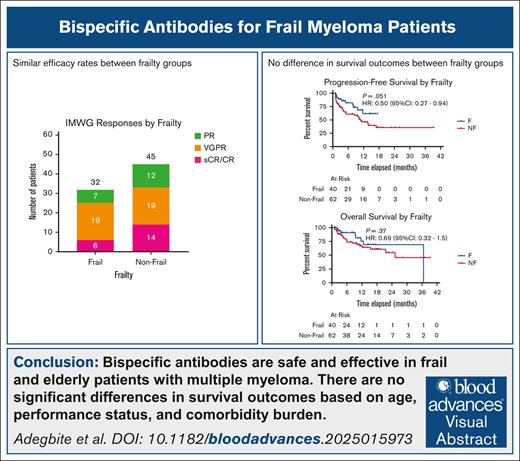
Key Points
BsAbs are safe and effective in frail and older patients with MM.
Advanced age, worse PS, and higher comorbidity burden do not predict inferior outcomes with BsAbs in myeloma.
Visual Abstract
Patients with relapsed/refractory (R/R) multiple myeloma (MM) are often frail with preexisting comorbidities and poor performance status (PS). To evaluate clinical characteristics and outcomes based on frailty, we conducted a single-center retrospective study of patients with R/R MM who received B-cell maturation antigen (BCMA)–directed bispecific antibodies (BsAbs). Frailty was defined using the simplified frailty index based on age, Eastern Cooperative Oncology Group (ECOG) PS, and Charlson comorbidity index (CCI; nonfrail score 0-1 and frail score ≥2). Of 102 patients analyzed (age range, 40-88 years), 40 (39%) were considered frail. The frail group had more patients aged ≥70 years (73% vs 29%; P < .001), with ECOG PS ≥2 (36% vs 0%; P < .001), and worse median CCI (2 vs 1; P < .001). Patients in the frail group experienced similar rates of all-grade cytokine release syndrome (58% vs 60%; P = .99), immune effector cell–associated neurotoxicity syndrome (15% vs 8%; P = .44), and treatment-related mortality (13% vs 21%; P = .27) compared to the nonfrail group. The best overall response rate was 80% (stringent complete response [sCR]/CR, 15%; very good partial response [VGPR], 48%) in the frail group vs 73% (sCR/CR, 23%; VGPR, 31%) in the nonfrail group (P = .40). With a median follow-up of 8.6 months (range, 3-14), there was no significant difference in median progression-free survival (not reached vs 11 months; P = .051) or overall survival (37 vs 25 months; P = .37) between the frail and nonfrail groups. Hence, BsAbs were deemed safe and effective for older and frail patients with R/R MM.
Introduction
Multiple myeloma (MM) is primarily a disease affecting older adults, with a median age of 69 years at the time of diagnosis.1 In addition to advanced age, many of these patients are frail with preexisting comorbidities, borderline performance status (PS), and impaired organ function. Given concerns for excessive toxicities, older as well as frail patients have been historically excluded from clinical trials evaluating therapies in newly diagnosed and relapsed/refractory (R/R) settings.2-5 However, several real-world studies have shown that chronological age and frailty should not be a barrier toward effective treatment modalities, including autologous stem cell transplant and chimeric antigen receptor (CAR) T-cell therapy for MM.6,7 Instead, frailty scores should be incorporated into screening and eligibility assessments, with concentrated efforts to optimize physical fitness and organ function before and during the course of therapy. The simplified frailty index (SFI) is one such frailty assessment tool that combines evaluation of PS, comorbidity burden, and age and has been developed and used previously to identify frail patients at risk for inferior outcomes with frontline therapy for transplant-ineligible newly diagnosed MM.8,9 Although there is growing evidence for real-world safety and efficacy of bispecific antibodies (BsAbs) for R/R MM, in which many of the patients were deemed ineligible for clinical trials, there is limited literature focusing on older and frail patients to guide decision-making and treatment selection. Hence, we conducted a retrospective analysis to study the impact of frailty, as per SFI, on outcomes with BsAbs for patients with R/R MM.
Methods
Our analysis included 102 adult patients with R/R MM who had received B-cell maturation antigen (BCMA)–directed BsAbs (teclistamab or elranatamab) between December 2017 and August 2024 at Memorial Sloan Kettering Cancer Center. The study was approved by the institutional review board and was compliant with the terms from the Declaration of Helsinki. All data underwent peer-based quality check for completeness and internal consistency. All patients except 1 had received BsAb with step-up dosing in the inpatient settings followed by outpatient administration, with supportive care and infectious prophylaxis as per the institutional guidelines. All patients had received at least 1 full step-up dose and had at least 1 month follow-up data available for efficacy and safety analyses. Chart review of electronic medical records was used to determine the SFI for each patient. SFI was calculated based on chronological age + Eastern Cooperative Oncology Group (ECOG) PS at the time of treatment + Charlson comorbidity index (CCI; based on retrospective review of each patient’s medical history), with a score of 0 to 1 indicating nonfrail and a score ≥2 indicating frail. CCI scores were calculated without incorporating age to reduce redundancy in the frailty score. Descriptive statistics (Mann-Whitney and χ2 tests) were used to highlight clinical characteristics (such as age, PS, and comorbidities), efficacy outcomes, and safety events (Table 1). High-risk cytogenetics were defined by the presence of deletion 17p/monosomy 17, t(4;14), and t(14;16) at any time point before BsAb treatment. Key data end points included incidence and severity of cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), grade ≥3 cytopenias, grade ≥3 infections, treatment-related mortality (TRM), response rates (Figure 1), progression-free survival (PFS), and overall survival (OS). Response rates were determined using the International Myeloma Working Group (IMWG) criteria.10 Adverse events were graded based on the American Society for Transplantation and Cellular Therapy criteria11 for CRS and ICANS and Common Terminology Criteria for Adverse Events v5.0 criteria for other adverse events. TRM was defined as an event without myeloma progression, with relapse as a competing event. Kaplan-Meier analysis was used for PFS and OS assessments between frail and nonfrail patients (Figure 2). The log-rank test was used to determine hazard ratios (HRs) in the PFS and OS curves (Figures 2 and 3). Cox proportional hazard regression model was used to determine patient- and disease-related predictors of PFS and OS in the entire patient population as a multivariate analysis (Table 2).
Baseline patient characteristics, safety, and efficacy stratified per frailty status in patients receiving BsAbs for MM
| . | Frail group (N = 40) . | Nonfrail group (N = 62) . | P value . |
|---|---|---|---|
| Patient characteristics | |||
| Age, median (range), y | 78 (69-81) | 65 (59-70) | <.001 |
| Age ≥70 years, n (%) | 29 (73) | 18 (29) | <.001 |
| Male sex, n (%) | 16 (40) | 26 (42) | 1 |
| Race, non-Hispanic White, n (%) | 20 (50) | 30 (48) | 1 |
| ECOG PS ≥2, n (%) | 10 (36) | 0 | <.001 |
| CCI score, median (range) | 2 (1-3) | 1 (0-1) | <.001 |
| CrCl ≤60 mL/min, n (%) | 23 (62) | 13 (22) | <.001 |
| High-risk cytogenetics, n (%) | 20 (57) | 30 (55) | .98 |
| Prior lines of therapy, median (range) | 6 (4-7) | 5 (4-7) | .44 |
| Extramedullary disease, n (%) | 12 (36) | 17 (30) | .73 |
| Triple-class refractory disease, n (%) | 32 (80) | 42 (75) | .74 |
| Receiving teclistamab, n (%) | 32 (80) | 39 (63) | .11 |
| Prior anti-BCMA therapy, n (%) | 13 (33) | 20 (33) | 1 |
| Safety outcomes | |||
| CRS, any grade, n (%) | 23 (58) | 37 (60) | .99 |
| CRS, grade ≥3, n (%) | 0 | 2 (3) | .68 |
| ICANS, any grade, n (%) | 6 (15) | 5 (8) | .44 |
| ICANS, grade ≥3, n (%) | 1 (3) | 0 | .82 |
| Grade ≥3 cytopenia <30 days, n (%) | 16 (40) | 27 (44) | .83 |
| Grade ≥3 infection <90 days, n (%) | 11 (28) | 20 (32) | .77 |
| Length of hospital stay, median (range), d | 7 (7-8) | 7 (5-9) | .58 |
| TRM, n (%) | 5 (13) | 13 (21) | .27 |
| Causes of death, n (%) | |||
| All cause deaths | 8 (20) | 21 (34) | .13 |
| Disease progression | 3 (8) | 8 (13) | .39 |
| Infection | 3 (8) | 7 (11) | .53 |
| Other | 2 (5) | 6 (10) | .39 |
| Efficacy outcomes | |||
| Best ORR, n (%) | 32 (80) | 45 (73) | .40 |
| sCR/CR, n (%) | 6 (15) | 14 (23) | .35 |
| VGPR, n (%) | 19 (48) | 19 (31) | .086 |
| . | Frail group (N = 40) . | Nonfrail group (N = 62) . | P value . |
|---|---|---|---|
| Patient characteristics | |||
| Age, median (range), y | 78 (69-81) | 65 (59-70) | <.001 |
| Age ≥70 years, n (%) | 29 (73) | 18 (29) | <.001 |
| Male sex, n (%) | 16 (40) | 26 (42) | 1 |
| Race, non-Hispanic White, n (%) | 20 (50) | 30 (48) | 1 |
| ECOG PS ≥2, n (%) | 10 (36) | 0 | <.001 |
| CCI score, median (range) | 2 (1-3) | 1 (0-1) | <.001 |
| CrCl ≤60 mL/min, n (%) | 23 (62) | 13 (22) | <.001 |
| High-risk cytogenetics, n (%) | 20 (57) | 30 (55) | .98 |
| Prior lines of therapy, median (range) | 6 (4-7) | 5 (4-7) | .44 |
| Extramedullary disease, n (%) | 12 (36) | 17 (30) | .73 |
| Triple-class refractory disease, n (%) | 32 (80) | 42 (75) | .74 |
| Receiving teclistamab, n (%) | 32 (80) | 39 (63) | .11 |
| Prior anti-BCMA therapy, n (%) | 13 (33) | 20 (33) | 1 |
| Safety outcomes | |||
| CRS, any grade, n (%) | 23 (58) | 37 (60) | .99 |
| CRS, grade ≥3, n (%) | 0 | 2 (3) | .68 |
| ICANS, any grade, n (%) | 6 (15) | 5 (8) | .44 |
| ICANS, grade ≥3, n (%) | 1 (3) | 0 | .82 |
| Grade ≥3 cytopenia <30 days, n (%) | 16 (40) | 27 (44) | .83 |
| Grade ≥3 infection <90 days, n (%) | 11 (28) | 20 (32) | .77 |
| Length of hospital stay, median (range), d | 7 (7-8) | 7 (5-9) | .58 |
| TRM, n (%) | 5 (13) | 13 (21) | .27 |
| Causes of death, n (%) | |||
| All cause deaths | 8 (20) | 21 (34) | .13 |
| Disease progression | 3 (8) | 8 (13) | .39 |
| Infection | 3 (8) | 7 (11) | .53 |
| Other | 2 (5) | 6 (10) | .39 |
| Efficacy outcomes | |||
| Best ORR, n (%) | 32 (80) | 45 (73) | .40 |
| sCR/CR, n (%) | 6 (15) | 14 (23) | .35 |
| VGPR, n (%) | 19 (48) | 19 (31) | .086 |
Boldface indicates statistically significant values.
CrCl, creatinine clearance; ORR, overall response rate; sCR, stringent CR; VGPR, very good partial response.
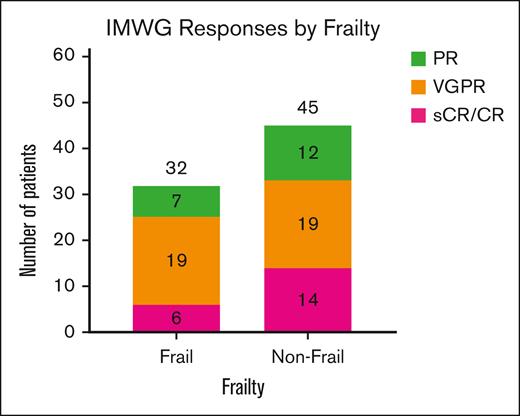
Distribution of IMWG hematologic responses based on frailty. Overall, 32 of the 40 frail patients (80%) and 45 of the 62 nonfrail patients (73%) showed at least a ≥50% reduction in circulating monoclonal protein levels based on IMWG response criteria. Among frail patients, 19 (48%) had VGPR, and 6 (15%) had either CR or sCR. Among nonfrail patients, 19 (31%) had VGPR, and 14 (23%) had either CR or sCR. There were no statistically significant differences in the distribution of response rates in each of the groups. PR, partial response; sCR, stringent CR; VGPR, very good partial response.
Distribution of IMWG hematologic responses based on frailty. Overall, 32 of the 40 frail patients (80%) and 45 of the 62 nonfrail patients (73%) showed at least a ≥50% reduction in circulating monoclonal protein levels based on IMWG response criteria. Among frail patients, 19 (48%) had VGPR, and 6 (15%) had either CR or sCR. Among nonfrail patients, 19 (31%) had VGPR, and 14 (23%) had either CR or sCR. There were no statistically significant differences in the distribution of response rates in each of the groups. PR, partial response; sCR, stringent CR; VGPR, very good partial response.
Multivariate analyses for predictors of PFS and OS in patients receiving BsAbs for MM
| . | HR . | 95% CI . | P value . |
|---|---|---|---|
| PFS multivariable analysis | |||
| Frailty | 1.7 | 0.54-12 | .58 |
| Age ≥70 years | 1.6 | 0.49-5 | .45 |
| ECOG ≥2 | 2.3 | 0.38-14 | .36 |
| CCI ≥2 | 1.2 | 0.23-6.6 | .8 |
| CrCl ≤60 | 0.88 | 0.19-4 | .87 |
| EMD | 1.1 | 0.38-3.1 | .88 |
| High-risk cytogenetics | 1 | 0.44-2.4 | .95 |
| Triple-class refractory disease | 0.49 | 0.15-1.6 | .24 |
| Grade ≥3 cytopenia <30 days, n (%) | 1.4 | 0.53-3.7 | .5 |
| Grade ≥3 infection <90 days, n (%) | 1.9 | 0.8-4.7 | .14 |
| Prior lines of therapy ≥4 | 2.4 | 0.49-12 | .28 |
| Prior anti-BCMA therapy | 1.3 | 0.53-3.2 | .55 |
| OS multivariable analysis | |||
| Frailty | 1.1 | 0.12-11 | .92 |
| Age ≥70 years | 3.5 | 0.75-17 | .11 |
| ECOG ≥2 | 4.6 | 0.41-51 | .22 |
| CCI ≥2 | 0.34 | 0.046-2.5 | .29 |
| CrCl ≤60 | 1.2 | 0.18-7.5 | .88 |
| EMD | 1.4 | 0.36-5.8 | .6 |
| High-risk cytogenetics | 1.6 | 0.42-6 | .48 |
| Triple-class refractory disease | 0.65 | 0.11-3.8 | .63 |
| Grade ≥3 cytopenia <30 days, n (%) | 2 | 0.49-7.8 | .34 |
| Grade ≥3 infection <90 days, n (%) | 1.8 | 0.52-6.5 | .34 |
| Prior lines of therapy ≥4 | 1.5 | 0.14-16 | .74 |
| Prior anti-BCMA therapy | 2 | 0.58-6.9 | .27 |
| . | HR . | 95% CI . | P value . |
|---|---|---|---|
| PFS multivariable analysis | |||
| Frailty | 1.7 | 0.54-12 | .58 |
| Age ≥70 years | 1.6 | 0.49-5 | .45 |
| ECOG ≥2 | 2.3 | 0.38-14 | .36 |
| CCI ≥2 | 1.2 | 0.23-6.6 | .8 |
| CrCl ≤60 | 0.88 | 0.19-4 | .87 |
| EMD | 1.1 | 0.38-3.1 | .88 |
| High-risk cytogenetics | 1 | 0.44-2.4 | .95 |
| Triple-class refractory disease | 0.49 | 0.15-1.6 | .24 |
| Grade ≥3 cytopenia <30 days, n (%) | 1.4 | 0.53-3.7 | .5 |
| Grade ≥3 infection <90 days, n (%) | 1.9 | 0.8-4.7 | .14 |
| Prior lines of therapy ≥4 | 2.4 | 0.49-12 | .28 |
| Prior anti-BCMA therapy | 1.3 | 0.53-3.2 | .55 |
| OS multivariable analysis | |||
| Frailty | 1.1 | 0.12-11 | .92 |
| Age ≥70 years | 3.5 | 0.75-17 | .11 |
| ECOG ≥2 | 4.6 | 0.41-51 | .22 |
| CCI ≥2 | 0.34 | 0.046-2.5 | .29 |
| CrCl ≤60 | 1.2 | 0.18-7.5 | .88 |
| EMD | 1.4 | 0.36-5.8 | .6 |
| High-risk cytogenetics | 1.6 | 0.42-6 | .48 |
| Triple-class refractory disease | 0.65 | 0.11-3.8 | .63 |
| Grade ≥3 cytopenia <30 days, n (%) | 2 | 0.49-7.8 | .34 |
| Grade ≥3 infection <90 days, n (%) | 1.8 | 0.52-6.5 | .34 |
| Prior lines of therapy ≥4 | 1.5 | 0.14-16 | .74 |
| Prior anti-BCMA therapy | 2 | 0.58-6.9 | .27 |
CrCl, creatinine clearance; EMD, extramedullary disease.
Results
Of the 102 patients evaluated (age range, 40-88 years), 40 (40%) were considered frail based on the SFI at the time of BCMA-directed BsAb treatment (P < .001). A total of 71 patients received teclistamab (70%), and the remaining received elranatamab. Furthermore, 32 of the 40 frail patients (80%) received teclistamab, whereas the remaining 8 (20%) received elranatamab. As expected, the frail group had a higher proportion of patients with age ≥70 years (73% vs 29%; P < .001), renal insufficiency (creatinine clearance <60 mL/min; 62% vs 22%; P < .001), ECOG PS ≥2 (36% vs 0%; P < .001), and worse median CCI (2 vs 1; P < .001; Table 1). However, the distribution of patients with extramedullary disease (36% vs 30%; P = .73), number of prior lines of therapy (6 vs 5; P = .44), high-risk cytogenetics (57% vs 55%; P = .98), triple-class refractory disease (80% vs 75%; P = .74), receiving teclistamab therapy (80% vs 63%; P = .11), and prior anti-BCMA therapy (33% vs 33%; P = 1) was similar between the frail and nonfrail groups.
Patients in the frail group experienced similar rates of all-grade CRS (58% vs 60%; P = .99) and ICANS (15% vs 8%; P = .44) compared to the nonfrail group. Incidence of grade ≥3 CRS (frail 0% vs nonfrail 3%; P = .68) and grade ≥3 ICANS (frail 3% vs nonfrail 0%; P = .82) were also similar between the 2 groups. Similarly, there were no differences in the incidence of grade ≥3 cytopenia within 30 days (40% vs 44%; P = .83) or grade ≥3 infection within 90 days (28% vs 32%; P = .77) between the frail and nonfrail groups. TRM was observed in 5 patients (13%) in the frail group compared to 13 (21%) in the nonfrail group (P = .27), with disease progression being the most common cause of death. The best overall response rate was 80% (15% stringent complete response [CR]/CR; 48% very good partial response) in the frail group vs 73% (23% stringent CR/CR; 31% very good partial response) in the nonfrail group (P = .40; Figure 1). With a median follow-up of 8.6 months (range, 3-14) for the entire population, the median PFS and OS were 5.5 (range, 2.1-12) and 9.4 months (range, 3.1-15), respectively. Using the hazard model analysis, the median PFS was not reached (NR; range, 12 to NR) in the frail group and 11 months (range, 5 to NR) in the nonfrail group (HR, 0.50; 95% confidence interval [CI], 0.27-0.94; P = .051). The median OS was 37 months (range, NR to NR) in the frail group and 25 months (range, 16 to NR) in the nonfrail group (HR, 0.69; 95% CI, 0.32-1.5; P = .37; Figure 2). There were no statistically significant differences in PFS or OS based on individual components of SFI, including age, comorbidity burden, and PS (Figure 3). Importantly, patients with no prior anti-BCMA therapy had better PFS (17 months; range, 10 to NR) than those with prior anti-BCMA therapy (8 months; range, 3 to NR; HR, 0.48; 95% CI, 0.24-0.94; P = .016), with no significant difference in OS. However, multivariable analyses of patient and disease characteristics did not reveal any statistically significant association with PFS or OS, including prior anti-BCMA therapy (Table 2).
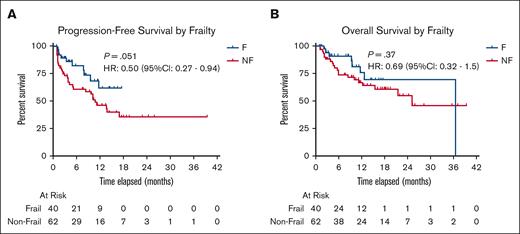
PFS and OS stratified by frailty. Kaplan-Meier curves were used to show the probability of survival without disease progression or death (A) as well as survival without death alone (B) based on frailty status. Both curves did not show statistically significant difference in median survival probability when HR calculations were performed: median PFS was NR (range, 12 to NR) in the frail group and 11 months (range, 5 to NR) in the nonfrail group (P = .051); median OS was 37 months (range, NR to NR) in the frail group and 25 months (range, 16 to NR) in the nonfrail group (P = .37). F, frail; NF, nonfrail.
PFS and OS stratified by frailty. Kaplan-Meier curves were used to show the probability of survival without disease progression or death (A) as well as survival without death alone (B) based on frailty status. Both curves did not show statistically significant difference in median survival probability when HR calculations were performed: median PFS was NR (range, 12 to NR) in the frail group and 11 months (range, 5 to NR) in the nonfrail group (P = .051); median OS was 37 months (range, NR to NR) in the frail group and 25 months (range, 16 to NR) in the nonfrail group (P = .37). F, frail; NF, nonfrail.
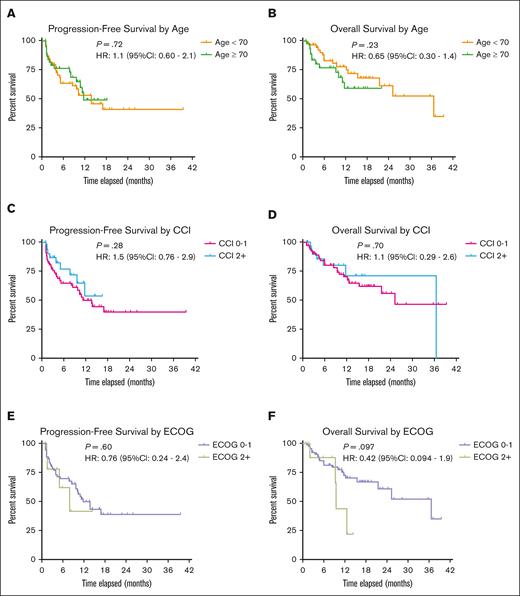
PFS and OS stratified by age, comorbidity index, and PS. Kaplan-Meier survival curves were used to compare survival probabilities of PFS and OS based on age (patients aged <70 years vs ≥70 years), CCI score (patients with scores of 0 or 1 vs ≥2), and ECOG PS (patients with ECOG of 0 or 1 vs ≥2). No statistically significant difference was found in median survival probability for each stratified group.
PFS and OS stratified by age, comorbidity index, and PS. Kaplan-Meier survival curves were used to compare survival probabilities of PFS and OS based on age (patients aged <70 years vs ≥70 years), CCI score (patients with scores of 0 or 1 vs ≥2), and ECOG PS (patients with ECOG of 0 or 1 vs ≥2). No statistically significant difference was found in median survival probability for each stratified group.
Discussion
This single-center retrospective study underscores the tolerable safety and reasonable efficacy of BsAbs for frail patients with MM in real-world practice, highlighting that frailty should not be a barrier to the use of BsAb due to concerns about excessive toxicities or inferior efficacy. High-risk disease features including extramedullary disease, high-risk cytogenetics, and number of prior lines of therapy were equally distributed between the 2 groups, and frailty appeared to be largely determined by patient age, physical fitness, and comorbidities. Despite having worse PS and higher comorbidity burden, frailty at the time of treatment initiation was not associated with increased risk of mortality or major prohibitive toxicities. Although older and frail patients are often deemed ineligible for effective therapies based on subjective assessment in routine practice or strict eligibility criteria in clinical trials, our study advocates expanding access to BsAbs for all patients regardless of age or frailty, with appropriate supportive and preventative measures to mitigate toxicities. Contrary to previously published studies showing inferior outcomes in frail patients,7,9,12,13 we found no significant difference in either PFS or OS between the 2 groups. Although there certainly could have been unmeasured selection bias for “less frail” patients with “more favorable” disease biology receiving BCMA-directed BsAbs in this patient population treated at a large academic center serving as a referral site, our study did show comparable efficacy even after adjusting for predictors of aggressive disease biology in the multivariable analysis. Similarly, we identified better PFS for patients who had not received prior ani-BCMA therapy, which can likely be explained by a host of factors, including T-cell exhaustion, potential loss of target antigen, and worse disease biology in a more heavily pretreated patient population. This finding was not corroborated in the multivariable analysis after adjusting for other risk factors.
As shown in previous real-world studies evaluating transplant and CAR T-cell therapy,6,14,15 this study affirms the efficacy and safety of BsAbs in older patients (age ≥70 years). With no difference in outcomes seen on the basis of age, the study emphasizes that age alone should not preclude patients from consideration for this treatment modality. Although there may be some selection bias, in which fitter older individuals with less aggressive biology were referred to and eventually received BsAbs, these findings nevertheless support the need for using alternative tools and assessments of patient- and disease-related characteristics instead of chronological age to determine BsAb eligibility in clinical trials and real-world practice. It is important to highlight that poor PS and organ dysfunction seen in patients at the time of disease relapse is often related to prior therapy and disease progression. However, these patients reflect a real-world patient population in need of effective treatments. The presence of ≥1 of these features in the clinical trial exclusion criteria should not be viewed by providers or insurance companies as a barrier to receiving effective salvage therapies, especially if treatment is considered safe with manageable toxicities, as per our analysis.
There is significant heterogeneity in physical fitness and comorbidity burden in older patients with R/R MM, compounded by physician bias in assessing functional capacity (ie, frailty) and eligibility for therapy. In this regard, SFI, comprising chronological age, physician-determined PS, and pertinent comorbidities, has emerged as a reliable and validated tool for point-of-care assessment of frailty. Although its role has been previously evaluated in clinical trials and real-world studies to determine trial eligibility and clinical outcomes,8,9,12,16 our analysis serves as a benchmark to incorporate frailty assessments using this index for patients receiving BCMA-directed BsAbs and non-BCMA–directed BsAbs in future trials and routine clinical practice. As studies compare the role of CAR T-cell therapy and BsAb in earlier lines of treatment, the use of such assessments is going to be important in interpreting the risk for treatment-related toxicities among older patients receiving either treatment modality. Moreover, frailty-based screening assessments for treatment selection and randomization are needed to provide more insight into treatment outcomes in this patient population. Although the frailty score used in our study is based on parameters that are routinely assessed and available in clinical practice and is therefore practical for clinical use, the use of comprehensive frailty assessments that more accurately reflect biological or functional frailty will remain important for further optimization of treatment strategies for frail patients.17 A physically active older individual (>75 years) with well-managed medical comorbidity (diabetes mellitus) considered frail per SFI may be fit per IMWG frailty score.
There are several limitations to this study. We included many patients who were referred for consultation and eventually underwent BsAb treatment at our center. Although the cohort is a fair representation of the older and frail real-world patient population, there is potential bias of excluding many patients who either deferred or were deemed ineligible for BsAbs by referring providers owing to potential barriers of frailty, medical comorbidities, and poor PS. Evaluation of ECOG PS was physician dependent and subject to intraobserver and interobserver bias in a retrospective analysis, compared with the activity of daily living and instrumental activities of daily living scales used in IMWG frailty scoring system.17 Retrospective CCI calculations were based on reported medical history, which may contain missing data and result in underestimating or overestimating the number of patients in the frail group. Due to the limitations of a small sample size, we were unable to investigate special subgroups, including very old (age >75 years), ECOG PS ≥3, and worse organ dysfunction (CCI >3). This, in turn, might have masked subgroup heterogeneity in the associations of age, frailty, and organ dysfunction with clinical outcomes of BsAbs, and results may not be generalizable to the very older and extremely frail patients. Nonetheless, this is, to our knowledge, the first study to report the role of a readily accessible and simple-to-use frailty assessment tool in determining outcomes after BsAb treatment for R/R MM and fills the knowledge gap in an area of unmet need. Moreover, this data set represents a real-world patient population and is supportive of appropriate decision-making and treatment selection for a patient population considered to be at risk for excessive toxicities and limited therapeutic benefit. There were adequate numbers of patients in this study to allow for meaningful multivariable analyses and determine the impact of frailty after adjusting for clinically significant risk factors. Although our findings support offering equitable access to effective treatment modalities including BsAbs for older and frail patients, further research is needed to confirm these findings and elucidate any potential subgroup differences in outcomes in a larger group of patients with longer follow-up.
Conclusions
Our findings, although based on retrospective assessment of frailty, support the use of BsAbs as a safe and effective treatment option for older and frail patients with R/R MM, with outcomes comparable to younger and nonfrail patients. When evaluating patients for therapy, age, comorbidities, and PS should be carefully assessed and optimized, and these should not be barriers toward enrollment in clinical trials because these patients continue to benefit from effective treatments without excessive toxicities.
Authorship
Contribution: B.A. and H. Hashmi designed the study; B.A., A.D., and D.N. analyzed data; B.A., C.R.T., and H. Hashmi wrote the first draft of the manuscript; and all authors reviewed the manuscript and provided feedback.
Conflict-of-interest disclosure: C.R.T. received honoraria from Sanofi and Janssen and research funding from Takeda and Janssen. T.S. reports consultancy with Roche-Genentech. A.M.L. is currently an employee with Memorial Sloan Kettering Cancer Center; reports consultancy with F. Hoffmann-La Roche Ltd, Janssen, Leerink, Arcellx, and Pfizer; reports honoraria from F. Hoffmann-La Roche Ltd, Janssen, SVB Leerink, Arcellx, and Pfizer; reports patents and royalties associated with Serametrix, Inc; and reports research funding from Pfizer. G.L.S. receives research funding from Janssen, Amgen, Beyond Spring, Bristol Myers Squibb (BMS), and GPCR, and is on a data safety monitoring board for Arcellx. N.K. reports membership on an entity’s board of directors or advisory committees for Janssen; Remedy Health 8/2022: other: part of (Patient Power); reports consultancy for Clinical Care Oncology, OncLive, and Intellisphere; and reports research funding from Amgen, Janssen, Epizyme, and AbbVie. H.J.L. reports consultancy for AbbVie, Immix Biopharma, Legend Biotech, Alexion, and Prothena, and research funding from Nexcella, Janssen, Alexion, Protego, and Prothena. M.S. reports honoraria from MJH Life Sciences (CancerNetwork), IDEOlogy, and Medscape; research funding from Sanofi, Angiocrine Bioscience, Inc, Amgen, and Omeros Corporation; and consultancy for Kite (a Gilead Company), Miltenyi Biotec, and Omeros Corporation. H. Hassoun received research funding from Janssen and Takeda. U.A.S. reports honoraria from Sanofi and Janssen, and research funding from Janssen and BMS. M.H. received research funding from AbbVie, GlaxoSmithKline (GSK), SpringWorks Therapeutics, Daiichi Sankyo, and Cosette Pharmaceuticals, and reports consultancy fees and honoraria from Curio Science LLC, Intellisphere LLC, Janssen, BMS, and GSK. S.M. received research funding from BMS, Johnson & Johnson (J&J), GSK, and SpringWorks Therapeutics. S.Z.U. reports consultancy for Oncopeptides, BMS-Celgene, BMS, Genentech, Pfizer, Sanofi, Seagen, Gracell, AbbVie, GSK, Amgen, Secura Bio, EdoPharma, Takeda, SkylineDx, TeneoBio, and J&J-Janssen, and received research funding from BMS-Celgene, BMS, Array Biopharma, Gilead, Sanofi, AbbVie, Merck, GSK, Pharmacyclics, Seagen, Amgen, Takeda, SkylineDx, and J&J-Janssen. H. Hashmi reports consultancy for Karyopharm, Amgen, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Hamza Hashmi, Myeloma and Cell Therapy Service, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: hashmih1@mskcc.org; and Carlyn Rose Tan, Myeloma and Cell Therapy Service, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: tanc4@mskcc.org.
References
Author notes
C.R.T., S.Z.U., and H. Hashmi contributed equally as joint senior authors.
Data are available on request from the corresponding authors, Hamza Hashmi (hashmih1@mskcc.org) and Carlyn Rose Tan (tanc4@mskcc.org).