Severe aplastic anemia (SAA) is a rare and life-threatening bone marrow failure disorder. Immunosuppressive therapy (IST) with antithymocyte globulin and cyclosporine has long been a frontline treatment option in SAA; however, its limited durability and risk of long-term complications such as secondary malignancies remain a drawback in this treatment modality. Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative option with significantly improved outcomes over the long term, particularly with HLA-matched related donors. However, the use of alternative donors, such as haploidentical, mismatched, or matched unrelated donors, has previously been limited due to increased transplant-related morbidity, particularly graft-versus-host disease (GVHD). HCTs have therefore been limited to young recipients and those with HLA-matched related donors, creating significant disparity for older adults and those who lack matched donor options. Nevertheless, more recent advances in HCT, such as posttransplant cyclophosphamide for GVHD prophylaxis, have led to improved outcomes of HCT with alternative donors; however, alternative donor HCT remains underused as up-front therapy, in part because of limited multicenter trial data. This review discusses current SAA treatment approaches, including both IST and HCT, and highlights remaining gaps. It also discusses how ongoing clinical trials such as CureAA and TransIT could help address these gaps. Furthermore, we discuss the importance of stakeholder engagement and implementation science in the integration of research-based evidence into clinical practice. Bridging these gaps is necessary for achieving equitable access for patients historically excluded from frontline HCT, including older adults and racially or ethnically diverse populations.
Introduction
Aplastic anemia (AA) is a rare, life-threatening bone marrow failure disorder characterized by a hypocellular bone marrow and pancytopenia due to either an inherited or an acquired etiology, leading to the destruction of hematopoietic stem cells. Since it was first described in the late 18th and early 19th century, significant progress has been made in understanding the pathophysiology of this disease.1 There are 2 key mechanisms for the destruction of hematopoietic stem cells. First, inherited syndromes such as Fanconi anemia, dyskeratosis congenita, or GATA2 deficiency and associated underlying germ line mutations affect DNA or telomere repair mechanisms, self-renewal pathways, or immune regulation. In contrast, acquired AA can be caused by direct marrow damage or by cytotoxic T cell immune-mediated destruction of hematopoietic stem cells. The latter may be triggered by an identifiable exposure to drugs and/or radiation, underlying infection, or may be idiopathic.
Idiopathic acquired severe AA (SAA), defined by an absolute neutrophil count of <0.5 × 109/L, platelet count of <20 × 109/L, absolute reticulocyte count of <60 × 109/L, and bone marrow cellularity of <25%, is universally fatal if patients do not receive therapy in a timely manner. For >40 years, immunosuppressive therapy (IST) with cyclosporine (CsA) and rabbit antithymocyte globulin (ATG) has been standard initial therapy and leads to reasonable response rates of ∼60% to 80%2,3; however, long-term durability is variable. The use of allogeneic hematopoietic cell transplantation (HCT) also gained significant momentum over the last 5 decades.4 With improvements in HLA-typing techniques, development of reduced toxicity conditioning regimens, and better supportive care, outcomes after HCT for SAA significantly improved over time.5
Most studies showing improved outcomes after HCT focused on patients treated with HLA-matched related donor (MRD).6-10 Enthusiasm for use of alternative donors, such as a related haploidentical donor, HLA-matched unrelated donor (MUD), or mismatched unrelated donor (MMUD), was diminished by the higher incidence of transplantation-related morbidity, including graft-versus-host disease (GVHD), especially in older patients.3,11 Consequently, the role of HCT in the management of treatment-naïve (TN) SAA has remained largely limited to younger patients with an available MRD, which creates a significant disparity. In the last decade, advances have been made in reducing HCT-related toxicities, including the risk of GVHD, especially with regimens containing posttransplantation cyclophosphamide (PTCy).12-14 These advances have increased the use of alternative donor HCT in patients with SAA and provide an opportunity to change the treatment paradigm for this population.6,15-20
Despite promising outcomes in these studies, alternative donor HCT is still not generally incorporated into SAA treatment algorithms as up-front therapy.3,21,22 This may be due to the lack of multicenter clinical trials assessing its role in this setting. Such trials are now ongoing. Although strong evidence is often expected to change clinical practice, the translation of research findings takes an average of 17 years to be implemented into clinical practice.23-25 To expedite this process, several groups have recognized the importance of integrating implementation science in the clinical trial framework and raising awareness among key stakeholders.26-28
The objective of our review is twofold. First, we summarize the current treatment landscape of SAA to illustrate gaps and suggest how up-front alternative donor HCT can help fill those gaps. We highlight the strengths and limitations of both IST and HCT and ongoing efforts in this field. Second, we emphasize how proactive integration of stakeholder engagement and an implementation framework may drive meaningful changes in the treatment of patients with SAA based on clinical trial data.
Strengths and shortcomings of current treatment approaches in TN SAA
Previous studies of IST using ATG and CsA as frontline therapy for patients with SAA show that approximately two-thirds of treated patients respond.3,29-31 However, low rates of sustained response are a major gap in the management of SAA.32 In contrast, HCT using an immunoablative regimen is usually curative33 and consistently shows improved failure-free survival compared to IST (80%-90% vs 30%-60%; Table 1).6,15-17 Nevertheless, transplant-related complications, especially with alternative donors, have historically been a barrier to widespread use.3,6-11,34,35
Studies showing comparison of outcomes between up-front alternative donor HCT with IST and salvage HCT
| Study . | No. of patients . | Age at diagnosis/HCT, median (range), y . | Comparison up-front HCT vs IST . | Comparison up-front MUD vs salvage HCT . |
|---|---|---|---|---|
| Dufour et al6 | Up-front HCT: 29 ∗IST: 58 ∗Salvage HCT: 24 | At diagnosis Up-front: 7.9 (0.5-18.6) At HCT Up-front: 8.4 (1.7-19.1) | 2-year OS Up-front HCT: 96% ± 4% IST: 94% ± 3% (P = .68) 2-year EFS Up-front HCT: 92% ± 5% IST: 40% ± 7% (P = .0001) | 2-year OS and EFS Up-front MUD HCT: 95% ± 5% Salvage MUD HCT: 74% ± 9% (P = .02) |
| Choi et al16 | Up-front HCT: 23 IST: 19 Salvage HCT: 11 | At diagnosis Up-front HCT: 9.3 (0.6-17.2) IST 8.5 (1.3-14.1) Salvage HCT: 6.8 (1.3-11.6) At HCT Up-front: 11.5 (1.1-17.7) Salvage: 8.1 (2.7-14.9) | OS Up-front HCT: 91.3% IST: 71.2% (P = .187) FFS HCT: 91.3% IST: 30.7% (P < .001) | 5-year EFS Up-front HCT: 91.3% Salvage HCT: 50.9% (P = .015) |
| Cheng et al15 | Up-front HCT: 28 IST: 24 | At diagnosis HCT: 8 (2-17) IST: 6 (4-16) | 10-year OS Up-front HCT: 89.3% ± 5.8% IST: 73.4% ± 12.6% (P = .806) FFS HCT: 89.3% ± 5.8% IST: 52.6% ± 10.5% (P = .008) | N/A |
| Xu et al17 | Up-front HCT: 76 IST: 37 | At diagnosis HCT: 28 (18-49) IST: 32 (18-62) | 8-year OS Up-front HCT: 83.7% IST: 75.6% (P = .328) FFS HCT: 83.7% ± 4.8% IST: 38.5% ± 13.2% (P = .001) | N/A |
| Xu et al36 | Up-front HCT: 23 Salvage HCT: 29 | All pediatric patients (<18 years) | N/A | OS: P = .698 FFS: P = .899 |
| Yang et al18 | Up-front HCT: 20 IST: 29 | At diagnosis Up-front HCT: 13 (4-18) IST: 12 (4-17) Salvage HCT: 12 (4-17) | 3-year OS Up-front HCT: 85.0% ± 8.0% IST: 79.3% ± 7.5% (P = .740) 3-year FFS HCT: 80.0% ± 8.9% IST: 35.9% ± 10.9% (P = .043) | EFS Up-front HCT: 80.0% ± 8.9% Salvage HCT: 41.7% ± 14.2% (P = .046) |
| Wu et al37 | Up-front HCT: 114 IST: 99 | At diagnosis Up-front HCT: 27.5 (15-54) IST: 31 (15-55) | OS Up-front HCT: 86.5% (95% CI, 79.7-93.8) IST: 92.4% (95% CI, 87.2-98.0) FFS HCT: 86.5% (95% CI, 79.7-93.8) | N/A |
| Study . | No. of patients . | Age at diagnosis/HCT, median (range), y . | Comparison up-front HCT vs IST . | Comparison up-front MUD vs salvage HCT . |
|---|---|---|---|---|
| Dufour et al6 | Up-front HCT: 29 ∗IST: 58 ∗Salvage HCT: 24 | At diagnosis Up-front: 7.9 (0.5-18.6) At HCT Up-front: 8.4 (1.7-19.1) | 2-year OS Up-front HCT: 96% ± 4% IST: 94% ± 3% (P = .68) 2-year EFS Up-front HCT: 92% ± 5% IST: 40% ± 7% (P = .0001) | 2-year OS and EFS Up-front MUD HCT: 95% ± 5% Salvage MUD HCT: 74% ± 9% (P = .02) |
| Choi et al16 | Up-front HCT: 23 IST: 19 Salvage HCT: 11 | At diagnosis Up-front HCT: 9.3 (0.6-17.2) IST 8.5 (1.3-14.1) Salvage HCT: 6.8 (1.3-11.6) At HCT Up-front: 11.5 (1.1-17.7) Salvage: 8.1 (2.7-14.9) | OS Up-front HCT: 91.3% IST: 71.2% (P = .187) FFS HCT: 91.3% IST: 30.7% (P < .001) | 5-year EFS Up-front HCT: 91.3% Salvage HCT: 50.9% (P = .015) |
| Cheng et al15 | Up-front HCT: 28 IST: 24 | At diagnosis HCT: 8 (2-17) IST: 6 (4-16) | 10-year OS Up-front HCT: 89.3% ± 5.8% IST: 73.4% ± 12.6% (P = .806) FFS HCT: 89.3% ± 5.8% IST: 52.6% ± 10.5% (P = .008) | N/A |
| Xu et al17 | Up-front HCT: 76 IST: 37 | At diagnosis HCT: 28 (18-49) IST: 32 (18-62) | 8-year OS Up-front HCT: 83.7% IST: 75.6% (P = .328) FFS HCT: 83.7% ± 4.8% IST: 38.5% ± 13.2% (P = .001) | N/A |
| Xu et al36 | Up-front HCT: 23 Salvage HCT: 29 | All pediatric patients (<18 years) | N/A | OS: P = .698 FFS: P = .899 |
| Yang et al18 | Up-front HCT: 20 IST: 29 | At diagnosis Up-front HCT: 13 (4-18) IST: 12 (4-17) Salvage HCT: 12 (4-17) | 3-year OS Up-front HCT: 85.0% ± 8.0% IST: 79.3% ± 7.5% (P = .740) 3-year FFS HCT: 80.0% ± 8.9% IST: 35.9% ± 10.9% (P = .043) | EFS Up-front HCT: 80.0% ± 8.9% Salvage HCT: 41.7% ± 14.2% (P = .046) |
| Wu et al37 | Up-front HCT: 114 IST: 99 | At diagnosis Up-front HCT: 27.5 (15-54) IST: 31 (15-55) | OS Up-front HCT: 86.5% (95% CI, 79.7-93.8) IST: 92.4% (95% CI, 87.2-98.0) FFS HCT: 86.5% (95% CI, 79.7-93.8) | N/A |
CI, confidence interval; EFS, event-free survival; FFS, failure-free survival; N/A, not applicable; OS, overall survival.
Matched historical controls.
Many groups have attempted to increase the response rate and durability of responses with IST by adding other agents, such as methylprednisolone, mycophenolate mofetil, danazol, sirolimus, or Cy, without success.38-44 Most recently, eltrombopag, a thrombopoietin-receptor agonist, used in combination with ATG and CsA, showed improvement in overall response rates to ∼70% of patients, with only ∼30% of patients achieving a complete response by 6 months; however, the long-term durability of response remained suboptimal with a 24-month event-free survival of only 46%.45,46 Moreover, the improvement in overall response rate was not seen in the pediatric population.47
Apart from the lack of response durability, the risks of subsequent neoplasms after IST and long-term organ dysfunction remain concerns.33,48,49 In contrast, by halting immune-mediated destruction of hematopoietic stem cells, HCT mitigates long-term complications of SAA, including the risk of clonal evolution.33 In addition, using lower conditioning intensity, several groups have demonstrated low risks of long-term malignant and nonmalignant HCT-related late effects.50-52 In a very long-term follow-up study of up to 30 years comparing frontline IST vs HCT, the development of secondary myelodysplastic syndrome or acute myeloid leukemia was 16% in patients receiving IST vs 1.3% in the HCT group. Additionally, iron overload (18% vs 4%) and cardiovascular events (11% vs 1%) were significantly worse in the IST group.53
The timing of therapy is another consideration. Initiating HCT takes longer than IST due to the need for donor identification, graft procurement, and pre-HCT patient workup. However, ongoing efforts from the National Marrow Donor Program54 and improvements in supportive care have helped shorten the time to HCT and reduce infection risk during the pretransplant workup period. These improvements have contributed to significant progress in HCT outcomes.5,35
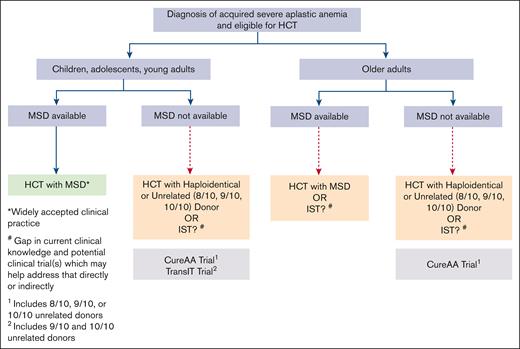
HCT with an MRD has long been considered the standard first-line therapy for young patients with SAA.21,22,55 The use of MUD HCT in TN SAA is also increasing, especially in younger patients or those needing rapid hematopoietic recovery due to underlying infection.21,22,55,56 However, few alternative donor transplants are done in TN patients aged >40 years or those who lack an MRD.21,22,55,56 This represents a substantial proportion of patients with SAA and specifically affects racially or ethnically diverse patients, given the heterogeneity in HLA and the limited diversity of donors in existing registries.57 This barrier to HCT represents a critical gap in the treatment of SAA, underscoring the need for additional studies to bridge this divide (Figure 1).
Current treatment paradigm in patients diagnosed with SAA: standard of care and gaps in knowledge. MSD, matched sibling donor.
Current treatment paradigm in patients diagnosed with SAA: standard of care and gaps in knowledge. MSD, matched sibling donor.
Evolving literature in the last decade challenges the traditional ineligibility criteria for up-front HCT, such as older age or the absence of a matched donor. Improved prognostication to identify patients with low risks of regimen-related toxicities, along with the successful use of reduced-intensity conditioning regimens and PTCy to mitigate the risk of treatment-related morbidities, even with alternative donors, is reshaping our perspective on the role of HCT as first-line treatment for SAA.58-63
Although alternative HCT is often recommended as an option for patients who fail up-front therapy with IST, patients undergoing salvage HCT tend to have worse outcomes than those who receive up-front HCT,6,64 possibly due to the extended time from diagnosis to HCT with multiple intercurrent infections and an increasing transfusion burden.65
Alternative donor HCT in patients with SAA
Several studies in the contemporary era using alternative donor HCT for SAA show promising overall and failure-free survival rates, but less-than-desirable rates of acute and chronic GVHD remain a deterrent to its widespread use.36,66-68 These studies predominantly used calcineurin inhibitors, mycophenolate mofetil, and methotrexate-based GVHD prophylaxis. With the successful application of PTCy-based GVHD prophylaxis in HCT regimens,69 some groups have also tested its efficacy for SAA.
Initial studies focusing on alternative donor HCT in patients with SAA in the United States were primarily limited to those with relapsed or refractory disease. The first study from Johns Hopkins used a conditioning regimen of ATG, fludarabine, low-dose Cy, and 2-Gy total body irradiation (TBI) in combination with PTCy-based GVHD prophylaxis (Baltimore regimen) in 16 patients with relapsed or refractory SAA.14 All had sustained engraftment. Overall survival was 100%, and only 2 patients developed mild chronic GVHD. A follow-up Blood and Marrow Transplant Clinical Trials Network (BMT CTN) single-arm phase 2 trial, using a similar preparative regimen and patient population, reported 81% overall survival at 1 year.12 More than 40% of enrolled patients were non-White, and >60% were from groups underrepresented in donor registries.12 In a long-term study to determine the optimal Cy dosing for unrelated donor HCT in SAA, Cy doses of 50 mg/kg and 100 mg/kg with 2-Gy TBI, ATG, and fludarabine were both found to be effective conditioning regimens.70 The 8-year probabilities of survival were 85% and 75.6% after the Cy 50 mg/kg and 100 mg/kg doses, respectively.70 Another study from the European Society of Blood and Marrow Transplantation reported outcomes of 33 patients with relapsed or refractory SAA who underwent haploidentical donor HCT.71 Half of the patients (n = 16) received the Baltimore regimen, which was associated with significantly better overall survival than non-Baltimore regimens (93% vs 64%; P = .03). Chronic GVHD rates were low at 10%, with no extensive chronic GVHD in patients who received PTCy-based prophylaxis. Similar low rates of chronic GVHD with PTCy were also seen in a study from the Brazilian Society of Bone Marrow Transplantation.72 In comparison, a recent analysis from the European Society of Blood and Marrow Transplantation SAA Working Party reported lower overall and event-free survival rates among patients who underwent HCT using haploidentical donor or MMUD than those who underwent HCT with MUD for either TN or relapsed/refractory SAA.73 It is important to note that these groups were heterogeneous in time from diagnosis to HCT, graft source, and conditioning regimen, which could have affected the outcomes. The most common cause of death was infection, and rates of chronic GVHD were similar between donor types at 18% to 20%.
Subsequently, DeZern et al reported outcomes of HCT in 37 patients with either relapsed/refractory or TN SAA.14 After observing initial high rates of graft failure in the TN population, the TBI dose was increased from 2 to 4 Gy. Among 10 patients who received the higher TBI dose, none developed graft failure, and none of the patients with TN SAA developed chronic GVHD. This study highlighted the need for further research to evaluate the efficacy of this regimen in the TN SAA setting. A follow-up single-center phase 2 trial assessing the feasibility and safety of up-front alternative donor HCT in 27 patients with TN SAA showed 92% overall survival and a low (4%) chronic GVHD rate.13 Ten patients (37%) were of a race or ethnicity other than non-Hispanic White, addressing an important barrier to HCT access. These studies also included a handful of patients aged >60 years, demonstrating the potential feasibility and efficacy of this regimen in older adults often considered ineligible for HCT. Although these trials did not compare outcomes between up-front alternative donor HCT and IST, other groups have conducted such comparisons, reporting similar or improved overall and failure-free survival with up-front alternative donor HCT (Table 1).
The evidence provided by these studies is prompting a reconsideration of traditional recommendations on the role of HCT for SAA. The latest American Society of Transplantation and Cellular Therapy guidelines suggest up-front HCT with either MUD or haploidentical donor rather than IST for patients lacking an MRD, albeit as a conditional recommendation based on very low- or low-quality evidence.55 Other practice guidelines still do not endorse this approach,21,22 recommending alternative donor HCT only in the relapsed or refractory setting.74-77 High-quality evidence, ideally from large multicenter trials, is needed to definitively establish the role of up-front alternative HCT in TN SAA.
Current clinical trials’ potential to influence clinical practice for TN SAA
Recognizing the need for robust multicenter trials to fill existing gaps in the current treatment paradigm of TN SAA, the 2021 BMT CTN State of the Science symposium highlighted the importance of conducting studies to assess the role of up-front alternative donor HCT as a crucial next step in this field.78 Consequently, a BMT CTN phase 2 trial (NCT06517641) CureAA (BMT CTN 2207: “Clinical Trial of Upfront Haploidentical or Unrelated Donor Bone Marrow Transplantation to Restore Normal Hematopoiesis in Aplastic Anemia”), currently enrolling across multiple US sites, was designed building upon prior BMT CTN experience in the relapsed/refractory setting and testing alternative donor HCT (haploidentical donor, MUD, or MMUD) as up-front therapy for all patients with SAA. The CureAA trial will use the 4-Gy TBI, Cy, fludarabine, and ATG-based conditioning backbone with PTCy, tacrolimus, and mycophenolate mofetil-based GVHD prophylaxis, successfully piloted by Johns Hopkins (see “Alternative donor HCT in patients with SAA” section). The primary objective of the CureAA trial is to assess GVHD-free, failure-free survival among TN patients receiving this regimen, which, because of the use of multiple alternative donor types, will, if successful, provide broad access to HCT. In addition, the trial includes recipients aged up to 75 years, increasing the consideration of access for older patients with SAA. The CureAA trial will also assess patients’ quality of life by measuring patient-reported outcomes, offering valuable perspectives on the impact of up-front HCT on patients’ physical, emotional, social, and financial health, as well as health care utilization. Simultaneously, another ongoing phase 3 trial (NCT05600426) TransIT (“A Trial Comparing Unrelated Donor BMT with IST for Pediatric and Young Adult Patients with Severe Aplastic Anemia”) aims to understand the role of MUD or MMUD HCT in TN SAA. After the excellent HCT outcomes in a previous pilot feasibility trial,79 this study will recruit patients aged up to 25 years with TN SAA lacking an MRD and randomize between up-front IST with CsA and ATG or MUD/MMUD HCT using 2-Gy TBI, Cy, fludarabine, and ATG-based conditioning backbone with CsA- and methotrexate-based GVHD prophylaxis. The BMT CTN is collaborating on this trial (BMT CTN 2202). The TransIT trial’s primary objective is to compare the time from randomization to treatment failure or death from any cause between study arms. It also has several secondary objectives including the comparison of quality of life using patient-reported outcome measures, salvage with rescue procedures, measures of gonadal function after treatment, assessment of evolving clonality in each arm to predict the development of secondary malignancies, and a novel and important measure of failure-free/immune suppression–free survival with adequate count comparison at 2 years.
In addition to these 2 multicenter trials, a handful of ongoing single-center trials are assessing the role of alternative donor HCT in the TN or relapsed/refractory SAA settings. These include a phase 2 trial (NCT06752694) aiming to study the role of a ruxolitinib-based GVHD prophylaxis regimen in TN older adults (age >40 years) or young adults (18-40 years) with high comorbidity undergoing HCT using MRD, MUD, or MMUD. Another phase 2 trial (NCT04558736) aims to assess the role of T-cell receptor αβ–positive T-cell–depleted haploidentical donor HCT in relapsed/refractory patients aged ≤21 years. Another phase 1 to 2 trial (NCT03173937) aims to study HCT in relapsed/refractory patients (aged 4-60 years) using an ex vivo expanded umbilical cord blood product (omidubicel), with interim analyses showing promising results.80 Lastly, CK0801, an allogeneic umbilical cord blood–derived T regulatory cell therapy product has shown safety and effectiveness in achieving transfusion independence in patients with relapsed/refractory bone marrow failure syndromes, including SAA, underscoring the need for studies in a larger cohort.81 In parallel, other groups are working to improve the effectiveness of IST in TN SAA, including a phase 2a/b trial (NCT06430788) assessing the addition of emapalumab (anti–interferon gamma antibody) to an ATG and CsA-based IST regimen in patients aged ≤25 years. This trial is simultaneously assessing the role of emapalumab as a bridge to HCT. Although all these trials focus on different questions, their overarching aims hold significant potential to change the practice paradigm for patients with TN SAA (Figure 1).
The role of stakeholder engagement and implementation framework
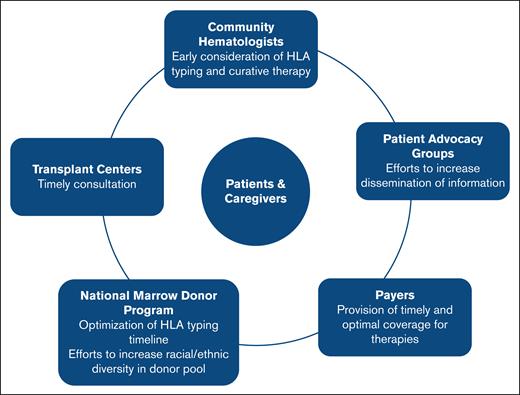
Realizing the full potential of any clinical trial depends on several factors. Previously published studies show that even with strong data from rigorous clinical trials, the translation of research findings into clinical practice remains limited.23,24,82,83 Several impactful and practice-changing multi-institutional studies were conducted in the field of HCT in the last 2 decades84; however, the successful translation of these findings into clinical practice varied.24,85 Based on these experiences, we now know that the strength of evidence alone is insufficient; equal emphasis must be placed on ensuring its implementation by identifying and engaging relevant stakeholders. Consequently, the field of dissemination and implementation science has gained momentum,26,27,86 emphasizing the need for proactive integration of implementation frameworks and stakeholder engagement in ongoing trials to bridge the gap between evidence and practice, if the trials meet end points (Figure 2).
Stakeholder awareness and engagement to improve dissemination and implementation of trials assessing the role of up-front alternative donor HCT in patients with SAA.
Stakeholder awareness and engagement to improve dissemination and implementation of trials assessing the role of up-front alternative donor HCT in patients with SAA.
The first step in stakeholder engagement involves increasing awareness among community hematologists and HCT physicians on existing gaps in the SAA treatment landscape and the potential relative advantage of up-front alternative donor HCT to fill these gaps. Discussions of potential long-term complications associated with the use of TBI87 and Cy,88 which could possibly deter clinicians from offering HCT, must be weighed against the potential for infections, clonal progression, and treatment failure with IST, as well as worse outcomes with subsequent salvage HCT. Discussions should also include opportunities to participate in current trials so that the trials can reach target accruals. The latter can be difficult in rare diseases such as SAA.
National organizations such as the Aplastic Anemia & Myelodysplastic Syndrome International Foundation and the North American Pediatric Aplastic Anemia Consortium play a pivotal role in increasing clinician awareness. Improved clinician awareness can ensure timely HLA typing, earlier consultations for curative therapy, and thorough discussion regarding risks and benefits of up-front HCT vs IST, especially for older individuals and those without matched donor options. Consequently, increasing clinicians’ knowledge of extant literature and ongoing clinical trials’ objectives, while acknowledging uncertainties around long-term complications in those treated with up-front HCT vs IST, may foster enthusiasm for trial referral and enrollment. These patient advocacy groups can increase awareness of clinical trials among the population of patients with SAA. Other important stakeholders include the National Marrow Donor Program, to facilitate timely donor procurement, and insurers, to facilitate coverage for the therapy.
In alignment with this objective, the BMT CTN, a US-based national network established in 2001 to advance clinical trials focused on adult and pediatric transplant and cellular therapy outcomes (bmtctn.net), is actively working to embed implementation science frameworks into its research. This effort is being led by the newly formed Dissemination and Implementation Committee, which aims to enhance the real-world impact and uptake of BMT CTN–supported trials. This committee collaborates closely with ongoing and upcoming BMT CTN protocol teams to formally assess implementation outcomes throughout the trials. Key components include adoption (the proportion of clinicians offering trials to eligible patients), penetration (the proportion of eligible patients being offered the trials and that of nonconsenting/withdrawn patients), sustainability (ongoing interest from the perspectives of clinicians and patients), and acceptability (patient logistics and overall burden) during the conduct of clinical trials.26,27,89 It would be important to have periodic assessments to understand how these efforts affect posttrial adoption in real-world practice. For the CureAA trial, the BMT CTN Dissemination and Implementation Committee is dedicated to engaging with community practices to raise awareness about the trial and to identify potential barriers to patient referral and trial implementation. In support of this effort, a survey is being conducted in collaboration with Aplastic Anemia & Myelodysplastic Syndrome International Foundation and the North American Pediatric Aplastic Anemia Consortium, targeting community hematologists and BMT physicians who treat SAA. The survey aims to gather insights on current treatment practices, including transplant referral patterns, and perceived challenges to patient enrollment.
Conclusions and future directions
In conclusion, through the tireless efforts of researchers worldwide, the treatment landscape for SAA has evolved, and treatment outcomes have improved, although gaps remain. HCT is now broadly accepted as an option for TN SAA; however, barriers related to its use in older patients and those without matched donors continue to affect a significant proportion of patients with SAA and remain a cause for health care disparity. Ongoing research efforts focus on both optimizing the role of IST as well as making alternative donor HCT a safe option for these patients. Whether the ongoing trials will change the SAA treatment landscape remains to be seen, but their successful completion relies heavily on proactive stakeholder engagement and a robust implementation framework.
Acknowledgments
The authors specially thank Michael Pulsipher, David Williams, and Bronwen Shaw from the TransIT (NCT05600426) trial team for their insightful input on the manuscript.
The Blood and Marrow Transplant Clinical Trials Network is supported in part by grants from the National Heart, Lung, and Blood Institute and the National Cancer Institute (U01HL069294 and U24HL138660).
Authorship
Contribution: N.S.B., A.B., Y.E., L.B., M.J., N.K., M.M.H., and A.E.D. conceived and designed the review; N.S.B., A.B., and A.E.D. wrote the first draft of the manuscript; and all other authors critically reviewed and edited the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.E.D. reports advisory board fees and/or consultancy fees and honoraria from Bristol Myers Squibb, Agios, and Novartis; and serving on clinical trial committees or data safety monitoring boards for Novartis, AbbVie, Kura, Geron, Servier, Keros, Shattuck Labs, and Bristol Myers Squibb. M.M.H. reports research funding from Sobi and Incyte. N.K. reports consulting fees from Incyte. A.B. reports speakers’ bureau and advisory board fees from Janssen. B.H. reports ad hoc advisory board fees from Sanofi, Incyte, Rigel, and MaaT Pharma; consultancy fees from ACI Group; data safety monitoring committee fees from Angiocrine; and adjudication committee fees from CSL Behring. B.R. reports advisory board fees from Astellas, Pfizer, Genentech, and Syndax. S.W. reports education bureau speaker fees from Sobi; advisory board participation (completed 11 December 2023) for MorphoSys; and research support for conducting clinical trials as site principal investigator from CTI Biopharma, Incyte, Telios, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Neel S. Bhatt, Clinical Research Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, M2-B230 Seattle, WA 98109; email: nbhatt@fredhutch.org.
References
Author notes
N.S.B. and A.B. are joint first authors.