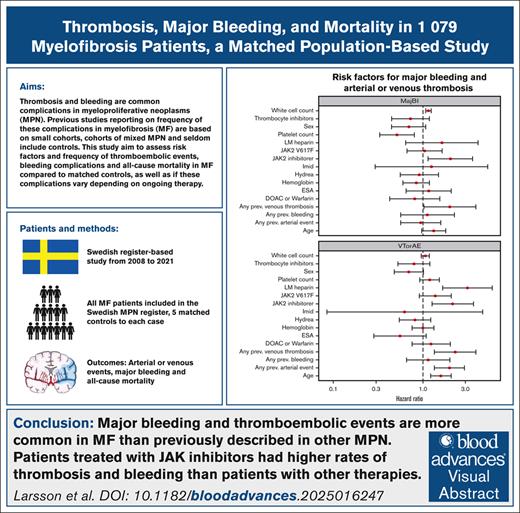
Key Points
This nationwide study shows that major bleeding and thromboembolic events are more frequent in MF than previously described in other MPNs.
The use of JAKis, a previous thrombosis, and older age were associated with an increased risk of a new thromboembolic event.
Visual Abstract
Bleeding and thrombotic events are known complications in myeloproliferative neoplasms (MPNs), but few studies have exclusively focused on patients with myelofibrosis (MF). In this nationwide population-based study, we assessed the frequency of major bleeding, thrombotic events, and all-cause mortality in 1079 patients diagnosed with MF and 5395 matched controls using multiple Swedish health care registers. Major bleeding, arterial, and venous events were seen at a rate of 2.55, 2.59, and 1.06 events per 100 years, respectively, in patients with MF. Compared to controls, the rates of bleedings, arterial events, venous events, and mortality were increased, with hazard ratios of 3.78 (95% confidence interval [CI], 2.98-4.79; P < .001), 1.73 (95% CI, 1.40-2.12; P < .001), 2.75 (95% CI, 1.93-3.90; P < .001), and 3.92 (95% CI, 3.50-4.40; P < .001), respectively. Patients treated with JAK inhibitors (JAKis) had higher rates of major bleeding (5.33), arterial events (4.67), and venous events (1.56) than patients with no ongoing symptom–directed therapy (rates, 2.32, 2.15, and 0.79) or hydroxyurea (rates, 2.05, 2.35, and 1.27, respectively). The use of JAKis or low-molecular-weight heparin, previous arterial or venous events, and older age were identified as independent risk factors for new arterial or venous events. A previous venous event, higher leukocyte count at diagnosis, and ongoing JAKi treatment were associated with an increased risk of major bleeding. This study shows that patients with MF have higher rates of thromboembolic events and major bleeding than described in other MPNs, and thromboembolic complications and major bleeding diverge in the different treatment groups.
Introduction
Myelofibrosis (MF) is a Philadelphia-negative myeloproliferative neoplasm (MPN) characterized by various degrees of bone marrow fibrosis, clonal proliferation of myeloid cells, and extramedullary hematopoiesis.1 MF occurs de novo (primary MF [PMF]) or secondary (secondary MF [SMF]) to polycythemia vera (PV; post-PV MF) or essential thrombocythemia (ET; post-ET MF). The clinical presentation of MF varies, ranging from asymptomatic, with only thrombocytosis as a sign of the disease in early-stage prefibrotic MF, to massive infiltration of bone marrow fibrosis in overt MF, with severe constitutional symptoms such as hepatosplenomegaly, fatigue, night sweats, and progressive pancytopenia.2 Thromboembolic events and bleeding are known complications in MF,3-5 but previous studies reporting on the frequency of thrombosis and bleeding have often been based on small cohorts, with populations of mixed MPN diagnoses, and seldom included controls.6 The aim of this nationwide study is to assess the frequency of arterial and venous events, major bleeding, all-cause stroke, and all-cause mortality (ACM) in PMF and SMF compared to matched controls. Secondly, we investigate whether the rates of the outcomes vary across the different therapies used in MF, and lastly, we seek to identify risk factors for thromboembolic events and major bleeding among patients with MF.
Methods
Data sources
The Swedish Blood Cancer Register was established in 2007 and contains information of all patients diagnosed with blood cancer or lymphoma in Sweden. In 2008, the Swedish MPN Register was founded as a part of the Swedish Blood Cancer Register, and because it is mandatory by Swedish law to report patients to the cancer registers, it has a high reported coverage of 99%.7 The registration of mutational status to the MPN register changed in 2016, with the addition of CALR, MPL, and JAK2 exon 12 mutations. In October 2020, prefibrotic MF was added as a diagnosis in the register.8 The National Patient Register (NPR) holds information on all inpatient somatic and psychiatric care since 1987 and outpatient care since 2001. The NPR includes information on diagnoses according to International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10), hospital admissions, surgical procedures, and discharge dates.9 The Swedish national quality register of stroke care, the Stroke Register, contains information on >94% of all acute strokes in Sweden. The register has a high validity for stroke diagnosis because it registers the index stroke once and collects the exact date of diagnosis rather than depending on discharge dates.10 All dispensations at Swedish pharmacies since 2006 are registered in the Swedish Prescribed Drug Register (PDR) according to Anatomical Therapeutic Chemical (ATC) classification, and the coverage of this register is 100%.11 The Cause of Death Register holds information on dates and causes of death of all deceased individuals in Sweden, with a coverage of >99%.12
Study population and outcomes
From the MPN register, all adult patients registered with a diagnosis of MF from 03 January 2008 to 29 December 2021 were included. Five controls, matched by gender, geographic region, and age at MF diagnosis, were randomly selected from the Population Register by Statistics Sweden (SCB). The sources of outcomes are listed in supplemental Data 1. Data from the MPN register were merged with information from the NPR, the Stroke Register, the PDR, and the Cause of Death Register by the National Board of Health and Welfare. Patients and controls were followed from the date of MF diagnosis to death or the end of study. To avoid double registration in the NPR, a recurrent event of the same type within 14 days was excluded. The outcomes of ischemic stroke, intracranial bleeding, and all-cause stroke were collected from the Stroke Register, in which all diagnoses are validated, and therefore, a new event within 14 days was not excluded as described previously. Data on treatment periods were obtained from the PDR, and the length of therapy was calculated using an approximate standard dosing for each drug. Treatment with interferon alfa (IFN), radioactive phosphorus (P32), transfusions, and phlebotomy were collected from the MPN register. The study was conducted according to the Declaration of Helsinki and approved by the Swedish Ethics Review Authority (registration number 2021-02227).
Statistical methods
Baseline characteristics are described with continuous variables presented as median and interquartile range and categorical variables presented as percentage of the sample. Mutational status is presented before and after 1 June 2016, because of the adjustment of registration of mutational status in the MPN register at this date. The results are presented as rates (events per time, in which time is measured in 100-year units) and hazard ratios (HRs) with 95% confidence intervals (CIs). P values <.05 were considered significant. Cox regression was used to compare outcomes, and results were visualized with Kaplan-Meier curves. To analyze the impact of the different MF therapies on outcome risk, a multivariable analysis was performed. The model was adjusted for sex, age, presence of JAK2 mutation, hemoglobin (Hb), white blood cell (WBC) count, platelet count, previous arterial or venous events, previous major bleeding, and different therapies, including antithrombotic treatment. Age, Hb, WBC count, and platelet count were used as continuous variables. Data were processed using R version 4.2.
Results
Baseline patient characteristics
A total of 1079 patients with MF and 5395 controls were included, with a median age at MF diagnosis of 72 years (Table 1). Of the patients with MF, 939 were classified as PMF, 29 as prefibrotic MF, and 108 as SMF (post-PV MF, n = 42; post-ET MF, n = 65; previous MPN Unclassified, n = 1). More than one-third of the patients (40.7%) had International Prognostic Scoring System (IPSS) score of intermediate-2 or high risk. Of the patients diagnosed after 1 June 2016, up to 53.6% had a JAK2 V617F mutation, 19.1% had a CALR mutation, 5.9% had a MPL mutation, 0.42% had a JAK2 exon 12 mutation, and 12.5% had no mutation (triple negative, Table 2). An arterial or venous event preceding the MF diagnosis was documented in 24.8% and 8.0% of the patients, respectively, and 8.5% of the patients had a major bleeding event before MF diagnosis (Table 1).
Baseline characteristics at diagnosis of 1079 patients with MF and their corresponding controls
| Variables . | MF, N = 1079 . | Controls, N = 5395 . |
|---|---|---|
| Demographics | ||
| Age, median (Q1-Q3), y | 72 (63.2-78.8) | 72 (63.2-78.9) |
| Female | 465 (43.1) | 2325 (43.1) |
| Medical history | ||
| Hypertension | 383 (35.5) | 1651 (30.6) |
| Diabetes mellitus | 123 (11.4) | 551 (10.2) |
| Atrial fibrillation | 112 (10.4) | 572 (10.6) |
| Congestive heart failure | 93 (8.6) | 339 (6.3) |
| COPD | 55 (5.1) | 220 (4.1) |
| Chronic renal failure | 39 (3.6) | 100 (1.9) |
| Obesitas | 24 (2.2) | 137 (2.5) |
| Liver disease | 27 (2.5) | 59 (1.1) |
| Non-hematological cancer | 245 (22.7) | 1022 (18.9) |
| Hematological cancer | 51 (4.7) | 75 (1.4) |
| Previous arterial event | ||
| Any arterial event | 268 (24.8) | 1035 (19.2) |
| Ischemic stroke | 88 (8.2) | 378 (7.0) |
| Ischemic heart disease | 188 (17.4) | 762 (14.1) |
| PAD | 19 (1.8) | 26 (0.48) |
| Previous venous event | ||
| Any venous thrombosis | 86 (8.0) | 200 (3.7) |
| Pulmonary embolism | 36 (3.3) | 102 (1.9) |
| Deep venous thrombosis | 37 (3.4) | 116 (2.2) |
| Splanchnic vein thrombosis | 19 (1.8) | 2 (0.037) |
| Cerebral venous thrombosis | 1 (0.093) | 1 (0.019) |
| Previous major bleeding | ||
| Any major bleeding | 92 (8.5) | 301 (5.6) |
| Cerebral hemorrhage | 14 (1.3) | 59 (1.1) |
| Gastrointestinal bleeding | 80 (7.4) | 247 (4.6) |
| Antithrombotic treatment | ||
| Any antithrombotic treatment | 516 (47.8) | 1 613 (29.9) |
| Antiplatelet agents | 405 (37.5) | 1 149 (21.3) |
| Direct oral anticoagulants | 48 (4.4) | 196 (3.6) |
| Vitamin K antagonist | 59 (5.5) | 279 (5.2) |
| Low-molecular-weight heparin | 42 (3.9) | 77 (1.4) |
| Variables . | MF, N = 1079 . | Controls, N = 5395 . |
|---|---|---|
| Demographics | ||
| Age, median (Q1-Q3), y | 72 (63.2-78.8) | 72 (63.2-78.9) |
| Female | 465 (43.1) | 2325 (43.1) |
| Medical history | ||
| Hypertension | 383 (35.5) | 1651 (30.6) |
| Diabetes mellitus | 123 (11.4) | 551 (10.2) |
| Atrial fibrillation | 112 (10.4) | 572 (10.6) |
| Congestive heart failure | 93 (8.6) | 339 (6.3) |
| COPD | 55 (5.1) | 220 (4.1) |
| Chronic renal failure | 39 (3.6) | 100 (1.9) |
| Obesitas | 24 (2.2) | 137 (2.5) |
| Liver disease | 27 (2.5) | 59 (1.1) |
| Non-hematological cancer | 245 (22.7) | 1022 (18.9) |
| Hematological cancer | 51 (4.7) | 75 (1.4) |
| Previous arterial event | ||
| Any arterial event | 268 (24.8) | 1035 (19.2) |
| Ischemic stroke | 88 (8.2) | 378 (7.0) |
| Ischemic heart disease | 188 (17.4) | 762 (14.1) |
| PAD | 19 (1.8) | 26 (0.48) |
| Previous venous event | ||
| Any venous thrombosis | 86 (8.0) | 200 (3.7) |
| Pulmonary embolism | 36 (3.3) | 102 (1.9) |
| Deep venous thrombosis | 37 (3.4) | 116 (2.2) |
| Splanchnic vein thrombosis | 19 (1.8) | 2 (0.037) |
| Cerebral venous thrombosis | 1 (0.093) | 1 (0.019) |
| Previous major bleeding | ||
| Any major bleeding | 92 (8.5) | 301 (5.6) |
| Cerebral hemorrhage | 14 (1.3) | 59 (1.1) |
| Gastrointestinal bleeding | 80 (7.4) | 247 (4.6) |
| Antithrombotic treatment | ||
| Any antithrombotic treatment | 516 (47.8) | 1 613 (29.9) |
| Antiplatelet agents | 405 (37.5) | 1 149 (21.3) |
| Direct oral anticoagulants | 48 (4.4) | 196 (3.6) |
| Vitamin K antagonist | 59 (5.5) | 279 (5.2) |
| Low-molecular-weight heparin | 42 (3.9) | 77 (1.4) |
Data are presented as n (%), unless otherwise specified.
COPD, chronic pulmonary obstructive disease; PAD, peripheral arterial disease; Q1, first quartile; Q3, third quartile.
Clinicogenetic characteristics of the patients with MF, presented as n (%) unless otherwise specified
| Variables . | MF, all, N = 1079 . | Prefibrotic MF, n = 29 . | PMF, n = 969 . | SMF, n = 108 . | Post-PV MF, n = 40 . | Post-ET MF, n = 65 . |
|---|---|---|---|---|---|---|
| Laboratory characteristics | ||||||
| Hb, median (Q1-Q3), g/dL | 11.6 (10.1-13.2) | 13.2 (11.5-15.0) | 11.8 (10.1-13.3) | 10.7 (9.8-11.8) | 10.7 (9.65-11.9) | 10.7 (9.9-11.8) |
| WBC count, median (Q1-Q3), × 109/L | 9.4 (6.4-14.4) | 12.4 (8.4-14.0) | 9.4 (6.5-14.3) | 8.5 (4.9-15.8) | 10.0 (5.1-19.5) | 8.2 (4.8-14.6) |
| Platelets count, median (Q1-Q3), × 109/L | 437 (215-748) | 697 (513-838) | 470 (215-763.5) | 340 (203.5-446.5) | 255 (188.5-370) | 374 (215-558) |
| Mutational status, patients diagnosed prior 31 May 2016∗ | ||||||
| JAK2 V617F | 296 (56.6) | 0 | 270 (56.7) | 26 (57.8) | 12 (100) | 13 (43.3) |
| CALR | 16 (3.1) | 0 | 14 (9.2) | 2 (4.4) | 0 | 2 (6.7) |
| MPL | 1 (0.19) | 0 | 1 (0.21) | 0 | 0 | 0 |
| Other mutation | 1 (0.19) | 0 | 1 (0.21) | 0 | 0 | 0 |
| No mutation | 201 (38.4) | 1 (100) | 185 (38.9) | 14 (31.1) | 0 | 12 (40.0) |
| Mutational status, patients diagnosed after 1 June 2016∗ | ||||||
| JAK2 V617F | 253 (53.6) | 22 (78.6) | 218 (53.7) | 13 (34.2) | 10 (71.4) | 3 (12.5) |
| JAK2 exon 12 | 2 (0.42) | 0 | 2 (0.49) | 0 | 0 | 0 |
| CALR | 90 (19.1) | 4 (14.3) | 78 (19.2) | 8 (21.2) | 0 | 8 (33.3) |
| MPL | 28 (5.9) | 2 (7.1) | 24 (5.9) | 2 (5.3) | 0 | 2 (8.3) |
| Other mutation | 31 (6.6) | 1 (3.6) | 23 (6.4) | 4 (10.5) | 2 (14.3) | 2 (8.3) |
| No mutation | 59 (12.5) | 1 (3.6) | 55 (13.5) | 3 (7.9) | 0 | 3 (12.5) |
| Symptoms at diagnosis | ||||||
| Weight loss | 115 (10.7) | 3 (10.3) | 97 (10.3) | 15 (13.9) | 13 (32.5) | 1 (1.5) |
| Pruritus | 29 (2.7) | 1 (3.4) | 25 (2.7) | 3 (2.8) | 3 (7.5) | 0 |
| Fever | 30 (2.8) | 0 | 23 (2.4) | 7 (6.5) | 5 (12.5) | 2 (3.1) |
| Night sweats | 92 (8.5) | 0 | 78 (8.3) | 14 (13.0) | 7 (17.5) | 7 (10.8) |
| Splenomegaly | 214 (19.8) | 3 (10.3) | 180 (19.2) | 31 (28.7) | 19 (47.5) | 11 (16.9) |
| IPSS category | ||||||
| Low risk | 155 (18.9) | 2 (22.2) | 141 (19.3) | 12 (15.4) | 2 (7.1) | 10 (20.4) |
| Intermediate-1 | 330 (40.3) | 5 (55.6) | 302 (41.3) | 23 (29.5) | 8 (28.6) | 15 (30.6) |
| Intermediate-2 | 197 (24.1) | 2 (22.2) | 171 (23.4) | 24 (30.8) | 10 (35.7) | 14 (28.6) |
| High risk | 136 (16.6) | 0 | 117 (16.0) | 19 (24.4) | 8 (28.6) | 10 (20.4) |
| Variables . | MF, all, N = 1079 . | Prefibrotic MF, n = 29 . | PMF, n = 969 . | SMF, n = 108 . | Post-PV MF, n = 40 . | Post-ET MF, n = 65 . |
|---|---|---|---|---|---|---|
| Laboratory characteristics | ||||||
| Hb, median (Q1-Q3), g/dL | 11.6 (10.1-13.2) | 13.2 (11.5-15.0) | 11.8 (10.1-13.3) | 10.7 (9.8-11.8) | 10.7 (9.65-11.9) | 10.7 (9.9-11.8) |
| WBC count, median (Q1-Q3), × 109/L | 9.4 (6.4-14.4) | 12.4 (8.4-14.0) | 9.4 (6.5-14.3) | 8.5 (4.9-15.8) | 10.0 (5.1-19.5) | 8.2 (4.8-14.6) |
| Platelets count, median (Q1-Q3), × 109/L | 437 (215-748) | 697 (513-838) | 470 (215-763.5) | 340 (203.5-446.5) | 255 (188.5-370) | 374 (215-558) |
| Mutational status, patients diagnosed prior 31 May 2016∗ | ||||||
| JAK2 V617F | 296 (56.6) | 0 | 270 (56.7) | 26 (57.8) | 12 (100) | 13 (43.3) |
| CALR | 16 (3.1) | 0 | 14 (9.2) | 2 (4.4) | 0 | 2 (6.7) |
| MPL | 1 (0.19) | 0 | 1 (0.21) | 0 | 0 | 0 |
| Other mutation | 1 (0.19) | 0 | 1 (0.21) | 0 | 0 | 0 |
| No mutation | 201 (38.4) | 1 (100) | 185 (38.9) | 14 (31.1) | 0 | 12 (40.0) |
| Mutational status, patients diagnosed after 1 June 2016∗ | ||||||
| JAK2 V617F | 253 (53.6) | 22 (78.6) | 218 (53.7) | 13 (34.2) | 10 (71.4) | 3 (12.5) |
| JAK2 exon 12 | 2 (0.42) | 0 | 2 (0.49) | 0 | 0 | 0 |
| CALR | 90 (19.1) | 4 (14.3) | 78 (19.2) | 8 (21.2) | 0 | 8 (33.3) |
| MPL | 28 (5.9) | 2 (7.1) | 24 (5.9) | 2 (5.3) | 0 | 2 (8.3) |
| Other mutation | 31 (6.6) | 1 (3.6) | 23 (6.4) | 4 (10.5) | 2 (14.3) | 2 (8.3) |
| No mutation | 59 (12.5) | 1 (3.6) | 55 (13.5) | 3 (7.9) | 0 | 3 (12.5) |
| Symptoms at diagnosis | ||||||
| Weight loss | 115 (10.7) | 3 (10.3) | 97 (10.3) | 15 (13.9) | 13 (32.5) | 1 (1.5) |
| Pruritus | 29 (2.7) | 1 (3.4) | 25 (2.7) | 3 (2.8) | 3 (7.5) | 0 |
| Fever | 30 (2.8) | 0 | 23 (2.4) | 7 (6.5) | 5 (12.5) | 2 (3.1) |
| Night sweats | 92 (8.5) | 0 | 78 (8.3) | 14 (13.0) | 7 (17.5) | 7 (10.8) |
| Splenomegaly | 214 (19.8) | 3 (10.3) | 180 (19.2) | 31 (28.7) | 19 (47.5) | 11 (16.9) |
| IPSS category | ||||||
| Low risk | 155 (18.9) | 2 (22.2) | 141 (19.3) | 12 (15.4) | 2 (7.1) | 10 (20.4) |
| Intermediate-1 | 330 (40.3) | 5 (55.6) | 302 (41.3) | 23 (29.5) | 8 (28.6) | 15 (30.6) |
| Intermediate-2 | 197 (24.1) | 2 (22.2) | 171 (23.4) | 24 (30.8) | 10 (35.7) | 14 (28.6) |
| High risk | 136 (16.6) | 0 | 117 (16.0) | 19 (24.4) | 8 (28.6) | 10 (20.4) |
All percentages are calculated from the total number of cases with available data. Data are presented as n (%), unless otherwise specified.
Q1, first quartile; Q3, third quartile.
In 2016, the registration of mutational status to the Swedish MPN register was changed with the addition CALR, MPL, and JAK2 exon12 mutations, and therefore, the data are presented in 2 separate time periods.
Treatments during follow-up
The most frequently used symptom-directed treatment in the MF cohort was hydroxyurea (HU), which was prescribed to 672 patients during a time period of 2040 patient-years. JAK inhibitors (JAKis) were used in 238 patients during an aggregated time of 450 patient-years, while 82 and 55 patients were given IFN and immunomodulatory drugs (IMiDs), respectively. The patients treated with IFN were younger (mean age, 55.7 years) than those in other treatment groups: HU (73.9 years), JAKis (69.5 years), IMiDs (72.3 years), erythropoietin-stimulating agents (74.7 years), and no symptom-directed therapy (67.7 years).
Antiplatelet agents were administered to 757 patients (treatment time, 2210 years), and 176, 115, and 201 patients with MF were treated with direct oral anticoagulants, warfarin, and low-molecular weight heparin (LMWH). For a time period of 2010 patient-years (41.6% of the total follow-up time for all patients), 1019 patients with MF had no ongoing antithrombotic treatment (Table 3).
Number of (possibly repeated) events and rates thereof in different treatment periods
| Treatment . | . | Patients, n . | Age, y . | Time . | Arterial event, n (rate) . | HR (95% CI) . | P value . | VTE, n (rate) . | HR (95% CI) . | P value . | Major bleeding, n (rate) . | HR (95% CI) . | P value . | All-cause mortality, n (rate) . | HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All time | MF | 1079 | 70.3 | 48.3 | 125 (2.59) | 1.73 (1.40-2.12) | <.001 | 51 (1.06) | 2.75 (1.95-3.90) | <.001 | 123 (2.55) | 3.78 (2.98-4.79) | <.001 | 532 (11.01) | 3.92 (3.50-4.40) | <.001 |
| Controls | 5395 | 69.4 | 223.8 | 337 (1.51) | 86 (0.38) | 152 (0.68) | 630 (2.81) | |||||||||
| No symptom-directed treatment | MF | 720 | 67.6 | 17.7 | 38 (2.15) | 1.49 (1.04-2.15) | .031 | 16 (0.79) | 2.85 (1.53-5.31) | <.001 | 41 (2.32) | 3.31 (2.22-4.94) | <.001 | 209 (11.81) | 4.31 (3.57-5.20) | <.001 |
| Controls | 3517 | 66.6 | 82.2 | 119 (1.45) | 26 (0.32) | 58 (0.71) | 227 (2.76) | |||||||||
| HU | MF | 672 | 73.9 | 20.4 | 48 (2.35) | 1.47 (1.06-2.03) | .020 | 26 (1.27) | 3.17 (1.92-5.21) | <.001 | 42 (2.05) | 3.14 (2.12-4.64) | <.001 | 204 (10.00) | 3.29 (2.75-3.95) | <.001 |
| Controls | 3319 | 73.1 | 93.9 | 150 (1.59) | 38 (0.40) | 62 (0.66) | 283 (3.01) | |||||||||
| IFN | MF | 82 | 55.7 | 2.7 | 1 (0.37) | 0.54 (0.069-4.30) | .56 | 1 (0.37) | 1.66 (0.17-15.98) | .66 | 3 (1.11) | 14.97 (1.56-143.95) | .019 | 6 (2.22) | 3.68 (1.28-10.61) | .016 |
| Controls | 408 | 55.5 | 13.4 | 9 (0.67) | 3 (0.77) | 1 | 8 (0.77) | |||||||||
| JAKis | MF | 238 | 69.5 | 4.5 | 21 (4.67) | 3.59 (2.03-6.35) | <.001 | 7 (1.56) | 4.10 (1.49-11.31) | .0064 | 24 (5.33) | 8.08 (4.18-15.63) | <.001 | 66 (14.67) | 5.81 (4.05-8.34) | <.001 |
| Controls | 1134 | 68.7 | 20.9 | 27 (1.29) | 8 (0.38) | 14 (0.67) | 53 (2.54) | |||||||||
| IMiDs | MF | 55 | 72.3 | 0.46 | 1 (2.17) | 1.15 (0.13-10.29) | .90 | 0 | - | - | 2 (4.35) | 3.22 (0.54-19.27) | .20 | 19 (41.30) | 23.26 (7.91-68.38) | <.001 |
| Controls | 270 | 72.3 | 2.2 | 4 (1.82) | 2 (0.89) | 3 (1.36) | 4 (1.82) | |||||||||
| ESAs | MF | 308 | 74.7 | 4.8 | 15 (3.13) | 1.75 (0.96-3.17) | .066 | 4 (0.84) | 1.01 (0.34-3.00) | .99 | 18 (3.75) | 4.85 (2.50-9.41) | <.001 | 92 (19.17) | 5.25 (3.89-7.08) | <.001 |
| Controls | 1501 | 74.0 | 21.7 | 39 (1.80) | 17 (0.78) | 17 (0.78) | 80 (3.69) | |||||||||
| No antithrombotic treatment | MF | 1019 | 67.4 | 20.1 | 41 (2.04) | 1.66 (1.16-2.37) | .0053 | 19 (0.94) | 2.50 (1.43-4.36) | .0012 | 64 (3.18) | 5.06 (3.56-7.20) | <.001 | 296 (14.73) | 6.33 (5.32-7.53) | <.001 |
| Controls | 5056 | 66.7 | 95.1 | 117 (1.23) | 36 (0.38) | 60 (0.63) | 222 (2.33) | |||||||||
| Antiplatelet agents | MF | 757 | 71.9 | 22.1 | 56 (2.53) | 1.63 (1.20-2.21) | .0017 | 14 (0.63) | 1.76 (0.95-3.27) | .072 | 37 (1.67) | 2.60 (1.74-3.88) | <.001 | 144 (6.51) | 2.14 (1.75-2.61) | <.001 |
| Controls | 3760 | 71.0 | 101.4 | 159 (1.57) | 36 (0.36) | 66 (0.65) | 308 (3.04) | |||||||||
| DOACs | MF | 176 | 75.6 | 3.0 | 12 (4.00) | 1.83 (0.93-3.58) | .080 | 13 (4.33) | 5.83 (2.55-13.30) | <.001 | 11 (3.67) | 4.02 (1.77-9.12) | <.001 | 45 (15.00) | 4.37 (2.89-6.61) | <.001 |
| Controls | 812 | 74.5 | 13.3 | 29 (2.18) | 10 (0.75) | 12 (0.90) | 45 (3.38) | |||||||||
| Warfarin | MF | 115 | 72.5 | 3.0 | 16 (5.33) | 2.29 (1.25-4.19) | .0070 | 6 (2.00) | 3.02 (1.07-8.48) | .036 | 8 (2.67) | 3.00 (1.23-7.34) | .016 | 42 (14.00) | 3.43 (2.29-5.12) | <.001 |
| Controls | 561 | 71.5 | 13.4 | 31 (2.31) | 9 (0.67) | 12 (0.89) | 55 (4.10) | |||||||||
| LMWH | MF | 201 | 66.9 | 1.4 | 10 (7.14) | 4.64 (1.93-11.16) | <.001 | 13 (9.29) | 60.52 (7.91-462.80) | <.001 | 10 (7.14) | 12.10 (3.80-38.59) | <.001 | 24 (17.14) | 7.58 (3.98-14.46) | <.001 |
| Controls | 965 | 66.1 | 6.4 | 10 (1.56) | 1 (0.16) | 4 (0.63) | 15 (2.34) |
| Treatment . | . | Patients, n . | Age, y . | Time . | Arterial event, n (rate) . | HR (95% CI) . | P value . | VTE, n (rate) . | HR (95% CI) . | P value . | Major bleeding, n (rate) . | HR (95% CI) . | P value . | All-cause mortality, n (rate) . | HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All time | MF | 1079 | 70.3 | 48.3 | 125 (2.59) | 1.73 (1.40-2.12) | <.001 | 51 (1.06) | 2.75 (1.95-3.90) | <.001 | 123 (2.55) | 3.78 (2.98-4.79) | <.001 | 532 (11.01) | 3.92 (3.50-4.40) | <.001 |
| Controls | 5395 | 69.4 | 223.8 | 337 (1.51) | 86 (0.38) | 152 (0.68) | 630 (2.81) | |||||||||
| No symptom-directed treatment | MF | 720 | 67.6 | 17.7 | 38 (2.15) | 1.49 (1.04-2.15) | .031 | 16 (0.79) | 2.85 (1.53-5.31) | <.001 | 41 (2.32) | 3.31 (2.22-4.94) | <.001 | 209 (11.81) | 4.31 (3.57-5.20) | <.001 |
| Controls | 3517 | 66.6 | 82.2 | 119 (1.45) | 26 (0.32) | 58 (0.71) | 227 (2.76) | |||||||||
| HU | MF | 672 | 73.9 | 20.4 | 48 (2.35) | 1.47 (1.06-2.03) | .020 | 26 (1.27) | 3.17 (1.92-5.21) | <.001 | 42 (2.05) | 3.14 (2.12-4.64) | <.001 | 204 (10.00) | 3.29 (2.75-3.95) | <.001 |
| Controls | 3319 | 73.1 | 93.9 | 150 (1.59) | 38 (0.40) | 62 (0.66) | 283 (3.01) | |||||||||
| IFN | MF | 82 | 55.7 | 2.7 | 1 (0.37) | 0.54 (0.069-4.30) | .56 | 1 (0.37) | 1.66 (0.17-15.98) | .66 | 3 (1.11) | 14.97 (1.56-143.95) | .019 | 6 (2.22) | 3.68 (1.28-10.61) | .016 |
| Controls | 408 | 55.5 | 13.4 | 9 (0.67) | 3 (0.77) | 1 | 8 (0.77) | |||||||||
| JAKis | MF | 238 | 69.5 | 4.5 | 21 (4.67) | 3.59 (2.03-6.35) | <.001 | 7 (1.56) | 4.10 (1.49-11.31) | .0064 | 24 (5.33) | 8.08 (4.18-15.63) | <.001 | 66 (14.67) | 5.81 (4.05-8.34) | <.001 |
| Controls | 1134 | 68.7 | 20.9 | 27 (1.29) | 8 (0.38) | 14 (0.67) | 53 (2.54) | |||||||||
| IMiDs | MF | 55 | 72.3 | 0.46 | 1 (2.17) | 1.15 (0.13-10.29) | .90 | 0 | - | - | 2 (4.35) | 3.22 (0.54-19.27) | .20 | 19 (41.30) | 23.26 (7.91-68.38) | <.001 |
| Controls | 270 | 72.3 | 2.2 | 4 (1.82) | 2 (0.89) | 3 (1.36) | 4 (1.82) | |||||||||
| ESAs | MF | 308 | 74.7 | 4.8 | 15 (3.13) | 1.75 (0.96-3.17) | .066 | 4 (0.84) | 1.01 (0.34-3.00) | .99 | 18 (3.75) | 4.85 (2.50-9.41) | <.001 | 92 (19.17) | 5.25 (3.89-7.08) | <.001 |
| Controls | 1501 | 74.0 | 21.7 | 39 (1.80) | 17 (0.78) | 17 (0.78) | 80 (3.69) | |||||||||
| No antithrombotic treatment | MF | 1019 | 67.4 | 20.1 | 41 (2.04) | 1.66 (1.16-2.37) | .0053 | 19 (0.94) | 2.50 (1.43-4.36) | .0012 | 64 (3.18) | 5.06 (3.56-7.20) | <.001 | 296 (14.73) | 6.33 (5.32-7.53) | <.001 |
| Controls | 5056 | 66.7 | 95.1 | 117 (1.23) | 36 (0.38) | 60 (0.63) | 222 (2.33) | |||||||||
| Antiplatelet agents | MF | 757 | 71.9 | 22.1 | 56 (2.53) | 1.63 (1.20-2.21) | .0017 | 14 (0.63) | 1.76 (0.95-3.27) | .072 | 37 (1.67) | 2.60 (1.74-3.88) | <.001 | 144 (6.51) | 2.14 (1.75-2.61) | <.001 |
| Controls | 3760 | 71.0 | 101.4 | 159 (1.57) | 36 (0.36) | 66 (0.65) | 308 (3.04) | |||||||||
| DOACs | MF | 176 | 75.6 | 3.0 | 12 (4.00) | 1.83 (0.93-3.58) | .080 | 13 (4.33) | 5.83 (2.55-13.30) | <.001 | 11 (3.67) | 4.02 (1.77-9.12) | <.001 | 45 (15.00) | 4.37 (2.89-6.61) | <.001 |
| Controls | 812 | 74.5 | 13.3 | 29 (2.18) | 10 (0.75) | 12 (0.90) | 45 (3.38) | |||||||||
| Warfarin | MF | 115 | 72.5 | 3.0 | 16 (5.33) | 2.29 (1.25-4.19) | .0070 | 6 (2.00) | 3.02 (1.07-8.48) | .036 | 8 (2.67) | 3.00 (1.23-7.34) | .016 | 42 (14.00) | 3.43 (2.29-5.12) | <.001 |
| Controls | 561 | 71.5 | 13.4 | 31 (2.31) | 9 (0.67) | 12 (0.89) | 55 (4.10) | |||||||||
| LMWH | MF | 201 | 66.9 | 1.4 | 10 (7.14) | 4.64 (1.93-11.16) | <.001 | 13 (9.29) | 60.52 (7.91-462.80) | <.001 | 10 (7.14) | 12.10 (3.80-38.59) | <.001 | 24 (17.14) | 7.58 (3.98-14.46) | <.001 |
| Controls | 965 | 66.1 | 6.4 | 10 (1.56) | 1 (0.16) | 4 (0.63) | 15 (2.34) |
Bold P values are those of statistical significance. Treatment periods are defined from the registry and pharma data for the case group, but each control gets the same period put in for comparison. Treatment periods are not exclusive, for example, the time on one therapy can overlap another treatment. Time is measured in 100-year units.
DOACs, direct oral anticoagulants; ESAs, erythropoietin-stimulating agents; VTE, venous thromboembolism.
Thromboembolic and bleeding events during follow-up
During the follow-up time (4830 patient-years), 125 arterial and 51 venous events occurred in the MF cohort, with rates of 2.59 and 1.06 events per 100 patient-years, respectively, compared to 337 arterial (rate, 1.51) and 86 venous events (rate, 0.38) among controls (HRs, 1.73 [95% CI, 1.40-2.12; P < .001] and 2.75 [95% CI, 1.95-3.90; P < .001], respectively; Table 3). When dividing the patients into PMF and SMF, the rates of arterial and venous events were higher among patients with SMF, at 2.49 and 1.25, respectively, than 2.27 and 0.96 in PMF (supplemental Table 2). In total, 80 cases of acute myocardial infarction (rate, 1.66), 40 ischemic strokes (rate, 0.83), and 38 pulmonary emboli (rate, 0.79) were documented in the patients with MF. Four events of splanchnic vein thrombosis were found among patients with MF (rate, 0.083) compared to 2 events in the control group (rate, 0.0089; supplemental Table 3). No cases of cerebral venous thrombosis were seen among patients or controls.
The rate of major bleeding in patients with MF were 2.55 (123 events) compared to 0.68 (152 events) in the controls (HR, 3.87; 95% CI, 2.98-4.79; P < .001; Table 3). In total, 38 events of intracranial hemorrhages occurred in the MF group (supplemental Table 3). The rate of major bleeding was higher in SMF (3.10) than PMF (2.14; supplemental Table 2).
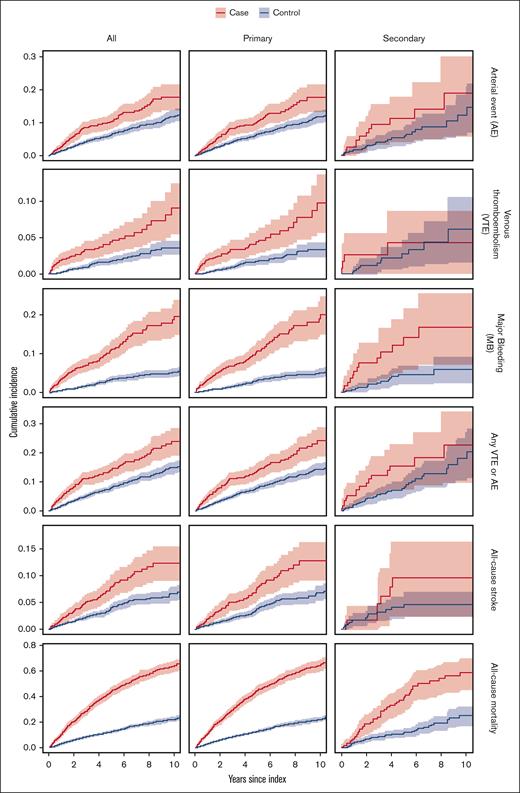
The cumulative incidence of arterial and venous events, major bleeding, and all-cause stroke was significantly higher in all patients with MF as well as in the PMF group than in corresponding controls (Figure 1).
Kaplan-Meier curves for the different outcomes in all patients with MF, PMF, and SMF. Cases are marked as red lines, and controls are marked in blue. Shaded areas represent 95% CIs.
Kaplan-Meier curves for the different outcomes in all patients with MF, PMF, and SMF. Cases are marked as red lines, and controls are marked in blue. Shaded areas represent 95% CIs.
All-cause mortality during follow-up
The rate of ACM was significantly higher in patients with MF (rate, 11.01) than controls (rate, 2.81), with an HR of 3.92 (95% CI, 3.50-4.40; Table 3). Patients with SMF had a rate of ACM of 15.22 compared to 10.57 in those with PMF (supplemental Table 2). The highest rate of ACM was seen among patients treated with IMiDs (rate, 41.30), and the lowest rate was seen in patients treated with IFN (rate, 2.22). The cumulative incidence of ACM was significantly higher in patients with MF than controls, and the statistical significance persisted when dividing patients with MF into PMF and SMF (Figure 1).
Events in the treatment groups
Patients with MF with no ongoing symptom-directed therapy had a rate of arterial events of 2.15, which was lower than the rates seen in patients treated with HU (rate, 2.35) and JAKi (rate, 4.67). Among the 82 patients treated with IFN, only 1 arterial event occurred during follow-up (rate, 0.37; Table 3).
The rate of venous thromboembolism ranged from 0 (patients treated with IMiDs) to 1.56 (patients treated with JAKi). Patients with no symptom-directed treatment and those treated with IFN had lower rates of venous thromboembolism (0.79 and 0.37, respectively) than patients treated with HU (rate, 1.27; Table 3).
The highest rates of major bleeding occurred in patients prescribed JAKi (rate, 5.33) and IMiDs (rate, 4.35). Patients with IFN treatment had the lowest rate of major bleeding (1.11), which was even lower than in patients with no ongoing symptom-directed treatment (rate, 2.32). In all patients treated with antithrombotic therapy, the highest rates of major bleeding were seen in patients with MF treated with LMWH (rate, 7.14), compared to patients prescribed direct oral anticoagulants (rate, 3.67), warfarin (rate, 2.67), and antiplatelet agents (rate, 1.67; Table 3).
The highest rate of all-cause stroke was seen among patients treated with IMiDs (rate, 2.17) and JAKi (rate, 2.00), whereas patients treated with IFN had the lowest rates of all-cause stroke (rate, 0.37; supplemental Table 3).
In patients treated with JAKi, a higher rate of arterial events occurred in patients harboring a JAK2 mutation (rate, 5.19) than in patients with no mutation (triple negative; rate, 1.22; Table 4). Major bleeding and venous events were more frequent among triple-negative patients treated with JAKis (rates, 8.77 and 2.53, respectively) compared to MF patients with JAK2 mutation and JAKi therapy (rates, 4.15 and 1.51). JAK2-mutated patients who were prescribed HU had higher rates of both arterial events (rate, 2.70) and major bleeding (rate, 2.46) than the triple-negative patients who were treated with HU (rates, 1.10 and 0.74, respectively; Table 4).
Rates of first event by treatment, mutational status, and previous event at diagnosis
| Treatment . | Event . | All Patients with MF . | JAK2 mutation . | Triple negative . | Previous arterial event . | Previous venous event . | Previous major bleeding . |
|---|---|---|---|---|---|---|---|
| All patients | AE | 2.28 (105/46.0; 1079) | 2.74 (62/22.6; 549) | 1.77 (26/14.7; 260) | 5.15 (45/8.7; 268) | 4.20 (13/3.1; 86) | 4.09 (13/3.2; 92) |
| VTE | 0.96 (45/47.1; 1079) | 1.13 (26/23.1; 549) | 0.72 (11/15.2; 260) | 1.16 (11/9.5; 268) | 2.48 (8/3.2; 86) | 1.81 (6/3.3; 92) | |
| MajBl | 2.27 (105/46.3; 1079) | 2.46 (56/22.8; 549) | 1.80 (27/15.0; 260) | 2.83 (26/9.2; 268) | 4.42 (14/3.2; 86) | 3.66 (12/3.3; 92) | |
| No treatment | AE | 2.49 (45/18.1; 750) | 2.63 (20/7.6; 366) | 2.64 (18/6.8; 206) | 7.40 (20/2.7; 179) | 8.38 (8/1.0; 54) | 3.07 (4/1.3; 64) |
| VTE | 0.75 (14/18.7; 750) | 0.91 (7/7.7; 366) | 0.55 (4/7.2; 206) | 1.34 (4/3.0; 179) | 1.86 (2/1.1; 54) | 0.72 (1/1.4; 64) | |
| MajBl | 2.28 (42/18.4; 750) | 2.24 (17/7.6; 366) | 1.97 (14/7.1; 206) | 2.03 (6/3.0; 179) | 4.79 (5/1.0; 54) | 2.20 (3/1.4; 64) | |
| Hydroxyurea | AE | 2.02 (40/19.8; 672) | 2.70 (30/11.1; 392) | 1.10 (6/5.4; 144) | 3.66 (17/4.6; 185) | 3.62 (5/1.4; 54) | 4.01 (5/1.2; 53) |
| VTE | 1.15 (23/20.0; 672) | 1.23 (14/11.4; 392) | 1.13 (6/5.3; 144) | 1.04 (5/4.8; 185) | 3.64 (5/1.4; 54) | 3.26 (4/1.2; 53) | |
| MajBl | 1.95 (39/20.0; 672) | 2.46 (28/11.4; 392) | 0.74 (4/5.4; 144) | 3.43 (16/4.7; 185) | 2.08 (3/1.4; 54) | 5.54 (7/1.3; 53) | |
| Interferon | AE | 0.37 (1/2.7; 82) | 1.19 (1/0.8; 38) | 0.00 (0/1.2; 23) | 0.00 (0/0.2; 10) | 0.00 (0/0.3; 10) | 0.00 (0/0.0; 2) |
| VTE | 0.37 (1/2.7; 82) | 1.19 (1/0.8; 38) | 0.00 (0/1.2; 23) | 0.00 (0/0.2; 10) | 0.00 (0/0.3; 10) | 0.00 (0/0.0; 2) | |
| MajBl | 0.74 (2/2.7; 82) | 1.17 (1/0.9; 38) | 0.83 (1/1.2; 23) | 0.00 (0/0.2; 10) | 0.00 (0/0.3; 10) | 0.00 (0/0.0; 2) | |
| JAK inhibitors | AE | 3.89 (17/4.4; 238) | 5.19 (14/2.7; 145) | 1.22 (1/0.8; 43) | 5.41 (6/1.1; 52) | 2.07 (1/0.5; 21) | 3.73 (2/0.5; 25) |
| VTE | 1.63 (7/4.3; 238) | 1.51 (4/2.6; 145) | 2.53 (2/0.8; 43) | 1.78 (2/1.1; 52) | 2.40 (1/0.4; 21) | 1.78 (1/0.6; 25) | |
| MajBl | 5.05 (21/4.2; 238) | 4.15 (11/2.6; 145) | 8.77 (6/0.7; 43) | 2.69 (3/1.1; 52) | 7.31 (3/0.4; 21) | 3.57 (2/0.6; 25) | |
| IMiDs | AE | 2.27 (1/0.4; 55) | 0.00 (0/0.3; 27) | 8.02 (1/0.1; 17) | 0.00 (0/0.1; 10) | 0.00 (0/0.0; 3) | 19.71 (1/0.1; 6) |
| VTE | 0.00 (0/0.5; 55) | 0.00 (0/0.3; 27) | 0.00 (0/0.1; 17) | 0.00 (0/0.1; 10) | 0.00 (0/0.0; 3) | 0.00 (0/0.1; 6) | |
| MajBl | 4.36 (2/0.5; 55) | 7.73 (2/0.3; 27) | 0.00 (0/0.1; 17) | 15.92 (1/0.1; 10) | 85.34 (1/0.0; 3) | 0.00 (0/0.1; 6) | |
| ESAs | AE | 2.56 (12/4.7; 308) | 2.70 (6/2.2; 160) | 1.19 (2/1.7; 78) | 3.84 (4/1.0; 72) | 2.49 (1/0.4; 34) | 9.35 (3/0.3; 35) |
| VTE | 0.85 (4/4.7; 308) | 0.90 (2/2.2; 160) | 0.59 (1/1.7; 78) | 0.92 (1/1.1; 72) | 2.49 (1/0.4; 34) | 3.08 (1/0.3; 35) | |
| MajBl | 3.43 (16/4.7; 308) | 2.70 (6/2.2; 160) | 4.22 (7/1.7; 78) | 1.90 (2/1.1; 72) | 5.07 (2/0.4; 34) | 3.07 (1/0.3; 35) |
| Treatment . | Event . | All Patients with MF . | JAK2 mutation . | Triple negative . | Previous arterial event . | Previous venous event . | Previous major bleeding . |
|---|---|---|---|---|---|---|---|
| All patients | AE | 2.28 (105/46.0; 1079) | 2.74 (62/22.6; 549) | 1.77 (26/14.7; 260) | 5.15 (45/8.7; 268) | 4.20 (13/3.1; 86) | 4.09 (13/3.2; 92) |
| VTE | 0.96 (45/47.1; 1079) | 1.13 (26/23.1; 549) | 0.72 (11/15.2; 260) | 1.16 (11/9.5; 268) | 2.48 (8/3.2; 86) | 1.81 (6/3.3; 92) | |
| MajBl | 2.27 (105/46.3; 1079) | 2.46 (56/22.8; 549) | 1.80 (27/15.0; 260) | 2.83 (26/9.2; 268) | 4.42 (14/3.2; 86) | 3.66 (12/3.3; 92) | |
| No treatment | AE | 2.49 (45/18.1; 750) | 2.63 (20/7.6; 366) | 2.64 (18/6.8; 206) | 7.40 (20/2.7; 179) | 8.38 (8/1.0; 54) | 3.07 (4/1.3; 64) |
| VTE | 0.75 (14/18.7; 750) | 0.91 (7/7.7; 366) | 0.55 (4/7.2; 206) | 1.34 (4/3.0; 179) | 1.86 (2/1.1; 54) | 0.72 (1/1.4; 64) | |
| MajBl | 2.28 (42/18.4; 750) | 2.24 (17/7.6; 366) | 1.97 (14/7.1; 206) | 2.03 (6/3.0; 179) | 4.79 (5/1.0; 54) | 2.20 (3/1.4; 64) | |
| Hydroxyurea | AE | 2.02 (40/19.8; 672) | 2.70 (30/11.1; 392) | 1.10 (6/5.4; 144) | 3.66 (17/4.6; 185) | 3.62 (5/1.4; 54) | 4.01 (5/1.2; 53) |
| VTE | 1.15 (23/20.0; 672) | 1.23 (14/11.4; 392) | 1.13 (6/5.3; 144) | 1.04 (5/4.8; 185) | 3.64 (5/1.4; 54) | 3.26 (4/1.2; 53) | |
| MajBl | 1.95 (39/20.0; 672) | 2.46 (28/11.4; 392) | 0.74 (4/5.4; 144) | 3.43 (16/4.7; 185) | 2.08 (3/1.4; 54) | 5.54 (7/1.3; 53) | |
| Interferon | AE | 0.37 (1/2.7; 82) | 1.19 (1/0.8; 38) | 0.00 (0/1.2; 23) | 0.00 (0/0.2; 10) | 0.00 (0/0.3; 10) | 0.00 (0/0.0; 2) |
| VTE | 0.37 (1/2.7; 82) | 1.19 (1/0.8; 38) | 0.00 (0/1.2; 23) | 0.00 (0/0.2; 10) | 0.00 (0/0.3; 10) | 0.00 (0/0.0; 2) | |
| MajBl | 0.74 (2/2.7; 82) | 1.17 (1/0.9; 38) | 0.83 (1/1.2; 23) | 0.00 (0/0.2; 10) | 0.00 (0/0.3; 10) | 0.00 (0/0.0; 2) | |
| JAK inhibitors | AE | 3.89 (17/4.4; 238) | 5.19 (14/2.7; 145) | 1.22 (1/0.8; 43) | 5.41 (6/1.1; 52) | 2.07 (1/0.5; 21) | 3.73 (2/0.5; 25) |
| VTE | 1.63 (7/4.3; 238) | 1.51 (4/2.6; 145) | 2.53 (2/0.8; 43) | 1.78 (2/1.1; 52) | 2.40 (1/0.4; 21) | 1.78 (1/0.6; 25) | |
| MajBl | 5.05 (21/4.2; 238) | 4.15 (11/2.6; 145) | 8.77 (6/0.7; 43) | 2.69 (3/1.1; 52) | 7.31 (3/0.4; 21) | 3.57 (2/0.6; 25) | |
| IMiDs | AE | 2.27 (1/0.4; 55) | 0.00 (0/0.3; 27) | 8.02 (1/0.1; 17) | 0.00 (0/0.1; 10) | 0.00 (0/0.0; 3) | 19.71 (1/0.1; 6) |
| VTE | 0.00 (0/0.5; 55) | 0.00 (0/0.3; 27) | 0.00 (0/0.1; 17) | 0.00 (0/0.1; 10) | 0.00 (0/0.0; 3) | 0.00 (0/0.1; 6) | |
| MajBl | 4.36 (2/0.5; 55) | 7.73 (2/0.3; 27) | 0.00 (0/0.1; 17) | 15.92 (1/0.1; 10) | 85.34 (1/0.0; 3) | 0.00 (0/0.1; 6) | |
| ESAs | AE | 2.56 (12/4.7; 308) | 2.70 (6/2.2; 160) | 1.19 (2/1.7; 78) | 3.84 (4/1.0; 72) | 2.49 (1/0.4; 34) | 9.35 (3/0.3; 35) |
| VTE | 0.85 (4/4.7; 308) | 0.90 (2/2.2; 160) | 0.59 (1/1.7; 78) | 0.92 (1/1.1; 72) | 2.49 (1/0.4; 34) | 3.08 (1/0.3; 35) | |
| MajBl | 3.43 (16/4.7; 308) | 2.70 (6/2.2; 160) | 4.22 (7/1.7; 78) | 1.90 (2/1.1; 72) | 5.07 (2/0.4; 34) | 3.07 (1/0.3; 35) |
Data in the table are reported as rate (number of events/patient time; number of patients), in which time is measured in 100-year units.
AE, arterial event; ESAs, erythropoietin-stimulating agents; IMiDs, immunomodulatory drugs; MajBl, major bleeding; VTE, venous thromboembolism.
Among patients with a medical history of arterial events, the rates of recurrent arterial events varied across treatment groups: JAKi (5.41), erythropoietin-stimulating agents (3.84), and HU (3.66). The highest rate was seen among the patients with no ongoing treatment (7.40; Table 4).
Patients with Hb <11 g/dL at diagnosis had a higher rate of arterial events (3.18) than patients with Hb >11 g/dL (1.86), and the same pattern was seen among patients treated with JAKis (rates, 5.92 and 2.40, respectively). Among patients treated with HU and having low Hb at diagnosis, the rate of arterial event was 1.83, whereas in the group of patients with HU and having Hb >11 g/dL, the rate of arterial event was marginally higher at 2.04 (Table 5).
Rates of first event by treatment, Hb level, and WBC count at diagnosis
| Treatment . | Events . | Hb <11.0 g/dL . | Hb >11.0 g/dL . | WBC <11 × 109/L . | WBC >11 × 109/L . |
|---|---|---|---|---|---|
| All patients | AE | 3.18 (46/14.5; 434) | 1.86 (58/31.2; 639) | 1.98 (61/30.8; 655) | 2.88 (43/14.9; 418) |
| VTE | 0.67 (10/14.9; 434) | 1.10 (35/31.8; 639) | 0.83 (26/31.4; 655) | 1.24 (19/15.3; 418) | |
| MajBl | 3.69 (54/14.6; 434) | 1.63 (51/31.4; 639) | 2.05 (64/31.2; 655) | 2.77 (41/14.8; 418) | |
| No treatment | AE | 3.36 (22/6.5; 318) | 2.01 (23/11.4; 427) | 2.55 (34/13.3; 483) | 2.37 (11/4.6; 262) |
| VTE | 0.44 (3/6.8; 318) | 0.94 (11/11.7; 427) | 0.65 (9/13.9; 483) | 1.07 (5/4.7; 262) | |
| MajBl | 3.26 (22/6.7; 318) | 1.73 (20/11.5; 427) | 2.05 (28/13.6; 483) | 3.02 (14/4.6; 262) | |
| HU | AE | 1.83 (8/4.4; 216) | 2.04 (31/15.2; 451) | 1.37 (16/11.7; 352) | 2.92 (23/7.9; 315) |
| VTE | 0.92 (4/4.4; 216) | 1.24 (19/15.4; 451) | 0.94 (11/11.7; 352) | 1.49 (12/8.1; 315) | |
| MajBl | 3.94 (17/4.3; 216) | 1.43 (22/15.4; 451) | 1.44 (17/11.8; 352) | 2.75 (22/8.0; 315) | |
| IFN | AE | 0.00 (0/0.3; 18) | 0.00 (0/0.2; 50) | 0.00 (0/0.1; 42) | 0.00 (0/0.1; 22) |
| VT | 0.00 (0/0.1; 14) | 5.03 (1/0.2; 50) | 7.33 (1/0.1; 42) | 0.00 (0/0.1; 22) | |
| MajBl | 39.38 (2/0.1; 14) | 0.00 (0/0.2; 50) | 6.98 (1/0.1; 42) | 8.69 (1/0.1; 22) | |
| JAKis | AE | 5.92 (11/1.9; 115) | 2.40 (6/2.5; 121) | 2.42 (7/2.9; 140) | 6.81 (10/1.5; 96) |
| VTE | 1.55 (3/1.9; 115) | 1.69 (4/2.4; 121) | 1.79 (5/2.8; 140) | 1.33 (2/1.5; 96) | |
| MajBl | 4.80 (9/1.9; 115) | 5.30 (12/2.3; 121) | 4.33 (12/2.8; 140) | 6.58 (9/1.4; 96) | |
| IMiDs | AE | 2.54 (1/0.4; 46) | 0.00 (0/0.0; 9) | 2.99 (1/0.3; 38) | 0.00 (0/0.1; 17) |
| VTE | 0.00 (0/0.4; 46) | 0.00 (0/0.0; 9) | 0.00 (0/0.4; 38) | 0.00 (0/0.1; 17) | |
| MajBl | 4.87 (2/0.4; 46) | 0.00 (0/0.0; 9) | 5.69 (2/0.4; 38) | 0.00 (0/0.1; 17) | |
| ESAs | AE | 2.45 (8/3.3; 212) | 2.83 (4/1.4; 95) | 2.87 (9/3.1; 197) | 1.94 (3/1.5; 110) |
| VTE | 0.31 (1/3.3; 212) | 2.06 (3/1.5; 95) | 0.31 (1/3.2; 197) | 1.97 (3/1.5; 110) | |
| MajBl | 2.76 (9/3.3; 212) | 5.00 (7/1.4; 95) | 4.10 (13/3.2; 197) | 2.02 (3/1.5; 110) |
| Treatment . | Events . | Hb <11.0 g/dL . | Hb >11.0 g/dL . | WBC <11 × 109/L . | WBC >11 × 109/L . |
|---|---|---|---|---|---|
| All patients | AE | 3.18 (46/14.5; 434) | 1.86 (58/31.2; 639) | 1.98 (61/30.8; 655) | 2.88 (43/14.9; 418) |
| VTE | 0.67 (10/14.9; 434) | 1.10 (35/31.8; 639) | 0.83 (26/31.4; 655) | 1.24 (19/15.3; 418) | |
| MajBl | 3.69 (54/14.6; 434) | 1.63 (51/31.4; 639) | 2.05 (64/31.2; 655) | 2.77 (41/14.8; 418) | |
| No treatment | AE | 3.36 (22/6.5; 318) | 2.01 (23/11.4; 427) | 2.55 (34/13.3; 483) | 2.37 (11/4.6; 262) |
| VTE | 0.44 (3/6.8; 318) | 0.94 (11/11.7; 427) | 0.65 (9/13.9; 483) | 1.07 (5/4.7; 262) | |
| MajBl | 3.26 (22/6.7; 318) | 1.73 (20/11.5; 427) | 2.05 (28/13.6; 483) | 3.02 (14/4.6; 262) | |
| HU | AE | 1.83 (8/4.4; 216) | 2.04 (31/15.2; 451) | 1.37 (16/11.7; 352) | 2.92 (23/7.9; 315) |
| VTE | 0.92 (4/4.4; 216) | 1.24 (19/15.4; 451) | 0.94 (11/11.7; 352) | 1.49 (12/8.1; 315) | |
| MajBl | 3.94 (17/4.3; 216) | 1.43 (22/15.4; 451) | 1.44 (17/11.8; 352) | 2.75 (22/8.0; 315) | |
| IFN | AE | 0.00 (0/0.3; 18) | 0.00 (0/0.2; 50) | 0.00 (0/0.1; 42) | 0.00 (0/0.1; 22) |
| VT | 0.00 (0/0.1; 14) | 5.03 (1/0.2; 50) | 7.33 (1/0.1; 42) | 0.00 (0/0.1; 22) | |
| MajBl | 39.38 (2/0.1; 14) | 0.00 (0/0.2; 50) | 6.98 (1/0.1; 42) | 8.69 (1/0.1; 22) | |
| JAKis | AE | 5.92 (11/1.9; 115) | 2.40 (6/2.5; 121) | 2.42 (7/2.9; 140) | 6.81 (10/1.5; 96) |
| VTE | 1.55 (3/1.9; 115) | 1.69 (4/2.4; 121) | 1.79 (5/2.8; 140) | 1.33 (2/1.5; 96) | |
| MajBl | 4.80 (9/1.9; 115) | 5.30 (12/2.3; 121) | 4.33 (12/2.8; 140) | 6.58 (9/1.4; 96) | |
| IMiDs | AE | 2.54 (1/0.4; 46) | 0.00 (0/0.0; 9) | 2.99 (1/0.3; 38) | 0.00 (0/0.1; 17) |
| VTE | 0.00 (0/0.4; 46) | 0.00 (0/0.0; 9) | 0.00 (0/0.4; 38) | 0.00 (0/0.1; 17) | |
| MajBl | 4.87 (2/0.4; 46) | 0.00 (0/0.0; 9) | 5.69 (2/0.4; 38) | 0.00 (0/0.1; 17) | |
| ESAs | AE | 2.45 (8/3.3; 212) | 2.83 (4/1.4; 95) | 2.87 (9/3.1; 197) | 1.94 (3/1.5; 110) |
| VTE | 0.31 (1/3.3; 212) | 2.06 (3/1.5; 95) | 0.31 (1/3.2; 197) | 1.97 (3/1.5; 110) | |
| MajBl | 2.76 (9/3.3; 212) | 5.00 (7/1.4; 95) | 4.10 (13/3.2; 197) | 2.02 (3/1.5; 110) |
Data in table are reported as rate (number of events/patient time; number of patients), in which time is measured in 100-year units.
AE, arterial event; ESAs, erythropoietin-stimulating agents; HU, hydroxyurea; IFN, interferon, IMiDs, immunomodulatory drugs; JAKis, JAK inhibitors; MajBl, major bleeding; VTE, venous thromboembolism.
Patients with MF with a WBC count >11 × 109/L at diagnosis had a higher rate of arterial events (rate, 2.88) than patients with a WBC count <11 × 109/L (rate, 1.98), and the same correlation was seen among patients treated with HU (rates, 2.92 and 1.37) and JAKis (rates, 6.81 and 2.42, respectively; Table 5).
Risk of arterial and venous events during treatment
In multivariable analysis, treatment with JAKis was associated with an increased risk of arterial or venous events (HR, 2.16; 95% CI, 1.27-3.68; P = .0046). Ongoing treatment with LMWH was also identified as an independent risk factor for arterial or venous events (HR, 3.16; 95% CI, 1.67-5.96; P < .001; Figure 2; supplemental Table 4). Previous arterial and venous events and older age were also associated with an increased risk of an arterial or venous event, with HRs of 1.98 (95% CI, 1.35-2.92; P < .001), 2.30 (95% CI, 1.35-3.93; P = .0022), and 1.59 (95% CI, 1.22-2.07; P < .001), respectively (supplemental Table 3). The presence of a JAK2 V617F mutation failed to reach statistical significance as a risk factor for arterial or venous events (HR, 1.38; 95% CI, 0.92-2.07; P = .12; supplemental Table 4).
Forest plot illustrating risk factors for major bleeding and arterial or venous event in MF. The model is adjusted for age, sex, presence of JAK2 V617F mutation, levels of Hb, WBC count, platelet count, previous thrombotic event or bleeding, symptom-directed therapy, and antithrombotic treatment. DOACs, direct oral anticoagulants; ESA, erythropoietin-stimulating agent; IMiDs, immunomodulatory drugs; LMWH, low-molecular weight heparin, VTE, venous thromboembolism.
Forest plot illustrating risk factors for major bleeding and arterial or venous event in MF. The model is adjusted for age, sex, presence of JAK2 V617F mutation, levels of Hb, WBC count, platelet count, previous thrombotic event or bleeding, symptom-directed therapy, and antithrombotic treatment. DOACs, direct oral anticoagulants; ESA, erythropoietin-stimulating agent; IMiDs, immunomodulatory drugs; LMWH, low-molecular weight heparin, VTE, venous thromboembolism.
Risk of major bleeding during treatment
Treatment with JAKis was identified as an independent risk factor for major bleeding in our cohort (HR, 2.04; 95% CI, 1.15-3.63; P = .015; Figure 2; supplemental Table 4). The use of antiplatelet agents, anticoagulants, or LMWH did not reach statistical significance as risk factors for major bleeding, with HRs of 0.73 (95% CI, 0.45-1.18; P = .20), 0.81 (95% CI, 0.43-1.55; P = .53), and 1.64 (95% CI, 0.64-4.17; P = .30), respectively. A high WBC count and a medical history of previous venous thrombosis were associated with an increased risk of major bleeding, with HRs of 1.16 (95% CI, 1.08-1.24; P < .001) and 2.01 (95% CI, 1.03-3.90; P = .040), respectively, whereas a high platelet count had a protective effect against major bleeding (HR, 0.51; 95% CI, 0.33-0.80; P = .0034; Figure 2; supplemental Table 4).
Discussion
In this nationwide population-based study including 1079 patients with PMF and SMF and matched controls, we conclude that the rate of venous and arterial events and major bleeding are significantly higher in MF than in corresponding controls and exceeds the rates of the same events in PV and ET.13 Treatment with JAKis was identified as an independent risk factor for arterial and venous events and major bleeding, a result that, to our knowledge, has not been previously shown.
In total 125 arterial events (rate, 2.59), 51 venous events (rate, 1.06), and 123 events of major bleeding (rate, 2.55) occurred during follow-up in our cohort, rates lightly higher than those previously described by Hernández-Boluda et al5 but lower than the rates presented in 743 patients with PMF in Korea.14 Both this study and the study by Hur et al14 are based on large health care registries, which gives a more complete coverage of events during follow-up and could explain the higher rates.
In our cohort, patients treated with JAKis had a higher rate of arterial events than described in the study from the Spanish Registry of MF.5 This could again be explained by the fact that our data were collected from the NPR, which has an almost complete coverage of thromboembolic events in inpatient and outpatient care. In Sweden, treatment with JAKis is subsidized only for patients classified as intermediate-2 or high-risk category by the IPSS, which reflects both the number of treated patients and the higher rate of complications expected among patients with higher IPSS score.5 Although this study was not designed to compare the different therapies head to head, the diverging rates of arterial events, ranging from 0.37 to 4.67 events per 100 patient-years, in the different treatment groups are quite striking. Concerns of increased risk of major adverse cardiovascular events have been raised for JAKis used in rheumatoid arthritis and other inflammatory conditions,15 and this led to a boxed warning by the US Food and Drug Administration in 2021. No boxed warning has been issued for ruxolitinib or fedratinib used in MF, and this is supported by previous randomized trials on ruxolitinib, which showed no indication of an increased risk of major adverse cardiovascular events.16,17 The low rates of events in patients treated with IFN could partly be explained by lower age in this group. IMiDs have, in a recent Spanish study, been associated with an increased risk of thrombotic events,5 whereas, in our cohort, only 1 arterial event and no venous event occurred in the 55 patients treated with IMiDs, and no association between this therapy and an increased risk of arterial or venous event could be established. In total, 76% of the patients treated with IMiDs in this cohort were also prescribed antiplatelet agents, anticoagulants, or LMWH, which could have prevented the development of thromboembolic events.
Prior studies have shown that the presence of a JAK2 mutation is associated with an increased risk of thrombosis in MF.5,18 Of the 238 patients treated with JAKis, 145 patients harbored a JAK2 mutation, and the rate of arterial events were higher in this group (rate, 5.19) than in triple-negative patients (rate, 1.22). On the contrary, venous events were more frequent in the triple-negative group treated with JAKis (rate, 2.53) than JAK2-mutated patients with the same therapy (rate, 1.51). In multivariable analysis, the presence of a JAK2 mutation did not reach statistical significance as a risk factor for venous or arterial events during follow-up in this study.
Leukocytosis as a risk factor for thrombosis in MPNs has been a matter of discussion, and previous studies have shown diverging results.5,18,19 In our cohort, we could not establish that a higher WBC at diagnosis was associated with an increased risk of arterial or venous events.
A previous venous or arterial event was identified as a risk factor for a recurrent venous or arterial event, which is consistent with earlier reports.5,20,21
Patient-specific traits such as thrombocytopenia, variceal bleeding secondary to portal hypertension, and acquired von Willebrand syndrome due to extremely high platelets, as well as ongoing antithrombotic treatment, can affect the occurrence of major bleeding in MF, but few studies have assessed the actual frequency and risk factors of these events.5,14,22 This study shows that major bleeding occurred at a rate of 2.55 events per 100 patient-years in the entire MF cohort, and patients treated with JAKis had the highest rates of bleeding events (rate, 5.33). Triple-negative patients with ongoing JAKi therapy had higher rates of major bleeding (rate, 8.77) than JAK2-mutated patients with JAKi treatment (rate, 4.15). Use of anticoagulants has been shown to be associated with an increased risk of major bleeding5; but in our cohort, use of anticoagulants failed to predict major bleeding. A higher WBC count at diagnosis, JAKi therapy, and previous venous thrombosis were identified as independent risk factors for major bleeding in this study, a result that, to our knowledge, has not been shown previously.
The high rate of all-cause mortality in the group of patients treated with IMiDs, despite few arterial and venous events and major bleeding occurring during the limited treatment time of 46 years, could represent the progressive nature of the disease and the use of IMiDs in the later stages of MF when all other therapies have failed.
Limitations of this study are mainly derived from its registry-based data retrieval, in which all data in the MPN register are manually reported at each local center; therefore, registrational errors cannot be ruled out and data cannot be confirmed on an individual level. This study does not hold data on smoking habits and hyperlipidemia, which are 2 major risk factors for cardiovascular events. Patients treated with HU and JAKis demonstrated higher rates of thrombosis than patients receiving IFN. IFN has known immunomodulatory effects, which could be one of the causes of lower rates of events in IFN–treated patients. Our study does not include data on inflammatory markers, and therefore, we cannot confirm such a correlation.
In conclusion, this population-based study shows that thromboembolic events and major bleeding are more frequent in MF than reported in other MPNs, and the rates of events diverge in the different treatment groups, with JAKi-treated patients having higher rates of arterial events than patients treated with other therapies. In Sweden, JAKi therapy is only subsidized for patients with higher IPSS score, which could explain the higher rates of complications. Treatment with JAKi, LMWH, previous venous or arterial events, and older age were all identified as independent risk factors for a recurrent arterial and venous thrombosis. A high WBC count at diagnosis, ongoing JAKi therapy, and previous venous events were associated with an increased risk of major bleeding. These findings could serve as a useful tool in clinical practice when choosing symptom-directed therapy and concomitant antithrombotic treatment based on the clinical presentation of each patient with MF. Further studies on thromboembolic events and major bleeding during the different therapies used in MF are needed to minimize the risk of disabling and life-threatening complications in patients with MF.
Acknowledgments
The authors thank all patients with myeloproliferative neoplasm (MPN) in Sweden who participated in the Swedish MPN register as well as all colleagues who contribute and report data to the Swedish MPN register.
This study was supported by FoU Region Norrbotten, Visare Norr, The Swedish Stroke Fund, Emil Andersson Fund for medical research, and the JC Kempe Memorial Scholarship Foundation.
Authorship
Contribution: A.S. and A.E.L. designed the study; H.R. performed statistical analysis; A.S., A.E.L., B.A., H.H., and M.L. analyzed the clinical data; A.E.L. wrote the first draft of the manuscript; and all authors analyzed and interpreted the data, and revised and gave the final approval of the manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anneli Enblom Larsson, Department of Public Health and Clinical Medicine, Umeå University, 90187 Umeå, Sweden; email: anneli.enblom-larsson@umu.se.
References
Author notes
Because of the sensitive nature of the data that support the findings of this study, data cannot be shared publicly for ethical reasons. The data will be shared on reasonable request to the corresponding author, Anneli Enblom-Larsson (anneli.enblom-larsson@umu.se), after permission from the National Board of Health and Welfare managing the included registers.
The full-text version of this article contains a data supplement.