Key Points
Enhancement of PEA and targeting p38-MAPK ameliorates hyperalgesia in sickle cell mice.
PEA exerts neuroprotective effects in central nervous system and peripheral neurons and reduces inflammation.
Visual Abstract
Chronic and acute pain are major comorbidities of sickle cell disease (SCD). Both peripheral and central nervous system mechanisms underlie SCD pain. We investigated the potential roles of endogenous fatty acyl derivatives with endocannabinoid-like activity; using targeted lipidomics of fatty acid ethanolamides and monoacylglycerols, we observed significant reduction of spinal palmitoylethanolamide (PEA) in the humanized, transgenic sickle (HbSS)–BERK mouse model of SCD when compared with healthy HbAA control mice. PEA is a paracannabinoid lipid mediator that acts as a putative mast cell stabilizer and has been evaluated clinically for mechanical, cold, and joint pain, which are all features of SCD pain. Increased p38-MAPK activity in the spinal cord and dorsal root ganglia (DRG) neurons, and cutaneous mast cell activation, contribute to chronic hyperalgesia in sickle cell mice. We provide the first evidence that inhibiting endogenous PEA degradation and administration of exogenous PEA, reduces measures of chronic hyperalgesia via inhibition of peripheral and central mechanisms of pain hypersensitivity in sickle cell mice. PEA alleviated spinal inflammation, oxidative stress, and p38-MAPK activity; in the periphery, inhibited p38-MAPK phosphorylation and nuclear translocation in DRG neurons, reduced mast cell extracellular trap formation, and markers of inflammation in HbSS mice. In addition, PEA treatment prevented and ameliorated acute hyperalgesia incited by hypoxia/reoxygenation challenge in sickle cell mice. Pain may persist even after hydroxyurea and curative therapies in SCD. PEA displayed safety in several clinical studies and has the translational potential to treat pain in SCD without the burden of additional medications.
Introduction
Sickle cell disease (SCD) is a monogenetic, autosomal recessive disorder of the β-globin gene resulting in sickle hemoglobin (HbS), which is characterized by hemolysis, ischemia/reperfusion injury, oxidative stress, inflammation, and vaso-occlusion, and life-threatening comorbidities including end-organ damage and intractable pain.1,2 Unpredictable and recurrent episodes of acute pain are unique to SCD and require hospitalization and opioid use, and reduce survival3; additionally, persistent chronic pain may affect most individuals.4 Pain treatment in SCD relies on opioids, which remains suboptimal because of liabilities including risk of tolerance and side effects.5 Humanized, transgenic sickle cell mice expressing human HbS recapitulate many of the clinical features of SCD and have provided insight into the mechanisms underlying SCD pain.6,7
The endocannabinoids, arachidonoylethanolamide (anandamide [AEA]) and 2-arachidonoyl-sn-glycerol (2-AG), modulate pain via both central and peripheral mechanisms.8,9 Preclinical studies indicate that activating cannabinoid receptors (CBRs), CB1R and/or CB2R, reduces hyperalgesia in sickle cell mice.10-12 CBR agonist, THC (Δ9-tetrahydrocannabinol), alleviated pressure and cold hypersensitivity in sickle cell mice.13 The antinociceptive effects of AEA are enhanced in a synergistic manner by palmitoylethanolamide (PEA),14 a member of the paracannabinoid family of lipid mediators; these agents share biogenetic and degradative pathways with AEA but do not productively interact with CBRs.15 PEA produces marked antinociceptive effects in animal models engaging the nuclear receptor, peroxisome proliferator activated receptor α (PPAR-α) rather than CB1R or CB2R.16,17 Additionally, clinical studies suggest that PEA may have analgesic efficacy in various human pain conditions, without the intoxicating effect of THC.18
Hydroxyurea, bone marrow transplant, and gene therapy are used to treat SCD, but these may not ameliorate pain.19,20 Recent US Food and Drug Administration–approved medications, crizanlizumab and voxelotor, failed to show effectiveness in a phase 3 clinical trial and caused side effects, respectively.21,22 In this study, we investigated the contribution of PEA in the modulation of hyperalgesia in humanized transgenic sickle (HbSS) mice expressing >99% human sickle Hb and their congenic (HbAA) controls expressing normal human HbA. Targeted lipidomic analyses revealed that spinal cord PEA concentrations are significantly lower in HbSS mice of both sexes than in age-matched HbAA controls. No such difference was observed in the levels of endocannabinoids, AEA and its ω-3 congener docosahexaenoyl ethanolamide (DHEA). Regardless of biological sex, systemic administration of PEA or a selective inhibitor of the PEA-degrading enzyme, NAAA (N-acyl-ethanolamine acid amidase), produced profound antinociceptive effects in HbSS mice, which were mediated by a combination of central and peripheral mechanisms. The results identify a dysfunction in PEA-mediated paracannabinoid signaling in SCD, which may be targeted by PEA therapy.
Materials and methods
Detailed methods are provided in the supplemental Data.
Mouse model of SCD
Male and female humanized transgenic homozygous BERK sickle cell (HbSS-BERK) and control (HbAA-BERK) mice were used. BERK transgenic mice are on a mixed genetic background (FVB/N, 129, DBA/2, C57BL/6, and Black Swiss) with complete knockout of murine α and β globin (α−/−, β−/−). HbSS-BERK express >99% human sickle Hb, and control HbAA-BERK express normal human HbA. Mice were bred in-house with ad libitum food and water on a 12-hour light/dark cycle in conventional housing. Spinal cords were collected at end point for characterization of fatty acid ethanolamide (FAE) and monoacylglycerol levels using liquid chromatography tandem mass spectrometry. All mouse experiments were approved by the institutional animal care and use committee and complied with National Institutes of Health guidelines. No human data or patients were used in this study.
Treatments
NAAA inhibitor ARN19702 (ARN) was synthesized in-house, and prepared in 15% PEG400, 15% Tween-80, and 70% distilled water. PEA (catalog no. 90350, Cayman Chemicals, Ann Arbor, MI) was prepared in 7.5% dimethyl sulfoxide, 7.5% Tween-20, and 85% sterile saline. Neflamapimod (Nef; catalog no. 3915, Tocris Bioscience, Bristol, United Kingdom) was diluted in 1% weight-to-volume pluronic acid in H2O.
Pain-related behaviors
Mechanical hyperalgesia
Paw withdrawal frequency evoked by a 1.0 g (4.08 mN) calibrated von Frey (Semmes-Weinstein) monofilament (Stoelting Co, Wood Dale, IL) was recorded for 10 repeated applications to the plantar surface of each hind paw.
Musculoskeletal hyperalgesia
The tensile force of peak forelimb exertion (grip force) was measured using a computerized grip force meter (SA Maier Co, Milwaukee, WI). Grip force measurements were normalized to individual body weights in grams.
Cold hyperalgesia
Mice were placed onto a cold plate (4°C; Ugo Basile model 35100, Collegeville, PA), and paw withdrawal frequency was recorded over a 2-minute period.
Cold avoidance test
Mice were transferred to cold avoidance chambers at 30°C. One of the chambers was then cooled to 23°C, and mice were reintroduced to the apparatus. The amount of time spent in the warm (30°C) and cold (23°C) chambers were recorded for a 5-minute period.
Hypoxia/reoxygenation (H/R)
Mice were placed into the hypoxia chamber (8% O2 and 92% N2; ProOx P110, BioSpherix, Parish, NY) for 3 hours followed by reoxygenation in room air (∼21% O2) for 1 hour.
Protein and other measurements
Spinal cords and blood plasma were collected at euthanasia and snap frozen in liquid N2; skin releasates were snap frozen immediately in liquid N2. Serum amyloid P (SAP; catalog no. MPTX20, R&D Systems, Minneapolis, MN), interleukin-6 (IL-6; catalog no. DY406-05, R&D Systems), tryptase (catalog no. DY2059, R&D Systems), IL-1β (catalog no. 900-K47, PeproTech, Thermo Fisher), active caspase-3 (catalog no. CASP3C-1KT, Sigma-Aldrich), Rho-associated protein kinase (ROCK) activity (catalog no. CSA001, Millipore Sigma), and malondialdehyde (MDA; MAK085-1KT, Sigma-Aldrich) were measured using colorimetric assays. Phosphorylated p38-MAPK (phospho-p38-MAPK) and total p38-MAPK were analyzed using western immunoblotting.
Cell culture
Immortalized rat pheochromocytoma (PC12) cells, primary dorsal root ganglia (DRG) neurons and mast cells (MCs) were used.
Statistical analysis
Data are shown as mean ± standard deviation. P < .05 was considered statistically significant. Data were analyzed using GraphPad Prism 10 (GraphPad, Boston, MA).
Results
HbSS mice exhibit constitutively reduced spinal cord PEA levels
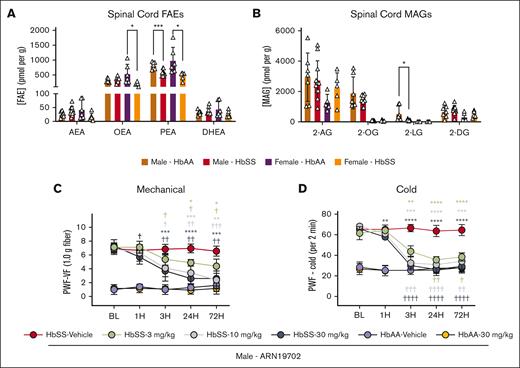
Targeted lipidomic analysis of the endocannabinoidome revealed the presence of both endocannabinoid and paracannabinoids in spinal cords of HbSS and HbAA mice (Figure 1A). Endocannabinoids included AEA, DHEA, and 2-AG, whereas paracannabinoids included PEA and oleoylethanolamide (OEA)23 and the GPR-119 agonist 2-OG (2-oleoyl-sn-glycerol).24 In male and female HbSS mice, spinal cord PEA concentrations were ∼30% (P < .001) and ∼50% (P < .05) lower, respectively, than sex-matched HbAA mice. OEA was reduced only in female HbSS mice (P < .05 vs female HbAA), but AEA and DHEA were not affected (Figure 1A). Similarly, no difference was observed in 2-AG, 2-OG, and 2-DG (2-docosahexaenoyl-sn-glycerol), whereas 2-LG (2-linoleoyl-sn-glycerol) was significantly reduced in male HbSS mice (P < .05 vs male HbAA) but undetectable in female HbSS and HbAA mice (Figure 1B). Fatty acid chain lengths and their chemistry direct the specificity of these lipid signaling molecules (supplemental Table 1). These findings prompted us to ask whether enhancing PEA-mediated activity may normalize nociception in HbSS mice.
HbSS mice have constitutively reduced spinal PEA compared with HbAA control mice. (A) HbSS mice exhibited a constitutive reduction in spinal cord PEA, regardless of sex, and female HbSS mice showed reduced OEA compared with HbAA mice, measured with liquid chromatography tandem mass spectrometry. (B) Male HbSS mice had significantly reduced spinal 2-LG compared with HbAA mice. (C-D) Targeting the PEA degradative enzyme, NAAA, with ARN (IP) dose-dependently reduced mechanical and cold hyperalgesia in the 72-hour period after a single treatment in male HbSS mice compared with baseline (BL) recordings and vehicle treatment. Mean ± standard deviation (SD). In panels A-B, data were analyzed with an unpaired Student 2-tailed t test; and in panels C-D, data were analyzed with repeated measures 2-way analysis of variance (ANOVA) and the Tukey post hoc multiple comparisons test. ∗Indicates a difference compared with time-matched vehicle; and †indicates a difference compared with respective BL. ∗,†P < .05; ∗∗,††P < .01; ∗∗∗,†††P < .001; ∗∗∗∗,††††P < .0001. Age: 3.5 to 5.0 months. Panels A-B: male HbAA, n = 7; male HbSS, n = 11; female HbAA, n = 6; female HbSS, n = 6; panels C-D: ARN, HbSS vehicle, n = 9; HbSS 3 mg/kg, n = 4; HbSS 10 mg/kg, n = 5; HbSS 30 mg/kg, n = 5; HbAA vehicle, n = 5; HbAA 30 mg/kg, n = 6. 2-DG, 2-docosahexaenoyl-sn-glycerol; 2-LG, 2-linoleoyl-sn-glycerol; 2-OG, 2-oleoyl-sn-glycerol; BW, body weight; MAG, monoacylglycerols; PWF, paw withdrawal frequency; VF, von Frey.
HbSS mice have constitutively reduced spinal PEA compared with HbAA control mice. (A) HbSS mice exhibited a constitutive reduction in spinal cord PEA, regardless of sex, and female HbSS mice showed reduced OEA compared with HbAA mice, measured with liquid chromatography tandem mass spectrometry. (B) Male HbSS mice had significantly reduced spinal 2-LG compared with HbAA mice. (C-D) Targeting the PEA degradative enzyme, NAAA, with ARN (IP) dose-dependently reduced mechanical and cold hyperalgesia in the 72-hour period after a single treatment in male HbSS mice compared with baseline (BL) recordings and vehicle treatment. Mean ± standard deviation (SD). In panels A-B, data were analyzed with an unpaired Student 2-tailed t test; and in panels C-D, data were analyzed with repeated measures 2-way analysis of variance (ANOVA) and the Tukey post hoc multiple comparisons test. ∗Indicates a difference compared with time-matched vehicle; and †indicates a difference compared with respective BL. ∗,†P < .05; ∗∗,††P < .01; ∗∗∗,†††P < .001; ∗∗∗∗,††††P < .0001. Age: 3.5 to 5.0 months. Panels A-B: male HbAA, n = 7; male HbSS, n = 11; female HbAA, n = 6; female HbSS, n = 6; panels C-D: ARN, HbSS vehicle, n = 9; HbSS 3 mg/kg, n = 4; HbSS 10 mg/kg, n = 5; HbSS 30 mg/kg, n = 5; HbAA vehicle, n = 5; HbAA 30 mg/kg, n = 6. 2-DG, 2-docosahexaenoyl-sn-glycerol; 2-LG, 2-linoleoyl-sn-glycerol; 2-OG, 2-oleoyl-sn-glycerol; BW, body weight; MAG, monoacylglycerols; PWF, paw withdrawal frequency; VF, von Frey.
PEA ameliorates chronic hyperalgesia
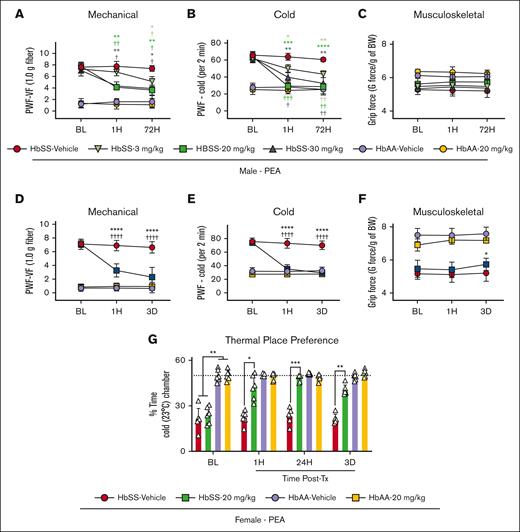
As a first test of this possibility, we used the compound ARN, a selective inhibitor of the PEA degradative enzyme NAAA,17 shown to increase PEA levels in murine blood and spinal cord.25 We found that systemic ARN administration (3, 10, and 30 mg/kg for 3 days) produced a dose-dependent reduction in mechanical and cold hyperalgesia in male HbSS mice. The effect appeared 3 hours after the first injection and was sustained for 3 days during treatment (P < .05; Figure 1C-D). Similarly, PEA dose-dependently (3, 20, and 30 mg/kg per day) reduced mechanical and cold hyperalgesia in male HbSS mice 1 hour after initial dose and maintained for 72 hours with daily treatment vs baseline and vehicle treatment (Figure 2A-C). No effects on hyperalgesia measures were observed in male or female control mice (Figure 2A-C). Female HbSS mice show constitutively increased cold hyperalgesia compared with male HbSS mice (supplemental Figure 1). Female HbSS mice treated with the optimal dose (20 mg/kg per day), showed a similar reduction to male HbSS in mechanical and cold hyperalgesia (P < .0001 for both vs baseline and vehicle; Figure 2D-E). Spontaneous, musculoskeletal hyperalgesia showed a modest but significant improvement in female HbSS mice treated with PEA compared with vehicle 72 hours after start of treatment (P < .05; Figure 2F). Females treated with PEA showed a concomitant reduction in cold aversion in a nonevoked cold avoidance test (P < .05; Figure 2G). PEA reduced hyperalgesia to a similar extent as CP55,940 in female HbSS mice (supplemental Figure 2). Thus, endogenously produced PEA modulates nociception in HbSS mice involving a spinal cord–mediated mechanism, and exogenously administered PEA can mitigate chronic hyperalgesia in both male and female HbSS sickle cell mice, without any effect on control HbAA mice.
PEA ameliorates chronic hyperalgesia in HbSS mice. (A-B) PEA intraperitoneal (IP) dose-dependently reduced hyperalgesia in male HbSS mice in response to mechanical and cold stimulation with maximal effect at 1 hour and 72 hours of daily treatment with 20 mg/kg per day dose. (C) PEA did not affect measures of musculoskeletal hyperalgesia in male HbSS or HbAA mice. (D-F) Optimized PEA dose (IP, 20 mg/kg per day) in female mice significantly ameliorated mechanical and cold hyperalgesia 1 hour and 72 hours after start of treatment compared with vehicle and BL measures, and musculoskeletal hyperalgesia was reduced at 72 hours in PEA-treated mice compared with vehicle. (G) PEA (IP, 20 mg/kg per day) treatment significantly ameliorated nonevoked cold hyperalgesia in a thermal place preference test, indicated by increased time spent in a cold chamber, 1 hour after initial dose and after 3 days of daily treatment compared with vehicle-treated HbSS mice. No effects were observed in male or female control mice with treatments. Mean ± SD. In panels A-G, data were analyzed using repeated measures 2-way ANOVA and the Tukey post hoc multiple comparisons test. ∗Indicates a difference compared with time-matched vehicle; †indicates a difference compared with respective BL. ∗,†P < .05; ∗∗,††P < .01; ∗∗∗,†††P < .001; ∗∗∗∗,††††P < .0001. Age: 3.5 to 5.0 months. For panels A-C: PEA male, HbSS vehicle, n = 4; HbSS 3 mg/kg, n = 4; HbSS 20 mg/kg, n = 4; HbSS 30 mg/kg, n = 5; HbAA vehicle, n = 5; HbAA 30 mg/kg, n = 6; panels D-G: PEA female, HbAA vehicle, n = 11; HbAA 20 mg/kg, n = 6; HbSS vehicle, n = 8; HbSS 20 mg/kg, n = 12. BW, body weight; PWF, paw withdrawal frequency; Tx, treatment; VF, von Frey.
PEA ameliorates chronic hyperalgesia in HbSS mice. (A-B) PEA intraperitoneal (IP) dose-dependently reduced hyperalgesia in male HbSS mice in response to mechanical and cold stimulation with maximal effect at 1 hour and 72 hours of daily treatment with 20 mg/kg per day dose. (C) PEA did not affect measures of musculoskeletal hyperalgesia in male HbSS or HbAA mice. (D-F) Optimized PEA dose (IP, 20 mg/kg per day) in female mice significantly ameliorated mechanical and cold hyperalgesia 1 hour and 72 hours after start of treatment compared with vehicle and BL measures, and musculoskeletal hyperalgesia was reduced at 72 hours in PEA-treated mice compared with vehicle. (G) PEA (IP, 20 mg/kg per day) treatment significantly ameliorated nonevoked cold hyperalgesia in a thermal place preference test, indicated by increased time spent in a cold chamber, 1 hour after initial dose and after 3 days of daily treatment compared with vehicle-treated HbSS mice. No effects were observed in male or female control mice with treatments. Mean ± SD. In panels A-G, data were analyzed using repeated measures 2-way ANOVA and the Tukey post hoc multiple comparisons test. ∗Indicates a difference compared with time-matched vehicle; †indicates a difference compared with respective BL. ∗,†P < .05; ∗∗,††P < .01; ∗∗∗,†††P < .001; ∗∗∗∗,††††P < .0001. Age: 3.5 to 5.0 months. For panels A-C: PEA male, HbSS vehicle, n = 4; HbSS 3 mg/kg, n = 4; HbSS 20 mg/kg, n = 4; HbSS 30 mg/kg, n = 5; HbAA vehicle, n = 5; HbAA 30 mg/kg, n = 6; panels D-G: PEA female, HbAA vehicle, n = 11; HbAA 20 mg/kg, n = 6; HbSS vehicle, n = 8; HbSS 20 mg/kg, n = 12. BW, body weight; PWF, paw withdrawal frequency; Tx, treatment; VF, von Frey.
PEA ameliorates spinal oxidative stress and inflammation
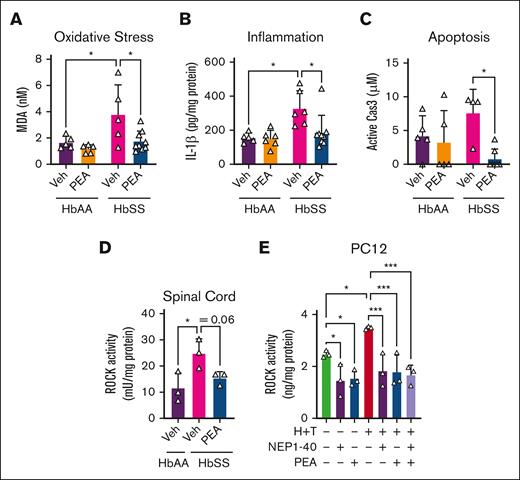
Spinal cord inflammation and oxidative stress are elevated in sickle cell mice.26 MDA, a marker of oxidative stress via lipid peroxidation, was significantly increased in female HbSS vs female HbAA mice, and PEA treatment (20 mg/kg per day) for 14 days significantly reduced spinal MDA vs vehicle (∼51%; P < .05; Figure 3A). MDA production in the spinal cord is downstream of spinal IL-1β signaling, which is produced by the enzymatic activity of caspase-3.27 IL-1β is increased in the spinal cord of female HbSS compared with female HbAA mice, and 14-day PEA treatment (20 mg/kg per day) significantly reduced spinal cord IL-1β concentrations and caspase-3 activity (∼43% [P < .05] and ∼26% [P < .05] vs vehicle-treated HbAA and vehicle-treated HbSS, respectively; Figure 3B-C). HbSS spinal cords show glial activation,26,28,29 which precedes the caspase-3 signaling, and PEA may be acting directly upon glia to reduce spinal oxidative stress and inflammation associated with pain.
PEA reduces markers of spinal cord inflammation and neuronal degeneration. After 14-day PEA treatment (IP 20 mg/kg per day), spinal cords of female HbSS mice compared with vehicle (Veh) show a significant reduction in (A) MDA, a marker of lipid peroxidation and oxidative stress; (B) IL-1β, a marker of inflammation; and (C) Cas3, marker of apoptosis. (D) ROCK activity is significantly increased in HbSS spinal cords compared with HbAA mice, and this was abrogated with 14-day PEA (IP 20 mg/kg per day) treatment. (E) Incitement of a sickle microenvironment with TNF-α (1 ng/mL) and hemin (40 μM) significantly increases ROCK activity in differentiated PC12 cells, which was ameliorated with NEP1-40 (2 μM) or PEA (30 μM). Mean ± SD. In panels A-C, data were analyzed using 2-way ANOVA and the Tukey post hoc multiple comparisons test. In panels D-E, data were analyzed with unpaired Student 2-tailed t test. ∗Indicates a difference compared with Veh. ∗P < .05; ∗∗∗P < .001. Age: 3.5 to 5.0 months. For panels A-C, n = 6 per condition; and panels D-E, n = 3 per condition. Cas3, caspase-3.
PEA reduces markers of spinal cord inflammation and neuronal degeneration. After 14-day PEA treatment (IP 20 mg/kg per day), spinal cords of female HbSS mice compared with vehicle (Veh) show a significant reduction in (A) MDA, a marker of lipid peroxidation and oxidative stress; (B) IL-1β, a marker of inflammation; and (C) Cas3, marker of apoptosis. (D) ROCK activity is significantly increased in HbSS spinal cords compared with HbAA mice, and this was abrogated with 14-day PEA (IP 20 mg/kg per day) treatment. (E) Incitement of a sickle microenvironment with TNF-α (1 ng/mL) and hemin (40 μM) significantly increases ROCK activity in differentiated PC12 cells, which was ameliorated with NEP1-40 (2 μM) or PEA (30 μM). Mean ± SD. In panels A-C, data were analyzed using 2-way ANOVA and the Tukey post hoc multiple comparisons test. In panels D-E, data were analyzed with unpaired Student 2-tailed t test. ∗Indicates a difference compared with Veh. ∗P < .05; ∗∗∗P < .001. Age: 3.5 to 5.0 months. For panels A-C, n = 6 per condition; and panels D-E, n = 3 per condition. Cas3, caspase-3.
PEA targets mechanisms of axonal injury and central sensitization
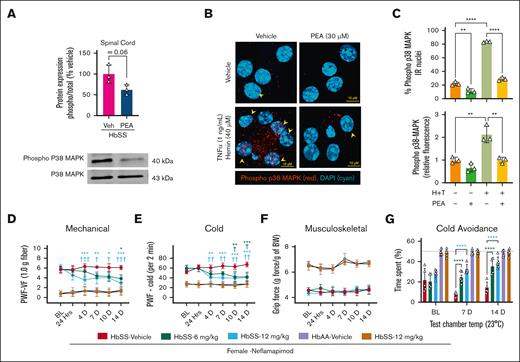
The sickle microenvironment contributes to spinal microglial activation and neural injury.10,29 PEA reduced spinal cord ROCK activity in female HbSS mice, which was significantly elevated compared with female HbAA mice (P < .05, vs HbAA; Figure 3D). PC12 pheochromocytoma cells differentiated with nerve growth factor, a model for immortalized neuronal cells,30 displayed equivalent reductions in baseline ROCK activity after treatment with PEA (30 μM) or the neurite outgrowth inhibitor receptor antagonist, NEP1-40, a putative enhancer of neurite formation (P < .05 vs vehicle; Figure 3E).31 Incitement of a sickle microenvironment with 1 ng/mL tumor necrosis factor α (TNF-α) and 40 μM hemin (representing free heme released from hemolysis; H+T) significantly increased ROCK activity in PC12 cells, which was completely abrogated by treatment with NEP1-40, PEA, or both (P < .001 vs H+T with NEP1-40, PEA, or both; Figure 2E). PEA (20 mg/kg per day) significantly reduced spinal p38-MAPK phosphorylation (Figure 4A), which is involved in chronic pain sensitization. DRG neurons are first order neurons that transmit stimuli to second order spinal cord neurons, and their dysregulation contributes to central sensitization and chronic hyperalgesia in SCD in a p38-MAPK–dependent manner.32,33 H+T significantly increased p38-MAPK phosphorylation and nuclear translocation in primary DRG neurons from HbSS mice (∼276%; P < .0001 vs vehicle; Figure 4B-C); nuclear translocation of phospho-p38-MAPK is an important step of transcriptional regulation by p38-MAPK.34 HbSS DRG neurons in culture displayed constitutive phospho-p38-MAPK nuclear translocation, which was significantly reduced by PEA treatment (P < .01 vs vehicle 30 μM); H+T substantially increased total and nuclear phospho-p38-MAPK, which was almost completely abrogated by PEA cotreatment (30 μM; Figure 4B-C). Moreover, inhibiting p38-MAPK phosphorylation with Nef improved measures of mechanical and cold hyperalgesia in a dose-dependent manner, without affecting musculoskeletal hyperalgesia over a 14-day period (oral, 6 and 12 mg/kg twice daily; Figure 4D-F). Nef also improved cold tolerance in a nonevoked cold avoidance test after 7 and 14 days of treatment (P < .01 and P < .001, respectively, vs vehicle; Figure 4J). Thus, PEA ameliorates central and peripheral mechanisms of sickle cell pain, which involves p38-MAPK signaling.
Inhibition of p38-MAPK ameliorates chronic hyperalgesia in HbSS mice. (A) Western immunoblotting revealed decreased p38-MAPK phosphorylation in the spinal cord of HbSS mice treated with PEA (IP, 14 days, 20 mg/kg per day). (B) PEA (30 μM) reduced phosphorylation of p38-MAPK and nuclear translocation of phosphorylated p38-MAPK in primary DRG neurons collected from female HbSS mice. Representative images of DRG neurons treated with Veh (complete media), TNF-α (1 ng/mL)/hemin (40 μM), and/or PEA (30 μM) immunolabeled with primary rabbit anti-mouse phospho-p38-MAPK (1:100; catalog no. 9211S, Cell Signaling Technology), secondary Cy3 AffiniPure donkey anti-rabbit immunoglobulin G (H+L), Absorption max: 550 nm and Excitation max: 570 nm (1:500; catalog no. 711-165-152, Jackson ImmunoResearch, West Grove, PA) antibodies, and DAPI (4′,6-diamidino-2-phenylindole) nuclear counterstain (1:25 000; catalog no. D1306, Invitrogen, Thermo Fisher). (B-C) DRG neurons showing p38-MAPK phosphorylation and phospho-p38-MAPK nuclear colocalization were enumerated and averaged in 6 fields of view per subject. Incitement of a sickle microenvironment significantly increased phospho-p38-MAPK nuclear colocalization, which was completely attenuated with PEA cotreatment. Z-stacks of 10× 0.5-μm images were acquired on a laser scanning confocal microscope (Zeiss LSM 900, Carl Zeiss AG) using a plan-apochromat 63× oil M27 objective lens. (D-G) Targeting p38-MAPK with Nef dose-dependently (oral 6 or 12 mg/kg twice daily) reduced PWF in response to mechanical and cold stimuli and reduced cold aversion at days 7 and 14 of treatment, without affecting grip force in female HbSS mice, suggesting reduced hyperalgesia. Mean ± SD. In panels A-C, data were analyzed with unpaired Student 2-tailed t test. In panels D-G, data were analyzed with 2-way ANOVA and the Tukey post hoc multiple comparisons test. ∗Indicates difference compared with Veh; †indicates difference compared with corresponding BL. ∗,†P < .05; ∗∗,††P < .01; ∗∗∗,†††P < .001; ∗∗∗∗P < .0001. Age: 3.5 to 5.0 months. For panels A-C, n = 3 per condition; panels D-G; female HbSS Veh, n = 5; female HbSS Nef 6 mg/kg, n = 5; female HbSS Nef 12 mg/kg, n = 5; female HbAA Veh, n = 6; female HbAA Nef 12 mg/kg, n = 6. BW, body weight; IR, immunoreactivity; PWF, paw withdrawal frequency; Temp, temperature; VF, von Frey.
Inhibition of p38-MAPK ameliorates chronic hyperalgesia in HbSS mice. (A) Western immunoblotting revealed decreased p38-MAPK phosphorylation in the spinal cord of HbSS mice treated with PEA (IP, 14 days, 20 mg/kg per day). (B) PEA (30 μM) reduced phosphorylation of p38-MAPK and nuclear translocation of phosphorylated p38-MAPK in primary DRG neurons collected from female HbSS mice. Representative images of DRG neurons treated with Veh (complete media), TNF-α (1 ng/mL)/hemin (40 μM), and/or PEA (30 μM) immunolabeled with primary rabbit anti-mouse phospho-p38-MAPK (1:100; catalog no. 9211S, Cell Signaling Technology), secondary Cy3 AffiniPure donkey anti-rabbit immunoglobulin G (H+L), Absorption max: 550 nm and Excitation max: 570 nm (1:500; catalog no. 711-165-152, Jackson ImmunoResearch, West Grove, PA) antibodies, and DAPI (4′,6-diamidino-2-phenylindole) nuclear counterstain (1:25 000; catalog no. D1306, Invitrogen, Thermo Fisher). (B-C) DRG neurons showing p38-MAPK phosphorylation and phospho-p38-MAPK nuclear colocalization were enumerated and averaged in 6 fields of view per subject. Incitement of a sickle microenvironment significantly increased phospho-p38-MAPK nuclear colocalization, which was completely attenuated with PEA cotreatment. Z-stacks of 10× 0.5-μm images were acquired on a laser scanning confocal microscope (Zeiss LSM 900, Carl Zeiss AG) using a plan-apochromat 63× oil M27 objective lens. (D-G) Targeting p38-MAPK with Nef dose-dependently (oral 6 or 12 mg/kg twice daily) reduced PWF in response to mechanical and cold stimuli and reduced cold aversion at days 7 and 14 of treatment, without affecting grip force in female HbSS mice, suggesting reduced hyperalgesia. Mean ± SD. In panels A-C, data were analyzed with unpaired Student 2-tailed t test. In panels D-G, data were analyzed with 2-way ANOVA and the Tukey post hoc multiple comparisons test. ∗Indicates difference compared with Veh; †indicates difference compared with corresponding BL. ∗,†P < .05; ∗∗,††P < .01; ∗∗∗,†††P < .001; ∗∗∗∗P < .0001. Age: 3.5 to 5.0 months. For panels A-C, n = 3 per condition; panels D-G; female HbSS Veh, n = 5; female HbSS Nef 6 mg/kg, n = 5; female HbSS Nef 12 mg/kg, n = 5; female HbAA Veh, n = 6; female HbAA Nef 12 mg/kg, n = 6. BW, body weight; IR, immunoreactivity; PWF, paw withdrawal frequency; Temp, temperature; VF, von Frey.
PEA attenuates MC activation and markers of inflammation in a sickle microenvironment
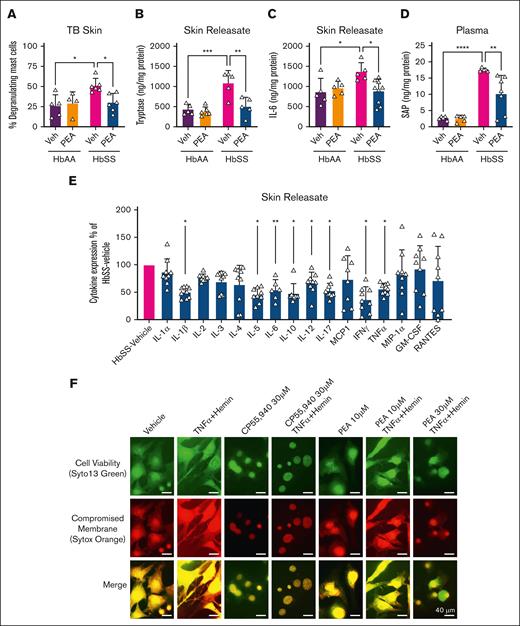
MC activation has been observed in the skin of HbSS mice compared with HbAA,35 which contributes to hyperalgesia. Toluidine blue–stained skin biopsies revealed significantly increased MC degranulation in female HbSS vs female HbAA mice treated with vehicle (P < .05; Figure 5A). PEA (20 mg/kg per day for 14 days) significantly reduced the percentage of degranulating cutaneous MCs (P < .05 vs vehicle-treated HbSS; Figure 5A). Concomitant with MC degranulation, HbSS mice showed significantly higher tryptase in skin biopsy–conditioned media (skin releasate), which was significantly reduced by PEA treatment (P < .001 vs vehicle-treated HbAA and P < .01 PEA-treated HbSS, respectively; Figure 5B). In addition to neuronal mechanisms of injury and inflammation, HbSS mice display features of global inflammation, which contribute to the prohyperalgesic milieu. Compared with HbAA, HbSS mice have constitutively elevated IL-6 in skin releasate (∼152%; P < .05; Figure 5C) and circulating SAP (∼564%; P < .0001; Figure 5D), an acute phase protein marker of inflammation and injury, which were significantly reduced in HbSS mice by 14-day PEA treatment (intraperitoneal, 20 mg/kg per day) vs vehicle (P < .01 and P < .05, respectively; Figure 5C-D). Evaluation of skin releasate with a cytokine microarray revealed a significant reduction in mediators of MC activation and inflammation in IL-1β (P < .05), IL-5 (P < .05), IL-6 (P < .01), IL-17 (P < .05), interferon gamma (P < .05), and TNF-α (P < .05), as well as pleiotropic IL-10 (P < .05) and IL-12 (P < .05) in HbSS mice treated for 14 days with PEA (intraperitoneal, 20 mg/kg per day) vs vehicle-treated HbSS mice (Figure 5E). To confirm the inhibitory effect of PEA on MC activation, we examined its effect directly on primary MCs isolated from the dorsal skin of HbSS mice. Earlier, we had observed that incitement of a sickle microenvironment with H+T for 4 hours led to robust activation of MCs in vitro and formation of MC extracellular traps (MCETs) characterized by extracellular release of DNA after chromatin disruption.35 MCET formation was attenuated in a dose-dependent manner by coincubation with PEA during incitement with H+T (Figure 5F). The attenuation of MCETs by PEA in a sickle microenvironment was similar to the preventive effect of CP55,940 (30 μM), a synthetic, mixed CB1R/CB2R agonist (Figure 5F). Thus, PEA inhibits MC activation and inflammation in the periphery, which may underlie hyperalgesia in HbSS mice.
PEA reduces MC degranulation, inflammation, and extracellular trap formation. (A) PEA (IP, 20 mg/kg per day) administration for 14 days significantly reduced percentage of degranulating MCs observed with TB staining in the skin of female HbSS mice compared with Veh treatment. (B-C) PEA significantly reduced tryptase and IL-6 in skin releasate of HbSS mice compared with Veh treatment, which is constitutively elevated compared with HbAA mice. (D) SAP is constitutively increased in the circulation of HbSS mice compared with HbAA mice, and SAP is significantly reduced after 14-day PEA (IP, 20 mg/kg per day) administration. (E) Cytokine microarray revealed a significant reduction in pro-inflammatory and mast cell–mediating cytokines IL-1ß, IL-5, IL-6, IL-10, IL-12, IL-17, IFN-γ, and TNFα in skin releasate of female PEA-treated HbSS mice compared to female vehicle-treated HbSS mice. (F) Overnight treatment of MCs from HbSS mouse skin with TNF-α (1 ng/mL) and hemin (40 μM) increased MCET formation, indicated by explosion of nuclear content observed with Syto 13 and Sytox Orange staining of DNA. Mixed CB1 and CB2 agonist CP55,940 (30 μM) attenuated MCET formation. PEA treatment (10 and 30 μM) dose-dependently reduced MCET formation. Mean ± SD. In panels A-D, data were analyzed using 2-way ANOVA and the Tukey post hoc multiple comparisons test. In panel E, data were analyzed using unpaired Student 2-tailed t test. In panel F, scale bar = 40 μm. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Age: 3.5 to 5.0 months. For panels A-D, n = 4-6; panel E, n = 9 per condition. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; MCP1, monocyte chemoattractant protein; MIP-1α, macrophage inflammatory protein-1; RANTES, regulated on activation normal T-cell expressed and secreted protein; TB, toluidine blue.
PEA reduces MC degranulation, inflammation, and extracellular trap formation. (A) PEA (IP, 20 mg/kg per day) administration for 14 days significantly reduced percentage of degranulating MCs observed with TB staining in the skin of female HbSS mice compared with Veh treatment. (B-C) PEA significantly reduced tryptase and IL-6 in skin releasate of HbSS mice compared with Veh treatment, which is constitutively elevated compared with HbAA mice. (D) SAP is constitutively increased in the circulation of HbSS mice compared with HbAA mice, and SAP is significantly reduced after 14-day PEA (IP, 20 mg/kg per day) administration. (E) Cytokine microarray revealed a significant reduction in pro-inflammatory and mast cell–mediating cytokines IL-1ß, IL-5, IL-6, IL-10, IL-12, IL-17, IFN-γ, and TNFα in skin releasate of female PEA-treated HbSS mice compared to female vehicle-treated HbSS mice. (F) Overnight treatment of MCs from HbSS mouse skin with TNF-α (1 ng/mL) and hemin (40 μM) increased MCET formation, indicated by explosion of nuclear content observed with Syto 13 and Sytox Orange staining of DNA. Mixed CB1 and CB2 agonist CP55,940 (30 μM) attenuated MCET formation. PEA treatment (10 and 30 μM) dose-dependently reduced MCET formation. Mean ± SD. In panels A-D, data were analyzed using 2-way ANOVA and the Tukey post hoc multiple comparisons test. In panel E, data were analyzed using unpaired Student 2-tailed t test. In panel F, scale bar = 40 μm. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Age: 3.5 to 5.0 months. For panels A-D, n = 4-6; panel E, n = 9 per condition. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; MCP1, monocyte chemoattractant protein; MIP-1α, macrophage inflammatory protein-1; RANTES, regulated on activation normal T-cell expressed and secreted protein; TB, toluidine blue.
PEA ameliorates acute hyperalgesia in HbSS mice
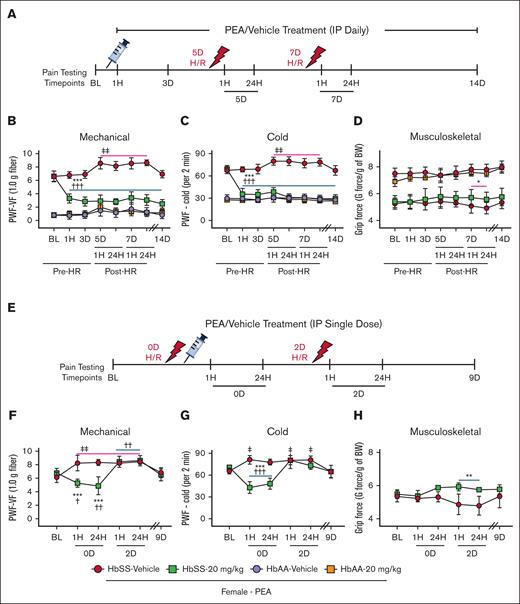
In addition to chronic pain, SCD may present with bouts of severe acute pain and vaso-occlusive crises (VOCs).36 Incitement of H/R causes acute hyperalgesia in sickle cell mice, which can model acute VOC pain.11 Mice were pretreated with PEA for 5 days before incitement of H/R. After 48 hours of H/R, a second H/R was incited (Figure 6A). PEA treatment (20 mg/kg per day) was continued through pre-H/R and post-H/R for a total of 14 days (Figure 6A). Incitement of H/R significantly exacerbated mechanical and cold hyperalgesia in female HbSS mice, compared with baseline (P < .01 vs baseline; Figure 6B-C). Pretreatment with PEA blocks H/R-evoked hyperalgesia and maintains effectiveness throughout the 14-day treatment period (P < .001 for both vs baseline and vehicle; Figure 6B-C). After the second incitement of H/R on day 7, PEA-treated female HbSS mice show significantly greater grip force, indicative of reduced musculoskeletal hyperalgesia, compared with vehicle-treated HbSS mice (P < .05; Figure 6D). Typically, people with SCD do not receive treatment until presenting with pain in the emergency department. To replicate this situation, HbSS mice were treated with a single dose of PEA after incitement of H/R (Figure 6E). PEA treatment in female HbSS mice significantly reduced mechanical and cold hyperalgesia when given immediately after first incitement of H/R compared with vehicle-treated HbSS mice (P < .001; Figure 6F-G), although this effect did not persist after second incitement of H/R, for which no treatment was given, suggesting that PEA is required as a continuous treatment regimen to combat bouts of acute episodic pain. The single dose of PEA maintained improved grip force after the second incitement of H/R compared with vehicle treatment, suggesting a disease-modifying effect on nonevoked, spontaneous pain that persists (P < .01; Figure 6H). Together, these data suggest that PEA treatment may have a preventive effect on acute pain and may also reduce acute pain once it has been evoked.
PEA administration ameliorates acute hyperalgesia in HbSS mice. (A) Pretreatment schema: mice are treated with PEA (IP, 20 mg/kg per day) for 14 days; H/R, hypoxia (3 hours at 8% O2) followed by reoxygenation (1 hour at ∼21% O2, normoxia), is incited on days 5 and 7; and hyperalgesia testing is performed at BL, 1 hour and 72 hours after start of daily treatment, 1 hour and 24 hours after first and second incitement of H/R, and finally on day 14 after start of treatment. (B-C) PEA treatment significantly reduces mechanical and cold hyperalgesia and prevents H/R-induced acute hyperalgesia, which is significantly increased in Veh-treated HbSS mice compared with BL. (D) PEA-treated HbSS mice also show increased grip force suggestive of less musculoskeletal hyperalgesia after second incitement of H/R compared with Veh treatment. (E) H/R-responsive treatment schema: mice are treated with a single dose of PEA (IP, 20 mg/kg) immediately after first incitement of H/R; H/R is incited on days 0 and 2; and hyperalgesia testing is performed at BL, 1 hour and 24 hours after first and second incitement of H/R, and finally on day 9 after start of experiment. (F-G) PEA treatment significantly reduces H/R-evoked mechanical and cold hyperalgesia 1 hour and 24 hours after incitement compared with Veh treatment and BL, however the second incitement of H/R significantly increases mechanical and cold hyperalgesia compared with BL regardless of treatment. (H) PEA treatment also shows increased grip force suggestive of less musculoskeletal hyperalgesia after second incitement of H/R in sickle cell mice compared with Veh treatment. Mean ± SD. In panels B-D,F-H, data were analyzed using repeated measures 2-way ANOVA and the Tukey post hoc multiple comparisons test. ∗Indicates difference compared with time-matched Veh; †indicates difference compared with SS-PEA BL; ‡indicates difference compared with SS-Veh BL. ∗,†,‡P < .05; ∗∗,††,‡‡P < .01; ∗∗∗,†††P < .001. Age: 3.5 to 5.0 months. For panels B-D: PEA female, HbAA Veh, n = 9; HbAA 20 mg/kg, n = 6; HbSS Veh, n = 10; HbSS 20 mg/kg, n = 10; panels F-H: HbSS Veh, n = 5; HbSS 20 mg/kg, n = 6. BW, body weight; HR, hypoxia/reoxygenation; PWF, paw withdrawal frequency; VF, von Frey.
PEA administration ameliorates acute hyperalgesia in HbSS mice. (A) Pretreatment schema: mice are treated with PEA (IP, 20 mg/kg per day) for 14 days; H/R, hypoxia (3 hours at 8% O2) followed by reoxygenation (1 hour at ∼21% O2, normoxia), is incited on days 5 and 7; and hyperalgesia testing is performed at BL, 1 hour and 72 hours after start of daily treatment, 1 hour and 24 hours after first and second incitement of H/R, and finally on day 14 after start of treatment. (B-C) PEA treatment significantly reduces mechanical and cold hyperalgesia and prevents H/R-induced acute hyperalgesia, which is significantly increased in Veh-treated HbSS mice compared with BL. (D) PEA-treated HbSS mice also show increased grip force suggestive of less musculoskeletal hyperalgesia after second incitement of H/R compared with Veh treatment. (E) H/R-responsive treatment schema: mice are treated with a single dose of PEA (IP, 20 mg/kg) immediately after first incitement of H/R; H/R is incited on days 0 and 2; and hyperalgesia testing is performed at BL, 1 hour and 24 hours after first and second incitement of H/R, and finally on day 9 after start of experiment. (F-G) PEA treatment significantly reduces H/R-evoked mechanical and cold hyperalgesia 1 hour and 24 hours after incitement compared with Veh treatment and BL, however the second incitement of H/R significantly increases mechanical and cold hyperalgesia compared with BL regardless of treatment. (H) PEA treatment also shows increased grip force suggestive of less musculoskeletal hyperalgesia after second incitement of H/R in sickle cell mice compared with Veh treatment. Mean ± SD. In panels B-D,F-H, data were analyzed using repeated measures 2-way ANOVA and the Tukey post hoc multiple comparisons test. ∗Indicates difference compared with time-matched Veh; †indicates difference compared with SS-PEA BL; ‡indicates difference compared with SS-Veh BL. ∗,†,‡P < .05; ∗∗,††,‡‡P < .01; ∗∗∗,†††P < .001. Age: 3.5 to 5.0 months. For panels B-D: PEA female, HbAA Veh, n = 9; HbAA 20 mg/kg, n = 6; HbSS Veh, n = 10; HbSS 20 mg/kg, n = 10; panels F-H: HbSS Veh, n = 5; HbSS 20 mg/kg, n = 6. BW, body weight; HR, hypoxia/reoxygenation; PWF, paw withdrawal frequency; VF, von Frey.
Discussion
Clinical and preclinical evidence suggest that pain in SCD is multifactorial, involving neuropathy, inflammation, and nociception engaging both peripheral and central mechanisms including neural injury and/or chronic inflammation, oxidative stress, and MC activation. Current pain management in SCD largely relies on opioids, which remain suboptimal because of side effects. Cannabinoid use is hindered by its intoxicating properties, and cardiovascular risks. PEA, an endogenous paracannabinoid, shows promise in treating neuropathic pain and modulating MC activation. We demonstrate that PEA targets the central and peripheral mechanisms underlying hyperalgesia in humanized HbSS-BERK mice.
PEA levels are significantly reduced in the spinal cord of HbSS mice compared with HbAA controls, irrespective of sex. Although we also observed reductions in OEA and 2-LG levels, these changes were more modest and sex specific, leading us to focus primarily on PEA. Previous studies have reported decreased 2-AG levels in the DRG of HbSS mice but did not examine the central nervous system. Central sensitization is a key contributor to chronic pain in SCD.32,37 Notably, spinal cord PEA concentrations in HbAA mice (∼1 μM) approach the 50% effective concentration of PEA at PPAR-α (3 μM), whereas these levels are markedly lower in HbSS mice (∼0.4 μM), suggesting a potential loss of endogenous PEA-mediated protection in SCD. The serine hydrolase, fatty acid amide hydrolase, is responsible for breaking down FAEs and has a high affinity for AEA.38 In contrast, the cysteine hydrolase NAAA preferentially degrades PEA over other FAEs.17 NAAA inhibitors, such as ARN, have shown promise in treating neuropathic and inflammatory pain.17,39 In HbSS mice, ARN administration reduced hypersensitivity to mechanical and cold stimuli in a dose-dependent manner, indicating that enhancing PEA-mediated activity could be an effective strategy for mitigating chronic pain in SCD. Our findings suggest a dysregulation in the molecular mechanisms responsible for PEA production and degradation in SCD.
PEA has neuroprotective effects, likely through the inhibition of oxidative stress, reduction of proinflammatory cytokines, and promotion of neuronal repair. Mechanisms of neuronal repair are dysregulated in HbSS mice,40 and we observed increased ROCK activity suggesting enhanced neuronal pruning. Exposure of neuronal cells to a sickle microenvironment in vitro increased ROCK activity, which was ameliorated by either PEA or the neurite pruning inhibitor NEP1-40. Coadministration of PEA and NEP1-40 did not produce additive effects in vitro, implying either a shared pathway or a maximal therapeutic effect from either treatment alone. This improvement in neuronal health, observed in both spinal cord tissue and neuronal-like cells, was further supported by studies of primary DRG neurons from HbSS mice. PEA treatment reduced phospho-p38-MAPK nuclear translocation in these neurons, indicating a potential reduction in nociceptive signaling and central sensitization. The role of p38-MAPK in chronic hyperalgesia was confirmed through dose-dependent inhibition using Nef, a selective p38-MAPK inhibitor, marking the first evidence of p38-MAPK’s involvement in mechanical and thermal hyperalgesia in HbSS mice. Neuroimmune activity and neuronal health in the spinal cord may precede the onset of pain in SCD. Glial cell activation and oxidative stress have been observed in the spinal cord of HbSS mice.26,29 MDA, a marker of lipid peroxidation, is elevated in the spinal cords of HbSS mice compared with HbAA controls, and this elevation is mitigated by PEA treatment, which also reduces upstream proinflammatory and proapoptotic markers, IL-1β and caspase-3, respectively. PEA treatment was further shown to reduce p38-MAPK phosphorylation in vivo in the spinal cord and in vitro in DRG neurons, highlighting its potential to alleviate both peripheral and central nociceptive signaling and hyperalgesia.
MC activation, degranulation, and extracellular trap formation play crucial roles in SCD pain by generating proinflammatory signals that recruit immune cells and interact directly with blood vessels and nerve fibers.35,41,42 The sickle microenvironment, characterized by cell-free heme and inflammatory cytokines such as TNF-α, may drive MC activation, and PEA treatment mitigates these effects on MC degranulation and trap formation.43 This stabilization of MCs by PEA may be mediated through various PPAR subtypes (α, β, and γ) or CB2 receptors, which exhibit anti-inflammatory properties and modulate MC activity. Support for this is provided by a reduction in SAP, an acute phase protein that responds to injury and illness, thereby initiating the innate immune response. Skin releasate showed reduced levels of tryptase and MC-derived cytokines after PEA treatment. IL-6, a key driver in MC degranulation and neuropathic pain, was downregulated, suggesting that PEA may influence MC autocrine signaling.44 PEA inhibited the release of proinflammatory cytokines IL-1β, TNF-α, and IL-17, all of which are produced by activated MCs and contribute to neutrophil recruitment, inflammation and pain individually or in concert.45-48 Additionally, IL-5, which promotes eosinophil recruitment and activation, is also released by activated MCs. Interferon gamma acts on MCs to stimulate IL-17 production in a degranulation-independent manner, whereas IL-5 enhances MC-specific surface markers, increasing MC activity.49 Thus, PEA may act upstream, alongside its direct effects on MCs, to mediate inflammation. These data suggest that PEA may reduce MC activation in vivo through downregulation of proinflammatory cytokines released by, and around, MCs. These factors may work independently or in synchrony to elicit their effects, although concomitant release may be because of shared upstream pathways and exemplifies the need to examine the contribution of individual cytokines and chemokines in SCD pain.
The reduction of cold hypersensitivity after exogenous PEA administration is particularly promising, because it demonstrates beneficial effects on both evoked and nonevoked cold hyperalgesia, indicating sensory improvements. Additionally, the modest improvement observed in spontaneous musculoskeletal hyperalgesia suggests a potential disease-modifying effect. Currently, avascular necrosis is considered the primary source of joint pain and weakened grip strength in SCD, yet no effective treatments address this type of pain.50 CP55,940, a mixed CB1R and CB2R agonist, resulted in a similar degree of improvement in pain-like behaviors over a 2-week period in HbSS mice, suggesting that PEA can be a nonintoxicating alternative to cannabinoids (supplemental Figure 2).
Episodes of unpredictable debilitating acute VOC pain are unique to SCD, requiring hospitalization. Pretreatment with PEA before H/R-evoked acute pain prevents the onset of acute hyperalgesia, and a single dose of PEA after H/R significantly reduces acute hyperalgesia. Thus, PEA can have a preventive effect as well as an analgesic effect on acute hyperalgesia in sickle cell mice. PEA may be alleviating acute hyperalgesia vis a vis mechanisms involving acute nociception, neuroinflammation, and MC activation. Earlier studies from our laboratory showed that genetic deletion and pharmacologic blockade of MCs with imatinib attenuated H/R-evoked acute mechanical, thermal, and deep-tissue hyperalgesia.42 It is likely that the MC inhibition by PEA may underlie its inhibitory effect on acute hyperalgesia after H/R. The acute effects in DRG neurons suggest direct interactions between PEA and afferent nociceptive inputs (Figure 4), although our observations that PEA attenuates MC activation suggest tonic control of inflammation. Substance P, a vasoactive and neuroinflammatory peptide, is increased in persons with SCD as well as sickle cell mice.10,51 PEA has been demonstrated to ameliorate substance P–induced MC activation.43 VOC is associated with neutrophil extracellular traps and inflammatory cytokines.52 We have recently reported the impact of H/R challenge on MCET formation,35 and herein we observed an amelioration of MC traps with concomitant reduction in MC degranulation and tryptase release after PEA treatment. These antinociceptive and anti-inflammatory effects may exist simultaneously to reduce acute painful neurotransmission.
PEA has been shown to prevent anoxia-ischemia induced cognitive impairment in neonatal rats by reducing neuroinflammation and astrogliosis.53 As demonstrated in Figure 2, PEA inhibits inflammation in the central nervous system and the periphery. It is noteworthy that circulating glial fibrillary acidic protein (GFAP) levels are significantly higher in children with SCD than healthy children. Additionally, SCD children with acute brain ischemia had an approximate twofold elevated plasma GFAP than children with SCD with normal magnetic resonance imaging.54 GFAP is a marker of astroglial activation and is also increased in the spinal cords of sickle cell mice.26 Thus, PEA may be inhibiting astroglial activation, neuroinflammation, and inflammation that may underlie acute and chronic hyperalgesia concomitant with MC activation in SCD. These data suggest that PEA is effective both as a preventive measure and in response to acute pain associated with SCD.
Clinical studies support analgesic effects of PEA in chronic neuropathic pain, fibromyalgia, and chronic pelvic pain.55,56 PEA-treated HbSS mice sustained reductions in hyperalgesia for 14 days. These findings complement the clinical evaluation of PEA's safety across various disease states, in which both topical and oral administration did not cause irritation, sensitization, or adverse effects.55,57 PEA has been assessed for potential teratogenic effects in preclinical models, showing no toxicity to dams, embryos, or fetuses, even at doses up to 1000 mg/kg in Wistar rats (equivalent to >9.7 g/dL in humans).58 This is particularly significant for individuals with SCD, because pregnancy precludes hydroxyurea treatment.59 The rapid and sustained reduction in hyperalgesia after PEA treatment suggests involvement of both neural and immune mechanisms, supported by preclinical and clinical studies of PEA in chronic diseases and acute injury models.
In conclusion, we provide evidence of antihyperalgesic activity of PEA enhancement or supplementation and a novel role for p38-MAPK in chronic hyperalgesia in transgenic humanized sickle cell mice. These findings are further supported by experimentation into the neuroprotective and anti-inflammatory basis of ameliorating hyperalgesia with PEA. Future studies using a greater dose range of PEA or as an adjuvant treatment to currently approved analgesics will provide insight for its full potential as a pain-relieving and disease-modifying agent, as well as inform any potential risks for tolerance and adverse effects, both of which are absent at the current doses. This study is limited by its evaluation of mice and cells. The need for safe, effective methods to address pain in SCD is paramount, given the recent withdrawal of US Food and Drug Administration–approved voxelotor, because of its risks vastly outweighing its benefits.21 Evaluation of PEA as a treatment has not been performed in a clinical SCD cohort, but these results may inform future prospective clinical trials to assess this promising compound.
Acknowledgments
The authors acknowledge the technical support provided by Nia Sanchez, Natalie Garcia, and Julia Nguyen. The authors thank Stacy Kiven and Reina Lomeli for assistance with mice breeding; Graham J Velasco for skin sectioning; Mya Arellano for data organization; and Joni Ricks-Oddie at the University of California, Irvine Institute for Clinical and Translational Science for statistical support.
This work has been supported by the University of California President’s Postdoctoral Fellowship (D.A.A.), an A.P. Giannini Foundation Fellowship (D.A.A.), the National Institutes of Health (NIH) grants from the following institutes: National Center for Complementary and Integrative Health (NCCIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Heart, Lung, and Blood Institute (NHLBI), National Cancer Institute (NCI), and National Institute of Biomedical Imaging and Bioengineering (NIBIB) (NCCIH K99 AT012494 [D.A.A.]; NIDDK R01 DK119498 [N.V.D.]; and NHLBI and NCI RO1s HL147562, CA263806, NCCIH U24 AT012868, and NIBIB U18 EB029354 [K.G.]), and a Susan Samueli Scholar Award (K.G.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: D.A.A. and K.G. conceptualized the study; D.A.A., Y.G., D.P., N.V.D., and K.G. developed the methodology; D.A.A., B.A., Y.G., H.T., and Y.F. performed the investigation; D.A.A., Y.G., and K.G. were responsible for visualization; D.A.A., D.P., N.V.D., and K.G. acquired funding; K.G. was responsible for project administration; D.P., N.V.D., and K.G. supervised the study; D.A. and K.G. wrote the original draft; and D.A., D.P., N.V.D., and K.G. reviewed and edited the manuscript.
Conflict-of-interest disclosure: K.G. reports research grants from Novartis, Grifols, Zilker LLC, and University of California, Irvine (UCI) Foundation, not related to this work. The remaining authors declare no competing financial interests.
Correspondence: Kalpna Gupta, Division of Hematology/Oncology, Department of Medicine, University of California, Irvine Medical Center, 200 S Manchester Ave, Suite 400, Room 414, Zot 4061, Orange, CA 92868; email: kalpnag@hs.uci.edu.
References
Author notes
All data are included in the article and/or supporting information. Data are available on request from the corresponding author, Kalpna Gupta (kalpnag@hs.uci.edu).
The full-text version of this article contains a data supplement.