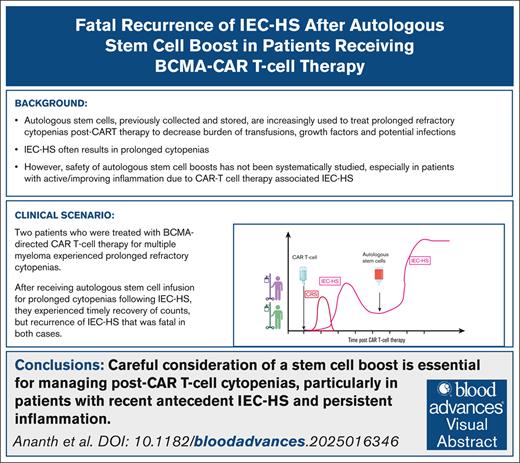
Visual Abstract
TO THE EDITOR:
B-cell maturation antigen (BCMA)–targeted chimeric antigen receptor (CAR) T cells have improved outcomes for relapsed/refractory multiple myeloma (RRMM), with US Food and Drug Administration–approved idecabtagene vicleucel (ide-cel)1 and ciltacabtagene autoleucel (cilta-cel)2 demonstrating high response rates (98% in CARTITUDE-1,3 89% in real-world study4). CAR T-cell therapy carries unique risks, including early cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome, and immune effector cell–associated hemophagocytic lymphohistiocytosis–like syndrome (IEC-HS), as well as prolonged cytopenias and infection risk.5-11,12,13 Severe cytopenias (grade 3 or greater, persistent >30 days after infusion) affect >20% of patients,1,2,4 often requiring frequent transfusions, growth factors, or rarely autologous hematopoietic stem cell (HSC) boost. Real-world multicenter studies in patients receiving BCMA-CAR T-cell therapy have shown about 5% to 27% of patients receive HSC boosts with good recovery5-7,12-14; however, caution is needed, as we observed a fatal rebound of IEC-HS in 2 patients who received HSC boost for persistent cytopenias and infections following resolution of initial IEC-HS. The study was approved by the Stanford Institutional Review Board in accordance with the Declaration of Helsinki, and all patients provided written informed consent.
Case 1
A 57-year-old man with RRMM, received cilta-cel as seventh-line therapy. Bridging with multiagent chemotherapy (dexamethasone, cyclophosphamide, etoposide, and cisplatin) resulted in stable disease, but he progressed before lymphodepletion (supplemental Table 1). With no alternative options, he proceeded with CAR T-cell therapy. Pre-CAR T-cell bone marrow (BM) showed 50% plasma cells, and serum involved-uninvolved light chain difference was 323.3 mg/dL.
After standard cyclophosphamide and fludarabine lymphodepletion, he received 0.7 × 106/kg CAR+ T cells. He developed grade 2 CRS (day 6) and IEC-HS (day 8), managed with tocilizumab, dexamethasone, and anakinra. By day 19, his inflammatory markers and cytopenias had improved, and anticytokine therapy was discontinued.
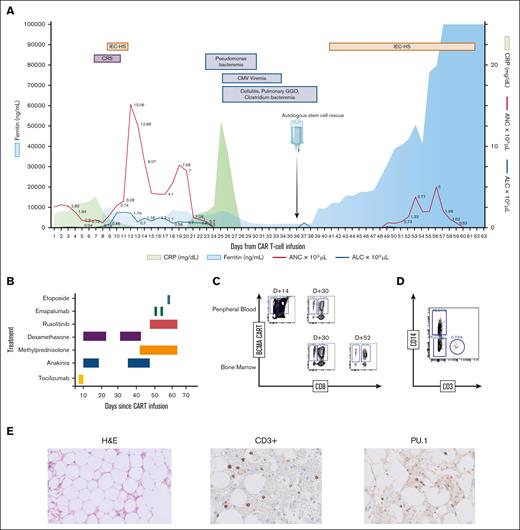
On day 21, he developed worsening neutropenia, leading to septic shock from pseudomonas bacteremia. Despite antimicrobial and growth factor support, he remained profoundly neutropenic and developed multiple infections, including cytomegalovirus viremia and pneumonia (Figure 1A). A BM biopsy on day 30 showed severe aplasia (<5% cellularity) with no residual plasma cells. Flow cytometry of BM aspirate showed increased CAR T-cells (47% of CD3+ T cells; CD8+ 40%, CD4+ 7%; Figure 1C).
Clinical, biochemical and correlative analysis for patient 1. (A) Time course of events following CAR T-cell infusion, including adverse events, infectious complications, stem cell infusion, and trends of inflammatory markers and blood counts. (B) Therapeutic strategies employed for managing CAR T-cell–related toxicities. For the first episode of CRS and IEC-HS, the patient received tocilizumab 8 mg/kg for 2 days, 10 mg every 6 hours of dexamethasone tapered over 10 days (tapered), and anakinra 100 mg every 6 hours tapered over 7 days. In the second episode of IEC-HS, the patient received 1 g of methylprednisolone for 3 days with a prolonged taper, along with a dexamethasone taper. Anakinra was started at 100 mg every 6 hours and tapered to every 24 hours over 1 week. Ruxolitinib was initiated at 5 mg daily and increased to twice daily, and was stopped after a week because of concerns about gastrointestinal bleeding. Two doses of emapalumab (1 mg/kg) were administered after high doses of steroids and anakinra failed to improve the clinical course. Finally, the patient received etoposide 50 mg/m2 3 days before death. (C) Immunophenotyping of BM and peripheral blood, showing CAR T-cell expansion, gated on CD3+ viable lymphocytes. (D) Immunophenotyping on day 62 (1 day before the patient died), demonstrating the expansion of mononuclear type cells. (E) H&E staining of BM on day 30 after CAR T-cell therapy but before stem cell boost, showing marked paucicellularity (<5%) (left) and immunohistochemistry demonstrating scattered CD3+ T cells (middle), and PU.1+ cells, which are overexpressed in monocytes, dendritic cells, and histiocytes (right). ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CMV, cytomegalovirus; CRP, C-reactive protein; GGO, ground glass opacity; H&E, hematoxylin and eosin.
Clinical, biochemical and correlative analysis for patient 1. (A) Time course of events following CAR T-cell infusion, including adverse events, infectious complications, stem cell infusion, and trends of inflammatory markers and blood counts. (B) Therapeutic strategies employed for managing CAR T-cell–related toxicities. For the first episode of CRS and IEC-HS, the patient received tocilizumab 8 mg/kg for 2 days, 10 mg every 6 hours of dexamethasone tapered over 10 days (tapered), and anakinra 100 mg every 6 hours tapered over 7 days. In the second episode of IEC-HS, the patient received 1 g of methylprednisolone for 3 days with a prolonged taper, along with a dexamethasone taper. Anakinra was started at 100 mg every 6 hours and tapered to every 24 hours over 1 week. Ruxolitinib was initiated at 5 mg daily and increased to twice daily, and was stopped after a week because of concerns about gastrointestinal bleeding. Two doses of emapalumab (1 mg/kg) were administered after high doses of steroids and anakinra failed to improve the clinical course. Finally, the patient received etoposide 50 mg/m2 3 days before death. (C) Immunophenotyping of BM and peripheral blood, showing CAR T-cell expansion, gated on CD3+ viable lymphocytes. (D) Immunophenotyping on day 62 (1 day before the patient died), demonstrating the expansion of mononuclear type cells. (E) H&E staining of BM on day 30 after CAR T-cell therapy but before stem cell boost, showing marked paucicellularity (<5%) (left) and immunohistochemistry demonstrating scattered CD3+ T cells (middle), and PU.1+ cells, which are overexpressed in monocytes, dendritic cells, and histiocytes (right). ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CMV, cytomegalovirus; CRP, C-reactive protein; GGO, ground glass opacity; H&E, hematoxylin and eosin.
Because of uncontrollable infections and persistent cytopenias, stored HSCs were infused on day 38, which comprised 13.4 × 106 CD34+ cells per kg with a total nucleated cell count (TNC) of 21.4 × 109. Following HSC infusion, he experienced marked elevation in inflammatory markers with worsening coagulopathy and liver function concerning for recurrent IEC-HS (supplemental Table 2). He was treated with high-dose steroids, anakinra, and ruxolitinib (Figure 1B) while receiving broad-spectrum antimicrobials with piperacillin-tazobactam, foscarnet, along with prophylactic antiviral/fungal agents. Despite initial engraftment within 2 weeks, he rapidly deteriorated with multiorgan failure. Repeat BM aspirate showed persistent pancytopenia and lingering CAR T-cells by flow cytometry (CD3+ T cells were 2.4% of CD45+ cells, out of which CD4+ CAR T-cells were 8.5% and CD8+ CAR T-cells were 67%). Given his worsening condition, emapalumab and etoposide were administered. By day 62, CAR T-cells were undetectable in blood by flow cytometry; however, there was increased expansion of CD14+/CD3− cells (46%), suggesting a high presence of monocytes/macrophages (Figure 1D). Despite continued aggressive treatment, he succumbed to multiorgan failure. Autopsy revealed multiorgan ischemic necrosis and profoundly hypocellular marrow (<1%) with rare macrophages and possible erythrophagocytosis. However, no dysplasia was seen, and hence, molecular testing for myeloid clonal mutations was not done. Immunohistochemistry showed scattered macrophages and CD3+ T cells (CD8+ cells were greater than CD4+ cells) in the BM (Figure 1E).
Case 2
A 67-year-old man with RRMM, received cilta-cel as ninth-line therapy following bridging with multiagent chemotherapy (dexamethasone, cyclophosphamide, etoposide, and cisplatin plus doxorubicin), which initially induced response but failed with monoclonal protein of 9 g/dL and 95% plasma cells in the BM.
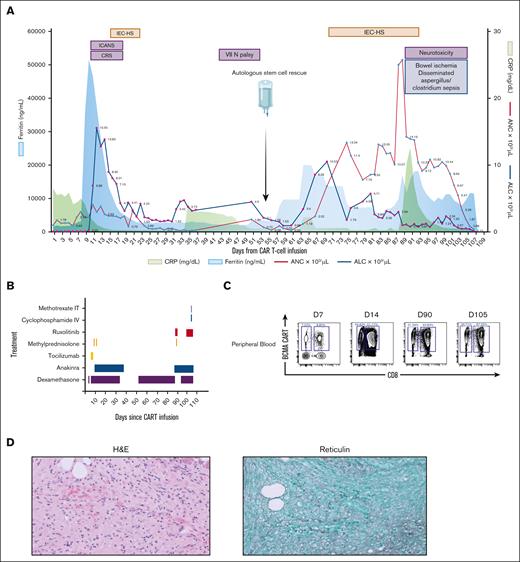
After lymphodepletion, he received 0.75 × 106/kg CAR+ T cells, developing grade 1 CRS (day 4), grade 3 immune effector cell–associated neurotoxicity syndrome (day 4), and grade 2 IEC-HS (day 9), managed with tocilizumab, steroids, and anakinra (Figure 2A-B). A BM biopsy (day 28) showed no measurable residual disease but severe fibrosis. Persistent cytopenias led to biweekly transfusions, prompting autologous HSC infusion on day 54, containing 20.8 × 106 CD34+ cells per kg with TNC of 22.2 × 109. He achieved engraftment 10 days after HSC boost, but developed recurrent IEC-HS with rising inflammatory markers and liver enzymes (supplemental Table 2). He was restarted on dexamethasone and anakinra and was initiated on ruxolitinib. However, he developed ischemic colitis requiring resection. Postoperative course was complicated by altered mental status with parkinsonian features (bradykinesia, masked facies, and resting tremors). Cerebrospinal fluid revealed 96% CAR+ T cells, elevated white blood cell (9 x 103/μL, 99% lymphocytes), and protein (400 mg/dL), and peripheral blood revealed continued persistence of BCMA CD8+ CAR T-cells (Figure 2C). Magnetic resonance imaging showed leptomeningeal enhancement in the cerebellar vermis, and he received intrathecal methotrexate and cyclophosphamide, but symptoms worsened. He remained on broad-spectrum antimicrobials including antibacterial, antifungal, Pneumocystis jiroveci pneumonia, and zoster prophylaxis; however, infectious workup revealed Clostridium bacteremia. He continued to deteriorate with no improvement in mental status or his inflammatory markers and ultimately died. Autopsy revealed evidence of disseminated Clostridium sepsis and Aspergillus infection. BM showed trilineage hematopoiesis with no obvious hemophagocytosis, and there were no signs of residual myeloma nor dysplasia (Figure 2D).
Clinical, biochemical and correlative analysis for patient 2. (A) Time course of events following CAR T-cell infusion, including adverse events, infectious complications, stem cell infusion, and trends of inflammatory markers and blood counts. (B) Therapeutic strategies employed for managing CAR T-cell–related toxicities. For the first episode of CRS, immune effector cell–associated neurotoxicity syndrome (ICANS), and IEC-HS, the patient received 2 doses of tocilizumab (8 mg/kg), followed by methylprednisolone (1 g), and a dexamethasone taper for ICANS, along with anakinra (100 mg every 6 hours) for 5 days, which was then tapered over 10 days. For recurrent IEC-HS, treatment was initially started with dexamethasone (10 mg every 6 hours) but was later escalated to methylprednisolone (1 g) because of a refractory clinical course. Anakinra (100 mg every 6 hours) and ruxolitinib (5 mg twice daily) were also added. In addition, the patient received intrathecal methotrexate (12 mg) and IV cyclophosphamide (500 mg/m2) 2 weeks before death. (C) Immunophenotyping of peripheral blood, showing CAR T-cell expansion, gated on CD3+ viable lymphocytes. (D) H&E staining of BM on day 28 (after cilta-cel infusion) (left), demonstrating hypocellular marrow (<10%) with extensive marrow fibrosis in trichrome staining (right). ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CRP, C-reactive protein; VII N palsy, seventh cranial nerve palsy.
Clinical, biochemical and correlative analysis for patient 2. (A) Time course of events following CAR T-cell infusion, including adverse events, infectious complications, stem cell infusion, and trends of inflammatory markers and blood counts. (B) Therapeutic strategies employed for managing CAR T-cell–related toxicities. For the first episode of CRS, immune effector cell–associated neurotoxicity syndrome (ICANS), and IEC-HS, the patient received 2 doses of tocilizumab (8 mg/kg), followed by methylprednisolone (1 g), and a dexamethasone taper for ICANS, along with anakinra (100 mg every 6 hours) for 5 days, which was then tapered over 10 days. For recurrent IEC-HS, treatment was initially started with dexamethasone (10 mg every 6 hours) but was later escalated to methylprednisolone (1 g) because of a refractory clinical course. Anakinra (100 mg every 6 hours) and ruxolitinib (5 mg twice daily) were also added. In addition, the patient received intrathecal methotrexate (12 mg) and IV cyclophosphamide (500 mg/m2) 2 weeks before death. (C) Immunophenotyping of peripheral blood, showing CAR T-cell expansion, gated on CD3+ viable lymphocytes. (D) H&E staining of BM on day 28 (after cilta-cel infusion) (left), demonstrating hypocellular marrow (<10%) with extensive marrow fibrosis in trichrome staining (right). ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CRP, C-reactive protein; VII N palsy, seventh cranial nerve palsy.
IEC-HS is a potentially life-threatening toxicity associated with CAR T-cell therapy, with its incidence varying by CAR T-cell product and disease type, often emerging after CRS resolves. In the cilta-cel recipient, IEC-HS has been reported in 1% to 3% of patients.2,4 However, to our knowledge, there are no previously reported cases of recurrent IEC-HS following resolution of an initial episode, nor established risk factors for its recurrence. Severe and prolonged cytopenias are known complications after CAR T-cell therapy, but the role of IEC-HS in exacerbating these cytopenias remains unclear.
Recent recommendations from the European Hematology Association and the European Society for Blood and Marrow Transplantation advocate for autologous HSC boost in patients with persistent grade ≥3 cytopenias beyond 14 days of CAR T-cells or those refractory to granulocyte colony-stimulating factor.15 Prior reports indicate that HSC boost can promote successful hematologic recovery in the setting of severe cytopenias after CAR T-cell therapy, even when attributed to prolonged marrow suppression after high-grade CRS.14 However, as IEC-HS is a relatively new entity, to our knowledge, there is no study evaluating the efficacy and safety of HSC boost for IEC-HS–associated cytopenias.
The 2 cases presented here illustrate the challenges of managing persistent cytopenias following IEC-HS. Among 62 cilta-cel recipients at our institution, 3 received HSC boost, whereas none of the 48 ide-cel recipients did. Two of these 3 cases are detailed here, who experienced recurrent IEC-HS following HSC boost despite initial hematologic recovery. Although both achieved engraftment at expected timeframes, their subsequent decline in blood counts coincided with rising inflammatory markers (Figures 1A and 2A; supplemental Tables 1 and 2).
The recurrence of IEC-HS following stem cell engraftment suggests a potential link to cytokine changes, warranting caution in using stem cell boosts in recently recovered IEC-HS, in which residual inflammation may persist. Additional mechanisms may include infection-driven inflammation or cytokine-mediated dysregulation triggered by a high HSC and TNC dose. Notably, both patients received a higher than typical number of HSCs (13.4 × 106 and 20.8 × 106 CD34+ cells per kg, respectively; range of previous reports: 1.76 × 106 to 23.55 × 106 CD34+ cells per kg).5-7,14,13 The dosing was primarily driven by logistical factors, as all remaining HSCs were stored in a single cryopreserved bag. To maximize the potential benefit of stem cell support in the setting of refractory cytopenias, the entire contents of the bag were infused.
Beyond IEC-HS recurrence, these cases also highlight the risks associated with CAR T-cell infusion in patients with high disease burden. Both patients progressed on high-dose chemotherapy as bridging and had no alternative bridging options at the time of infusion. With the increasing availability of CAR T-cell therapy earlier in treatment lines and the emergence of novel bridging strategies such as talquetamab, it is anticipated that fewer patients will require CAR T-cell therapy at the time of high disease burden.
Moreover, these cases highlight the importance of carefully considering stem cell boosts, especially in patients with antecedent IEC-HS. Stem cell boosts should be carefully considered until inflammation has resolved. During this period, supportive care, including antimicrobial therapy, transfusions, and granulocyte colony-stimulating factor or other growth factors, is crucial for managing cytopenias and preventing complications.
In conclusion, these cases highlight the urgent need for further understanding of the pathophysiology of IEC-HS, the mechanisms of persistent cytopenias, and the interaction with infused autologous HSCs. Understanding of these would likely improve the management of prolonged cytopenias associated with IEC-HS.
Contribution: S.A., N.E.T., H.H., and S.S. prepared the manuscript; S.F.-P. and D.S. reviewed pathology; B.S. designed chimeric antigen receptor T-cell flow cytometry; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hitomi Hosoya, Division of Blood and Marrow Transplant and Cell Therapy, Department of Medicine, Stanford University, 780 Welch Rd, CJ250, Stanford, CA, 94304; email: hosoyah@stanford.edu; and Surbhi Sidana, Division of Blood and Marrow Transplant and Cell Therapy, Department of Medicine, Stanford University, 780 Welch Rd, CJ250C, Stanford, CA, 94304; email: surbhi.sidana@stanford.edu.
References
Author notes
S.A. and N.E.T. contributed equally to this study.
H.H. and S.S. contributed equally to this study.
Access to molecular profiles of cases in this study is available upon request from the corresponding authors Hitomi Hosoya (hosoyah@stanford.edu) and Surbhi Sidana (surbhi.sidana@stanford.edu).
The full-text version of this article contains a data supplement.