conformation, which is a novel homodimer of αIIbβ3 (Figure 6). This new αIIbβ3 conformation highlights the strength of the BaR method for analyzing crude samples from original sources with minimal manipulation. We believe that this is the key to preserving the unique homodimer confirmation. Furthermore, the additional homodimer interface formed by 2 αIIbβ3 molecules stabilizes the protein in its intermediate state before the full extension. Our working hypothesis is that homodimerization is a previously unknown self-regulatory mechanism to temporally regulate its ligand accessibility in the intermediate state (Figure 7). We have indirect evidence to support our hypothesis. Both extended αIIbβ3 molecules in the intermediate conformation and the homodimer conformation were unliganded, which is distinct from the bent state (supplemental Figure 3). Future studies are needed to validate this conformation in live cells and determine its impact on αIIbβ3 function. In addition, this homodimer conformation of αIIbβ3 may open a new avenue for antiplatelet therapy. Stabilizing the homodimer may decrease the αIIbβ3 activation and reduce platelet aggregation. The current US Food and Drug Administration–approved αIIbβ3 antagonists target the ligand-binding site and have an adverse risk of bleeding. Comparatively, regulating the conformational equilibrium of αIIbβ3 on the surface of resting platelets may be a safer approach.
Our results highlight cryo-EM with BaR for system structural proteomics. BaR is an unbiased, untargeted, bottom-up approach, as summarized in Figure 1, performing in silico purification on heterogeneous data sets to solve high-resolution cryo-EM maps and understand protein structures and mechanisms in their original environment. As demonstrated in our current and previous study, BaR provides an opportunity to (1) solve structures from multiple proteins, (2) solve structures of a protein in multiple conformations, (3) bypass the overexpressing and purifying process, (4) preserve native regulation cues, and (5) provide a chance to solve novel conformations. However, it has inherent limitations: (1) larger data sets are needed, which complicates data acquisition, storage, and processing; (2) low-abundant proteins in the crude sample are hard to solve; and (3) proteins with electron density maps at >4 Å resolution are challenging to identify. Advances in cryo-EM sample preparation and BaR methodology will help address these issues in future studies.
In conclusion, our study was the first to focus on platelets using a structural-omics approach that combines cryo-EM with BaR data analysis. Using this approach, we were able to demonstrate the highly structural dynamics of αIIbβ3 from native platelet membranes, which actively rearrange among 3 different states: bent, intermediate, and homodimer. In addition, we showed the strength of the BaR methodology in preserving natural regulatory cues of the proteins from their environment. Our study highlights the potential of creating a structural atlas of platelet membrane protein complexes, which will ultimately enrich our understanding of the foundation of platelet signaling circuitry in response to their surroundings.
Acknowledgments
M.T.N. receives research funding from the National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (HL098217 and HL154026). X.H. receives funding from the American Heart Association Postdoctoral Fellowships (897185) and the American Society of Hematology Fellow Scholar Award. This research was also supported in part by the grant AI145069 to E.Y. from the National Institute of Allergy and Infectious Diseases. The authors thank Belinda Willard and Ling Li at Lerner Research Institute’s Proteomics & Metabolomics Core for the acquisition of mass spectrometry data. The timsTOF Pro 2 mass spectrometer instrument was purchased via an NIH-shared instrument grant, S10 OD030398. The authors acknowledge the assistance of Kunpeng Li and Kyle Whiddon from the Case Western Reserve University School of Medicine Cryo-Electron Microscopy Facility and support by the NIH grant S10 OD032437-01.
Authorship
Contribution: X.H., E.Y., and M.T.N. conceived the study; X.H. and M.T.N. designed the experiments and wrote the manuscript; X.H., C.-C.S., Z.Z., M.L., M.M., and M.T.N. performed the experiments and analyzed the data; and C.-C.S., Z.Z., M.L., M.M., and E.Y. critically read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marvin T. Nieman, Department of Pharmacology, Case Western Reserve University, 2109 Adelbert Rd W309B, Cleveland, OH 44106-4965; email: marvin.nieman@case.edu; and Xu Han, Department of Pharmacology, Case Western Reserve University, 2109 Adelbert Rd W309B, Cleveland, OH 44106-4965; email: xxh112@case.edu.
References
Author notes
The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the Proteomics Identifications Database partner repository with the data set identifier PXD058005.
Original data are available on request from the corresponding authors, Marvin T. Nieman (marvin.nieman@case.edu) and Xu Han (xxh112@case.edu).
The full-text version of this article contains a data supplement.