Key Points
Although rare, critical ITP bleeding was fatal (47% of adults; 15% of children), with higher mortality in older cases and delayed treatment.
The combination of corticosteroids, IV immunoglobulin, and platelet transfusion was the most common treatment for critical ITP bleeding.
Visual Abstract
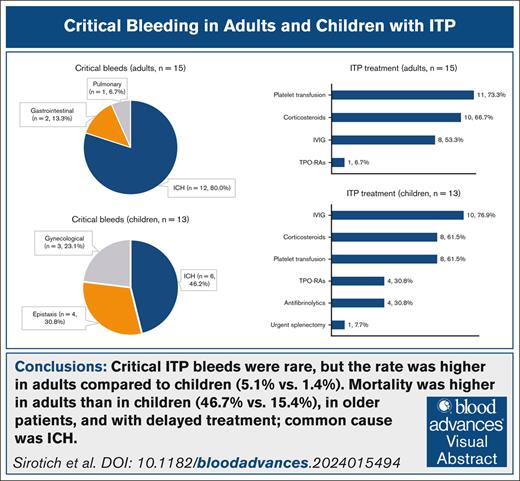
Critical bleeding in patients with immune thrombocytopenia (ITP) is a life-threatening hematologic emergency. This study aimed to describe the frequency, management, and outcomes of critical bleeds among adults and children with ITP. We conducted a retrospective cohort study of patients with ITP who presented to the emergency room with a platelet count <20 × 109/L across 7 centers in the United States and Canada between 2010 and 2019. Of 1226 patients (n = 296 adults; n = 930 children), 28 (2.3%) had critical bleeds (adults, n = 15 [median age, 68 years]; children, n = 13 [median age, 11 years]). Of patients with critical bleeds, 12 adults (80.0%) and 6 children (46.2%) had intracranial hemorrhage (ICH). For adults, the common interventions used to treat critical bleeds were platelet transfusions (n = 11 [73.3%]), corticosteroids (n = 10 [66.7%]), and IV immunoglobulin (n = 8 [53.3%]), and for children, common interventions were IV immunoglobulin (n = 10 [76.9%]), corticosteroids (n = 8 [61.5%]), platelet transfusions (n = 8 [61.5%]), thrombopoietin receptor agonists (n = 4 [30.8%]), and antifibrinolytic agents (n = 4 [30.8%]). For both adults and children, the most common treatment combination was corticosteroids, IV immunoglobulin, and platelet transfusion (n = 6 [40.0%] vs n = 6 [46.2%]). The median time from presentation to first treatment was 6.9 hours for adults and 3.5 hours for children. Overall, 9 patients (32.1%) with critical ITP bleeds died, including 7 adults (46.7%) and 2 children (15.4%). Critical bleeding in patients with ITP was rare but frequently fatal, especially among older adults with ICH and when treatments were delayed.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease that causes low platelet count and a variable increased risk of bleeding.1,2 The incidence of ITP is 2 to 5 per 100 000.1,3-6 Although most bleeding is minor and can be treated in an outpatient setting, major bleeding can be life-threatening and require urgent, multipronged treatments typically initiated in the emergency room (ER). Major bleeding has been previously defined as intracranial hemorrhage (ICH), pulmonary hemorrhage, excessive vaginal bleeding, macroscopic hematuria, overt gastrointestinal bleeding, epistaxis lasting >5 minutes, or multiple oral blood blisters.7,8 The reported rate of all non-ICH major bleeding in ITP is ∼10% in adults and up to 20% in children.2 Severe thrombocytopenia, specifically a platelet count <20 × 109/L,9 predisposes patients to major bleeding, and pooled estimates of the 5-year risk of death from bleeding in ITP are as high as 47.8% for patients aged >60 years.10
A subset of major bleeds are critical bleeds, defined as fatal, life-threatening, or causing significant morbidity.11 ICH, the most common critical bleed, has been reported to occur in 1.1% of adults and 0.7% of children hospitalized for ITP in the United States between 2007 and 2016.12 The overall mortality from ICH was 26.7% and higher for adults than children.12 The frequency, management, and outcomes of critical bleeds in patients with ITP have not been well described. Although there are evidence-based recommendations for the outpatient management of ITP,13 patients with critical bleeds have been excluded from guidelines. Treatment of these urgent, life-threatening events requires a rapid, coordinated approach that includes ER staff, hematologists, transfusion medicine specialists, intensivists, pharmacists, and laboratory medicine specialists. Interventions that rapidly raise the platelet count and restore hemostasis are required14,15; however, there is significant variability in practice,16 which can lead to treatment delays and omissions. A comprehensive description of critical ITP bleeds will aid in the identification and treatment of patients who require emergency management and inform new treatment guidelines.
This study aimed to describe critical bleeding in a large cohort of adults and children with ITP, including the treatments they received and clinical outcomes.
Methods
Study design
This multicenter retrospective cohort study included adults and children with ITP presenting to 7 centers in the United States and Canada, including 3 adult centers and 4 pediatric centers. Eligible patients were adults and children with ITP who presented to the ER between 1 January 2010 and 31 December 2019 with a platelet count of <20 × 109/L. Trained research personnel reviewed the medical records manually to assess eligibility and confirm the diagnosis of ITP based on established criteria.17 Patients with thrombocytopenia owing to other causes were excluded (eg, myelodysplastic syndrome, infection/sepsis, liver disease, disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, hematologic malignancy, and patients with malignancy who had received cytotoxic chemotherapy within 30 days).
Definition of critical ITP bleeds
We used the published definition of critical bleeding in patients with ITP, which is based on the following criteria: (1) a bleed in a critical anatomical site, including ICH, intraspinal, intraocular, retroperitoneal, pericardial, or intramuscular with compartment syndrome; or (2) an ongoing bleed that results in hemodynamic instability or respiratory compromise.11
Data collection
We collected data from the medical records of eligible patients using electronic data capture forms in REDCap, a secure web application for managing research databases. Data collection forms were piloted to ensure feasibility and consistency; then data collection was done in duplicate for the first 20 patients at each site and for all bleeding events. We collected demographics and medical history, including the date of diagnosis of ITP and previous ITP treatments, interventions administered in the hospital, and clinical outcomes for up to 14 days in the hospital. For patients who remained in hospital after 14 days, outcomes were assessed at the time of discharge or death.
Bleeding was assessed from the time of presentation to the ER. Discrete or recurrent bleeding episodes were captured as separate events. We collected laboratory and diagnostic imaging results related to bleeding, medical treatments, and procedural or surgical interventions used to treat bleeding. A content expert and a statistician centrally adjudicated bleeding events to verify the classification of bleeds as critical and causes of death.
Analysis
We summarized the characteristics, management, and outcomes of critical bleeding in adults and children with ITP using descriptive statistics. We avoided inferential statistics to prevent misleading interpretations because of the small number of critical bleeding events. We reported the proportion of patients who received specific treatments or treatment combinations and their outcomes. We used SAS 9.4M7 (SAS Institute, Cary, NC) for the analyses.
Ethical approval for this study was obtained from the Hamilton Integrated Research Ethics Board (project number 8119). Investigators at each site obtained appropriate local research ethics board approval before accessing patient personal health information. Potentially eligible patients were identified for screening, after which a master patient list was generated in which eligible patients were given a study identification number. The only place where identifying information and the study number were linked was on the master list, which was securely stored and accessed only by authorized study personnel. All data collected during the chart review were identified only by study identification number to maintain patient confidentiality. Any personal level data required to identify the study cohort for the manual chart review were accessed by authorized study team members, and files containing any identifiers were transferred via secure file transfer in accordance with institutional privacy policies. Given that this research design is a retrospective observational study and does not involve any active intervention, we did not seek individual informed consent.
Results
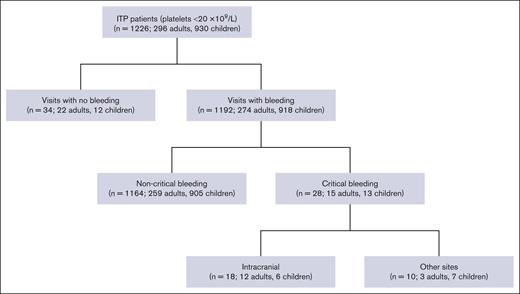
We identified 1226 patients (n = 296 adults; n = 930 children) with ITP who presented to the ER with a platelet count <20 × 109/L (Figure 1). Of those, 1192 patients (97.2%) had bleeding and 34 (2.8%) had no bleeding.
Patients with critical ITP bleeds
We identified 28 patients (2.3%) with a critical ITP bleed, including 15 adults (5.1%) and 13 children (1.4%; Table 1). Of the 15 adults with critical bleeds, 7 (46.7%) were female, and among 13 children, 9 (69.2%) were female. Among adults with critical bleeds, 12 had ICH (80.0%), and 3 (20.0%) had bleeding at other sites (2 had gastrointestinal and 1 had pulmonary) that resulted in hemodynamic instability or respiratory compromise. Among children with critical bleeds, 6 (46.2%) had ICH, and 7 (53.8%) had bleeding at other sites (3 had epistaxis, 3 had gynecological, and 1 had both epistaxis and gastrointestinal) that resulted in hemodynamic instability or respiratory compromise. In adults with critical bleeds, concomitant bleeding was present at the following sites: skin (n = 10 [66.7%]), oral (n = 6 [40.0%]), gastrointestinal (n = 5 [33.3%]), urinary (n = 4 [26.7%]), and epistaxis (n = 3 [20.0%]). In children, concomitant bleeding was present at the following sites: skin (n = 6 [46.2%]), oral (n = 1 [7.7%]), gastrointestinal (n = 2 [15.4%]), and urinary (n = 1 [7.7%]).
Characteristics of patients with ITP and critical bleeding at presentation to the ER
| Characteristics . | Adults, n = 15 (%) . | Children, n = 13 (%) . |
|---|---|---|
| Female | 7 (46.7) | 9 (69.2) |
| Age, median (IQR), y | 68 (45-74) | 11 (5-16) |
| Type of bleeds∗ | ||
| ICH | 12 (80.0) | 6 (46.2) |
| Gastrointestinal bleeding | 2 (13.3) | 1 (7.7) |
| Pulmonary bleeding | 1 (6.7) | — |
| Epistaxis | — | 4 (30.8) |
| Gynecological bleeding | — | 3 (23.1) |
| Noncritical concurrent sites of bleeding | ||
| None | 4 (26.7) | 6 (46.2) |
| Skin | 10 (66.7) | 6 (46.2) |
| Oral | 6 (40.0) | 1 (7.7) |
| Gastrointestinal | 5 (33.3) | 2 (15.4) |
| Epistaxis | 3 (20.0) | 0 (0.0) |
| Urinary | 4 (26.7) | 1 (7.7) |
| ITP disease stage | ||
| Newly diagnosed ITP (<3 months) | 8 (53.3) | 6 (46.2) |
| Persistent ITP (3-12 months) | 1 (6.7) | 3 (23.1) |
| Chronic ITP (>12 months) | 5 (33.3) | 3 (23.1) |
| Unknown | 1 (6.7) | 1 (7.7) |
| Race | ||
| Black/African/Afro-Caribbean | 3 (20.0) | 2 (15.4) |
| White | 2 (13.3) | 5 (38.5) |
| Unknown | 10 (66.7) | 6 (46.2) |
| Comorbidities† | 10 (66.7) | 2 (15.4) |
| Lowest platelets count during visit, median (IQR), ×109/L | 8 (3-15) | 3 (1-6) (n = 11) |
| Lowest hemoglobin during visit, median (IQR), g/L | 77 (63-106) | 79.5 (69-100) (n = 10) |
| No. of distinct ITP treatments previously received, median (IQR) | 3 (1.5-5) (n = 7) | 3 (2-5) (n = 9) |
| Previous use of TPO-RAs | 3 (20.0) | 4 (30.8) |
| History of splenectomy | 4 (26.7) | 1 (7.7) |
| Timing of critical bleed | ||
| Prevalent (on ER arrival or before) | 10 (66.7) | 12 (92.3) |
| Incident (day 2 onward) | 5 (33.3) | 1 (7.7) |
| Time from ER arrival to imaging for ICH patients, median (IQR), h | 11.5 (3-28.5) (n = 12) | 2.5 (0-5) (n = 6) |
| Critical bleed as initial presentation of ITP | 8 (53.3) | 4 (30.8) |
| Duration of ITP, median (IQR), mo | 78.5 (49-151.5) (n = 6 [40.0%]) | 10 (1-14) (n = 9 [69.2%]) |
| Subsequent critical bleed at a later visit | 1 (6.7) | 0 (0.0) |
| Characteristics . | Adults, n = 15 (%) . | Children, n = 13 (%) . |
|---|---|---|
| Female | 7 (46.7) | 9 (69.2) |
| Age, median (IQR), y | 68 (45-74) | 11 (5-16) |
| Type of bleeds∗ | ||
| ICH | 12 (80.0) | 6 (46.2) |
| Gastrointestinal bleeding | 2 (13.3) | 1 (7.7) |
| Pulmonary bleeding | 1 (6.7) | — |
| Epistaxis | — | 4 (30.8) |
| Gynecological bleeding | — | 3 (23.1) |
| Noncritical concurrent sites of bleeding | ||
| None | 4 (26.7) | 6 (46.2) |
| Skin | 10 (66.7) | 6 (46.2) |
| Oral | 6 (40.0) | 1 (7.7) |
| Gastrointestinal | 5 (33.3) | 2 (15.4) |
| Epistaxis | 3 (20.0) | 0 (0.0) |
| Urinary | 4 (26.7) | 1 (7.7) |
| ITP disease stage | ||
| Newly diagnosed ITP (<3 months) | 8 (53.3) | 6 (46.2) |
| Persistent ITP (3-12 months) | 1 (6.7) | 3 (23.1) |
| Chronic ITP (>12 months) | 5 (33.3) | 3 (23.1) |
| Unknown | 1 (6.7) | 1 (7.7) |
| Race | ||
| Black/African/Afro-Caribbean | 3 (20.0) | 2 (15.4) |
| White | 2 (13.3) | 5 (38.5) |
| Unknown | 10 (66.7) | 6 (46.2) |
| Comorbidities† | 10 (66.7) | 2 (15.4) |
| Lowest platelets count during visit, median (IQR), ×109/L | 8 (3-15) | 3 (1-6) (n = 11) |
| Lowest hemoglobin during visit, median (IQR), g/L | 77 (63-106) | 79.5 (69-100) (n = 10) |
| No. of distinct ITP treatments previously received, median (IQR) | 3 (1.5-5) (n = 7) | 3 (2-5) (n = 9) |
| Previous use of TPO-RAs | 3 (20.0) | 4 (30.8) |
| History of splenectomy | 4 (26.7) | 1 (7.7) |
| Timing of critical bleed | ||
| Prevalent (on ER arrival or before) | 10 (66.7) | 12 (92.3) |
| Incident (day 2 onward) | 5 (33.3) | 1 (7.7) |
| Time from ER arrival to imaging for ICH patients, median (IQR), h | 11.5 (3-28.5) (n = 12) | 2.5 (0-5) (n = 6) |
| Critical bleed as initial presentation of ITP | 8 (53.3) | 4 (30.8) |
| Duration of ITP, median (IQR), mo | 78.5 (49-151.5) (n = 6 [40.0%]) | 10 (1-14) (n = 9 [69.2%]) |
| Subsequent critical bleed at a later visit | 1 (6.7) | 0 (0.0) |
In adults: ICH (n = 9), ICH with respiratory compromise (n = 2), ICH with hemodynamic instability and respiratory compromise (n = 1), gastrointestinal bleeding with hemodynamic instability (n = 2), and pulmonary bleeding with respiratory compromise (n = 1). In children: ICH (n = 3), ICH with hemodynamic instability and respiratory compromise (n = 2), ICH with hemodynamic instability (n = 1), gastrointestinal bleeding with respiratory compromise (n = 1), epistaxis with hemodynamic instability (n = 3), and epistaxis with hemodynamic instability and respiratory compromise (n = 1).
In adults: comorbidities included myocardial infarction (n = 2), congestive heart failure (n = 5), peripheral vascular disease (n = 1), cerebral vascular accident or transient ischemic attack (n = 5), chronic obstructive pulmonary disease (n = 2), liver disease (n = 1), diabetes (n = 3), moderate to severe chronic kidney disease (n = 2), solid tumor (n = 1), leukemia (n = 1), lymphoma (n = 1), autoimmune hemolytic anemia (n = 2), and rheumatoid arthritis (n = 1). In children: comorbidities included diabetes (n = 1) and autoimmune hemolytic anemia (n = 1).
At the time of the critical bleed, the median age was 68 years (interquartile range [IQR], 45-74) for adults and 11 years (IQR, 5-16) for children. The median lowest platelet count at the time of the critical bleed was 8 × 109/L (IQR, 3 × 109 to 15 × 109/L) for adults and 3 × 109/L (IQR, 1× 109 to 6 × 109/L) for children. Of 15 adults, 8 (53.3%) had a critical bleed as their initial presentation of ITP; the others (n = 6) had ITP for a median of 78.5 months (IQR, 49-151.5). Of 13 children, 4 (30.8%) had a critical bleed as their initial presentation of ITP; the others (n = 9) had ITP for a median of 10 months (IQR, 1-14). Regarding previous treatments, 3 adults (20.0%) and 4 children (30.8%) had received thrombopoietin receptor agonists (TPO-RAs); 4 adults (26.7%) and 1 child (7.7%) had a history of splenectomy. One adult (6.7%) had a subsequent critical bleed at a later visit (Table 1). Four adults (26.7%) and 6 children (46.2%) required admission to the intensive care unit during critical bleed visits. Six patients (n = 5 adults; n = 1 child) developed an incident critical bleed during their hospital admission after a median of 1.5 days (IQR, 1-2; Table 2).
Characteristics of patients with incident critical bleeds (n = 6)
| Age (y) . | Sex . | ITP stage . | Initial platelets count (×109/L) . | ASA, NSAID, or anticoagulant at presentation or during visit . | Comorbidities . | Treatment history . | Noncritical concurrent sites of bleeding . | Sites of critical bleeding . | Time between initial presentation and critical bleed (d) . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 85 | Female | Chronic | 1 | Warfarin, ASA, and LMWH | CHF | Prednisone/prednisolone and other ITP treatment | Oral, epistaxis, gastrointestinal, urinary, skin, and purpura | ICH | 21 | Death∗ |
| 49 | Female | Chronic | 5 | None | Pulmonary sarcoidosis, CVID, and autoimmune hemolytic anemia | Dexamethasone, romiplostim, platelet transfusion, splenectomy, and other ITP treatment | Oral, gastrointestinal, skin, and coffee ground emesis | ICH | 2 | Alive |
| 50 | Female | Newly diagnosed | <20 | None | None | None | Skin | ICH | 2 | Alive |
| 74 | Male | Persistent | 19 | None | MI, diabetes, and CAD | IV immunoglobulin, prednisone/prednisolone, splenectomy | None | ICH | 1 | Death† |
| 37 | Female | Chronic | 16 | Other Anticoagulant | CVA or TIA and venous sinus thrombosis | IV immunoglobulin, prednisone/prednisolone, dexamethasone, methylprednisolone, eltrombopag, and splenectomy | Oral and skin | ICH with respiratory compromise | 1 | Alive |
| 3 | Male | Newly diagnosed | <20 | None | None | None | Skin | Epistaxis with hemodynamic instability | 1 | Alive |
| Age (y) . | Sex . | ITP stage . | Initial platelets count (×109/L) . | ASA, NSAID, or anticoagulant at presentation or during visit . | Comorbidities . | Treatment history . | Noncritical concurrent sites of bleeding . | Sites of critical bleeding . | Time between initial presentation and critical bleed (d) . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 85 | Female | Chronic | 1 | Warfarin, ASA, and LMWH | CHF | Prednisone/prednisolone and other ITP treatment | Oral, epistaxis, gastrointestinal, urinary, skin, and purpura | ICH | 21 | Death∗ |
| 49 | Female | Chronic | 5 | None | Pulmonary sarcoidosis, CVID, and autoimmune hemolytic anemia | Dexamethasone, romiplostim, platelet transfusion, splenectomy, and other ITP treatment | Oral, gastrointestinal, skin, and coffee ground emesis | ICH | 2 | Alive |
| 50 | Female | Newly diagnosed | <20 | None | None | None | Skin | ICH | 2 | Alive |
| 74 | Male | Persistent | 19 | None | MI, diabetes, and CAD | IV immunoglobulin, prednisone/prednisolone, splenectomy | None | ICH | 1 | Death† |
| 37 | Female | Chronic | 16 | Other Anticoagulant | CVA or TIA and venous sinus thrombosis | IV immunoglobulin, prednisone/prednisolone, dexamethasone, methylprednisolone, eltrombopag, and splenectomy | Oral and skin | ICH with respiratory compromise | 1 | Alive |
| 3 | Male | Newly diagnosed | <20 | None | None | None | Skin | Epistaxis with hemodynamic instability | 1 | Alive |
ITP stage: newly diagnosed ITP (<3 months); persistent ITP (3-12 months); and chronic ITP (>12 months).
ASA, aspirin; CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; CVID, common variable immunodeficiency; LMWH, low molecular weight heparin; MI, myocardial infarction; NSAID, nonsteroidal anti-inflammatory drug; TIA, transient ischemic attack.
Cause of death by CHF.
Cause of death by intracranial bleed.
Risk factors for critical ITP bleeds
Compared with patients without critical bleeds, patients with critical bleeds were older (for adults: ≥65 years, 9/104 [8.7%] vs <65 years, 6/191 [3.1%]; for children: ≥12 years, 6/200 [3.0%] vs <12 years, 7/725 [1.0%]), and were more commonly in the persistent or chronic phase of ITP (persistent/chronic vs newly diagnosed: adults, 6/89 [6.7%] vs 8/171 [4.7%]; children, 6/89 [4.1%] vs 6/744 [0.8%]). Adults with critical bleeds more often had previous myocardial infarction, congestive heart failure, cerebrovascular accidents, or transient ischemic attack than adults without critical bleeds, and they more commonly received anticoagulation (3/16 [18.6%] vs 12/277 [4.3%]; Table 3).
Risk factors for critical ITP bleeds in patients with ITP presenting to the ER with platelet counts less than 20 × 109/L (adults, n = 296; children, n = 930)
| Variables . | Adults . | Children . | ||
|---|---|---|---|---|
| Total patients with ITP . | Critical bleeding, n (%) . | Total patients with ITP . | Critical bleeding, n (%) . | |
| Overall | 296 | 15 (5.1) | 930 | 13 (1.4) |
| Age, y | ||||
| ≥65 | 104 | 9 (8.7) | — | — |
| <65 | 191 | 6 (3.1) | — | — |
| Age, y | ||||
| ≥12 | — | — | 200 | 6 (3.0) |
| <12 | — | — | 725 | 7 (1.0) |
| Sex | ||||
| Female | 173 | 7 (4.0) | 417 | 9 (2.2) |
| Male | 122 | 7 (5.7) | 508 | 4 (0.8) |
| ITP disease stage | ||||
| Newly diagnosed ITP (<3 months) | 171 | 8 (4.7) | 744 | 6 (0.8) |
| Persistent ITP (3-12 months) | 15 | 1 (6.7) | 53 | 3 (5.7) |
| Chronic ITP (>12 months) | 74 | 5 (6.8) | 92 | 3 (3.3) |
| Myocardial infarction | ||||
| Yes | 13 | 2 (15.4) | — | — |
| No | 283 | 13 (4.6) | — | — |
| CHF | ||||
| Yes | 18 | 5 (27.8) | — | — |
| No | 278 | 10 (3.6) | — | — |
| CVA or TIA | ||||
| Yes | 18 | 5 (27.8) | — | — |
| No | 278 | 10 (3.6) | — | — |
| COPD | ||||
| Yes | 14 | 2 (14.3) | — | — |
| No | 282 | 13 (4.6) | — | — |
| Rituximab in last 6 months | ||||
| Yes | 8 | 0 (0.0) | 5 | 2 (40.0) |
| No | 284 | 15 (5.3) | 921 | 11 (1.2) |
| ITP treatment at presentation | ||||
| Yes | 89 | 4 (4.5) | 98 | 6 (6.1) |
| No | 206 | 11 (5.3) | 828 | 7 (0.8) |
| Antiplatelets at presentation | ||||
| Yes | 40 | 1 (2.5) | 6 | 0 (0.0) |
| No | 252 | 14 (5.6) | 921 | 13 (1.4) |
| Anticoagulants at presentation | ||||
| Yes | 16 | 3 (18.6) | 1 | 0 (0.0) |
| No | 277 | 12 (4.3) | 917 | 13 (1.4) |
| Variables . | Adults . | Children . | ||
|---|---|---|---|---|
| Total patients with ITP . | Critical bleeding, n (%) . | Total patients with ITP . | Critical bleeding, n (%) . | |
| Overall | 296 | 15 (5.1) | 930 | 13 (1.4) |
| Age, y | ||||
| ≥65 | 104 | 9 (8.7) | — | — |
| <65 | 191 | 6 (3.1) | — | — |
| Age, y | ||||
| ≥12 | — | — | 200 | 6 (3.0) |
| <12 | — | — | 725 | 7 (1.0) |
| Sex | ||||
| Female | 173 | 7 (4.0) | 417 | 9 (2.2) |
| Male | 122 | 7 (5.7) | 508 | 4 (0.8) |
| ITP disease stage | ||||
| Newly diagnosed ITP (<3 months) | 171 | 8 (4.7) | 744 | 6 (0.8) |
| Persistent ITP (3-12 months) | 15 | 1 (6.7) | 53 | 3 (5.7) |
| Chronic ITP (>12 months) | 74 | 5 (6.8) | 92 | 3 (3.3) |
| Myocardial infarction | ||||
| Yes | 13 | 2 (15.4) | — | — |
| No | 283 | 13 (4.6) | — | — |
| CHF | ||||
| Yes | 18 | 5 (27.8) | — | — |
| No | 278 | 10 (3.6) | — | — |
| CVA or TIA | ||||
| Yes | 18 | 5 (27.8) | — | — |
| No | 278 | 10 (3.6) | — | — |
| COPD | ||||
| Yes | 14 | 2 (14.3) | — | — |
| No | 282 | 13 (4.6) | — | — |
| Rituximab in last 6 months | ||||
| Yes | 8 | 0 (0.0) | 5 | 2 (40.0) |
| No | 284 | 15 (5.3) | 921 | 11 (1.2) |
| ITP treatment at presentation | ||||
| Yes | 89 | 4 (4.5) | 98 | 6 (6.1) |
| No | 206 | 11 (5.3) | 828 | 7 (0.8) |
| Antiplatelets at presentation | ||||
| Yes | 40 | 1 (2.5) | 6 | 0 (0.0) |
| No | 252 | 14 (5.6) | 921 | 13 (1.4) |
| Anticoagulants at presentation | ||||
| Yes | 16 | 3 (18.6) | 1 | 0 (0.0) |
| No | 277 | 12 (4.3) | 917 | 13 (1.4) |
COPD, chronic obstructive pulmonary disease.
Treatments for critical ITP bleeds
For adults, interventions that were used to treat critical bleeds were platelet transfusions (n = 11 [73.3%]), corticosteroids (n = 10 [66.7%]), and IV immunoglobulin (n = 8 [53.3%]). One patient received TPO-RAs (6.7%). Supportive treatments included red blood cell transfusions (n = 7 [46.7%]) and plasma transfusions (n = 5 [33.3%]). For children, treatments included IV immunoglobulin (n = 10 [76.9%]), corticosteroids (n = 8 [61.5%]), platelet transfusions (n = 8 [61.5%]), TPO-RAs (n = 4 [30.8%]), antifibrinolytic agents (tranexamic acid or aminocaproic acid, n = 4 [30.8%]), and red blood cell transfusions (n = 6 [46.2%]). One patient (aged 20 years) underwent urgent splenectomy (Table 4). The median number of treatments was 3 for adults (IQR, 1.5-4) and 3 for children (IQR, 3-5). The most common treatment combination for both adults and children was corticosteroids, IV immunoglobulin, and platelet transfusion (n = 6 [40.0%] vs n = 6 [46.2%]). Time from arrival in ER to administration of the first ITP treatment was 6.9 hours (median [IQR, 4.3-9.6]) for adults (n = 11) and 3.5 hours (median [IQR, 2.7-7.8]) for children (n = 10).
ITP treatments administered in hospital for critical bleeds in patients with ITP
| ITP treatment . | Patients, n (%) . | Arrival in ER to first administration, median (IQR), h . | First dose, median (IQR) . |
|---|---|---|---|
| Adults (n = 15) | |||
| Platelet transfusion | 11 (73.3) | 5 (4-8) | 1 (1-1) units |
| Corticosteroids | 10 (66.7) | 19 (10-34) | — |
| Prednisone | 7 (46.7) | 28 (19-38) | 60 (50-80) mg |
| Methylprednisolone | 5 (33.3) | 14.5 (5-56) | 1 (0.1-1) g |
| Dexamethasone | 4 (26.7) | 15 (10-26.5) | 7 (4-25) mg |
| IV immunoglobulin | 8 (53.3) | 8 (7-50) | 70 (60-80) g |
| TPO-RAs | 1 (6.7) | 51 (31-71) | — |
| Eltrombopag | 1 (6.7) | 31 (31-31) | 75 (75-75) mg |
| Romiplostim | 1 (6.7) | 71 (71-71) | 100 (100-100) μg |
| Danazol | 1 (6.7) | 107 (107-107) | 200 (200-200) mg |
| Rh immunoglobulin | 1 (6.7) | 46 (20-72) | 300 (300-300) μg |
| Children (n = 13) | |||
| IV immunoglobulin | 10 (76.9) | 7 (4-9) | 42.5 (24-65) g |
| Corticosteroids | 8 (61.5) | 19.5 (4-51.5) | — |
| Methylprednisolone | 8 (61.5) | 5 (2-25) | 0.85 (0.061-92.5) g |
| Prednisone | 4 (30.8) | 53 (21-88.5) | 75 (45-80) mg |
| Dexamethasone | 1 (7.7) | 53 (53-53) | 4 (4-4) mg |
| Platelet transfusion | 8 (61.5) | 4 (2-21) | 1 (1-1) units |
| TPO-RAs | 4 (30.8) | 22 (21-23) | — |
| Romiplostim | 3 (23.1) | 22 (12-27) | 170 (155-250) μg |
| Eltrombopag | 2 (15.4) | 22 (21-23) | 50 (25-75) mg |
| Antifibrinolytics | 4 (30.8) | 35.5 (8-65) | — |
| IV aminocaproic acid | 2 (15.4) | 26.5 (0-53) | Not available |
| Oral aminocaproic acid | 2 (15.4) | 46.5 (18-75) | 3001 (2-6000) mg |
| IV tranexamic acid | 1 (7.7) | Not available | 400 (400-400) mg |
| Oral tranexamic acid | 2 (15.4) | 36.5 (8-65) | 1000 (1000-1000) mg |
| Rituximab | 1 (7.7) | 22 (22-22) | 258 (258-258) mg |
| Urgent splenectomy | 1 (7.7) | Not applicable | Not applicable |
| ITP treatment . | Patients, n (%) . | Arrival in ER to first administration, median (IQR), h . | First dose, median (IQR) . |
|---|---|---|---|
| Adults (n = 15) | |||
| Platelet transfusion | 11 (73.3) | 5 (4-8) | 1 (1-1) units |
| Corticosteroids | 10 (66.7) | 19 (10-34) | — |
| Prednisone | 7 (46.7) | 28 (19-38) | 60 (50-80) mg |
| Methylprednisolone | 5 (33.3) | 14.5 (5-56) | 1 (0.1-1) g |
| Dexamethasone | 4 (26.7) | 15 (10-26.5) | 7 (4-25) mg |
| IV immunoglobulin | 8 (53.3) | 8 (7-50) | 70 (60-80) g |
| TPO-RAs | 1 (6.7) | 51 (31-71) | — |
| Eltrombopag | 1 (6.7) | 31 (31-31) | 75 (75-75) mg |
| Romiplostim | 1 (6.7) | 71 (71-71) | 100 (100-100) μg |
| Danazol | 1 (6.7) | 107 (107-107) | 200 (200-200) mg |
| Rh immunoglobulin | 1 (6.7) | 46 (20-72) | 300 (300-300) μg |
| Children (n = 13) | |||
| IV immunoglobulin | 10 (76.9) | 7 (4-9) | 42.5 (24-65) g |
| Corticosteroids | 8 (61.5) | 19.5 (4-51.5) | — |
| Methylprednisolone | 8 (61.5) | 5 (2-25) | 0.85 (0.061-92.5) g |
| Prednisone | 4 (30.8) | 53 (21-88.5) | 75 (45-80) mg |
| Dexamethasone | 1 (7.7) | 53 (53-53) | 4 (4-4) mg |
| Platelet transfusion | 8 (61.5) | 4 (2-21) | 1 (1-1) units |
| TPO-RAs | 4 (30.8) | 22 (21-23) | — |
| Romiplostim | 3 (23.1) | 22 (12-27) | 170 (155-250) μg |
| Eltrombopag | 2 (15.4) | 22 (21-23) | 50 (25-75) mg |
| Antifibrinolytics | 4 (30.8) | 35.5 (8-65) | — |
| IV aminocaproic acid | 2 (15.4) | 26.5 (0-53) | Not available |
| Oral aminocaproic acid | 2 (15.4) | 46.5 (18-75) | 3001 (2-6000) mg |
| IV tranexamic acid | 1 (7.7) | Not available | 400 (400-400) mg |
| Oral tranexamic acid | 2 (15.4) | 36.5 (8-65) | 1000 (1000-1000) mg |
| Rituximab | 1 (7.7) | 22 (22-22) | 258 (258-258) mg |
| Urgent splenectomy | 1 (7.7) | Not applicable | Not applicable |
Outcomes of critical ITP bleeds
Mortality among patients with critical ITP bleeds was 9 of 28 (32.1%), including 7 of 15 adults (46.7%) and 2 of 13 children (15.4%). Mortality was higher among older patients: for adults, 5 of 9 patients (55.6%) aged ≥65 years died compared with 2 of 6 patients (33.3%) aged <65 years (2/6). For children, 2 of 7 (28.6%) aged ≥12 years died, whereas no deaths occurred in children <12 years. In adults, 5 of 12 patients with ICH died (41.7%) compared with 2 of 3 patients without ICH (66.7%). In children, 2 of 6 patients (33.3%) with ICH died compared with 0 of 7 without ICH. For adults, mortality was 3 of 5 (60.0%) for those whose first treatment was given later than 6.9 hours (median time to first treatment) compared with 3 of 6 (50.0%) for those who received treatment earlier. For children, mortality was 1 of 4 (25.0%) for those whose first treatment was later than 3.5 hours (median time to first treatment) and 0 of 6 for those who received treatment earlier (Table 5).
Mortality according to different variables in patients with critical bleeding with ITP (adults, n = 15; children, n = 13)
| Variables . | Adults . | Children . | ||
|---|---|---|---|---|
| Total patients with critical bleed . | Mortality, n (%) . | Total patients with critical bleed . | Mortality, n (%) . | |
| Overall | 15 | 7 (46.7) | 13 | 2 (15.4) |
| Age, y | ||||
| ≥65 | 9 | 5 (55.6) | — | — |
| <65 | 6 | 2 (33.3) | — | — |
| Age, y | ||||
| ≥12 | — | — | 7 | 2 (28.6) |
| <12 | — | — | 6 | 0 (0.0) |
| Sex | ||||
| Female | 7 | 3 (42.9) | 9 | 1 (11.1) |
| Male | 7 | 3 (42.9) | 4 | 1 (25.0) |
| Type of bleeds | ||||
| ICH | 12 | 5 (41.7) | 6 | 2 (33.3) |
| Others | 3 | 2 (66.7) | 7 | 0 (0.0) |
| Delayed treatment∗ | ||||
| Yes | 5 | 3 (60.0) | 4 | 1 (25.0) |
| No | 6 | 3 (50.0) | 6 | 0 (0.0) |
| Critical bleed as initial presentation of ITP | ||||
| Yes | 8 | 3 (37.5) | 4 | 1 (25.0) |
| No | 7 | 4 (57.1) | 9 | 1 (11.1) |
| Variables . | Adults . | Children . | ||
|---|---|---|---|---|
| Total patients with critical bleed . | Mortality, n (%) . | Total patients with critical bleed . | Mortality, n (%) . | |
| Overall | 15 | 7 (46.7) | 13 | 2 (15.4) |
| Age, y | ||||
| ≥65 | 9 | 5 (55.6) | — | — |
| <65 | 6 | 2 (33.3) | — | — |
| Age, y | ||||
| ≥12 | — | — | 7 | 2 (28.6) |
| <12 | — | — | 6 | 0 (0.0) |
| Sex | ||||
| Female | 7 | 3 (42.9) | 9 | 1 (11.1) |
| Male | 7 | 3 (42.9) | 4 | 1 (25.0) |
| Type of bleeds | ||||
| ICH | 12 | 5 (41.7) | 6 | 2 (33.3) |
| Others | 3 | 2 (66.7) | 7 | 0 (0.0) |
| Delayed treatment∗ | ||||
| Yes | 5 | 3 (60.0) | 4 | 1 (25.0) |
| No | 6 | 3 (50.0) | 6 | 0 (0.0) |
| Critical bleed as initial presentation of ITP | ||||
| Yes | 8 | 3 (37.5) | 4 | 1 (25.0) |
| No | 7 | 4 (57.1) | 9 | 1 (11.1) |
Delayed treatment was referred to when patients received their first ITP treatment after the median time to administration of the first ITP treatment for all patients.
Among adults, 4 of 12 patients (33.3%) who achieved a minimum platelet count response (>30 × 109/L) died, whereas 3 of 3 patients (100%) who did not reach this minimum platelet threshold died. Among children, 1 of 5 patients (20.0%) who achieved a minimum platelet count response died, whereas 1 of 8 patients (12.5%) who did not achieve a minimum platelet count response died. Two adults (13.3%) and 2 children (15.4%) developed neurological disability associated with a critical ITP bleed. The median number of days in hospital was 7 (IQR, 2-30) for adults and 3 (IQR, 2-6) for children.
Discussion
This study provides a comprehensive description of the characteristics, treatment approaches, and outcomes for adults and children with ITP who presented to the ER with severe thrombocytopenia and experienced a critical bleed. Our results highlight factors associated with critical bleeding in both adults and children and variables associated with higher mortality among patients who experienced a critical bleed. Our findings underscore the high mortality of these rare events, delays in time to first treatment, and the need for a standardized approach to management.
The frequency of critical bleeds in ITP was rare, occurring in 5.1% of adults and 1.4% of children who presented to the ER with a platelet count <20 × 109/L. Nevertheless, these events were associated with a high risk of death: 46.7% in adults and 15.4% in children. Although most critical bleeds were ICH (80.0% in adults and 46.2% in children), nearly one-fourth of critical bleeds in children were gynecological bleeds (23.1%). Adults and children in older age groups had a higher rate of critical bleeds. A previous systematic review also found that ICH was more common in adults than children (1.4% vs 0.4%),2 and previous studies have reported that older age was associated with more frequent and more severe bleeding.8,18-20 We found that critical bleeds occurred more often in patients during the chronic stage of ITP; however, for 12 of 28 patients (42.9%), critical bleeding was the initial presentation of ITP (n = 8 adults; n = 4 children). Adults with comorbidities, such as myocardial infarction, congestive heart failure, cerebrovascular accidents, or transient ischemic attack, and who were receiving anticoagulation had a higher rate of critical bleeds. This finding underscores the need for careful monitoring and risk assessment in these high-risk populations. In a French ITP registry (n = 302) that reported 20 major bleeds defined as gross hematuria, gastrointestinal, or intracranial bleeds, exposure to anticoagulants was associated with major bleeds.8
Mortality was higher in adults (46.7%) than in children (15.4%), and in both adults and children, the mortality was higher in older age groups. This disparity may reflect differences in underlying health conditions, response to treatments, or delays in initiating appropriate therapy. A similarly high mortality rate was observed in a study on ICH in children with ITP, in which 25.0% of 40 children died.21 Another study on ITP hospitalizations with ICH in the United States reported an overall mortality of 26.7%, with higher mortality in adults than in children.12 The occurrence of neurological disability in 13.3% of adults and 15.4% of children with critical bleeds further highlights the potential long-term consequences of critical bleeds, particularly ICH. A previous survey of 40 children with ITP and ICH reported that 25.0% had neurological sequelae.21 These results emphasize the need for rapid recognition and standardized management strategies to mitigate adverse health outcomes associated with these events.
Regarding treatment, corticosteroids, IV immunoglobulin, and platelet transfusions were the most commonly used interventions. A retrospective and prospective survey of 40 children with ICH reported similar results: 78% received IV immunoglobulin, 75% received platelet transfusions, 73% received corticosteroids, and 43% had urgent splenectomy.21 A retrospective cohort study of 29 adults with ITP presenting to the ER (32 visits) with severe thrombocytopenia (platelets <20 × 109/L) and major bleeding reported that patients received a median of 3 different treatment modalities (IQR, 2-4) including IV immunoglobulin in 90.6% of visits, corticosteroids in 81.3% of visits, and platelet transfusions in 75.0% of visits.22 Similarly, in a retrospective study of 58 children with ITP in 68 episodes of major bleeding, common treatments included corticosteroids in 27 bleeds (39.7%), IV immunoglobulin in 17 (25.0%), and corticosteroids plus IV immunoglobulin in 12 (17.6%).23 We also found that TPO-RAs and antifibrinolytic agents were used to treat critical bleeds. TPO-RAs may be used as an adjunctive treatment to stabilize and prevent rebleeding after initial management,14 and the use of antifibrinolytics such as tranexamic acid is supported by studies and guidelines to restore hemostasis.14,24,25 We noted a lag in time to the first ITP treatment, which was more pronounced in adults than children (6.9 vs 3.5 hours). Mortality was higher in those who received first ITP treatment later than the median time. Delayed time to first treatment represents an opportunity for improvement, given the association between timely treatment and improved outcomes in acute bleeding scenarios.26
In this multicenter study, we described the characteristics, management, and outcomes of a cohort of patients with ITP who experienced a critical bleed. Our data collection spans a long time horizon (10 years) and multiple centers in the United States and Canada. Strengths of this study include the strict eligibility criteria, careful methods for accurate data collection, and the use of a specific definition to identify the most severe bleeding events. The inclusion of both adults and children was justified by similar strategies that can be applied to both age groups. Characterizing critical bleeds and mortality according to different factors in adults and children will help identify the risk groups prone to developing critical bleeds and experiencing the worst outcomes. Limitations of this study include the lack of statistical inferences that can be made from this small data set and insufficient data to determine treatment effects. Although our sample frame was broad, it included only urban academic centers, which limits the generalizability of our findings to smaller ERs, where access to certain treatments, such as platelet transfusions and IV immunoglobulin, may be more limited, and to health care settings where patients bypass the ER and are directly admitted to hospital.
Our results highlight the variation in treatment approaches across patients, emphasizing the need for a standardized management protocol to reduce delays and optimize care. In addition, our data strengthen the existing evidence regarding treatment approaches used and provide valuable insights into treatment timeliness, patient characteristics, and associated outcomes, which are critical in clinical decision-making. The results from this cohort study will contribute to a clinical practice guideline for emergency management of critical bleeding in ITP patients, alongside systematic reviews of both randomized and observational studies.
In conclusion, critical bleeds in patients with ITP were rare, but the rate was higher in adults than children, representing 5.1% vs 1.4% of patients with ITP presenting to the ER with severe thrombocytopenia. The rate of critical bleeds was higher in adults with comorbidities and in both adults and children with chronic ITP. Treatments of critical bleeds included corticosteroids, IV immunoglobulin, and platelet transfusion often administered in combination and often with adjunctive use of TPO-RAs and tranexamic acid. Median time from presentation to ER to first ITP treatment was longer for adults than for children. Mortality was higher in adults than in children, in older patients, and in those whose treatment was delayed. A standardized approach to the management of critical bleeds in patients with ITP is needed to optimize treatment and avoid adverse health outcomes associated with this hematologic emergency.
Acknowledgments
The authors thank the patients, their families, and the study teams at the data collection sites.
This study was funded by the Canadian Institutes of Health Research (D.M.A.; funding reference number 165811) and the Platelet Disorder Support Association (E.S.). The Michael G. DeGroote Centre for Transfusion Research receives partial infrastructure funding from Canadian Blood Services and Health Canada. This research also received funding support from Canadian Blood Services (Canadian Blood Services Graduate Fellowship Award [S.R.C.; reference number GFP2023-SC]), funded by the federal government (Health Canada) and the provincial and territorial ministries of health. This publication was supported by the Einstein Foundation Berlin as part of the Einstein Foundation Award for Promoting Quality in Research (G.G.).
The contents are those of the authors and do not necessarily represent the official views of the Platelet Disorder Support Association, Canadian Blood Services, the federal, provincial, or territorial governments of Canada, the Einstein Foundation, or the award jury, nor does mention of any organizations imply endorsement by these entities. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation or approval of the manuscript; or the decision to submit the manuscript for publication.
Authorship
Contribution: E.S., S.R.C., G.G., and D.M.A. conceptualized and designed the study; E.S., D.M., M.S.J., A.C., C.E.B., R.F.G., J.W.Y., S.N., C.T.Q, A. Pfeiffer, M.P.L., K.P., A. Pishko, K.V., A.S., K.C., R.G., A.A., M.B., B.C., E.J.B., S.J.K., E.X., I.S., B.L., T.M., T.B., E.M.H., H.E.R., and J.L. contributed to data collection; E.S., D.M., M.S.J., D.G., L.M.V., K.E., and D.M.A. verified the acquired study data; S.R.C., K.E., D.K., and M.A.-M. conducted the analyses; E.S. and S.R.C. drafted the manuscript; D.M.A. supervised the study; and all authors provided major intellectual contributions to the manuscript, reviewed and revised its contents, and approved the final version.
Conflict-of-interest disclosure: A.C. reports personal fees from Synergy, Sanofi, Pfizer, MingSight, and UpToDate, outside the submitted work. R.F.G. reports research funding from Agios, Novartis, and Sobi; and had a consultancy role with Agios, Sanofi, and Sobi, outside the submitted work. M.P.L. reports personal fees from Novartis Dova, Principia, argenx, Rigel, Sobi, Sanofi, Janssen, and UpToDate; and research funding from Novartis Dova, Principia, argenx, Rigel, Sobi, Sanofi, and Janssen. A.P. served on an advisory board for Biomarin and received authorship royalties from UpToDate. D.M.A. reports research grants from Rigel; and personal fees from Rigel, Amgen, Medison, Daiichi Sankyo, Sobi, Principia, Chugai, Alpine, argenx, Sanofi, and UpToDate, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Donald M. Arnold, Michael G. DeGroote Centre for Transfusion Research, McMaster University, HSC-3H50, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; email: arnold@mcmaster.ca; and Saifur R. Chowdhury, Department of Health Research Methods, Evidence, and Impact, McMaster University, 1280 Main St West, Hamilton, ON L8S 4L8, Canada; email: saifur.rahm1994@gmail.com/ saifur@mcmaster.ca.
References
Author notes
E.S. and S.R.C. contributed equally as first authors to this study.
Original data are available on request from the corresponding authors, Donald M. Arnold (arnold@mcmaster.ca) and Saifur R. Chowdhury (saifur@mcmaster.ca; saifur.rahm1994@gmail.com).