Key Points
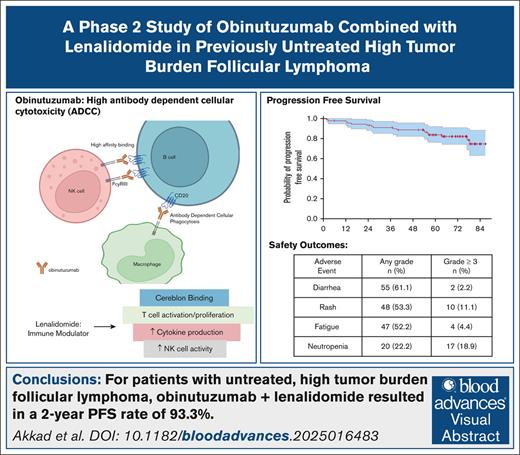
For patients with untreated high tumor burden follicular lymphoma, obinutuzumab and lenalidomide led to a 2-year PFS rate of 93.3%.
Obinutuzumab combined with lenalidomide was well tolerated with the most common AEs being diarrhea, rash, and fatigue.
Visual Abstract
Follicular lymphoma (FL) has a clinical course that is often characterized by high response rates to first-line therapy, followed by multiple relapses over a prolonged natural history. Currently, there are multiple possible approaches to frontline therapy for untreated advanced-stage FL, but there is an ongoing debate around what is the preferred approach. Based on the benefits seen with combining lenalidomide, an immunomodulatory agent, with rituximab, an anti-CD20 antibody, we aimed to evaluate the safety and efficacy of lenalidomide in combination with obinuzutumab, an anti-CD20 antibody with enhanced antibody-dependent cellular cytotoxicity. The eligibility criteria included a diagnosis of FL, grade 1 to 3a, stage II to IV, Eastern Cooperative Oncology Group performance status ≤ 2, adequate organ function, and high tumor burden according to the Groupe d’Étude des Lymphomes Folliculaires criteria. Participants received 6 cycles of induction with the combination, followed by 24 cycles of maintenance. Among 90 patients, the primary end point, 2-year progression-free survival (PFS), was 93.3% (95% confidence interval [CI], 88.2-98.6), and the median PFS was not reached with a median follow-up of 70.7 months. The complete response rate at 30 months was 89.7% (95% CI, 81.3-95.2). The most common adverse events (AEs) of any grade were diarrhea (61.1%), maculopapular rash (53.3%), and fatigue (52.2%). The most common AEs ≥ grade 3 were neutropenia (18.9%), maculopapular rash (11.1%), and pneumonia (6.7%). In this single-center trial, these findings indicate that, for patients with previously untreated, high tumor burden FL, obinutuzumab and lenalidomide led to robust and durable responses with a favorable 2-year PFS and manageable safety profile. This trial was registered at www.clinicaltrials.gov as #NCT02871219.
Introduction
Follicular lymphoma (FL) is the second most common subtype of lymphoma with an estimated 14 000 new cases diagnosed annually.1 FL often initially responds to first-line (1L) chemoimmunotherapy, but most patients eventually suffer multiple relapses that are characterized by an increasing resistance to therapy and a decreasing duration of response (DOR) in subsequent lines of therapy. Consequently, despite improvements in the response rates and progression-free survival (PFS), the major cause of death for patients with FL remains lymphoma or adverse events (AEs) associated with therapy.2 Currently, there are multiple possible approaches to chemoimmunotherapy as frontline therapy for untreated, advanced-stage FL, but there is ongoing debate about the preferred approach.3 Identifying frontline therapy that produces durable responses, limits toxicity, and preserves options for future treatment remains an important unmet need in this disease.
For patients with FL who require treatment, chemoimmunotherapy with rituximab, a monoclonal antibody directed against CD20, has demonstrated improvements in the response rates, PFS, and overall survival (OS) when compared with chemotherapy alone.4-7 Despite the high response rates and a median PFS of ∼10 years with the addition of 2 years of maintenance rituximab, most patients will anticipate a relapse.8 Among the treatment options in the frontline setting, the combination of bendamustine and rituximab has become a common approach. However, the negative impact on T cell function associated with bendamustine has raised concerns about the impact of frontline treatment on the subsequent use of T cell-focused therapy, like chimeric antigen receptor T cells and bispecific antibodies, in later lines of therapy.9,10 In addition, the AEs of chemoimmunotherapy, including infectious complications, organ dysfunction, and second cancers, raise questions as to what the optimal 1L treatment strategy for FL should be.
Obinutuzumab is a humanized, type 2, anti-CD20 monoclonal antibody that is glycoengineered for enhanced antibody-dependent cellular cytotoxicity when compared with rituximab because of the increased affinity for the Fc gamma receptor IIIa .11-13 The GALLIUM study evaluated obinutuzumab + chemotherapy (G-chemo) vs rituximab + chemotherapy (R-chemo) in patients with untreated, advanced-stage FL and showed improved 3-year PFS (80% vs 73.3%) for G-chemo when compared with R-chemo (hazard ratio, 0.66; 95% confidence interval [CI], 0.51-0.85).9 However, a lenalidomide containing arm was not included in the GALLIUM study. In addition, obinutuzumab has been studied in combination with lenalidomide in the relapsed setting in a single-arm study and has been shown to have a 2 year PFS of 65% (95% CI, 54-74) and a complete response rate (CRR) of 38% (95% CI, 28-50).14
Lenalidomide is an immunomodulatory agent that has been shown to activate natural killer cells, T cells, or both and to lead to the expansion of immune effector cells in vivo in non-Hodgkin lymphoma and chronic lymphocytic leukemia models.15 Preclinical models have also shown a synergistic anti-tumor effect when lenalidomide was combined with anti-CD20 molecules.16 The combination of rituximab and lenalidomide (R2) has been studied in patients with previously untreated and relapsed/refractory FL. In the RELEVANCE trial, R2 was compared with rituximab-based chemoimmunotherapy and led to favorable response rates and survival outcomes that were similar to standard chemoimmunotherapy (complete response [CR] rate at 120 weeks: 48% vs 53%) in high tumor burden, previously untreated FL.17 Despite the promise of nonchemotherapy 1L treatment in FL, R2 was not superior to chemoimmunotherapy. We therefore hypothesized that combining obinutuzumab, an anti-CD20 antibody with increased affinity for the FCgRIIIa receptor, with lenalidomide could enhance the efficacy with limited toxicity. As an initial test of this hypothesis, we performed a single-arm, single-center, open-label, phase 2 study to evaluate the efficacy and safety of obinutuzumab in combination with lenalidomide in patients with untreated, high tumor burden FL.
Methods
Study design
This was an investigator initiated, open-label, single-arm, phase 2 study that was conducted at The University of Texas MD Anderson Cancer Center. The key trial eligibility criteria included a diagnosis of FL, previously untreated, grade 1, 2, or 3a, and stage II to IV with high tumor burden disease according to the Groupe d’Étude des Lymphomes Folliculaires criteria.18 Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2 and adequate organ function. Creatinine clearance was required to be >30 mL/mi as calculated by the modified Cockcroft-Gault formula; dose reductions were incorporated for patients with a Creatinine clearance of <60 mL/min. Patients were excluded if they had evidence of central nervous system disease, grade 3b disease, evidence of transformation, or previous use of lenalidomide (the supplemental Materials contain the protocol and outlines the full inclusion and exclusion criteria). Patients received 20 mg lenalidomide on days 1 to 21, in combination with 1000 mg obinutuzumab (on day 1, 8, and 15 of cycle 1 and on day 1 from cycle 2 to 6), in a 28 day cycle for 6 cycles as induction. Patients who achieved at least a partial response (PR) continued maintenance treatment for 24 cycles, which consisted of lenalidomide (10 mg for patients with CR, 20 mg for patients with PR) from cycle 7 to 18, and obinutuzumab on day 1 of every other cycle from cycle 7 to 30. After cycle 30, patients were followed every 6 months for 3 follow-up visits, then annually for 2 years. The use of granulocyte colony–stimulating factor was permitted for the management of neutropenia in accordance with the American Society of Clinical Oncology guidelines.19 Dose modification, delay, or discontinuation was based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, toxicity grade and was specified in the protocol (supplemental Data). AE reporting started at the time of patient consent. This study was carried out in compliance with the principles of the International Conference on Harmonization, Good Clinical Practice, Declaration of Helsinki, and all applicable national and local regulations that governed clinical studies. This study was approved by the MD Anderson Institutional Review Board, and all authors had access to the primary clinical trial data. Patients were required to provide written informed consent before enrollment.
Outcomes
The primary objective of this study was to evaluate the efficacy of obinutuzumab in combination with lenalidomide in patients with previously untreated FL. The primary end point of this study was PFS rate at 2 years as assessed by the investigator.20,21 Two year PFS was selected as the primary end point based on historic outcomes in previous studies of patients with previously untreated FL across chemoimmunotherapy regimens, including the PRIMA,22 StiL,23 and BRIGHT24 studies, in which ∼20% of patients progressed within 2 years. Therefore, studies that evaluate chemoimmunotherapy regimens in untreated, advanced-stage FL should aim for a 2-year PFS estimate of >80%, as is the case in this study. This observation led to the analysis of the National LymphoCare Study, which showed that patients with untreated FL who experienced progressive disease within 24 months of initial treatment (POD24) had inferior OS with 5 year OS estimates of 50% vs 90% in the reference group, thereby establishing that outcomes in the first 2 years are informative for the prognosis and natural history of FL.20 Secondary end points included CRR at 120 weeks, best CRR, best overall response rate (ORR), DOR, event-free survival (EFS), transformation-free survival (TFS), and OS. Secondary end points also included an evaluation of safety, including the frequency and severity of AEs and the frequency of treatment-emergent AEs that required discontinuation of the study drug or dose reductions.
Assessment
Response assessments were completed by the principal investigator (L.J.N.) using the Lugano criteria (2014).25 Patients were evaluated for response using positron emission tomography/computed tomography on day 1 of cycle 4, 7, 10, 14, 20, 26, end of treatment, and every 6 months for 3 assessments, then annually until disease progression. A unilateral bone marrow biopsy was done at screening or up to 90 days before the first dose of study drug. For patients with positive bone marrow involvement, a bone marrow biopsy was repeated to confirm CR. The best ORR (CR + PR) and CRR at 120 weeks were assessed by the investigator. AEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
Descriptive statistics, including the mean, standard deviation, median, and range for continuous variables, such as age and laboratory measurements, and frequency counts and percentages for categorical variables, such as stage, response status, and AE type and grade, were reported. OS time was calculated from the treatment start date to death date. PFS was calculated from the treatment start date to relapse/progression date or death date, whichever happened earlier. Alive patients without progression were censored at the last follow-up date. The DOR was calculated from the patient’s first response date to relapse/progression date or death date, whichever happened earlier. EFS time was calculated from the treatment start date to progression date, transformation date, next lymphoma treatment date, or death date, whichever happened earliest. TFS time was calculated from the treatment start date to disease transformation date or death date, whichever happened earlier. The Kaplan-Meier method was used to estimate OS, PFS, DOR, EFS, and TFS. Patients were followed for at least 3 years or until death, and the final analysis was performed when the last patient was followed for 3 years. The statistical software programs SAS 9.4 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc, Palo Alto, CA) were used for all analyses and the analyses were carried out by L. Feng.
In designing this study, it was expected that this experimental regimen will achieve better efficacy than the standard of care for this population. Other studies that evaluated the current standard of care regimens in untreated FL have shown an estimated 2-year PFS rate of ∼77% to 87%.22-24 These regimens include bendamustine and rituximab (StiL23 and BRIGHT24 studies) and immunochemotherapy, followed by rituximab maintenance (PRIMA22 study). In a single center study it was reported that the standard of care of lenalidomide and rituximab in this patient population had a 2-year PFS rate of 86.5% (95% CI, 77-97.2).26 Given this, we assumed that the current regimen would be at least as good as the lower bound of the 95% CI for the 2-year PFS rate. With a sample size of 90 patients, the 2-sided 95% CI for 2-year PFS rate would extend 8.7% from the observed rate, leaving an expected rate of 77% (nQuery Advisor 7.0).
The baseline clinical, response, toxicity, and outcomes data were captured by the MD Anderson clinical research coordinators, nurses, and investigators. Data audits and validation were completed internally. De-identified data for specific research questions may be available on request from the corresponding author. The study protocol is included as a data supplement that is available with the online version of this article.
Results
Patient characteristics
From December 2016 to April 2019, 90 patients were enrolled in this study at the MD Anderson Cancer Center. The study flow is summarized in supplemental Figure 2. The patient characteristics are summarized in Table 1. The median age was 58 years (range, 33-84). The ECOG performance status ranged from 0 to 2 with the majority (68.9%) having an ECOG of 0. Lactate dehydrogenase (LDH) was elevated in most patients (76.7%). Most patients had advanced stage (90%) disease; 19 (21.1%) had an FL international prognostic index (FLIPI) score of 0 to 1, 34 (37.8%) had an FLIPI of 2, and 37 (41.1%) had an FLIPI of 3 to 5. All patients had a high tumor burden according to the Groupe d’Étude des Lymphomes Folliculaires criteria.
Patient characteristics
| Characteristics . | N = 90 . |
|---|---|
| Age, y | |
| Median | 58 |
| Range | 33-84 |
| Sex, n (%) | |
| Male | 47 (52.2) |
| Race, n (%) | |
| Asian | 5 (5.56) |
| African American | 4 (4.44) |
| American Indian | 1 (1.11) |
| White | 70 (77.8) |
| Hispanic | 9 (10) |
| Other | 1 (1.11) |
| ECOG, n (%) | |
| 0 | 62 (68.9) |
| 1 | 26 (28.9) |
| 2 | 2 (2.22) |
| Stage, n (%) | |
| II | 9 (10) |
| III-IV | 81 (90) |
| Grade, n (%) | |
| 1 or 2 | 72 (80) |
| 3 | 18 (20) |
| FLIPI, n (%) | |
| 0-1 | 19 (21.1) |
| 2 | 34 (37.8) |
| 3-5 | 37 (41.1) |
| LDH > ULN∗, (%) | 69 (76.7) |
| Median Hgb, g/dL | 13.70 |
| Range Hgb, g/dL | 8.00-16.70 |
| Median CrCl | 98.4 |
| Range CrCl | 36.8-21.7 |
| Characteristics . | N = 90 . |
|---|---|
| Age, y | |
| Median | 58 |
| Range | 33-84 |
| Sex, n (%) | |
| Male | 47 (52.2) |
| Race, n (%) | |
| Asian | 5 (5.56) |
| African American | 4 (4.44) |
| American Indian | 1 (1.11) |
| White | 70 (77.8) |
| Hispanic | 9 (10) |
| Other | 1 (1.11) |
| ECOG, n (%) | |
| 0 | 62 (68.9) |
| 1 | 26 (28.9) |
| 2 | 2 (2.22) |
| Stage, n (%) | |
| II | 9 (10) |
| III-IV | 81 (90) |
| Grade, n (%) | |
| 1 or 2 | 72 (80) |
| 3 | 18 (20) |
| FLIPI, n (%) | |
| 0-1 | 19 (21.1) |
| 2 | 34 (37.8) |
| 3-5 | 37 (41.1) |
| LDH > ULN∗, (%) | 69 (76.7) |
| Median Hgb, g/dL | 13.70 |
| Range Hgb, g/dL | 8.00-16.70 |
| Median CrCl | 98.4 |
| Range CrCl | 36.8-21.7 |
CrCl, creatinine clearance; Hgb, hemoglobin; ULN, upper limit of normal.
∗LDH ULN is 214.
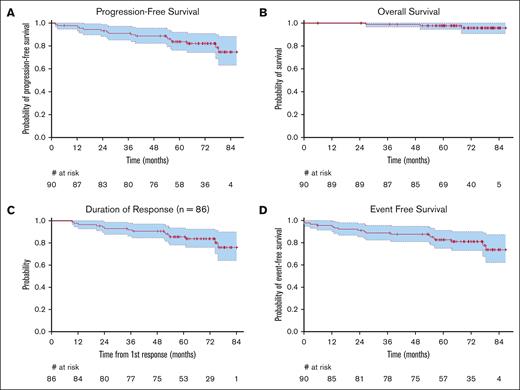
Efficacy
Among the 90 evaluable patients, the best CRR was 96.6% (95% CI, 90.4-99.3) and the best ORR was 97.7% (95% CI, 92.0-99.7; Table 2). The ORR and CRR at time of first evaluation (cycle 4, day 1) was 98.9% and 91.9%, respectively. The median time to first response was 2.8 months (range, 1.8-6.3). The CRR at 30 months or 120 weeks was 89.7% (95% CI, 81.3-95.2). The median DOR was not reached (Figure 1C). A total of 32 patients (35.6%) discontinued treatment before completing therapy. The reasons for discontinuation are summarized in Table 3. The most common reasons treatment was discontinued were infection, including COVID-19 pneumonia in 3 patients, followed by progression, patient preference, and cough. Of the 32 patients who discontinued treatment, 21 (66%) remained in remission during the study follow-up period. Of the 58 patients who did not discontinue treatment early, 52 (90%) remained in remission during the study follow-up period. Obinutuzumab dose reduction occurred in 1 case because of rash. The doses were held in 6.6% of instances with the most common reasons being neutropenia, upper respiratory infection, and dyspnea. The dose of lenalidomide was reduced on 7 occasions (1%), most commonly because of rash (4 instances), followed by neutropenia and fatigue, and doses were held in 5.5% of all instances with the most common reasons being neutropenia and rash.
Response rates
| Response . | n (%) . |
|---|---|
| Best response (n = 88) | |
| OR | 86 (97.7) |
| CR | 85 (96.6) |
| PR | 1 (1.1) |
| PD | 2 (2.27) |
| Response at 120 weeks (n = 87) | |
| CR | 78 (89.7) |
| Response at 12 weeks (n = 86) | |
| CR | 79 (91.9) |
| PR | 6 (7.0) |
| Response at 24 weeks (n = 84) | |
| CR | 84 (100) |
| Response . | n (%) . |
|---|---|
| Best response (n = 88) | |
| OR | 86 (97.7) |
| CR | 85 (96.6) |
| PR | 1 (1.1) |
| PD | 2 (2.27) |
| Response at 120 weeks (n = 87) | |
| CR | 78 (89.7) |
| Response at 12 weeks (n = 86) | |
| CR | 79 (91.9) |
| PR | 6 (7.0) |
| Response at 24 weeks (n = 84) | |
| CR | 84 (100) |
Reasons for discontinuation
| Reason for discontinuation . | n . |
|---|---|
| Infection | 7 |
| Progression | 4 |
| Patient preference∗ | 4 |
| Cough | 4 |
| Financial† | 3 |
| Second primary cancer‡ | 1 |
| Transformation | 1 |
| Arthritis | 1 |
| Investigator discretion | 1 |
| Sick sinus syndrome | 1 |
| Ineligible§ | 1 |
| Abdominal pain | 1 |
| Fatigue | 1 |
| Neutropenia | 1 |
| Poor wound healing | 1 |
| Reason for discontinuation . | n . |
|---|---|
| Infection | 7 |
| Progression | 4 |
| Patient preference∗ | 4 |
| Cough | 4 |
| Financial† | 3 |
| Second primary cancer‡ | 1 |
| Transformation | 1 |
| Arthritis | 1 |
| Investigator discretion | 1 |
| Sick sinus syndrome | 1 |
| Ineligible§ | 1 |
| Abdominal pain | 1 |
| Fatigue | 1 |
| Neutropenia | 1 |
| Poor wound healing | 1 |
Two patients had upper airway cough syndrome, 2 patients had concerns because of risk associated with COVID-19 pandemic.
One patient had a change in insurance and 2 patients lost insurance.
Noninvasive bladder cancer.
Patient with biopsy-proven transformation after having received 1 dose of study drug combination.
Survival
The median PFS was not reached with a median follow-up of 70.7 months (95% CI, 65.8-76.1), and ranged from 1.8 to 86.8 months. The 2-year PFS, the primary end point of the study, was 93.3% (95% CI, 88.2-98.6), thus exceeding the expected rate of 86.5% (Figure 1A). The 3-year and 5-year PFS estimates were 91% (95% CI, 85.3-97.2) and 83.7% (95% CI, 76.3-91.9), respectively. There was no difference in the PFS when the participants were stratified by FLIPI score (supplemental Figure 1), gender, race, ECOG, and grade (data not shown). The median OS, DOR, EFS, and time to next lymphoma treatment were not reached (Figure 1B-D). Six patients (7%) had progression of disease before 24 months (POD24).21 Five of these 6 patients were alive at the time of last follow-up, and 1 died 76.5 months from the date of consent. Of these 6, 1 had biopsy-proven transformation. All 6 received additional therapy. The patient who transformed received dose adjusted-R-EPOCH. The other 5 each received a different treatment including, bendamustine + rituximab, Obinutuzumab + rituximab, radiation, tazemetostat, or umbralisib. In this study, including the follow-up period, 12 patients received a second-line treatment, except those who transformed. The most common second-line treatment was bendamustine + rituximab (6 patients). In total, 4 patients (4.4%) experienced transformation to large cell lymphoma with 2 transformations occurring in the first month, 1 in month 5, and 1 in month 38. At the time of last follow-up, 3 of these 4 patients were still alive. There were 3 deaths, 1 owing to lymphoma, 1 owing to infection, and 1 owing to secondary acute myelogenous leukemia.
Safety
Among the 90 patients included in the safety analysis, AEs that occurred in more than 10% of patients are summarized in Table 4. The most common any grade AE was diarrhea (61.1%), and 2 patients had ≥grade 3 diarrhea. Other common any grade AEs included maculopapular rash (53.3%), fatigue (52.2%), constipation (48.9%), and myalgia (45.6%). The most common AEs that were grade 3 or higher included neutropenia (18.9%), maculopapular rash (11.1%), and fatigue (4.4%). There were 11 grade 4 AEs, including 10 grade 4 neutropenia cases and 1 grade 4 febrile neutropenia case. There were no grade 5 AEs reported. AEs were not associated with treatment discontinuation, and only 1 patient discontinued treatment because of fatigue and 1 because of neutropenia. AEs led to dose delays or dose reductions of obinutuzumab in 6.7% of patients and of lenalidomide in 6.4% of patients. Neutropenia was a common AE that was present in 22.2% of patients, and most were grade 3 or higher (17/20 patients). Seven patients had grade 3 neutropenia, 10 patients had grade 4 neutropenia, and 2 patients experienced febrile neutropenia. Only a few patients received growth factor support (13.3%). Infections were common and included upper respiratory infection (26.7%) and pneumonia (17.8%). Enrollment and treatment for this study occurred during the COVID-19 pandemic, and 3 patients discontinued treatment secondary to COVID-19 infection. Seven patients discontinued treatment for any infection. There were 4 infusion reactions that occurred during this study, and all were grade 1 or 2. Three thromboembolic events were reported with 1 being grade 1 and 2 being grade 2. In addition, 1 grade 1 portal vein thrombosis was reported. There were no cases of tumor lysis syndrome or tumor flare.
AEs
| AE (N = 90) . | Any grade n (%) . | Grade ≥ 3 n (%) . | Grade 4 n (%) . |
|---|---|---|---|
| Diarrhea | 55 (61.1) | 2 (2.2) | 0 (0) |
| Rash (maculopapular) | 48 (53.3) | 10 (11.1) | 0 (0) |
| Fatigue | 47 (52.2) | 4 (4.4) | 0 (0) |
| Constipation | 44 (48.9) | 0 (0) | 0 (0) |
| Myalgia | 41 (45.6) | 0 (0) | 0 (0) |
| Cough | 30 (33.3) | 2 (2.2) | 0 (0) |
| Nausea | 27 (30) | 0 (0) | 0 (0) |
| Dyspnea | 23 (25.6) | 0 (0) | 0 (0) |
| Upper respiratory infection | 23 (25.6) | 1 (1) | 0 (0) |
| Fever | 21 (23.3) | 1 (1) | 0 (0) |
| Headache | 20 (22.2) | 1 (1) | 0 (0) |
| Neutropenia | 20 (22.2) | 7 (7.8) | 10 (11.1) |
| Allergic rhinitis | 16 (17.8) | 0 (0) | 0 (0) |
| Lower extremity edema | 14 (15.6) | 0 (0) | 0 (0) |
| Dizziness | 13 (14.4) | 0 (0) | 0 (0) |
| Pneumonia | 10 (11.1) | 6 (6.7) | 0 (0) |
| Lymphedema | 10 (11.1) | 0 (0) | 0 (0) |
| Memory impairment | 10 (11.1) | 0 (0) | 0 (0) |
| Febrile neutropenia | 0 (0) | 1 (1) | 1 (1) |
| AE (N = 90) . | Any grade n (%) . | Grade ≥ 3 n (%) . | Grade 4 n (%) . |
|---|---|---|---|
| Diarrhea | 55 (61.1) | 2 (2.2) | 0 (0) |
| Rash (maculopapular) | 48 (53.3) | 10 (11.1) | 0 (0) |
| Fatigue | 47 (52.2) | 4 (4.4) | 0 (0) |
| Constipation | 44 (48.9) | 0 (0) | 0 (0) |
| Myalgia | 41 (45.6) | 0 (0) | 0 (0) |
| Cough | 30 (33.3) | 2 (2.2) | 0 (0) |
| Nausea | 27 (30) | 0 (0) | 0 (0) |
| Dyspnea | 23 (25.6) | 0 (0) | 0 (0) |
| Upper respiratory infection | 23 (25.6) | 1 (1) | 0 (0) |
| Fever | 21 (23.3) | 1 (1) | 0 (0) |
| Headache | 20 (22.2) | 1 (1) | 0 (0) |
| Neutropenia | 20 (22.2) | 7 (7.8) | 10 (11.1) |
| Allergic rhinitis | 16 (17.8) | 0 (0) | 0 (0) |
| Lower extremity edema | 14 (15.6) | 0 (0) | 0 (0) |
| Dizziness | 13 (14.4) | 0 (0) | 0 (0) |
| Pneumonia | 10 (11.1) | 6 (6.7) | 0 (0) |
| Lymphedema | 10 (11.1) | 0 (0) | 0 (0) |
| Memory impairment | 10 (11.1) | 0 (0) | 0 (0) |
| Febrile neutropenia | 0 (0) | 1 (1) | 1 (1) |
Discussion
This study evaluated the safety and efficacy of obinutuzumab and lenalidomide in patients with high tumor burden, untreated FL. This study met its primary end point, demonstrating a 2-year PFS rate of 93.3%. At 2 years, only 6 patients had progressed or died with this regimen. With a median follow up of 70.7 months, the median PFS and OS were not reached. Frontline obinutuzumab and lenalidomide also led to high CR rates, both after the induction phase of 6 cycles (CRR, 93.3%) and at 30 months (CRR, 89.7%), showing that the remissions achieved after the first 6 cycles persisted in most patients. Studies also have shown that the end of induction CR rates may be prognostic for PFS27 and that CR at 30 months may serve as a surrogate end point for PFS for 1L therapy among patients with FL treated with chemoimmunotherapy.28 Our data indicate that the combination of frontline obinutuzumab and lenalidomide is highly active in patients with previously untreated, high tumor burden FL with evidence of early responses and favorable 2-year PFS.
Previous randomized controlled trials have demonstrated the benefits of lenalidomide when combined with a CD20 antibody, and obinutuzumab as the preferred CD20 antibody, independently. GALLIUM demonstrated the superiority of obinutuzumab with chemotherapy vs rituximab with chemotherapy with 3-year PFS rates of 80% vs 73.3%, respectively.9 RELEVANCE showed similar CRRs at 120 weeks for rituximab with lenalidomide vs rituximab with chemotherapy (48% vs 53%), as well as a similar 3-year PFS (77% vs 78%), however, the safety profiles were different and less neutropenia was noted in the group that received rituximab and lenalidomide.17 Although a direct comparison cannot be made, the CRR of patients who received obinutuzumab and lenalidomide in this study at 120 weeks was 89.7%, the best CRR was 96.6%, and the 3-year PFS was 91%. In addition, the POD24 rate in this study was 7% and was reported to be 12% in the RELEVANCE study.29 This suggests that the increased affinity for the FCgRIIIa receptor, which leads to an increased antibody-dependent cellular cytotoxicity with obinutuzumab than with rituximab, may indicate that obinutuzumab is a superior partner for lenalidomide than rituximab. In addition, the difference in CRR is likely also attributable to the difference in the response criteria used with the RELEVANCE study using the 1999 International Working Group criteria and this study using the Lugano criteria (2014). However, a randomized study with a head-to-head comparison would be required to test this hypothesis.
Similar findings to our study were observed in a multicenter study that evaluated frontline obinutuzumab and lenalidomide in FL (LYSA study).30 This study did not reach its primary end point of end of induction CRR, most likely because one-third of patients did not undergo an end of induction bone marrow biopsy and therefore the true CRR was likely underestimated. The CRR at the end of treatment was 63%, and the best CRR was 77%.30 POD24 was reported as 14% in the LYSA study, higher than what was found in this study. The LYSA study used the 1999 International Working Group criteria to evaluate response, whereas our study used the Lugano criteria (2014). The Lugano criteria (2014) includes a larger role for positron emission tomography–computed tomography in staging and response and is therefore a more relevant set of criteria to assess the response to treatment among FL cases in the modern era.25 In addition, the LYSA study had a relatively high treatment discontinuation rate of 75 patients (75%) as opposed to 32 patients (35.6%) in this study. This study is also the only study, to our knowledge, that evaluated the efficacy and safety of this combination in a US population.
No new safety signals were identified in this study. The most common AEs were diarrhea and rash, whereas the most common serious AEs were neutropenia and rash. Neutropenia was a common AE in the GALLIUM study in the G-chemo arm and in the R2 arm of the RELEVANCE trial; these studies reported any grade neutropenia at rates of 50.6% and 75%, respectively. Similarly, in the GALEN study, grade ≥3 neutropenia was reported in 48% of patients. Our study noted lower rates of neutropenia when compared with these studies with 22.2% of patients having any grade neutropenia and 18.9% having ≥ grade 3 neutropenia. Two patients had febrile neutropenia in our study. The LYSA study permitted the use of growth factor for an absolute neutrophil count of <500/μL for up to 3 days, whereas our study followed the American Society of Clinical Oncology 2006 guidelines, which takes into account age, medical history, disease characteristics, and myelotoxicity of the regimen. This follows common clinical practice and may explain the difference in the incidence of neutropenia between the studies. Our study showed a relatively high rate of ≥ grade 3 rash (11.1%) when compared with the RELEVANCE and GALEN studies, which reported rates of 4% (in the R2 arm) and 2%, respectively, for ≥ grade 3 rash. Although lenalidomide has been shown to lead to an increased incidence of thromboembolic events when used in multiple myeloma, similar to other studies on lenalidomide for lymphoma, there were only a few thromboembolic events in this study (3 grade 1 or 2 thromboembolic events and 1 portal vein thrombosis).31,32 In the GALLIUM study, there were higher rates of infusion reactions in the G-chemo group than in the R-chemo group (6.6% vs 3.5%), and the LYSA study reported 28 (28%) infusion reactions with 3 being ≥ grade 3. In contrast, our study had only 4 (4.4%) infusion reactions, all of which were grade 1 or 2. Both the GALLIUM study and our study used premedications, including acetaminophen, diphenhydramine, and corticosteroids 60 minutes before obinutuzumab infusion. Premedications in the LYSA study were not reported, thus it is possible that the inclusion of premedications, as described above, may account for the low rate of obinutuzumab infusion reactions. Two second primary malignancies (SPMs) occurred in this study; 1 patient was found to have a noninvasive urothelial cancer after receiving 11 cycles of the combination, and another patient was diagnosed with acute myelogenous leukemia during the end of treatment follow-up period. The LYSA study reported 11 SPMs (11%), the GALLIUM study noted 43 SPMs in the G-chemo arm (7.2%), and the RELEVANCE study noted 38 SPMs (7%) in the R2 arm.
The limitations of this study include that it was performed at a single center, which can limit generalizability. However, even at a single center, this study enrolled a relatively large sample size with gender and racial diversity that is representative of the US FL population.33 In addition, because this was a phase 2 study, it did not include a comparator group and response was investigator determined. Furthermore, in this study, it was noted that 76.7% of patients had an elevated LDH, which is higher than what was previously reported in FL.34 LDH elevation was a characteristic that allowed for study entry. Consequently, this may have introduced a bias in this population, which was also served at a tertiary referral center. There was no central review of eligibility criteria or pathology, which led to the enrollment of 2 patients who had occult transformation to large cell lymphoma that was unknown before initiating treatment. Despite these limitations, this trial showed that obinutuzumab and lenalidomide were both active and safe as 1L treatment for patients with FL and a high tumor burden.
Both lenalidomide and obinutuzumab are also being studied in combination with other therapies for lymphoma. For example, obinutuzumab and lenalidomide were studied in combination with polatuzumab in the relapsed setting in a multicenter phase 1b/2 trial.35,36 This study showed favorable efficacy with an end of induction CRR of 61%, an ORR of 76%, and a landmark PFS of 53% (95% CI, 36-70) at 48 months in patients with relapsed/refractory FL, however, all patients experienced an AE and more than half experienced a serious AE, most commonly myelosuppression or infection. In the modern era of treatment with T-cell engagers, there are also upcoming studies that combined lenalidomide with bispecific antibodies. For example, epcoritamab, a CD20 × CD3 bispecific antibody has been studied in combination with rituximab and lenalidomide in untreated FL. This ongoing study (ClinicalTrials.gov identifier: NCT04663347) showed a CRR of 85% and an ORR of 95% at a median follow-up of 21.1 months with the most common AE being COVID-19 infection (63%), cytokine release syndrome (56%), and neutropenia (56%).37 Given the lower rates of neutropenia seen with obinutuzumab and lenalidomide in our study, it may be a good candidate to serve as a backbone with the addition of bispecifics in the future.
In conclusion, obinutuzumab, combined with lenalidomide, in untreated high tumor burden FL shows promising efficacy and manageable safety. The robust CRR, few progression events within 2 years, and few transformation events during an albeit modest follow-up period are encouraging. A randomized study would be necessary to understand where this treatment fits into the treatment landscape and in combinations with bispecific antibodies as they move forward as the experimental arm in randomized trials.
Acknowledgments
This research study was supported, in part, by The University of Texas MD Anderson Cancer Center and P30CA016672, research grants from Genentech/Roche and Bristol Meyers Squibb, and the Futcher Foundation. The MD Anderson Lymphoma Tissue Bank was used in this study and is supported by KW Cares. C.R.F. is a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research and was supported during this work by the Cancer Prevention and Research Institute of Texas RR190079 and National Cancer Institute K24CA208132. The salary of P.S. is supported by the Leukemia Lymphoma Society Scholar in Clinical Research Career Development Program, the Kite Gilead Scholar in Clinical Research Award, and the Sabin Family Fellowship Award.
Authorship
Contribution: N.A., L.J.N., C.R.F., D.C., and P.S. wrote the manuscript; L.J.N. and L. Feng designed the study; J.R.W., F.B.H., H.J.L., L. Fayad, S.A., R.N., M.A.R., M.W., N.H.F., S.S.N., and L.J.N. provided patients and/or resources to the study; L.J.N., L.C., and K.I. oversaw the conduct of the trial and data capture; all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: L.J.N. reports receiving honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb (BMS), Caribou Biosciences, Daiichi Sankyo, Genentech/Roche, Genmab, Gilead/Kite, Janssen, Incyte, Ipsen, Merck, Novartis, Regeneron, and Takeda; and research support from BMS, Caribou Biosciences, Daiichi Sankyo, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Ipsen, Janssen, Merck, Novartis, and Takeda. C.R.F. reports serving as a consultant for AbbVie, Bayer, BeiGene, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech/Roche, Genmab, Gilead, Karyopharm, N-Power Medicine, Pharmacyclics/Janssen, Seagen, and Spectrum; holding stock or stock options in Foresight Diagnostics and N-Power Medicine; and receiving research funding from 4D, AbbVie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, BostonGene, Celgene, Cellectis EMD, Gilead, Genentech/Roche, Guardant, Iovance, Janssen Pharmaceutical, Kite, Morphosys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, and Ziopharm, as well as from the Burroughs Wellcome Fund, Cancer Prevention and Research Institute of Texas RR190079: Cancer Prevention and Research Institute of Texas Scholar in Cancer Research, Eastern Cooperative Oncology Group, National Cancer Institute, and V Foundation. P.S. reports serving as a consultant for Roche-Genentech, AbbVie-Genmab, Ipsen, Kite/Gilead, Hutchison MediPharma, AstraZeneca-Acerta, ADC Therapeutics, Sobi, and TG Therapeutics; and receiving research funds from Sobi, AstraZeneca-Acerta, ALX Oncology, and ADC Therapeutics. J.R.W. reports receiving research funding from or serving on the advisory board of AbbVie, ADC therapeutics, Allogene, AstraZeneca, BMS, Genentech, Janssen, Kite/Gilead, Morphosys/Incyte, Novartis, Nurix, Pfizer, and Regeneron. D.C. reports receiving research funding from BMS, Genentech, Genmab, and Ono Pharmaceutical; and receiving an honorarium from Genmab. S.A. reports receiving research support to their institution for clinical trials from Nektar, Merck, Xencor, Chimagen, Genmab, Kite/Gilead, Janssen, and Caribou; and receiving consultancy fees/honoraria from ADC therapeutics, Kite/Gilead, and Janssen. M.W. reports receiving research funding from Loxo Oncology, Juno Therapeutics, Genentech, Molecular Templates, and Vincerx; receiving honoraria from WebMD, Oncology Specialty Group, Nurix, National Institutes of Health, Moffit Cancer Center, MJH Life Sciences, MD Education, Meeting Minds Experts, Medscape, IDEOlogy Health, i3Health, Eastern Virginia Medical School, CAHON, Bantam Pharmaceutical, OncLive, Studio ER Congressi, Scripps, and Practice Point Communications; receiving travel support and research funding from Celgene; receiving honoraria and research funding from Genmab; receiving honoraria and travel support from Dava Oncology and Physicians Education Resources; serving as a consultant for and receiving research funding from VelosBio, Oncternal, and Eli Lilly and Company; serving as a consultant for and receiving honoraria and research funding from Pharmacyclics, Janssen, BioInvent, BeiGene, and Acerta Pharma; serving as a consultant for Pepromene Bio, Parexel, Milken Institute, Miltenyi Biomedicine, InnoCare, Genentech, DTRM Biopharma (Cayman) Limited, Deciphera, Be Biopharma, Amphista Therapeutics Limited, and ADC Therapeutics America; serving as a consultant for and receiving honoraria from Merck, Leukemia & Lymphoma Society, BMS, and AbbVie; and serving as a consultant for and receiving honoraria, travel support, and research funding from Kite Pharma and AstraZeneca. S.S.N. reports receiving research support from Kite/Gilead, Allogene, Precision Biosciences, Adicet Bio, Sana Biotechnology, and Cargo Therapeutics; serving as an advisory board member or consultant for Kite/Gilead, Sellas Life Sciences, Allogene, Adicet Bio, BMS, Fosun Kite, Caribou, Astellas Pharma, Morphosys, Janssen, Chimagen, ImmunoACT, Takeda, Synthekine, Carsgen, Appia Bio, GlaxoSmithKline, Galapagos, ModeX Therapeutics, Jazz Pharmaceuticals, ADC Therapeutics, BioOra Limited, Arovella Therapeutics, Merck, Pfizer, and Poseida; and having intellectual property related to cell therapy. H.J.L. reports serving as a consultant for and receiving research funding from Oncternal; receiving research funding from Takeda and BMS; serving as a consultant for and receiving honoraria and research funding from AstraZeneca and Pfizer; and receiving honoraria from Curio and MJH Life Sciences. F.B.H. reports receiving honoraria from Roche. N.H.F. reports being an employee of BostonGene; and receiving research support from Celgene/BMS. M.G. reports receiving research funding from Sanofi, Allogene, and Kite/Gilead; receiving research funding and honoraria from AbbVie; and receiving honoraria from BMS, Daiichi Sankyo, and DAVA Oncology. R.N. reports serving on the advisory boards of ADC Therapeutics Lymphoma, 280 Bio, Inc, and Novartis. L. Fayad reports receiving research funding from Roche/Genentech. The remaining authors declare no competing financial interests.
Correspondence: Loretta J. Nastoupil, Southwest Oncology, 1 Mercado St Ste 100, Durango, CO 81301; email: loretta.nastoupil@commonspirit.org.
References
Author notes
Deidentified data for specific research questions may be available on request from the corresponding author, Loretta J. Nastoupil (loretta.nastoupil@commonspirit.org). The study protocol is included as a data supplement, available with the online version of this article.
The full-text version of this article contains a data supplement.