Key Points
BCL11A disruption impairs erythroid precursor expansion in vitro and after xenotransplantation into immunodeficient mice.
BCL11A regulates at least 25 genes directly and numerous others indirectly in erythroid precursors.
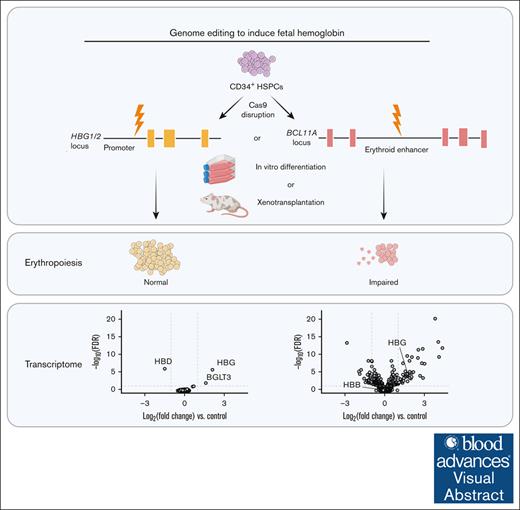
Visual Abstract
Genetic depletion of the transcriptional repressor BCL11A in red blood cell precursors alleviates β-hemoglobinopathies by inducing the fetal γ-globin genes. However, additional erythroid genes are regulated by BCL11A and the effects of its deficiency on erythropoiesis are insufficiently described. We discovered that Cas9 disruption of the BCL11A intron 2 erythroid enhancer in CD34+ hematopoietic stem and progenitor cells using a clinically approved strategy caused impaired expansion and apoptosis of erythroid precursors in vitro and reduced repopulation of the erythroid compartment after xenotransplantation into immunodeficient mice. Mutant colony-forming unit erythroid cells, proerythroblasts, and basophilic erythroblasts exhibited dysregulation of 94 genes (more than twofold change, false discovery rate < 0.05), 25 of which are likely direct targets of BCL11A. Differentially expressed genes were associated with a range of biological pathways that affect cell expansion and survival. Our findings reveal that BCL11A regulates additional aspects of erythropoiesis beyond γ-globin gene repression, with unknown clinical consequences.
Introduction
The β-like globin locus undergoes a perinatal developmental switch from γ-globin (HBG1 and HBG2) to β-globin (HBB) gene transcription, resulting in a shift from fetal hemoglobin (HbF, α2γ2) to adult hemoglobin (α2β2) expression in red blood cells (RBCs).1 Reversing the γ-to-β globin switch is effective for treating common β-hemoglobinopathies caused by HBB mutations, particularly sickle cell disease and β-thalassemia.2 Several therapeutic approaches to induce RBC HbF through genetic engineering of patient hematopoietic stem cells (HSCs) are based on altering the expression or function of BCL11A, a transcriptional repressor that is induced in adult-type erythroid precursors approximately during birth and binds to a cognate motif in the γ-globin gene promoters to inhibit their expression.3-5 In December 2023, exagamglogene autotemcel (exa-cel), a therapy using CRISPR/Cas9 to disrupt an erythroid-specific enhancer in BCL11A intron 2, received US Food and Drug Administration approval for treating sickle cell disease and β-thalassemia.2,6,7 Lentiviral vector-mediated, erythroid-specific expression of a short hairpin RNA targeting BCL11A messenger RNA also demonstrates efficacy for treating sickle cell disease.8 It is also possible to induce HbF therapeutically by genome editing-mediated disruption of a key BCL11A binding motif (TGACC) at position −118 to −114 of the γ-globin promoters.5,9-11
Suppression of BCL11A expression for therapeutic induction of HbF must be erythroid specific, as the gene is essential for the production of B cells, some T cells, dendritic cells, HSCs, and some neurons.12-18 In contrast, erythroid-specific disruption of the Bcl11a gene in mice has minimal effects on RBC development and BCL11A deficiency seems to be tolerated during in vitro erythroid differentiation of human CD34+ hematopoietic stem and progenitor cells (HSPCs).19-21 These results are consistent with findings that BCL11A regulates relatively few genes directly in erythroid cells.22 However, we noticed reduced expansion of erythroid precursors generated from CD34+ HSPCs harboring Cas9 disruptions of the BCL11A intron 2 enhancer, which is consistent with data reported in a meeting abstract and a recent publication.23,24 Considering the importance of BCL11A as a therapeutic target for β-hemoglobinopathies, we sought to evaluate this effect further.
Methods
Genome editing of CD34+ HSPCs using Cas9 RNP
Genome editing was performed using ExPERT GTx (MaxCyte, Inc, Rockville, MD) with electroporation program HSC3 or 4D-Nucelofector (Lonza, Basel, Switzerland) with electroporation program DS-130. The modified synthetic single-guide RNAs (sgRNAs) (supplemental Table 1) were purchased from Synthego (Menlo Park, CA) or BioSpring GmbH (Frankfurt, Germany). The 3× NLS-SpCas9 protein was manufactured by the St. Jude Protein Core (Memphis, TN). Cas9 and sgRNA were mixed at a 1:2 or 1:1.5 ratio and incubated for 15 minutes to make the ribonucleoprotein (RNP) complex.
For xenotransplantation into NBSGW mice, 6 to 10 ×106 cells were electroporated in 100 μL MaxCyte Electroporation Buffer in OC-100 cartridges using MaxCyte ExPERT GTx with program HSC3. For small-scale genome editing, ∼5 × 105 cells were electroporated in 20 μL using P3 Primary Cell 96-well Nucleofector Kit and Lonza 4D-Nucleofector with program DS-130. After electroporation, cells were incubated in the supplemented X-VIVO 10 media without penicillin/streptomycin for 24 hours.
Erythroid and myeloid differentiation of CD34+ HSPCs
Erythroid differentiation was performed using a 3-phase protoco1.25 CD34+ HSPCs were incubated for 7 days in phase 1 medium (IMDM, Thermo Fisher Scientific, Waltham, MA), 3% human AB serum (Atlanta Biologicals, Flowery Branch, GA, S40110), 2% human blood type AB plasma (SeraCare, Milford, MA), 1× penicillin/streptomycin, 3 U/mL erythropoietin (EPOGEN; Amgen, Thousand Oaks, CA, # 55513-144-01), 3 U/mL heparin (Sagent Pharmaceuticals, Schaumburg, IL, 25021-401-02), 200 μg/mL holo-transferrin (Millipore Sigma, Burlington, MA, T0665), 10 ng/mL human stem cell factor, and 1 ng/mL human interleukin-3 (IL-3; R&D Systems, Minneapolis, MN, 203-IL/CF). At days 7 to 13 (phase 2), phase 1 media without IL-3 were used. At days 13 to 18 (phase 3), phase 1 media without IL-3 and stem cell factor and with 1 mg/mL holo-transferrin were used. Myeloid differentiation was performed using myeloid media with StemSpan SFEM II (StemCell Technologies, Vancouver, Canada), StemSpan Myeloid Expansion Supplement (100×), and 1× penicillin/streptomycin. Cells were counted using Solution 18 (Acridine Orange/DAPI [4′,6-diamidino-2-phenylindole]) and NucleoCounter NC-3000 (ChemoMetec Inc, Allerod, Denmark).
Xenotransplantation studies
Mouse studies were approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital. The immunodeficient mouse strain, nonobese diabetic, B6.SCID.Il2rg–/–KitW41/W41 (NBSGW) was purchased from The Jackson Laboratory (Bar Harbor, ME). The 6- to 9-week-old female NBSGW mice were transplanted with 5 × 105 cells (for competition repopulation assay, 1:1 of edited 2.5 × 105 cells) per mouse through their tail vein. Mice were housed in the St. Jude Animal Research Center with food ad libitum and Baytril/Sulfatrim water for infection prophylaxis. After 16 to 17 weeks, mice were euthanized to collect bone marrow (BM) from femurs, tibiae, and hip bones for further analyses.
Statistical analysis
Outcomes were compared using paired t tests, exact Wilcoxon rank sum tests, or generalized estimating equation models as appropriate. In the generalized estimating equation model, we adjusted for donor and time effects. The Shapiro-Wilk test was used to determine whether the data followed a normal distribution. Logarithmic transformations were applied to some outcomes. False discovery rate (FDR) q values were reported as appropriate. In all figures, ∗, ∗∗, ∗∗∗, or ∗∗∗∗ refers to P < .05, P < .01, P < .001, or P < .0001, respectively.
Results
BCL11A disruption impairs the expansion of CD34+ HSPC-derived erythroid precursors
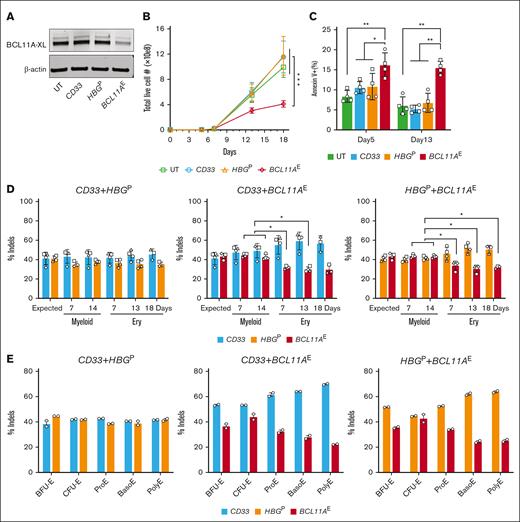
We electroporated peripheral blood–mobilized CD34+ HSPCs from 3 healthy donors with RNP consisting of Cas9 + sgRNA targeting the +58 DNase I hypersensitive site of the BCL11A erythroid enhancer (BCL11AE), according to the clinically approved exa-cel therapy.6,7 For comparison, we electroporated the same HSPCs with RNPs targeting the protein-coding region of the myeloid-expressed gene CD33 or the BCL11A binding motif at position -118 to -114 in the γ-globin gene promoters (HBGP).26,27 In a separate experiment, we electroporated CD34+ HSPCs from 2 different healthy donors with RNPs targeting BCL11AE, CD33, or the BCL11A protein-coding region in exon 2 (BCL11AEx). Gene targeted and control cells were grown in HSPC maintenance medium for 1 day and then switched to erythroid differentiation medium. Indel frequencies in HSPCs grown in maintenance medium for 4 days after electroporation were ≥78% for all targets (supplemental Figure 1A-B). Western blot analysis on day 7 of erythroid differentiation revealed that the BCL11A-XL isoform capable of suppressing γ-globin transcription was reduced after targeting the BCL11A erythroid enhancer and to a greater extent after targeting the protein-coding region (Figure 1A; supplemental Figure 1C). On days 13 and 18, the expansion of BCL11AE-disrupted erythroblasts was reduced by 50% to 55% and 35% to 41%, respectively, and accompanied by significantly increased apoptosis compared with CD33- or HBGP-targeted cells (Figure 1B-C). Disruption of BCL11A exon 2 (BCL11AEx) caused greater growth suppression and apoptosis than disruption of the intron 2 erythroid enhancer (BCL11AE), commensurate with more substantial reduction of BCL11A protein expression by the former (supplemental Figure 1D-E). The proportions of erythroid precursors at defined stages of maturation were not altered by BCL11AE disruption compared with controls (supplemental Figure 1F). As expected, disruption of BCL11AE or HBGP but not CD33 caused induction of HbF (supplemental Figure 1G).
Disruption of the BCL11A erythroid enhancer impairs in vitro human erythropoiesis. Healthy human adult donor peripheral blood mobilized CD34+ HSPCs were electroporated with RNP complexes consisting of Cas9 + sgRNA targeting either the CD33 protein coding region, the BCL11A intron 2 erythroid enhancer (BCL11AE), or the BCL11A binding motif in the γ-globin genes (HBGP) grown in HSPC maintenance medium for 24 hours and then switched to erythroid differentiation medium. (A) Western blot revealing BCL11A-XL and β-actin protein levels on day 7 of erythroid differentiation. (B) Live cell numbers on days 5, 7, 13, and 18 of erythroid differentiation. Symbols illustrate the average normalized values for 3 different CD34+ HSPC donors. Data are illustrated as mean ± standard error of the mean (SEM). ∗∗∗FDR-adjusted P < .001 determined by generalized estimating equation (GEE) models with post hoc comparisons. (C) Apoptosis measured by flow cytometry at days 5 and 13 of erythroid differentiation. Graph reveals %annexin V+, propidium iodide positive or negative cells from replicate experiments, each with 2 different CD34+ HSPC donors. Data are illustrated as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 indicate FDR-adjusted paired t tests or exact Wilcoxon tests with post hoc comparisons. (D) Equal numbers of cells targeted separately at 2 different loci (BCL11AE, CD33, or HBGP) were mixed and transferred to erythroid (Ery) or myeloid differentiation media. Graphs illustrate indel frequencies over time. The expected indel frequency at each target site was calculated as 50% of the indel frequency in cells targeted with each individual RNP (supplemental Figure 2B). Each symbol represents a separate experiment with unique donor CD34+ HSPCs. Data are illustrated as mean ± SD. ∗FDR-adjusted P < .05 determined by paired t tests or exact Wilcoxon tests with post hoc comparisons. (E) On days 4, 7, and 14 of erythroid differentiation, mixtures of cells targeted at different loci as described for panel D were fractionated into defined maturation stages by flow cytometry (supplemental Figure 2C) and indel frequencies of each targeted gene were determined. Erythroid precursors in order of increasing maturation stage are as follows: BFU-E, burst-forming unit erythroid; CFU-E, colony-forming unit erythroid; ProE, proerythroblast; BasoE, basophilic erythroblast; and PolyE, polychromatophilic erythroblast. Graphs show data from an experiment with duplicate using a healthy donor CD34+ HSPCs. UT, untreated.
Disruption of the BCL11A erythroid enhancer impairs in vitro human erythropoiesis. Healthy human adult donor peripheral blood mobilized CD34+ HSPCs were electroporated with RNP complexes consisting of Cas9 + sgRNA targeting either the CD33 protein coding region, the BCL11A intron 2 erythroid enhancer (BCL11AE), or the BCL11A binding motif in the γ-globin genes (HBGP) grown in HSPC maintenance medium for 24 hours and then switched to erythroid differentiation medium. (A) Western blot revealing BCL11A-XL and β-actin protein levels on day 7 of erythroid differentiation. (B) Live cell numbers on days 5, 7, 13, and 18 of erythroid differentiation. Symbols illustrate the average normalized values for 3 different CD34+ HSPC donors. Data are illustrated as mean ± standard error of the mean (SEM). ∗∗∗FDR-adjusted P < .001 determined by generalized estimating equation (GEE) models with post hoc comparisons. (C) Apoptosis measured by flow cytometry at days 5 and 13 of erythroid differentiation. Graph reveals %annexin V+, propidium iodide positive or negative cells from replicate experiments, each with 2 different CD34+ HSPC donors. Data are illustrated as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 indicate FDR-adjusted paired t tests or exact Wilcoxon tests with post hoc comparisons. (D) Equal numbers of cells targeted separately at 2 different loci (BCL11AE, CD33, or HBGP) were mixed and transferred to erythroid (Ery) or myeloid differentiation media. Graphs illustrate indel frequencies over time. The expected indel frequency at each target site was calculated as 50% of the indel frequency in cells targeted with each individual RNP (supplemental Figure 2B). Each symbol represents a separate experiment with unique donor CD34+ HSPCs. Data are illustrated as mean ± SD. ∗FDR-adjusted P < .05 determined by paired t tests or exact Wilcoxon tests with post hoc comparisons. (E) On days 4, 7, and 14 of erythroid differentiation, mixtures of cells targeted at different loci as described for panel D were fractionated into defined maturation stages by flow cytometry (supplemental Figure 2C) and indel frequencies of each targeted gene were determined. Erythroid precursors in order of increasing maturation stage are as follows: BFU-E, burst-forming unit erythroid; CFU-E, colony-forming unit erythroid; ProE, proerythroblast; BasoE, basophilic erythroblast; and PolyE, polychromatophilic erythroblast. Graphs show data from an experiment with duplicate using a healthy donor CD34+ HSPCs. UT, untreated.
Next, we electroporated CD34+ HSPCs with RNPs targeting BCL11AE, CD33, or HBGP, prepared 1:1 mixtures of cells targeted at 2 different loci individually, transferred them to media containing erythroid or myeloid cytokines, and measured indel frequencies over time (supplemental Figure 2A). The expected indel frequencies in the 1:1 mixtures were estimated to be 50% of the original indel frequency for input cells targeted at a single locus, which was >80% (supplemental Figure 2B). Mixtures of cells targeted at either CD33 or HBGP maintained the expected indel frequencies throughout in vitro myeloid or erythroid differentiation (Figure 1D, left panel). In contrast, the proportion of BCL11AE-targeted cells declined progressively in mixtures with CD33- or HBGP-targeted cells during erythroid but not myeloid differentiation (Figure 1D, middle and right panels). After 4, 7, and 14 days of erythroid differentiation, we purified erythroblasts at different maturation stages by flow cytometry (supplemental Figure 2C) and determined indel frequencies. As expected, mixtures of HBGP:CD33 targeted cells maintained expected indel frequencies for each targeted locus at all developmental stages (Figure 1E, left panel). In contrast, BCL11AE-targeted erythroid cells were progressively outgrown by CD33- or HBGP-targeted cells during erythroid maturation (Figure 1E, middle and right panels).
BCL11AE disruption impairs human erythropoiesis in a mouse xenotransplantation model
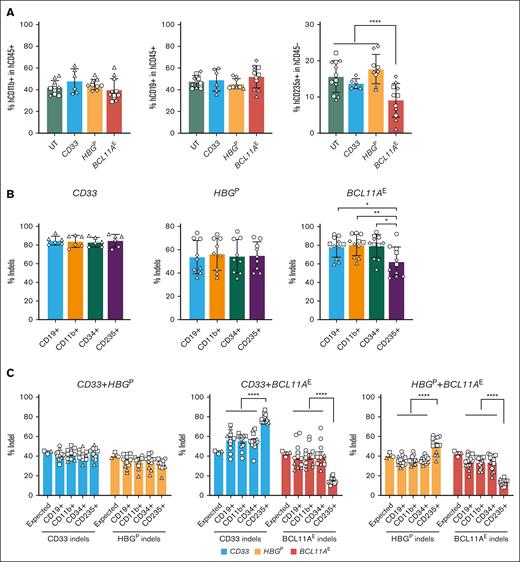
To study the effects of BCL11A deficiency on erythropoiesis in vivo, we disrupted BCL11AE, CD33, or HBGP individually in CD34+ HSPCs, transplanted them separately or in 1:1 mixtures into immunodeficient NBSGW mice, and examined donor cell progeny in host BM after 16 weeks (supplemental Figure 3A). Before cell mixing, the indel frequencies at each target were >78% (supplemental Figure 3B-C). Homogeneous BCL11AE-, CD33-, or HBGP-disrupted HSPCs generated similar levels of CD45+ hematopoietic cells, CD11b+ myeloid cells, and CD19+ B cells in recipient BM (supplemental Figure 3D; Figure 2A, left and middle panels). In contrast, BCL11AE-disrupted HSPCs generated ∼58% fewer erythroblasts than cells disrupted at the other loci (Figure 2A, right panel). Moreover, CD235+ erythroblasts generated after transplantation of BCL11AE-disrupted HSPCs exhibited a significant, selective reduction in indel frequency, indicating they are outcompeted by nontargeted cells (Figure 2B, compare left and middle panels with right panel). Erythroid maturation of human donor HSPCs was not altered by disruption of BCL11A or control genes (supplemental Figure 3E).
Disruption of the BCL11A erythroid enhancer impairs human erythropoiesis in vivo. (A) CD34+ HSPCs were electroporated with RNPs targeting either BCL11AE, CD33, or HBGP and then transplanted separately into immunodeficient NBSGW mice (supplemental Figure 3A, left panel). At 16 weeks, recipient BM was isolated and analyzed by flow cytometry for the proportions of human myeloid (hCD11b+), B cell (hCD19+), and erythroid (hCD235+) lineages. Data are illustrated as mean ± SD. ∗∗∗∗FDR-adjusted P < .0001 determined by GEE models with post hoc comparisons. (B) Percentages of indels in flow cytometry-purified human donor-derived CD19+ B cells, CD11b+ myeloid cells, CD34+ lineage− HSPCs, and CD235+ erythroid cells isolated from mice transplanted with BCL11AE-, CD33-, or HBGP-disrupted HSPCs. Each symbol datapoint represents an individual mouse transplanted with cells from a specific CD34+ HSPC donor represented by unique symbols. Data are illustrated as mean ± SD. ∗FDR-adjusted P < .05 or ∗∗P < .01 determined by paired t tests or exact Wilcoxon tests with post hoc comparisons. (C) CD34+ HSPCs were electroporated with RNPs targeting either BCL11AE, CD33, or HBGP. Equal numbers of cells targeted at 2 different loci were mixed and then transplanted into immunodeficient NBSGW mice (supplemental Figure 3A, right panel). At 16 weeks, indel frequencies were measured in human donor-derived CD19+ B cells, CD11b+ myeloid cells, CD34+ lineage− HSPCs, and CD235+ erythroid cells purified from mouse recipient BM. Graphs reveal %indels (mean ± SEM) in mixtures of cells targeted at the indicated loci. ∗∗∗∗FDR-adjusted P < .0001 determined by paired t tests or exact Wilcoxon tests with post hoc comparisons. UT, untreated.
Disruption of the BCL11A erythroid enhancer impairs human erythropoiesis in vivo. (A) CD34+ HSPCs were electroporated with RNPs targeting either BCL11AE, CD33, or HBGP and then transplanted separately into immunodeficient NBSGW mice (supplemental Figure 3A, left panel). At 16 weeks, recipient BM was isolated and analyzed by flow cytometry for the proportions of human myeloid (hCD11b+), B cell (hCD19+), and erythroid (hCD235+) lineages. Data are illustrated as mean ± SD. ∗∗∗∗FDR-adjusted P < .0001 determined by GEE models with post hoc comparisons. (B) Percentages of indels in flow cytometry-purified human donor-derived CD19+ B cells, CD11b+ myeloid cells, CD34+ lineage− HSPCs, and CD235+ erythroid cells isolated from mice transplanted with BCL11AE-, CD33-, or HBGP-disrupted HSPCs. Each symbol datapoint represents an individual mouse transplanted with cells from a specific CD34+ HSPC donor represented by unique symbols. Data are illustrated as mean ± SD. ∗FDR-adjusted P < .05 or ∗∗P < .01 determined by paired t tests or exact Wilcoxon tests with post hoc comparisons. (C) CD34+ HSPCs were electroporated with RNPs targeting either BCL11AE, CD33, or HBGP. Equal numbers of cells targeted at 2 different loci were mixed and then transplanted into immunodeficient NBSGW mice (supplemental Figure 3A, right panel). At 16 weeks, indel frequencies were measured in human donor-derived CD19+ B cells, CD11b+ myeloid cells, CD34+ lineage− HSPCs, and CD235+ erythroid cells purified from mouse recipient BM. Graphs reveal %indels (mean ± SEM) in mixtures of cells targeted at the indicated loci. ∗∗∗∗FDR-adjusted P < .0001 determined by paired t tests or exact Wilcoxon tests with post hoc comparisons. UT, untreated.
We next performed competitive repopulation studies by transplanting 1:1 mixtures of cells targeted separately at 2 loci. BM repopulation by human donor cells was >95% (supplemental Figure 3F). Similar to the results of in vitro studies, mixtures of HSPCs targeted at either HBGP or CD33 generated expected indel frequencies in B-cell (CD19+), myeloid (CD11b+), HSPC (CD34+), and erythroid (CD235+) progenies (Figure 2C, left panel). In contrast, the indel frequencies of CD235+ erythroblasts derived from BCL11AE-disrupted HSPCs were significantly lower than the expected frequency of ∼40% (Figure 2C, middle and right panels).
BCL11A regulates multiple genes indirectly through a smaller number of direct target genes
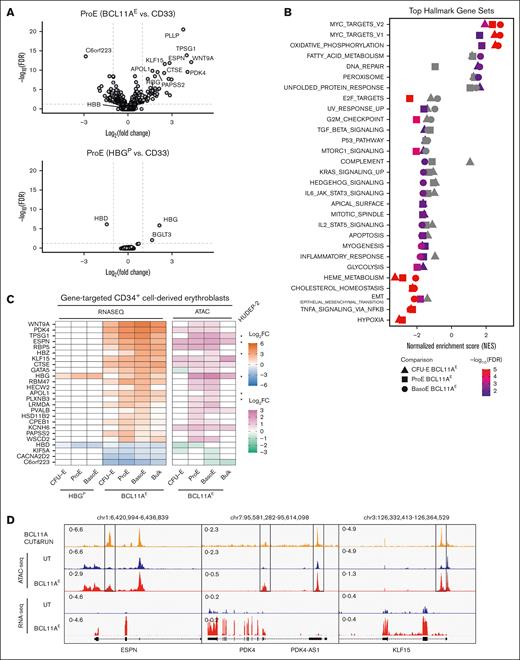
To investigate the transcriptomic changes caused by BCL11A disruption during erythropoiesis, we performed RNA sequencing (RNA-seq) on colony forming unit-erythroid precursors, proerythroblasts, and basophilic erythroblasts generated from BCL11AE-, HBGP-, CD33-disrupted and untreated CD34+ HSPCs (supplemental Figure 4A). No significant differentially expressed genes (DEGs) (more than twofold change, FDR < 0.05) were identified when comparing untreated erythroid cells with CD33-disrupted ones at any maturation stage (supplemental Figure 4B). Compared with CD33-disrupted erythroblasts, BCL11AE-disrupted ones exhibited significant differential gene expression in each erythroid precursor stage examined: 12 upregulated and 18 downregulated genes in colony forming unit-erythroid, 43 upregulated and 24 downregulated genes in proerythroblasts, and 35 upregulated and 16 downregulated genes in basophilic erythroblasts (Figure 3A; supplemental Figure 4C-D). In total, we identified 94 DEGs (52 upregulated and 42 downregulated) across the 3 maturation stages examined in BCL11AE-disrupted cells. In contrast, HBGP-disrupted HSPCs had only 5 DEGs (3 upregulated and 2 downregulated) across the stages, with 3 of these genes located within the β-globin gene cluster. Upregulated genes in BCL11AE-disrupted cells were significantly enriched in pathways including Myc targets, oxidative phosphorylation, and fatty acid metabolism (FDR < 0.05 and normalized enrichment score > 1.5 by gene set enrichment analysis; Figure 3B). Downregulated genes were associated with processes including cell cycle, P53 pathway, apoptosis, glycolysis, cholesterol homeostasis, and heme metabolism.
Genes regulated by BCL11A during erythropoiesis in vitro. (A) Gene expression changes identified by RNA-seq analysis of erythroblasts generated by in vitro differentiation of gene-targeted CD34+ HSPCs. Volcano plots illustrate DEGs in BCL11AE- (top panel) or HBGP-disrupted (bottom panel) proerythroblasts compared with CD33-disrupted ones. HBG refers to HBG1 + HBG2 transcripts. Dashed lines represent the 0.05 FDR statistical significance cutoff (y-axis) and log2 fold change of 1 or −1 (x-axis). (B) Gene set enrichment analysis (GSEA) from BCL11AE- vs CD33-disrupted CFU-E, proerythroblasts, and basophilic erythroblasts. Gene sets represented on the left and right halves of the graph are enriched in downregulated and upregulated genes, respectively. Gray shapes indicate that the gene set has an FDR ≥0.05 or normalized enrichment score ≤1.5. (C) Analysis of 25 genes regulated directly by BCL11A in erythroblasts. Left panel illustrates RNA expression heat map in HBGP- or BCL11AE-disrupted vs CD33-disrupted erythroblasts. Right panel illustrates heat map of assay for transposase-accessible chromatin using sequencing (ATAC-seq) signals reflecting changes in chromatin accessibility near BCL11A-bound regions in BCL11AE-disrupted vs untreated erythroblasts. Asterisks indicate direct BCL11A target genes that were identified in HUDEP-2 cells.22 (D) BCL11A Cleavage Under Targets & Release Using Nuclease, ATAC-seq, and RNA-seq signals around regulatory regions in 3 selected BCL11A target genes. Ranged values in upper left of tracks represent y-axis scaling of counts per million reads. BasoE, basophilic erythroblast; CFU-E, colony-forming unit erythroid; log FC, log fold change; ProE, proerythroblast.
Genes regulated by BCL11A during erythropoiesis in vitro. (A) Gene expression changes identified by RNA-seq analysis of erythroblasts generated by in vitro differentiation of gene-targeted CD34+ HSPCs. Volcano plots illustrate DEGs in BCL11AE- (top panel) or HBGP-disrupted (bottom panel) proerythroblasts compared with CD33-disrupted ones. HBG refers to HBG1 + HBG2 transcripts. Dashed lines represent the 0.05 FDR statistical significance cutoff (y-axis) and log2 fold change of 1 or −1 (x-axis). (B) Gene set enrichment analysis (GSEA) from BCL11AE- vs CD33-disrupted CFU-E, proerythroblasts, and basophilic erythroblasts. Gene sets represented on the left and right halves of the graph are enriched in downregulated and upregulated genes, respectively. Gray shapes indicate that the gene set has an FDR ≥0.05 or normalized enrichment score ≤1.5. (C) Analysis of 25 genes regulated directly by BCL11A in erythroblasts. Left panel illustrates RNA expression heat map in HBGP- or BCL11AE-disrupted vs CD33-disrupted erythroblasts. Right panel illustrates heat map of assay for transposase-accessible chromatin using sequencing (ATAC-seq) signals reflecting changes in chromatin accessibility near BCL11A-bound regions in BCL11AE-disrupted vs untreated erythroblasts. Asterisks indicate direct BCL11A target genes that were identified in HUDEP-2 cells.22 (D) BCL11A Cleavage Under Targets & Release Using Nuclease, ATAC-seq, and RNA-seq signals around regulatory regions in 3 selected BCL11A target genes. Ranged values in upper left of tracks represent y-axis scaling of counts per million reads. BasoE, basophilic erythroblast; CFU-E, colony-forming unit erythroid; log FC, log fold change; ProE, proerythroblast.
Notably, DEGs resulting from BCL11AE disruption were highly stage specific: 23 of 52 upregulated and 29 of 42 downregulated DEGs appeared in only 1 stage, whereas just 9 upregulated and 3 downregulated DEGs were shared across all 3 stages. We reasoned that these DEGs include both direct BCL11A targets and genes indirectly affected. To identify direct targets, we focused on DEGs containing regions exhibiting BCL11A occupancy near a cognate binding motif, with chromatin accessibility changes after BCL11AE disruption (supplemental Figure 4E). Of the identified 94 DEGs, 26% (25 genes) exhibited the features of direct BCL11A targets. Of these, 84% (21 genes) were upregulated and 16% (4 genes) were downregulated, consistent with the primary role of BCL11A as a transcriptional repressor (Figure 3C-D). Notably, direct BCL11A targets were highly enriched among DEGs shared across all 3 stages: 8 of the 9 shared upregulated genes (CTSE, ESPN, KLF15, PDK4, RBM47, RBP5, TPSG1, WNT9A) and 2 of the 3 shared downregulated genes (C6orf223, CACNA2D2) were direct targets. Six of the 25 genes identified to be targeted by BCL11A directly in this study overlapped with BCL11A targets identified in the erythroid cell line HUDEP-2.22 Overall, these findings suggest that BCL11A exerts its regulatory influence through a relatively small set of direct target genes acting on a larger number of stage-specific genes.
A recent study by Janoudi et al,24 which was published while the current study was under review, also demonstrated that BCL11AE disruption impairs erythropoiesis and erythroid gene expression. We compared the DEGs identified in that study with our findings, using the same analytical pipeline. Of the 35 upregulated DEGs in basophilic erythroblasts identified in the current study, 21 overlapped with DEGs reported by Janoudi et al.24 Notably, 15 of 21 (71.4%) of these overlapping DEGs are direct targets of BCL11A (supplemental Figure 5A-B).
Regulation of gene expression by BCL11A during erythropoiesis in vivo after xenotransplantation
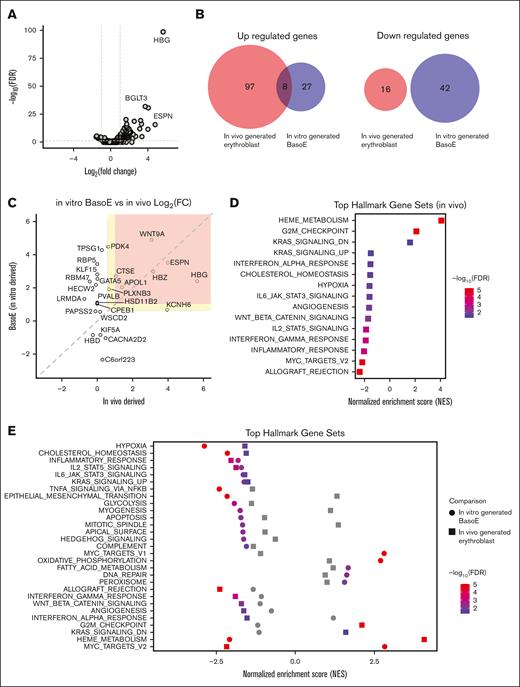
To assess the effect of BCL11AE disruption on erythropoiesis in vivo, we transplanted BCL11AE-disrupted CD34+ HSPCs into NBSGW mice. After 16 weeks, we performed RNA-seq analysis on CD235+ human erythroblasts purified from recipient BM by immunomagnetic bead selection. Erythroblasts derived from BCL11A enhancer–disrupted HSPCs exhibited 105 upregulated and 16 downregulated genes (more than twofold change, FDR < 0.05) compared with those derived from control untreated HSPCs (Figure 4A). When we compared those DEGs with those identified in basophilic erythroblasts generated by in vitro differentiation (the most closely matching maturation stage, compare supplemental Figures 2C and 3E), only 8 genes overlapped (Figure 4B). Notably, 7 of these 8 genes were direct BCL11A targets (Figure 4C).
Regulation of gene expression by BCL11A during in vivo erythropoiesis. CD34+ HSPCs were electroporated with RNP targeting BCL11AE and transplanted into NBSGW mice. Untreated CD34+ HSPCs were transplanted as a negative control. After 16 weeks, hCD235+ donor erythroblasts were isolated from recipient BM and analyzed by RNA-seq. (A) Volcano plot illustrating gene expression changes in BCL11AE-disrupted erythroblasts. Dashed lines represent the 0.05 FDR statistical significance cutoff (y-axis) and log2 fold change of 1 or −1 on the x-axis. (B) Overlap of DEGs (fold change > 2 and FDR < 0.05) between BCL11AE-disrupted vs control erythroblasts generated in vivo and BCL11AE-disrupted vs control basophilic erythroblasts generated in vitro. (C) Scatterplot illustrating the log2 fold changes in expression of 25 BCL11A target genes in BCL11AE-disrupted erythroblasts generated in vivo (x-axis) vs in vitro (y-axis). Colored sections represent thresholds for twofold change (red) and 1.5-fold change (yellow). (D) GSEA of altered gene expression in BCL11AE-disrupted vs control erythroblasts generated in vivo. Gene sets represented on the left and right halves of the graph are enriched in downregulated and upregulated genes, respectively. (E) GSEA comparing altered gene expression pathways in BCL11AE-disrupted erythroblasts generated in vivo and in vitro. BasoE, basophilic erythroblast; log FC, log fold change.
Regulation of gene expression by BCL11A during in vivo erythropoiesis. CD34+ HSPCs were electroporated with RNP targeting BCL11AE and transplanted into NBSGW mice. Untreated CD34+ HSPCs were transplanted as a negative control. After 16 weeks, hCD235+ donor erythroblasts were isolated from recipient BM and analyzed by RNA-seq. (A) Volcano plot illustrating gene expression changes in BCL11AE-disrupted erythroblasts. Dashed lines represent the 0.05 FDR statistical significance cutoff (y-axis) and log2 fold change of 1 or −1 on the x-axis. (B) Overlap of DEGs (fold change > 2 and FDR < 0.05) between BCL11AE-disrupted vs control erythroblasts generated in vivo and BCL11AE-disrupted vs control basophilic erythroblasts generated in vitro. (C) Scatterplot illustrating the log2 fold changes in expression of 25 BCL11A target genes in BCL11AE-disrupted erythroblasts generated in vivo (x-axis) vs in vitro (y-axis). Colored sections represent thresholds for twofold change (red) and 1.5-fold change (yellow). (D) GSEA of altered gene expression in BCL11AE-disrupted vs control erythroblasts generated in vivo. Gene sets represented on the left and right halves of the graph are enriched in downregulated and upregulated genes, respectively. (E) GSEA comparing altered gene expression pathways in BCL11AE-disrupted erythroblasts generated in vivo and in vitro. BasoE, basophilic erythroblast; log FC, log fold change.
To assess the robustness of our observations with different thresholds for DEGs, we applied a less stringent threshold of >1.5-fold change. Only 17 of 377 upregulated genes and 4 of 266 downregulated genes in erythroblasts generated in vivo overlapped with DEGs identified in erythroblasts generated in vitro (supplemental Figure 5C). Among the 17 overlapping upregulated genes, 11 are direct BCL11A targets (Figure 4C).
Many of the gene expression pathways altered after BCL11AE disruption in vivo, such as hypoxia and cholesterol homeostasis, are shared between in vivo- and in vitro-derived erythroblasts (Figure 4D-E). Conversely, pathways including allograft rejection and angiogenesis were only altered in vivo, whereas pathways such as DNA repair and apoptosis were only altered in vitro. Interestingly, some pathways were regulated in opposite directions in vivo and in vitro. For example, the heme pathway was enriched for upregulated genes in vivo but for downregulated genes in vitro, which may indicate that CD235+ cells from NBSGW mice are at a more mature stage (supplemental Figure 3E). Overall, our findings suggest that BCL11A regulates a small set of direct target genes, which influence broader gene networks according to stage- and environment-specific conditions.
Discussion
Because BCL11A is a clinically validated target for treating common β-hemoglobinopathies, it is important to evaluate more closely the molecular and functional effects of its depletion in preclinical models for erythropoiesis and ultimately in clinical studies. Loss of BCL11A has profound negative effects on HSCs, lymphocytes, and the nervous system.12-18 In contrast, this study reveals that BCL11A deficiency causes relatively subtle impairment of erythropoiesis. Specifically, we reveal that disruption of the erythroid-specific BCL11A intron 2 enhancer in CD34+ HSPCs caused reduced expansion and increased apoptosis of RBC precursors during in vitro differentiation and reduced contribution to the erythroid compartment after xenotransplantation. Several other studies have analyzed the relative contribution of BCL11A erythroid enhancer–disrupted CD34+ HSPCs to different blood lineages after xenotransplantation. Wu et al21 found no reduction in contribution to the erythroid compartment, although too few mice were evaluated to detect quantitative differences. Zeng et al28 observed variable and modest reduction of erythroid engraftment compared with other lineages, although the study was not focused on this issue. In agreement with this study, Janoudi et al24 revealed that BCL11A enhancer–disrupted CD34+ HSPCs exhibited a broader perturbation of the transcriptome after in vitro erythroid differentiation and selectively reduced contribution to the erythroid compartment after xenotransplantation compared with CD34+ HSPCs edited at the HBG1/HBG2 loci. Another study revealed that BCL11A is required for glucocorticoid-induced expansion of adult-type human erythroid precursors.29 Together, these findings indicate that BCL11A has additional roles in erythroid development beyond fetal γ-globin gene silencing.
One limitation of our human-to-mouse xenotransplantation studies is that circulating human reticulocytes and RBCs in mice are destroyed rapidly by host macrophage, which restricts studies of erythroid physiology.30 In addition, it is possible that incompatibilities between the mouse hematopoietic niche and human erythropoiesis exacerbate the effects of BCL11A deficiency after xenotransplantation. In another study, nonhuman primates reconstituted with HSCs harboring Cas9-mediated disruptions of BCL11AE exhibited normal hemoglobin level, normal reticulocyte count, and retention of high-level gene edits in erythroid precursors over several years of follow-up.31 Furthermore, these animals tolerated phlebotomy and hydroxyurea treatments without obvious defects in erythroid response, predicting that any effects of BCL11A deficiency on steady state or stress erythropoiesis are mild. Nonetheless, it may be interesting to revisit these nonhuman primate studies with our current findings in mind, for example, by determining whether BCL11AE disruption causes expansion of the BM erythroid compartment and/or alteration of its transcriptome.
A recent study revealed that BCL11A directly regulates a small set of target genes in the erythroid cell line HUDEP-2.22 Here, we reveal that BCL11A regulates a similar set of direct target genes and many indirect ones in primary erythroblasts, with biological consequences. Our finding that BCL11AE disruption perturbs the erythroid transcriptome to a substantially greater extent than HBGP disruption differs from 2 reports32,33 but is in agreement with the recent study by Janoudi et al.24 In our study, the effects of BCL11A depletion on the expression of its direct target genes tended to be similar in different erythroid populations. However, the indirect effects on gene expression varied according to maturation stage and between precursors generated in vitro vs in vivo. Although some of these differences may represent technical artifacts, they are more likely due to biological factors, such as stage-specific effects on transcription, cell selection after xenotransplantation, and differences between in vivo and in vitro environments. Overall, these findings suggest that BCL11A regulates a conserved set of direct erythroid target genes with distinct downstream transcriptional activities according to cellular context. Despite variation in DEGs observed in different BCL11AE-disrupted erythroid precursors, the overall effects on their expansion were similar, indicating that multiple distinct pathways led to common biological outcomes. Gene set enrichment analysis during BCL11A-deficient erythropoiesis identified G2M cell cycle checkpoint, MYC, glycolysis, oxidative phosphorylation, and hypoxia pathways, all of which can affect cell survival and expansion.
BCL11A is reported to exert pro-proliferative and anti-apoptotic functions in multiple cell types.18,34-36 In mouse lymphoid cells, Bcl11a deficiency was found to activate p53 by inhibiting the expression of its ubiquitin ligase gene Mdm2 and enhancing expression of the pro-apoptotic Bcl2.16 In a chronic lymphocytic leukemia cell line, suppression of BCL11A with small interfering RNA resulted in the differential expression of 1903 genes, including induction of pro-apoptotic BCL2 and BCL2L11.37 Disruption of Bcl11a in mouse HSCs caused impaired cell cycle entry with reduced expression of Cdk6 but did not induce apoptosis.14 The changes in erythroid gene expression resulting from BCL11A deficiency reported in this study are distinct from those observed in other hematopoietic lineages.
Understanding how BCL11A mediates erythroid precursor expansion and survival requires further investigation. We speculate that BCL11A may facilitate adaptation of erythropoiesis to the environmental changes that occur during transition from fetal to postnatal life. Most mammals use 1 of 3 different mechanisms to increase the oxygen affinity of fetal RBCs, which is thought to enhance oxygen delivery in the hypoxic uterine environment.38 For example, humans and Old World monkeys developed distinct γ-globin genes that generate high-oxygen affinity HbF. Expression of the γ-globin genes is activated directly by hypoxia inducible factor-1, a master regulator of hypoxic adaptation.25 Conversely, by repressing γ-globin expression approximately during birth, BCL11A facilitates β-globin expression and a switch to lower-oxygen affinity adult hemoglobin. Similarly, BCL11A may facilitate the transition from a continuous fetal nutrient supply through placental circulation to intermittent feeding of the newborn. A role for BCL11A in perinatal adaptation of erythropoiesis is consistent with its developmental expression pattern and the perturbation of hypoxia, glycolysis, and oxidative phosphorylation pathways in BCL11AE-disrupted erythroblasts. It may be possible to test this model in mice with erythroid-specific disruption of Bcl11a.19 However, some erythroid functions of BCL11A may reflect evolutionary adaptations that have not occurred in mice, similar to the existence of fetal γ-globin genes and their regulation by hypoxia inducible factor-1.25
The clinical implications of our findings are unknown. Humans with rare germline heterozygous BCL11A loss-of-function mutations and intact HBB alleles are reported to have elevated RBC HbF levels and normal blood counts.39 Our findings that erythropoiesis is impaired after disruption of the BCL11A erythroid enhancer contrast markedly with clinical studies revealing that the same perturbation in HSCs of individuals with sickle cell disease or β-thalassemia results in improved erythropoiesis.6,7 Both diseases are associated with ineffective erythropoiesis that is relieved by high-level γ-globin gene expression, which occurs with suppression of BCL11A.40,41 Thus, in β-hemoglobinopathies, it is highly likely that the proven benefits of the exa-cel cellular drug product harboring Cas9 disruptions of the BCL11A erythroid enhancer outweigh any potential negative effects of erythroid BCL11A deficiency.2,6,7 Early results of clinical trials reported in meeting abstracts indicate that reni-cell, an autologous cellular drug product in which the BCL11A binding motif in the γ-globin (HBG1/HBG2) promoters is disrupted by Cas12a, is effective for treating sickle cell disease and β-thalassemia.10,11 Long-term studies and comparisons of patients with β-hemoglobinopathy treated with exa-cel or Renizgamglogene autogedtemcel (reni-cel) should better elucidate the physiological differences (if any) between BCL11A depletion in erythroid cells or destruction of its cognate binding motif used for repressing γ-globin transcription.
Acknowledgments
The authors thank Xuili An (New York Blood Center) for allophycocyanin-conjugated anti-BAND3 antibody and staff at St. Jude Institutional Core Facilities, including the Center for Advanced Genome Engineering, the Flow Cytometry and Cell Sorting Shared Resource, and the Hartwell Center. The authors also thank David Cullins and Emilia Kooienga from Experimental Hematology Flow Cytometry and Cell Sorting core.
This work was supported by National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) grants U01 HL163983 (M.J.W.) and R01 156647 (M.J.W.), Assisi Foundation grant 94-000 R25 (M.J.W. and J.S.Y.), Gates Foundation grant 60005015- 5500002819 (M.J.W. and J.S.Y.), and American Lebanese Syrian Associated Charities. T.M. is currently supported by A DBT-Ramalingaswami re-entry fellowship, Government of India.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: Y.J., R.F., J.S.Y., and M.J.W. conceived and designed the experiments and wrote the manuscript with input from all authors; Y.J. performed tissue culture, flow cytometry, xenotransplantation experiments, and other molecular biology experiments; R.F. performed western blot, Cleavage Under Targets & Release Using Nuclease, and assay for transposase-accessible chromatin using sequencing; L.E.P., Y.C., S.Z., and J.X. performed bioinformatics data analysis and advised on the project; T.M. performed flow cytometry experiments; Y.Y. and K.M. performed xenotransplantation experiments; J.M.G. and G.K. performed statistical analysis and interpretation; and J.S.Y. and M.J.W. supervised the project.
Conflict-of-interest disclosure: M.J.W. is a consultant for Fulcrum Therapeutics and an equity owner of Cellarity. J.S.Y. is a consultant for Portal Bio and Orna Therapeutics and an equity owner of Beam Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Mitchell J. Weiss, Department of Hematology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS#355, Memphis, TN 38105-3678; email: mitch.weiss@stjude.org; and Jonathan S. Yen, Department of Hematology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS#355, Memphis, TN 38105-3678; email: jonathan.yen@stjude.org.
References
Author notes
Y.J. and R.F. contributed equally to this study.
The FASTQ files for RNA sequencing, assay for transposase-accessible chromatin using sequencing, and Cleavage Under Targets & Release Using Nuclease studies have been deposited in the NCBI Gene Expression Omnibus database (accession number: GSE283797).
The full-text version of this article contains a data supplement.