Key Points
RHOA G17V enhances CD3/CD28-induced NFAT transcriptional activity by binding p300 and enhancing its histone acetyltransferase activity.
RHOA G17V, a mutation altering the function of a signaling molecule, has epigenetics effects.
Visual Abstract
The RHOA G17V mutation is highly recurrent in T follicular helper (TFH) cell lymphoma of the angioimmunoblastic type (AITL; 60%-70% of cases) and frequently associated with mutations in other T-cell receptor signaling genes, including CD28. Here, we sought to elucidate how RHOA and CD28 variants may work in concert to sustain T-cell activation by generating stable Jurkat T-cell lines expressing wild-type (wt) RHOA or RHOA G17V with wt CD28 or CD28 T195P. Concomitant expression of RHOA G17V and CD28 T195P induced significantly higher levels of interleukin-2 (IL-2) production and NFAT nuclear factor of activated T cells (NFAT) and activator protein 1 (AP1) transcriptional activities than either variant alone upon T-cell activation with agonistic anti-CD3 and anti-CD28 antibodies. We identified the histone acetyltransferase p300 as a major interacting partner of RHOA G17V in our model and human primary T cells. p300 inhibition abolished the increased IL-2 secretion induced by CD3/CD28 stimulation in cells expressing RHOA G17V and/or CD28 T195P. Chromatin immunoprecipitations and immunofluorescence staining revealed an increase of p300-specific H3K18ac and H3K27ac marks at the IL-2 promoter and across whole genome, respectively, in cells expressing RHOA G17V. Finally, immunofluorescence staining of tumor samples from 4 patients with AITL carrying RHOA G17V variant and 4 carrying wt RHOA showed that neoplastic TFH cells with RHOA G17V have increased H3K18ac and H3K27ac levels compared with non-neoplastic T cells. Collectively, these findings uncover a new mechanism of action by which RHOA G17V potentiates CD28 T195P-induced NFAT and AP1 transcriptional activities by enhancing p300 histone acetyltransferase activity and expand the notion that epigenetic deregulation contributes to the pathogenesis of TFH lymphomas.
Introduction
Follicular helper T-cell lymphoma of angioimmunoblastic type (AITL), one of the most common peripheral T-cell lymphomas (PTCLs),1 comprises CD4+ neoplastic T cells with a T follicular helper (TFH) immunophenotype and an abundant reactive microenvironment.2 In addition, other PTCLs exhibiting a TFH immunophenotype and overlapping characteristics with AITL are considered part of the same disease spectrum.3-5 Prognosis of AITL remains poor, with a 5-year overall survival of ∼30%.6,7
Recurrent mutations in epigenetic regulators TET2, DNMT3A, and IDH2 are detected in TFH lymphomas8,9 but are unlikely sufficient to drive lymphomagenesis.10,11 Highly recurrent RHOA G17V mutation is detected in up to 70% of AITLs.12-14,RHOA mutations often co-occur with TET2 mutations, are detected at lower variant allelic frequencies, and represent a secondary event in multistep TFH lymphomagenesis.15-18 RHOA, a small guanosine triphosphatase (GTPase) activated downstream of T-cell receptor (TCR) engagement in mature T cells, is involved in cytoskeleton reorganization after T-cell activation.12,13 RHOA oscillates between an active GTP-bound state and an inactive guanosine diphosphate–bound state. Several studies provided evidence that RHOA G17V cannot bind GTP and has a dominant-negative effect on GTPase function of the endogenous protein,12,14,19-21 but how RHOA G17V contributes to lymphomagenesis remains controversial. Fujisawa et al showed that RHOA G17V binding to VAV1 enhances VAV1 adapter function, leading to increased TCR signaling.22 Mice engineered to express RHOA G17V in CD4+ cells in a TET2-deficient background develop T-cell lymphomas with AITL features.23-25 Moreover, these mice show polarization of CD4+ T cells toward TFH-cell phenotype and increased PI3K-AKT-mTOR pathway activity. In a model of adoptive transfer of murine TET2–/– T cells retrovirally transduced with RHOA wild type (wt) or G17V, TET2 loss and RHOA G17V synergize to modulate FoxO1 activity.25 More recently, and somewhat in opposition to previous studies, RHOA G17V alone, expressed under the LCK promoter in murine CD4+ T cells, induced TFH-cell specification and AITL-like lymphoma characterized by NF-κB pathway activation and subsequent increase of PON2 expression.26 Thus, RHOA G17V promotes T-cell activation, TFH specification, and lymphomagenesis, but a comprehensive understanding of the molecular target of RHOA G17V in AITL development is missing.
Accumulating evidence indicates that mutation-induced activation of TCR signaling is a common oncogenic mechanism in PTCLs, particularly in those derived from TFH cells. Activating mutations or gene fusions of costimulatory/TCR signaling genes such as CD28 or FYN were reported in approximately half of TFH lymphomas.12,19,20,22,27-32 In TFH lymphomas, besides RHOA G17V, CD28 is the most altered TCR-related gene (20/134 [15%]).32,CD28 alterations comprised mutually exclusive activating point mutations33 (T195P, D124D, and D124E in 11/134 cases [8.2%]) and fusions (9/134 [6.7%]).19,32,34,35 Notably, others14,31,33 and we32 observed co-occurrence of CD28 point mutations and RHOA G17V.
Here, we report that RHOA and CD28 variants work in concert to sustain T-cell activation by promoting p300-dependent histone acetylation and show that in lymphomas biopsies, neoplastic TFH cells carrying the RHOA G17V variant have increased H3K18ac and H3K27ac levels.
Methods
Details on antibodies, reagents, plasmids, quantitative polymerase chain reaction (qPCR) primers, cell lines, and assays used in this study are provided in supplemental Material. Mouse anti-human CD3 plus mouse anti-human CD28 and crosslinker (goat anti-mouse immunoglobulin G) stimulation will hereafter be called CD3/CD28 costimulation.
RHOA interactome and validation by coimmunoprecipitation
Jurkat cells stably transduced with empty vector, RHOA wt, or RHOA variants (G17V or K18N) were used to uncover RHOA interacting partners. Jurkat cells stably transduced with empty vectors or expressing wt or variants of CD28 and RHOA were used to confirm selected interactions with RHOA G17V.
qPCR
After serum starvation, 2 × 106 Jurkat cells expressing indicated forms of CD28 and RHOA were CD3/CD28 costimulated in OpTmizer for 0, 2, 4, 6, 8, and 10 hours at 37°C. RNA was extracted using RNeasy plus mini kit and quantified using Qubit. One microgram was used to perform retro-transcription with the first strand complementary DNA synthesis kit with random primers. Target gene expression levels were quantified using the TaqMan Fast Advanced Master Mix and TaqMan designed probes on a QuantStudio 5 machine.
ChIP-qPCR
Approximately 7 × 106 Jurkat cells expressing indicated forms of CD28 and RHOA were CD3/CD28 costimulated in OpTmizer for 4 hours at 37°C. Cells were crosslinked using 1% formaldehyde for 8 minutes and quenched in 125-mM glycine for 5 minutes. After washing with cold phosphate-buffered saline, cells were processed with iDeal chromatin immunoprecipitation (ChIP)–qPCR kit. Sonication was performed in a final volume of 700 μL on ice using a Branson digital sonifier for 3 minutes and 45 seconds (amplitude 37 %, pulse on 0.7 second, and pulse off 1.3 second). Chromatin shearing was assessed on 1.5 % agarose gel after ribonuclease (RNAse) treatment, reverse crosslinking, proteinase K treatment, and DNA isolation using QIAquick PCR purification kit. Ideal size was considered between 500 bp and 100 bp. Chromatin was incubated at 4°C under constant agitation overnight with antibodies linked to magnetic beads. Two percent of material used per ChIP was kept aside as input. Immunoprecipitated chromatin was analyzed by qPCR (primer pairs detailed in supplemental Table 1) using PowerUp SYBR green master mix on a QuantStudio 5 machine.
Isolation and electroporation of human primary T cells
Blood samples from healthy, informed, and consenting human donors were obtained from Interregional Blood Transfusion SRC Ltd (Epalinges, Switzerland). Peripheral blood mononuclear cells were isolated by gradient method using Ficoll-Paque Plus. Primary T cells were isolated using Pan T Cell Isolation kit. Untouched T cells were grown in OpTmizer enriched with 50 μg/mL of recombinant interleukin-2 (IL-2). Seventy-two hours before electroporation, 30 × 106 T cells were stimulated with Dynabeads human T-activator CD3/CD28. Cells were electroporated using Neon Transfection System 100 μL kit and Neon transfection machine with following protocol: 1600 volts, 10 milliseconds, and 3 pulses with pCMV6-RHOA-wt-HA or pCMV6-RHOA-G17V-HA vectors.
Results
RHOA G17V and CD28 T195P synergistically promote IL-2 secretion upon CD3/CD28 stimulation
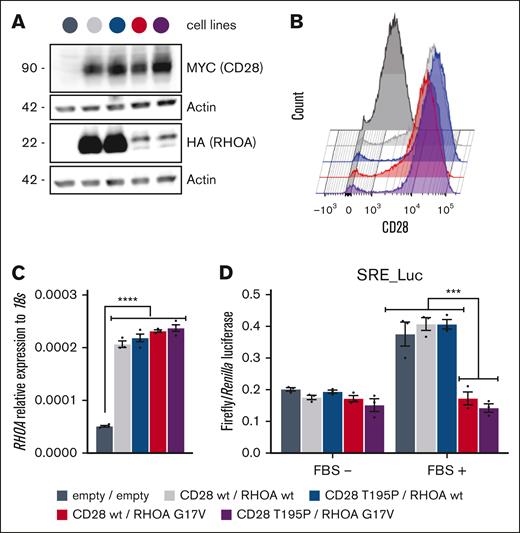
Five stable Jurkat T-cell lines bearing the 2 empty vectors or expressing either RHOA wt or its G17V mutant, in combination with CD28 wt or T195P mutant, were generated by sequential lentiviral infections. Transduced cells were selected by positive fluorescence-activated cell sorting for green fluorescent protein (RHOA expression) and CD28 expression at plasma membrane (supplemental Figure 1). CD28 T195P protein levels were slightly higher than those of CD28 wt, and cell surface levels of exogenous CD28 constructs were considerably higher than endogenous CD28, which is poorly expressed in the Jurkat clone E6-1 (Figure 1A-B). As previously reported,14,19,22 protein expression of RHOA G17V was much lower than wt form (Figure 1A), although all ectopic RHOA-expressing cell lines showed similar levels of RHOA messenger RNA by real-time qPCR (Figure 1C). RHOA G17V being characterized as a dominant negative for its GTPase activity,12-14,19 we confirmed that RHOA G17V–expressing cells showed inhibited serum–induced expression of luciferase reporter gene transcription driven by serum-responsive element (Figure 1D).
Characterization of the Jurkat cell lines used in this study. (A) Representative western blot from the 5 modified Jurkat cell lines. Expression of ectopic Myc-tagged CD28 and HA-tagged RHOA was revealed by anti-Myc and anti-HA, respectively. Anti-actin blotting serves as a loading control. Positions of molecular weight markers are indicated in kilodalton. (B) Representative flow cytometry analysis of the cell lines showing CD28 expression level at the plasma membrane. Expression levels of CD28 T195P were slightly higher than those of CD28 wt. (C) Relative RHOA expression measured by qPCR in all 5 cell lines. The 4 cell lines expressing exogenous RHOA showed similar levels of RHOA expression. Data are expressed as mean ± standard error of the mean (SEM) of 3 independent experiments. ∗∗∗∗P < 0.0001. (D) SRE luciferase reporter assay monitoring the activity of RHOA wt, compared with G17V mutant, which was previously characterized as dominant negative. Cells were stimulated or not with FBS for 6 hours. Data are represented as mean ± SEM from 3 independent experiments. Significant differences in activation were determined using 2-way analysis of variance (ANOVA) with Tukey multiple comparison test (∗∗∗P ≤ .001, compared with cells expressing RHOA wt). The color code used for the 5 Jurkat cell lines generated for this study is indicated at the bottom of the figure. FBS, fetal bovine serum; SRE, serum-responsive element.
Characterization of the Jurkat cell lines used in this study. (A) Representative western blot from the 5 modified Jurkat cell lines. Expression of ectopic Myc-tagged CD28 and HA-tagged RHOA was revealed by anti-Myc and anti-HA, respectively. Anti-actin blotting serves as a loading control. Positions of molecular weight markers are indicated in kilodalton. (B) Representative flow cytometry analysis of the cell lines showing CD28 expression level at the plasma membrane. Expression levels of CD28 T195P were slightly higher than those of CD28 wt. (C) Relative RHOA expression measured by qPCR in all 5 cell lines. The 4 cell lines expressing exogenous RHOA showed similar levels of RHOA expression. Data are expressed as mean ± standard error of the mean (SEM) of 3 independent experiments. ∗∗∗∗P < 0.0001. (D) SRE luciferase reporter assay monitoring the activity of RHOA wt, compared with G17V mutant, which was previously characterized as dominant negative. Cells were stimulated or not with FBS for 6 hours. Data are represented as mean ± SEM from 3 independent experiments. Significant differences in activation were determined using 2-way analysis of variance (ANOVA) with Tukey multiple comparison test (∗∗∗P ≤ .001, compared with cells expressing RHOA wt). The color code used for the 5 Jurkat cell lines generated for this study is indicated at the bottom of the figure. FBS, fetal bovine serum; SRE, serum-responsive element.
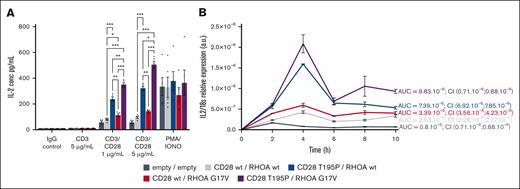
We monitored IL-2 secretion in cells stimulated either with immunoglobulin G (negative control), phorbol myristate acetate (PMA)/ionomycin (positive control), or a combination of agonistic anti-CD3 and anti-CD28 antibodies (Figure 2A). Cells harboring CD28 T195P showed significantly increased IL-2 secretion in response to CD3/CD28 costimulation compared with cells expressing CD28 wt. RHOA G17V alone induced a slight increase in IL-2 secretion, whereas the combined presence of RHOA G17V and CD28 T195P induced significantly higher levels of IL-2 secretion than either mutant alone upon CD3/CD28 stimulation (Figure 2A). Observed effects were more than additive, suggesting that RHOA G17V and CD28 T195P synergize to activate IL-2 transcription. PMA/ionomycin induced similar increase in IL-2 secretion across all cell lines, suggesting a possible role for the calcium-sensitive NFAT transcription factor downstream of CD28 and RHOA variants. Indeed, PMA/ionomycin directly activates protein kinase theta (PKCθ) and calcium release from endoplasmic reticulum, thereby mimicking strong T-cell activation.36,37 We then followed IL-2 messenger RNA levels between 0 hour and 10 hour after CD3/CD28 costimulation (Figure 2B). Cells with empty vectors peaked at 2 hours after stimulation, whereas cells expressing exogenous wt CD28 and wt RHOA peaked at 4 hours, showing significantly higher IL-2 expression. RHOA G17V mildly and CD28 T195P strongly further increased IL-2 expression, and the combination of both variants induced significantly higher and more persistent levels of IL-2 expression than the respective wt/mutant combinations (Figure 2B). These results suggested CD28 and RHOA variants affect IL-2 secretion through the control of IL-2 transcription.
CD28 and RHOA mutants synergize in promoting IL-2 transcription and IL-2 secretion. (A) In vitro secretion of IL-2. A total of 200 000 Jurkat cells per well, transduced with the lentiviruses indicated by the color code, were seeded and stimulated with either immunoglobulin G (IgG) control (5 μg/mL); anti-CD3 (5 μg/mL) with crosslinker (5 μg/mL); anti-CD3 and anti-CD28 and crosslinker (1 μg/mL or 5 μg/mL); or with PMA (20 ng/mL) and IONO (1 μM). Twenty-four hours later, supernatants were collected, and IL-2 was measured by enzyme-linked immunosorbent assay. Data are represented as mean ± SEM from 5 independent experiments conducted in quadruplicates. Significant differences in IL-2 secretion were determined using 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗∗P ≤ .01; ∗∗∗P ≤ .001). (B) Time course of IL-2 expression. A total of 2 × 106 Jurkat cells per well bearing the indicated lentiviruses were seeded and stimulated with anti-CD3, anti-CD28, and crosslinker at 5 μg/mL during indicated periods of time. RNAs were extracted, and reverse transcription was performed. qPCR was performed using TaqMan reagents and a QuantStudio 5 machine. Data are presented as mean ± SEM from 3 independent experiments. AUCs and 95% CIs are indicated. AUC, area under the curve; CI, confidence interval; IONO, ionomycin.
CD28 and RHOA mutants synergize in promoting IL-2 transcription and IL-2 secretion. (A) In vitro secretion of IL-2. A total of 200 000 Jurkat cells per well, transduced with the lentiviruses indicated by the color code, were seeded and stimulated with either immunoglobulin G (IgG) control (5 μg/mL); anti-CD3 (5 μg/mL) with crosslinker (5 μg/mL); anti-CD3 and anti-CD28 and crosslinker (1 μg/mL or 5 μg/mL); or with PMA (20 ng/mL) and IONO (1 μM). Twenty-four hours later, supernatants were collected, and IL-2 was measured by enzyme-linked immunosorbent assay. Data are represented as mean ± SEM from 5 independent experiments conducted in quadruplicates. Significant differences in IL-2 secretion were determined using 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗∗P ≤ .01; ∗∗∗P ≤ .001). (B) Time course of IL-2 expression. A total of 2 × 106 Jurkat cells per well bearing the indicated lentiviruses were seeded and stimulated with anti-CD3, anti-CD28, and crosslinker at 5 μg/mL during indicated periods of time. RNAs were extracted, and reverse transcription was performed. qPCR was performed using TaqMan reagents and a QuantStudio 5 machine. Data are presented as mean ± SEM from 3 independent experiments. AUCs and 95% CIs are indicated. AUC, area under the curve; CI, confidence interval; IONO, ionomycin.
RHOA G17V and CD28 T195P enhance T-cell activation by distinct means
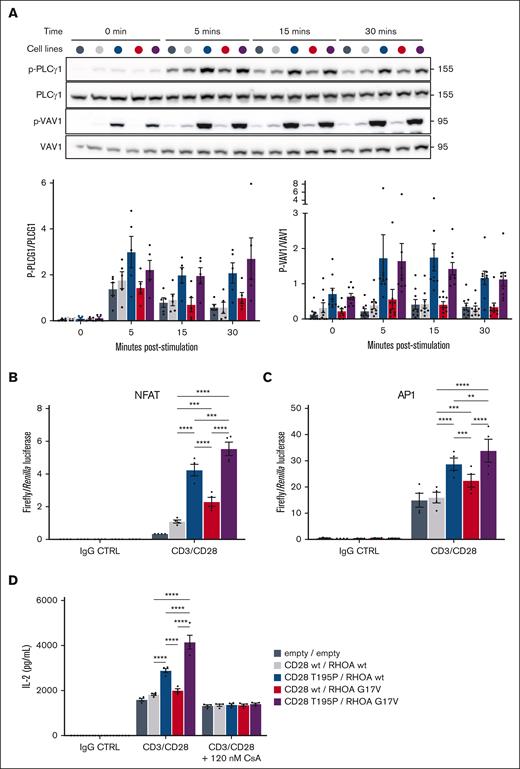
CD28 is a major costimulatory receptor in promoting full T-cell activation in the presence of TCR engagement.38-40 TCR/CD28 activation triggers increased Ca2+ fluxes and a cascade of phosphorylation events resulting in activation of transcription factors.40 RHOA G17V reportedly increases VAV1 adapter function through phosphorylation.22 Thus, we first sought for changes in the phosphorylation levels of key cytoplasmic transducers of CD28/TCR signaling pathways, as well as Ca2+ fluxes, after CD3/CD28 costimulation. Cells were serum starved overnight and CD3/CD28 costimulated for 0, 5, 15, and 30 minutes to assess phosphoinositide 3-kinase (PI3K), NF-κB/NFAT, and activator protein 1 (AP1) pathway activation by measuring the phosphorylation levels of S6K (target of mTOR), phospholipase C gamma 1 (PLCγ1) and IκBα, and JNK and extracellular signal–regulated kinase, respectively. We also assessed VAV1 phosphorylation, a proximal signaling event in NF-κB/NFAT and AP1 pathways, by western blot (Figure 3A; supplemental Table 3; supplemental Figure 2A). Jurkat cells are PTEN deficient, therefore S6K was uniformly phosphorylated in all stimulated conditions (supplementary Figure 2A). For all other substrates, phosphorylation was maximal at 5 minutes and decreased at 30 minutes. Because the phosphorylation patterns of IκBα, JNK, and extracellular signal–regulated kinase were similar to those of VAV1 and PLCγ1, we subsequently focused on the latter 2 (Figure 3A). CD3/CD28 costimulation induced PLCγ1 and VAV1 phosphorylation at each time point in all cell lines compared with unstimulated cells. Of note, CD28 T195P significantly increased PLCγ1 and VAV1 phosphorylation levels compared with CD28 wt. This increase was still detectable at 30 minutes after stimulation. In contrast, RHOA G17V did not increase proteins’ phosphorylation compared with cells harboring CD28 and RHOA wt forms. Unlike what we observed for IL-2 secretion and IL-2 transcription, the combination of CD28 T195P and RHOA G17V did not significantly increase the phosphorylation of signaling proteins of interest compared with CD28 mutant alone after costimulation. Besides, no significant difference was observed in Ca2+ flux after stimulation of cells with ectopic expression of CD28 and RHOA variants (supplemental Figure 2B). These results suggested that CD28 T195P enhances the phosphorylation of TCR transducers after CD3/CD28 costimulation, and RHOA G17V may act downstream of these, at the transcriptional level,41 to produce increased IL-2 levels observed when both CD28 and RHOA variants are present.
RHOA G17V and CD28 T195P synergize in promoting NFAT and AP1 transcriptional activity upon CD3/CD28 stimulation. (A) Representative western blot and densitometry quantification of PLCγ1 and VAV1 phosphorylation from cell lines stimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL during the indicated periods of time before protein extraction. The CD28 activating mutant, but not RHOA G17V, induced increased phosphorylation of PLCγ1 and VAV1. Total PLCγ1 and VAV1 served as controls (CTRLs) for the quantification of the phosphorylated forms. Positions of molecular weight markers are indicated in kilodalton. (B-C) Luciferase reporter assays monitoring the activities of NFAT (B) and AP1 (C) upon stimulation of cell lines either with IgG CTRL or anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 hour and 30 minutes. Data are represented as mean ± SEM from 4 to 5 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (D) In vitro secretion of IL-2 upon costimulation with anti-CD3, anti-CD28 and crosslinker at 2 μg/mL with or without a small dose of CsA (120 nM) for 24 hours. Data are represented as mean ± SEM from 4 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). Statistics not shown are indicated in the Supplemental Table 3.
RHOA G17V and CD28 T195P synergize in promoting NFAT and AP1 transcriptional activity upon CD3/CD28 stimulation. (A) Representative western blot and densitometry quantification of PLCγ1 and VAV1 phosphorylation from cell lines stimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL during the indicated periods of time before protein extraction. The CD28 activating mutant, but not RHOA G17V, induced increased phosphorylation of PLCγ1 and VAV1. Total PLCγ1 and VAV1 served as controls (CTRLs) for the quantification of the phosphorylated forms. Positions of molecular weight markers are indicated in kilodalton. (B-C) Luciferase reporter assays monitoring the activities of NFAT (B) and AP1 (C) upon stimulation of cell lines either with IgG CTRL or anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 hour and 30 minutes. Data are represented as mean ± SEM from 4 to 5 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (D) In vitro secretion of IL-2 upon costimulation with anti-CD3, anti-CD28 and crosslinker at 2 μg/mL with or without a small dose of CsA (120 nM) for 24 hours. Data are represented as mean ± SEM from 4 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). Statistics not shown are indicated in the Supplemental Table 3.
NFAT, AP1, and NF-κB being the major transcription factors involved in IL-2 transcription,42,43 we looked for changes in their transcriptional activities 5 hours after CD3/CD28 costimulation (Figure 3B-D). RHOA or CD28 variants expressed together or in combination with the RHOA or CD28 wt forms increased NF-κB transcriptional activity to similar levels (supplemental Figure 3A), suggesting that synergy is not achieved at the level of NF-κB. Meanwhile, CD28 T195P significantly increased the transcriptional activities of NFAT and AP1 compared with wt RHOA or CD28 cells. RHOA G17V also significantly increased, to a lesser extent, transcriptional activities of NFAT and AP1. Presence of both variants significantly increased NFAT and, to a lesser extent, AP1 transcriptional activities compared with mutants alone. These results suggest RHOA G17V promoted IL-2 transcription through modulation of NFAT and AP1 transcriptional activities without affecting the phosphorylation levels of TCR pathway signal transducers.
We measured IL-2 secretion by our cells after CD3/CD28 costimulation with or without cyclosporin A (CsA; calcineurin inhibitor) at a dose that does not blunt IL-2 secretion completely (Figure 3D). CsA abolished the differences in IL-2 secretion between cells expressing RHOA and CD28 wt forms and those expressing variants alone or combinations thereof (Figure 3D). Similar results were obtained blocking AP1 activity with the combination of 2 drug compounds inhibiting JNK (SP600125) and c-Fos (T-5224) activities (supplemental Figure 3B). These data suggest that the effects of CD28 and RHOA mutants on promoting IL-2 secretion upon CD3/CD28 stimulation hinged on calcium-dependent NFAT and AP1 activation.
RHOA G17V interacts with p300 in our Jurkat T-cell model and in human primary T cells
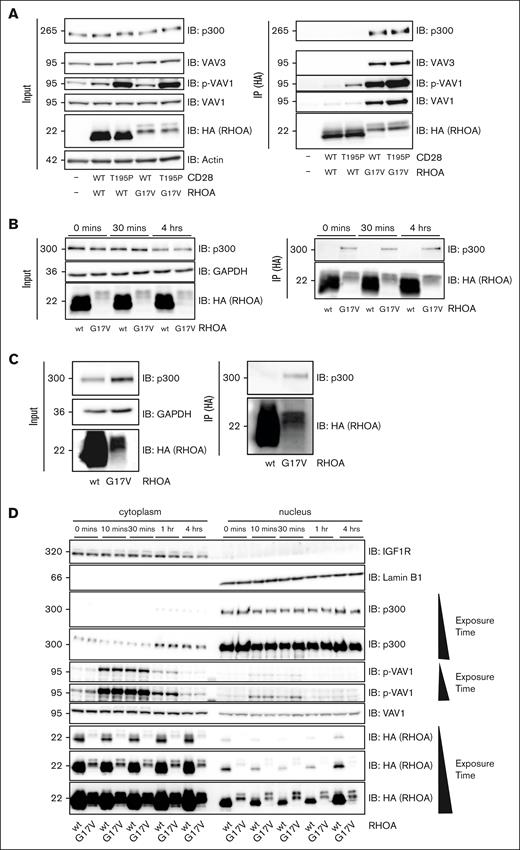
To understand how RHOA G17V modulates NFAT and AP1 activities without affecting the phosphorylation of key TCR transducers, we performed immunoprecipitations of ectopic RHOA followed by mass spectrometry to identify binding partners (supplemental Figure 4). Jurkat cells were transduced by lentiviruses expressing hemagglutinin (HA)-tagged RHOA, either wt, G17V or K18N, an activating form previously described in few patients with AITL.19 Cells were costimulated with agonistic anti-CD3/CD28 antibodies for 5 minutes before anti-HA immunoprecipitation. Identified partner proteins were categorized according to their specific interaction with RHOA wt (n = 18 candidates), G17V (n = 16 candidates), K18N (n = 3 candidates), or both mutants (n = 9 candidates) and ranked by decreased spectral counts (supplemental Table 2). As expected, no RHOA protein was found in immunoprecipitates (IPs) from cells expressing the empty vector. Moreover, RHOA wt, G17V, or K18N proteins were found in each corresponding IP from cells expressing RHOA wt, G17V, or K18N (upper part of the supplemental Table 2). We found expected binding partners of RHOA, including regulators guanine nucleotide exchange factor (GEF) proteins [ARHGEF1 and ARHGEF11]; inhibitor [RIPOR2]; and other [DAAM1]) and effectors (ROCK1, DIAPH1, and PNK2). We confirmed the previously reported interaction of RHOA G17V with VAV1 and, to a lesser extent, PLCγ1.22 We noticed that the wt and K18N forms of RHOA were also binding but at lower level to VAV1 (6 counts), suggesting a lower affinity. Furthermore, RHOA G17V and K18N were binding VAV3, with more counts for G17V (69) than for K18N (21). Interestingly, we identified the epigenetic modifier p300 (E1A-binding protein p300, also known as Ep300), a histone acetyltransferase (HAT), as a specific RHOA G17V binding partner in CD3/CD28-stimulated cells with 27 spectral counts. p300 was neither present in the negative control (empty IP) nor in the IPs of RHOA wt or K18N. We sought to confirm the interactions of RHOA G17V with p300, VAV1, and VAV3 by coimmunoprecipitation and western blot analysis using cells harboring empty vectors or CD28 and RHOA wt or variant forms (Figure 4). Both p300 and VAV3 coimmunoprecipitated only with RHOA G17V but not with RHOA wt, even though expression levels of RHOA G17V were much lower than RHOA wt (Figure 4A). These interactions were independent of the presence of CD28 wt or mutated forms (Figure 4A, right panel). Moreover, VAV1 and phosphorylated VAV1 (p-VAV1) coprecipitated with RHOA G17V and, to a lower extent, with RHOA wt. Interestingly, the presence of CD28 T195P promoted VAV1 phosphorylation (Figures 3A and 4A, left panel). p-VAV1 was therefore also detected in higher amounts in precipitates from CD28_T195P/RHOA (wt or G17V) than in those from CD28_wt/RHOA_ (wt or G17V cells) (Figure 4A, right panel). We also analyzed co-IPs of HA-tagged RHOA from cells stimulated for 30 minutes or 4 hours (Figure 4B). At either time point, RHOA wt did not bind to p300, whereas G17V did. Collectively, these results suggest that RHOA G17V is a gain-of-function mutant that has increased binding affinity for VAV1 and a newly acquired capacity of binding to p300 and VAV3 compared with RHOA wt.
RHOA G17V interacts with p300 and both colocalize. (A) RHOA G17V interacts with p300 in modified Jurkat cells. Representative western blot analysis of lysates (left) and anti-HA IP of ectopically expressed HA-tagged RHOA (right) for the indicated cell lines. Cells were costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 minutes. Whole-cell lysates (input, left) and (IP, right) were analyzed by immunoblotting (IB) using anti-HA, anti–p-VAV1, anti-VAV1, anti-VAVA3, and anti-p300 antibodies. Positions of molecular weight markers are indicated in kilodalton. The experiment was performed 3 times. (B) RHOA G17V interacts with p300 at every time point of costimulation. Representative western blot analysis of lysates (left) and anti-HA IPs of ectopically expressed HA-tagged RHOA (right) from the indicated cell lines. Cells were costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL during the indicated periods of time. Whole-cell lysates (input, left) and IPs (right) were analyzed by IB using anti-HA, anti-GAPDH, and anti-p300 antibodies. The experiment was performed 3 times. (C) RHOA G17V interacts with p300 in human primary T cells. Representative western blot analysis of lysates (left) and anti-HA IPs of ectopically expressed HA-tagged RHOA wt or G17V (right) from human primary T cells. Whole-cell lysates (input, left) and IPs (right) were analyzed by IB using anti-HA, anti-APDH, and anti-p300 antibodies. Positions of molecular weight markers are indicated in kilodalton. The experiment was performed twice. (D) Representative western blot analysis of cytoplasmic (left) and nuclear (right) cellular fractions of cell lines expressing ectopically either the wt or G17V form of RHOA. Cells were costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL during the indicated time periods. Whole-cell lysates were analyzed by IB using the indicated antibodies. IGF1R and Lamin B1 immunoblots show the separation between cytoplasmic and nuclear fractions. The experiment was performed twice. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
RHOA G17V interacts with p300 and both colocalize. (A) RHOA G17V interacts with p300 in modified Jurkat cells. Representative western blot analysis of lysates (left) and anti-HA IP of ectopically expressed HA-tagged RHOA (right) for the indicated cell lines. Cells were costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 minutes. Whole-cell lysates (input, left) and (IP, right) were analyzed by immunoblotting (IB) using anti-HA, anti–p-VAV1, anti-VAV1, anti-VAVA3, and anti-p300 antibodies. Positions of molecular weight markers are indicated in kilodalton. The experiment was performed 3 times. (B) RHOA G17V interacts with p300 at every time point of costimulation. Representative western blot analysis of lysates (left) and anti-HA IPs of ectopically expressed HA-tagged RHOA (right) from the indicated cell lines. Cells were costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL during the indicated periods of time. Whole-cell lysates (input, left) and IPs (right) were analyzed by IB using anti-HA, anti-GAPDH, and anti-p300 antibodies. The experiment was performed 3 times. (C) RHOA G17V interacts with p300 in human primary T cells. Representative western blot analysis of lysates (left) and anti-HA IPs of ectopically expressed HA-tagged RHOA wt or G17V (right) from human primary T cells. Whole-cell lysates (input, left) and IPs (right) were analyzed by IB using anti-HA, anti-APDH, and anti-p300 antibodies. Positions of molecular weight markers are indicated in kilodalton. The experiment was performed twice. (D) Representative western blot analysis of cytoplasmic (left) and nuclear (right) cellular fractions of cell lines expressing ectopically either the wt or G17V form of RHOA. Cells were costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL during the indicated time periods. Whole-cell lysates were analyzed by IB using the indicated antibodies. IGF1R and Lamin B1 immunoblots show the separation between cytoplasmic and nuclear fractions. The experiment was performed twice. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To assess whether the RHOA mutant is also able to bind endogenous p300 in primary T cells, RHOA wt or G17V forms were transiently expressed in CD3/CD28-activated human primary T cells. Coimmunoprecipitation confirmed that only RHOA G17V binds endogenous p300 in human primary T cells (Figure 4C).
Both cytoplasmic and nuclear compartments contained both RHOA wt and G17V and p300 (Figure 4D); although p300 was mainly present in the nucleus, whereas the 2 forms of RHOA were mainly cytoplasmic. Immunofluorescence staining of ectopically expressed RHOA wt and G17V showed that both proteins colocalized with p300 in the nucleus, and both forms of RHOA were additionally present in the cytoplasm in unstimulated cells (supplemental Figure 5). Interestingly, after 1 and 4 hours of stimulation, the cytoplasm-to-nuclear ratio of p300 increased (Figure 4D), suggesting CD3/CD28 triggering may favor the interaction of RHOA G17V with p300 by promoting its shuttling to the cytoplasm.
CD28 and RHOA mutants promote IL-2 secretion in a p300 acetyltransferase activity–dependent manner
To investigate whether VAV1 has a role in the effect of RHOA and CD28 mutants in promoting NFAT transcriptional activity, we assessed NFAT transactivation in a VAV1 knockdown condition vs in control conditions (Figure 5A; supplemental Table 4). VAV1 knockdown reduced NFAT activity across all cell lines, suggesting that VAV1 is required for full NFAT transcriptional activity after CD3/CD28 costimulation. Nevertheless, the effects of RHOA and CD28 variants on NFAT transactivation were still present, suggesting that VAV1 is not involved in the mutant-dependent increase of IL-2 secretion upon CD3/CD28 stimulation.
Induction of IL-2 secretion by CD28 and RHOA mutants requires p300 acetyltransferase activity. (A) Luciferase reporter assays monitoring the activity of NFAT in cell lines silenced for VAV1 (small interfering RNA [siRNA] VAV1) or CTRL cells (siRNA CTRL [siCTRL], scrambled), costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 hours and 30 minutes. Data are represented as mean ± SEM from 5 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05). Representative western blot of VAV1 knockdown by siRNA. Cells not used for luciferase assay were pooled according to siCTRL or siVAV1, and proteins were extracted and subjected to western blot. VAV1 signal was reduced between 24% and 29% depending on the experiment. It should be noted that only 15% to 20% of Jurkat cells are usually transduced by plasmids and siRNA by electroporation. (B) In vitro secretion of IL-2 upon stimulation with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 24 hours with or without the specific p300 acetyltransferase inhibitor A-485 at 5 μM. Cells were pretreated with 5 μM of A-485 for 48 hours. Data are represented as mean ± SEM from 6 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗∗P ≤ .01). Statistics not shown are indicated in supplemental Table 4.
Induction of IL-2 secretion by CD28 and RHOA mutants requires p300 acetyltransferase activity. (A) Luciferase reporter assays monitoring the activity of NFAT in cell lines silenced for VAV1 (small interfering RNA [siRNA] VAV1) or CTRL cells (siRNA CTRL [siCTRL], scrambled), costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 hours and 30 minutes. Data are represented as mean ± SEM from 5 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05). Representative western blot of VAV1 knockdown by siRNA. Cells not used for luciferase assay were pooled according to siCTRL or siVAV1, and proteins were extracted and subjected to western blot. VAV1 signal was reduced between 24% and 29% depending on the experiment. It should be noted that only 15% to 20% of Jurkat cells are usually transduced by plasmids and siRNA by electroporation. (B) In vitro secretion of IL-2 upon stimulation with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 24 hours with or without the specific p300 acetyltransferase inhibitor A-485 at 5 μM. Cells were pretreated with 5 μM of A-485 for 48 hours. Data are represented as mean ± SEM from 6 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗∗P ≤ .01). Statistics not shown are indicated in supplemental Table 4.
We then investigated the role of p300 acetyltransferase activity in the cooperation of RHOA and CD28 mutants. We measured IL-2 secretion upon CD3/CD28 costimulation with or without the small molecule A-485, a specific inhibitor of p300 acetyltransferase activity.44 Similar to CsA, A-485 abolished the effects of RHOA G17V and/or CD28 T195P in promoting CD3/CD28-induced IL-2 secretion (Figure 5B). These findings are consistent with a model in which, upon CD3/CD28 costimulation, CD28 T195P enhances NFAT and AP1 activation by increasing cytoplasmic signaling molecules’ phosphorylation, whereas RHOA G17V modulates NFAT and AP1 activities in a p300 acetyltransferase activity–dependent manner. Our data also suggest a pivotal role for p300 in regulating IL-2 transcription.
RHOA G17V expression induces an increased p300-dependent acetylation of the IL-2 promoter
Our previous data showed that p300 acetyltransferase activity is an essential mediator of RHOA G17V and CD28 effects on IL-2 secretion. Interestingly, p300 binds to NFAT and is part of the machinery driving IL-2 transcription.45 Therefore, we hypothesized that RHOA G17V may modulate p300 acetyltransferase activity and modify acetylation of histones at the IL-2 promoter. Thus, we performed ChIP experiments targeting different histone marks: (1) H3K4me3, whose peak near the transcription start site (TSS) is proportional to transcription levels; (2) H3K9ac that marks active gene transcription when increased near the TSS; and (3) p300-specific modifications, H3K18ac and H3K27ac. ChIP was followed by real-time PCR to compare the relative amounts of acetylated IL-2 promoter in cells expressing RHOA G17V vs RHOA wt. We compared enrichment of histone modifications in 4 regions localized around the IL-2 TSS: R1 (−677; −567); R2 (−226; −133); R3 (+2; +100); and R4 (+291; +386; Figure 6A-D). We observed higher H3K4me3 levels near IL-2 TSS (in the region R2) in cells expressing RHOA G17V than RHOA wt (Figure 6A). Higher levels of H3K9ac in R2, R3, and R4 regions were also observed in cells harboring G17V vs wt RHOA (Figure 6B). These observations are in accordance with a higher IL-2 transcription in cells expressing G17V vs wt RHOA. p300-specific H3K18ac and H3K27ac modifications showed significantly higher levels in regions R1/R2/R3 and R2/R3, respectively, in cells harboring G17V vs wt RHOA (Figure 6C-D). Thus, p300 acetyltransferase activity at the IL-2 promoter is higher in presence of RHOA G17V than RHOA wt.
RHOA G17V induces an increased p300-dependent acetylation. (A-D) The IL-2 promoter carries p300-specific marks of histone modification. In all figure panels, cells transduced with combinations of CD28 wt/RHOA wt (light gray) or CD28 wt/ RHOA G17V (red) were stimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 4 hours. Cells were then fixed, and ChIP was performed using IgG CTRL, anti-H3K4me3 (A), H3K9ac (B), H3K18ac (C), and H3K27ac (D) antibodies. The relative enrichment of 4 regions (R1 [−677; −567]; R2 [−226; −133]; R3 [+2; +100]; and R4 [+291; +386]) of the IL-2 promoter was assessed by qPCR using 4 pairs of primers. R2 and R3 regions are considered as proximal IL-2 promoter. Data are represented as mean ± SEM of 7 independent experiments. Significant differences in enrichment were determined using a 1-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (E) RHOA G17V expression induces increased p300-specific histone acetylation marks at whole-genome level. Sections of cell pellets (cytoblocs) were stained for H3K18ac (rabbit polyclonal ab1191; 1/800) or H3K27ac (rabbit polyclonal ab4729; 1/300) in combination with DAPI and assessed by immunofluorescence (upper). The mean intensity values of H3K18ac or H3K27ac and DAPI expression of segmented cells were quantified, and H3K18ac:DAPI and H3K27ac:DAPI ratios were calculated (lower). These ratios were compared between cells. Data are represented as mean ± SEM with the number of cells analyzed indicated in the figure. (F) TFH cells from patients with AITL carrying RHOA G17V show increased H3K18ac and H3K27ac p300-specific marks. Mean intensity ratios of H3K18ac:DAPI and H3K27ac:DAPI were compared between thousands of CD3+PD1+ (TFH) and CD3+PD1– (reactive) T cells for each of 4 patients with AITL carrying RHOA G17V alteration (1A, 1B, 1C, and 1D) and 4 patients carrying the wt RHOA (1E, 1F, 1G, and 1H). The means of each patient are plotted and compared. CD3+PD1+ cells showed increased H3K18ac:DAPI and H3K17ac:DAPI ratios compared with CD3+PD1– cells in patients with AITL carrying RHOA G17V mutation (∗P ≤ .05) but not in patients carrying wt RHOA.
RHOA G17V induces an increased p300-dependent acetylation. (A-D) The IL-2 promoter carries p300-specific marks of histone modification. In all figure panels, cells transduced with combinations of CD28 wt/RHOA wt (light gray) or CD28 wt/ RHOA G17V (red) were stimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 4 hours. Cells were then fixed, and ChIP was performed using IgG CTRL, anti-H3K4me3 (A), H3K9ac (B), H3K18ac (C), and H3K27ac (D) antibodies. The relative enrichment of 4 regions (R1 [−677; −567]; R2 [−226; −133]; R3 [+2; +100]; and R4 [+291; +386]) of the IL-2 promoter was assessed by qPCR using 4 pairs of primers. R2 and R3 regions are considered as proximal IL-2 promoter. Data are represented as mean ± SEM of 7 independent experiments. Significant differences in enrichment were determined using a 1-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (E) RHOA G17V expression induces increased p300-specific histone acetylation marks at whole-genome level. Sections of cell pellets (cytoblocs) were stained for H3K18ac (rabbit polyclonal ab1191; 1/800) or H3K27ac (rabbit polyclonal ab4729; 1/300) in combination with DAPI and assessed by immunofluorescence (upper). The mean intensity values of H3K18ac or H3K27ac and DAPI expression of segmented cells were quantified, and H3K18ac:DAPI and H3K27ac:DAPI ratios were calculated (lower). These ratios were compared between cells. Data are represented as mean ± SEM with the number of cells analyzed indicated in the figure. (F) TFH cells from patients with AITL carrying RHOA G17V show increased H3K18ac and H3K27ac p300-specific marks. Mean intensity ratios of H3K18ac:DAPI and H3K27ac:DAPI were compared between thousands of CD3+PD1+ (TFH) and CD3+PD1– (reactive) T cells for each of 4 patients with AITL carrying RHOA G17V alteration (1A, 1B, 1C, and 1D) and 4 patients carrying the wt RHOA (1E, 1F, 1G, and 1H). The means of each patient are plotted and compared. CD3+PD1+ cells showed increased H3K18ac:DAPI and H3K17ac:DAPI ratios compared with CD3+PD1– cells in patients with AITL carrying RHOA G17V mutation (∗P ≤ .05) but not in patients carrying wt RHOA.
Next, p300-dependent histone modifications were analyzed by multispectral immune-fluorescence microscopy. Cells expressing the CD28 wt form together with either wt or G17V forms of RHOA were CD3/CD28 costimulated for 4 hours (Figure 6E). H3K18ac and H3K27ac modifications and total chromatin content (DAPI [4′,6-diamidino-2-phenylindole]) levels were quantified by immunofluorescence staining, and mean fluorescence intensities of H3K18ac:DAPI and H3K27ac:DAPI ratios were determined. In accordance with ChIP-qPCRs results, comparison between RHOA wt and G17V cells revealed higher H3K18ac:DAPI (0.0758 ± 0.0004 vs 0.0969 ± 0.004; P < .0001) and H3K27ac:DAPI (0.0126 ± 0.00006 vs 0.0296 ± 0.0001; P < .0001) ratios in RHOA mutant cells. These findings indicate RHOA G17V expression induces increased p300 HAT activity at IL-2 promoter and across whole genome.
TFH cells in AITL biopsies from patients with RHOA G17V show increased H3K18ac and H3K27ac levels
Finally, we performed histological staining on samples from patients with AITL carrying the RHOA G17V mutation (n = 4) or the wt RHOA (n = 4; as demonstrated by targeted high-throughput sequencing performed in the setting of the diagnostic workup; Figure 6F; supplemental Figures 6A and 7A). We stained the samples for DAPI (reflecting total chromatin content), CD3, PD1, and either H3K18ac or H3K27ac. We measured the mean intensities of DAPI and histone marks and compared H3K18ac:DAPI and H3K27ac:DAPI ratios between thousands of CD3+PD1+ (neoplastic TFH) cells and CD3+PD1– (reactive T) cells in each sample. Both marks showed higher levels in CD3+PD1+ cells than in CD3+PD1– cells in each sample carrying the RHOA G17V mutation (P < .05), whereas no difference was observed between TFH cells and reactive T cells in patients with AITL carrying wt RHOA (Figure 6F; supplemental Figures 6B and 7B). These results corroborate those obtained in cell lines.
Discussion
We experimentally explored the mechanistic consequences of co-occurring CD28 activating and RHOA dominant-negative G17V variants in TFH lymphoma.19 We stably transduced Jurkat T cells with either empty vectors or combinations of expression constructs for wt or mutated CD28 and RHOA forms. Combination of CD28 T195P and RHOA G17V promoted IL-2 secretion and IL-2 transcription upon CD3/CD28 costimulation in a synergistic manner. This cooperation did not affect the phosphorylation level of TCR signaling molecules induced by the CD28 mutant alone but led to increased transcriptional activities of NFAT and AP1. We then identified RHOA G17V partner proteins, among which the most prevalent interactors were p300 and VAV1. We confirmed the previously reported coimmunoprecipitation of VAV1 and p-VAV1 with RHOA G17V by western blot22 and also observed (weaker) binding of VAV1 and p-VAV1 to RHOA wt. Although VAV1 was essential for full NFAT activity, it did not contribute to the additional NFAT transcriptional enhancement observed when both CD28 and RHOA variants were present.
A critical discovery was the specific interaction between RHOA G17V and p300, confirmed by coimmunoprecipitation in both modified Jurkat cells and transiently transduced human primary T cells. Using the p300 acetyltransferase inhibitor A-485, we demonstrated that p300 activity is crucial for the CD28 and RHOA mutants' effects on IL-2 secretion. This suggests that RHOA G17V modulates IL-2 transcription through p300 acetyltransferase activity–dependent NFAT and AP1 activation. ChIP followed by qPCR revealed that RHOA G17V expression leads to a significant increase in p300-specific histone acetylation marks (H3K18ac and H3K27ac) at the IL-2 promoter compared with RHOA wt. Furthermore, immunofluorescence analysis showed a genome-wide increase in these acetylation marks in the presence of RHOA G17V. Oncogenic RHOA Q63L reportedly exhibits a different spectrum of activity compared with the wt form under physiological stimulation.46 Similarly, our observations suggest that RHOA G17V acquires a new function in directly binding p300 and modifying its HAT activity at the IL-2 promoter and probably others. Conversely, activated RHOA wt may repress IL-2 transcription through its partner ROCK and unknown molecular players, aligning with its antagonistic role with p300 in different contexts. RHOA wt activation by growth hormone abrogates repression of STAT5A transcription activity by the HDAC6/p300 complex47; RHOA also inhibits, whereas its negative regulator RhoGDI enhances, the CBP/p300–mediated estrogen receptor (ER)–dependent transactivation.48 Our findings support a model in which the CD28 T195P activating mutation directly enhances NFAT and AP1 transcriptional activities by increasing the phosphorylation of signaling molecules downstream of TCR/CD28 coactivation. RHOA G17V may further amplify NFAT and AP1 activation and NFAT and AP1 target gene transcription by its binding to p300 and the increase of p300 HAT activity, indicating a shift in RHOA G17V’s role from signaling modulation to epigenetic regulation.
Previously,19,32 we observed that over half of patients with TFH lymphoma with RHOA G17V alteration (35/67) harbored co-occurrent activating mutations in TCR-related genes. These combined mutations likely contribute to lymphomagenesis by sustaining TFH-cell activation. Previous ChIP sequencing studies reported differences in the distribution of p300 DNA occupancy sites between T helper type 1 (Th1) and T helper type 2 (Th2) cells, mainly driven by STAT family members.49 p300 has also been involved in the differentiation of Th17 cells50 and regulatory T cells51 through its recruitment by FOXP3.52 Aberrations in histone after translational modifications, including those mediated by p300, are linked to various cancers,53-55 including TFH lymphoma, in which histone deacetylase inhibitors have shown therapeutic efficacy.56,57 Although RHOA G17V has been shown to induce TFH-cell specification in vivo when expressed in CD4+ T cells in mice,23,24 our data demonstrate that RHOA G17V modulates p300 acetyltransferase activity in vitro, a finding corroborated by AITL biopsies carrying the RHOA G17V mutation. Further studies will be needed to investigate whether the modulation of p300 is involved in how RHOA G17V may enhance TFH differentiation and promote TFH-derived malignancies.
p300 inhibitors hold potential as anticancer drugs. As a preclinical proof of concept, A-485 use in ER+ luminal breast cancer led to decreased H3K27 acetylation at enhancers of ER target genes and therefore to ERα signaling blockade.58 p300/CBP inhibitor CCS1477 was also used successfully on cellular models of castration-resistant prostate cancer.59 Finally, selective CREB-binding protein/p300 inhibitors are and will be used in phase 1 clinical trials in patients with metastatic castration-resistant prostate cancer only (ClinicalTrials.gov identifier NCT04575766) or with NUT carcinoma (ClinicalTrials.gov identifier NCT05488548). Our results suggest that targeting p300 acetyltransferase activity may open new avenues for TFH lymphoma therapies.
Acknowledgments
The authors thank the Protein Analysis Facility from the University of Lausanne for their expertise, set up, and liquid chromatography with tandem mass spectrometry shotgun analysis of the RHOA interactome. The authors are grateful to Catherine Chapuis Bernasconi and Emilie Lingre for laboratory support. The authors thank the biobank of Pathology Institute of the University Hospital of Lausanne (CHUV). The authors also acknowledge the helpful discussions with Baptiste Guey.
This work was funded by the CHUV, the University of Lausanne and the Swiss National Science Foundation (grant number 310030_172954 [L.d.L.]). M.T. acknowledges support by Swiss Cancer Research (grant number KFS-4095-02-2017-R) and the Emma Muschamp Foundation.
Authorship
Contribution: D.V., M.T., and L.d.L. conceived and designed the study; D.V., M.J., and K.I. developed the methodologies; D.V. acquired the data; D.V. and K.I. analyzed and interpreted the data; D.V., E.M., B.B., M.T., F.L., and L.d.L. wrote the manuscript; and L.d.L. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurence de Leval, Institute of Pathology Centre Hospitalier Universitaire Vaudois, Rue du Bugnon 25, CH-1011 Lausanne, Switzerland; email: laurence.deleval@chuv.ch; and David Vallois, Institute of Pathology, Centre Hospitalier Universitaire Vaudois, Rue du Bugnon 25, CH-1011 Lausanne, Switzerland; email: david.vallois@chuv.ch.
References
Author notes
Data are available on request from the corresponding authors, David Vallois (david.vallois@chuv.ch) and Laurence de Leval (laurence.deleval@chuv.ch).
The full-text version of this article contains a data supplement.


![Induction of IL-2 secretion by CD28 and RHOA mutants requires p300 acetyltransferase activity. (A) Luciferase reporter assays monitoring the activity of NFAT in cell lines silenced for VAV1 (small interfering RNA [siRNA] VAV1) or CTRL cells (siRNA CTRL [siCTRL], scrambled), costimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 5 hours and 30 minutes. Data are represented as mean ± SEM from 5 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗P ≤ .05). Representative western blot of VAV1 knockdown by siRNA. Cells not used for luciferase assay were pooled according to siCTRL or siVAV1, and proteins were extracted and subjected to western blot. VAV1 signal was reduced between 24% and 29% depending on the experiment. It should be noted that only 15% to 20% of Jurkat cells are usually transduced by plasmids and siRNA by electroporation. (B) In vitro secretion of IL-2 upon stimulation with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 24 hours with or without the specific p300 acetyltransferase inhibitor A-485 at 5 μM. Cells were pretreated with 5 μM of A-485 for 48 hours. Data are represented as mean ± SEM from 6 independent experiments conducted in quadruplicates. Significant differences in activation activity were determined using a 2-way ANOVA with Tukey multiple comparison test (∗∗P ≤ .01). Statistics not shown are indicated in supplemental Table 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/12/10.1182_bloodadvances.2024014671/3/m_blooda_adv-2024-014671-gr5.jpeg?Expires=1764974693&Signature=kmz5XMjgDQhpBmVwWRKiagJG4Y4-gJU8AAXMfRzPd6fwCsTFDwmYWMZfwQkOeQ72yczIZyGnKoe3M9eyVcfcZ3juwW1Hq0WZvRB7u2AecQ-1fb5wLnV097RhBp4fJOgGppAGtZKHf7evkKOsV-uVrGAhqFF2uB38Uxj98j0b07o8yKoh5Pu7ykDxpMujdFsp3LLSurVLaF7QVsvlkEbx1j3M6mOlbHk1zy5ZcBQnZEBKdQnXe7tAcnMzHRfMNkphXbk8Lt~4RqJdQj6yDYMSqIHtw41AMPuu-pjeHX-zDyxNq9ZHr7F5mwnMrspPRFaK4NZXayeXfqTMobohdpsT6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![RHOA G17V induces an increased p300-dependent acetylation. (A-D) The IL-2 promoter carries p300-specific marks of histone modification. In all figure panels, cells transduced with combinations of CD28 wt/RHOA wt (light gray) or CD28 wt/ RHOA G17V (red) were stimulated with anti-CD3, anti-CD28, and crosslinker at 2 μg/mL for 4 hours. Cells were then fixed, and ChIP was performed using IgG CTRL, anti-H3K4me3 (A), H3K9ac (B), H3K18ac (C), and H3K27ac (D) antibodies. The relative enrichment of 4 regions (R1 [−677; −567]; R2 [−226; −133]; R3 [+2; +100]; and R4 [+291; +386]) of the IL-2 promoter was assessed by qPCR using 4 pairs of primers. R2 and R3 regions are considered as proximal IL-2 promoter. Data are represented as mean ± SEM of 7 independent experiments. Significant differences in enrichment were determined using a 1-way ANOVA with Tukey multiple comparison test (∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001). (E) RHOA G17V expression induces increased p300-specific histone acetylation marks at whole-genome level. Sections of cell pellets (cytoblocs) were stained for H3K18ac (rabbit polyclonal ab1191; 1/800) or H3K27ac (rabbit polyclonal ab4729; 1/300) in combination with DAPI and assessed by immunofluorescence (upper). The mean intensity values of H3K18ac or H3K27ac and DAPI expression of segmented cells were quantified, and H3K18ac:DAPI and H3K27ac:DAPI ratios were calculated (lower). These ratios were compared between cells. Data are represented as mean ± SEM with the number of cells analyzed indicated in the figure. (F) TFH cells from patients with AITL carrying RHOA G17V show increased H3K18ac and H3K27ac p300-specific marks. Mean intensity ratios of H3K18ac:DAPI and H3K27ac:DAPI were compared between thousands of CD3+PD1+ (TFH) and CD3+PD1– (reactive) T cells for each of 4 patients with AITL carrying RHOA G17V alteration (1A, 1B, 1C, and 1D) and 4 patients carrying the wt RHOA (1E, 1F, 1G, and 1H). The means of each patient are plotted and compared. CD3+PD1+ cells showed increased H3K18ac:DAPI and H3K17ac:DAPI ratios compared with CD3+PD1– cells in patients with AITL carrying RHOA G17V mutation (∗P ≤ .05) but not in patients carrying wt RHOA.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/12/10.1182_bloodadvances.2024014671/3/m_blooda_adv-2024-014671-gr6.jpeg?Expires=1764974693&Signature=NW9nvV3h4T1Y5xCXbaydWMXAxRTtjNuDAg7riYDE6W~7FMpiBwIp10~KMUmSPQdgeJLGtABobBISsGmdUXmdCJA~5QTtJUJVaDbUUmO3qNiOswBvVZRdjR1fCBjTSctjEjGrI1k2xMuZWrt8DUC6lTJrx1kmwc5gCFev-s4kaog0N4cVTzus6WKzAZRlIGKu60-xVn80vngeAt63gGzU~mQP5fAg5~ht3rwc~6zZjQRQfNpB8zf2HAUwOabHvr8uQqQvg7g3xUPsa-ssdKXlw60pxirI-pu3LSdOxwLwqQ6SkcVypTm0jYHEz-9sa2E4i4zZ7eSyfVqRMsc2D5prew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)