Key Points
ADAMTS13 can be proteolytically degraded by hemostatic proteases, which may reduce its capacity to regulate VWF.
Protease-resistant ADAMTS13 is stable against coagulation, fibrinolytic, and neutrophil proteases, and retains its activity toward VWF.
Visual Abstract
Recombinant ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) was recently approved by the US Food and Drug Administration for the treatment of heritable thrombotic thrombocytopenic purpura, and preclinical studies have demonstrated its efficacy in treating other thrombotic conditions. However, the current ADAMTS13 product is susceptible to degradation by proteases, which may reduce its therapeutic efficacy. Protease-sensitive sites were mapped to the linker regions in ADAMTS13. The linkers were mutated to generate T4L/T8L-ADAMTS13, and an additional elastase cleavage site was also disrupted (T4L/T8L-ADAMTS13[I380G]). Degradation of each ADAMTS13 mutant was tested using purified coagulation or neutrophil proteases, activated neutrophils, or with plasma-based assays. FRETS-VWF73 and microfluidic flow assays were used to characterize their activity. Thrombin, factor Xa, factor XIa, kallikrein, and plasmin cleaved wild-type (WT)-ADAMTS13 at 2 sites. Mutation of both the T4- and T8-linkers protects against degradation at these sites over 3 hours. T4L/T8L-ADAMTS13(I380G) was resistant to elastase degradation. T4L/T8L-ADAMTS13 is stable in plasma thrombin generation assays and fibrinolysis assays, and T4L/T8L-ADAMTS13(I380G) exhibits improved stability to activated neutrophils. T4L/T8L-ADAMTS13 exhibited similar activity to WT-ADAMTS13 using FRETS-VWF73 and in a microfluidic VWF-platelet string cleavage assay. This work identifies prominent protease cleavage sites within ADAMTS13 and demonstrates that disruption of these sites does not impair its capacity to regulate VWF. Future work will explore the therapeutic efficacy of protease-resistant ADAMTS13 in vivo.
Introduction
ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) is a multidomain metalloprotease (∼180 kDa) and is the primary molecular regulator of von Willebrand Factor (VWF). It is produced by hepatic stellate cells and circulates at a concentration of ∼5 nM.1-3 The proteolysis of VWF by ADAMTS13 is regulated by shear stress, which exposes the Tyr1605-Met1606 scissile bond within the VWF A2 domain.4 Shear-activation of VWF exposes an extended binding interface for ADAMTS13 exosites within the disintegrin-like (D), cysteine-rich (C), and spacer (S) domains that accelerate proteolysis by the metalloprotease (M) domain. The interaction between the MDTCS domains (thrombospondin-1 domain [T]) of ADAMTS13 and the shear-unfolded A2 domain of VWF can be quantified and characterized by the minimal substrate, VWF73.5-7 However, cleavage of VWF73 does not directly reflect the capacity of ADAMTS13 to regulate multimeric VWF in physiological settings. Although ADAMTS13 lacking its C-terminal CUB (complement C1r/C1s, Uegf, Bmp1) domains exhibits increased activity toward VWF73,8 it has reduced capacity to cleave multimeric VWF in microfluidics-based assays.9-13 Similarly, mice lacking the C-terminal CUB domains exhibited faster occlusion time in a ferric chloride injury model of the mesenteric arteriole compared with mice with full-length ADAMTS13.14 Collectively, these data demonstrate the importance of intact full-length ADAMTS13 for optimal regulation of VWF.
ADAMTS13 is susceptible to limited proteolysis by coagulation and fibrinolytic proteases.15-20 Evidence of ADAMTS13 degradation has also been found in patients with sepsis-induced disseminated intravascular coagulation (DIC),21 and in a patient with α2-antiplasmin deficiency that experienced an episode of thrombotic thrombocytopenic purpura (TTP).16 Cleavage by thrombin, plasmin, and factor XIa (FXIa) impairs the capacity of ADAMTS13 to cleave VWF-platelet strings in a microfluidics-based assay17 due to removal of the C-terminal CUB domains. Such proteolytic regulation of ADAMTS13 may be important in hemostasis to stabilize VWF-platelet strings at sites of vascular injury but may also contribute to thrombosis risk in disease settings such as sepsis-induced DIC21 or trauma-induced coagulopathy.22 Additionally, elevated levels of plasmin-antiplasmin complex have been observed in some patients during acute episodes of TTP, suggesting a co-incidence of plasmin activity and the onset of TTP symptoms.23 Observations that ADAMTS13 was degraded after addition of urokinase or streptokinase to plasma20 suggest that pharmacologic administration of plasminogen activators in thrombolysis therapy may contribute to ADAMTS13 degradation in clinical settings.24-26
VWF and ADAMTS13 are now recognized as important contributors to a growing number of conditions in which vascular inflammation and thrombosis play a role, including cancer, atherosclerosis, sepsis, neurological conditions, and liver disease.27-36 Although recombinant ADAMTS13 has recently been approved by the US Food and Drug Administration for the treatment of heritable TTP, preclinical studies have shown its benefit in experimental models of stroke,37,38 myocardial infarction,39 and others,22,40-47 without increased risk of bleeding. Tissue-plasminogen activator (tPA) is the standard thrombolytic used to treat acute thrombosis but carries a high risk for intracerebral hemorrhage with a narrow therapeutic time window.48 Recombinant ADAMTS13 in combination with tPA may improve thrombolysis and extend the therapeutic window of thrombolysis therapy without increased risk of bleeding.24-26,37 However, the current ADAMTS13 product is not optimized for use as a thrombolytic agent with tPA because of its susceptibility to degradation.
Here, we describe the design and characterization of protease-resistant ADAMTS13 mutants. We provide evidence that mutating the T4- and T8-linker domains prevents the proteolytic degradation of ADAMTS13 while preserving its capacity to regulate VWF-platelet strings.
Materials and methods
Materials
Recombinant (r)ADAMTS13 and human ADAMTS13 Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN), kallikrein, FXIIa (Enzyme Research Laboratories, South Bend, IN), thrombin, FXa, FIXa, FXIa, FVIIa, activated protein C (APC), elastase, cathepsin G (Sigma-Aldrich, St Louis, MO), proteinase 3 (Enzo Life Sciences, Inc, Farmingdale, NY), FRETS-VWF73 (AnaSpec, Fremont, CA), AEBSF (4-[2-aminoethyl]benzenesulfonyl fluoride hydrochloride), Sivelestat (Tocris Bioscience, Bristol, United Kingdom), and cathepsin G inhibitor 1 (Cayman Chemical Company, Ann Arbor, MI).
In vitro and in silico analysis of ADAMTS13 cleavage sites
Cardiovascular proteases were surveyed for their capacity to degrade ADAMTS13. rADAMTS13 (100 nM) was incubated with 50 nM purified proteases at 37°C in ADAMTS13 reaction buffer (Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM CaCl2, 10 μM ZnCl2, 0.005% Tween 20, pH 7.4). This concentration of ADAMTS13 was selected to provide sufficient detection in this assay. Reactions were stopped at various time points using sodium dodecyl sulfate (SDS) loading dye containing β-mercaptoethanol and separated via SDS-PAGE (polyacrylamide gel electrophoresis) under reducing conditions. Gels were stained using SYPRO Ruby to visualize all protein or transferred to a nitrocellulose membrane and analyzed by western blotting using polyclonal anti-ADAMTS13 primary antibody (Abcam: ab28274) and goat anti-rabbit horseradish peroxidase–conjugated secondary antibody (BioRAD). Protease-sensitive sites were estimated by comparing band sizes with those from previous studies15,18,49 and with in silico analysis tools predicting cleavage sites of specific proteases (plasmin, thrombin, FXIa, kallikrein, cathepsin G, and elastase) in the ADAMTS13 peptide sequence: PROSPER (Monash University, Melbourne, Australia), Expasy PeptideCutter (SIB Swiss Institute of Bioinformatics, Vaud, Switzerland), and NEBcutter (version 2.0; New England Biolabs, Ipswich, MA).
Designing ADAMTS13 mutants
Wild-type (WT)-ADAMTS13 was cloned into pcDNA3.1 containing C-terminal V5 6× His tag, as previously described.8 The ADAMTS13 T4-linker (W868-A894) was replaced with GGS[GGGS]6, and the T8-linker (G1134-A1191) was replaced with [GGGS]14GS. These substitutions were made alone in the T4L or T8L mutant, or together in the T4L/T8L double mutant. Each ADAMTS13 mutant was synthesized into pcDNA 3.1(+) from Bio Basic Inc (Markham, ON, Canada). The I380G mutation was introduced into the T4L/T8L mutant using the Q5 site-directed mutagenesis kit (New England Biolabs, Oshawa, ON, Canada) according to manufacturer protocol using primers S1 (GCTGACCCCCGGAGCAGCAGTGC) and AS1 (TCCACCAGGGAGCGGCAGC). Reactions were transformed into NEB 5-alpha Competent Escherichia coli (C2987I), plated on 100 μg/mL ampicillin, miniprepped, and sent for Sanger sequencing to confirm mutation.
Expression and purification of ADAMTS13 mutants
WT-ADAMTS13 and each mutant (T4L, T8L, T4L/T8L, or T4L/T8L[I380G]) was transfected into HEK293T cells using transporter 5 (Polysciences Inc, Warrington, PA), as previously described.8 Protein was collected into FreeStyle 293 expression medium (Invitrogen, Waltham, MA) every 2 days for 3 to 5 collections. Proteins were purified via gravity column chromatography using Q Sepharose Fast Flow agarose beads, followed by HisPur Ni-NTA resin (Cytiva, Washington, DC), and buffer-exchanged to reaction buffer using PD-10 desalting columns.8
ADAMTS13 degradation by purified proteases
Each purified mutant (T4L, T8L, and T4L/T8L) and rADAMTS13 (50 nM) were incubated for 0 to 180 minutes with various recombinant proteases (50 nM or 100 nM) at 37°C in ADAMTS13 reaction buffer. Aliquots were removed at 0, 30, and 180 minutes, and reactions were stopped using SDS loading dye containing β-mercaptoethanol. Proteins were separated via SDS-PAGE using 4% to 20% polyacrylamide gels under reducing conditions and analyzed by western blot, as previously described.8
ADAMTS13 degradation in plasma
Ethics approval was obtained from the Hamilton integrated research ethics board for the use of human blood for research purposes (research ethics board no. 16605). For plasma coagulation experiments, 36 μL of pooled platelet-poor human plasma was incubated with 400 nM rADAMTS13, 30 μM phospholipid vesicles, 10 μL of 1:100 RecombiPlasTin (as a source of tissue factor; Werfen, Bedford, MA), and 1 mg/mL Gly-Pro-Arg-Pro (GPRP)-amide to prevent fibrin formation, at 37°C for 15 minutes in a black-bottom 96-well plate. Coagulation was initiated with 52 mM CaCl2 in 40 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) to a final volume of 100 μL. In a parallel plate, an identical reaction took place with the addition of 1 mM fluorogenic thrombin substrate (Z-Gly-Gly-Arg-AMC acetate; MedChemExpress, Monmouth Junction, NJ) to monitor thrombin formation. The reaction was read in a plate reader for 90 minutes in 1-minute intervals (λexcitation [λex] = 360 nm; λemission [λem] = 460 nm). A 2-μL aliquot was removed at 0 and 90 minutes to measure ADAMTS13 degradation by western blotting, as described earlier.
For fibrinolysis experiments, 50 μL of pooled platelet-poor human plasma was supplemented with 120 nM rADAMTS13 or T4L/T8L-ADAMTS13 and varying concentrations of tPA (Activase). Plasma was clotted with 30 mM CaCl2 and 1:100 RecombiPlasTin (as a source of tissue factor). Clot formation and lysis was monitored at an optical density of 405 nm in 30-second intervals; 2-μL aliquots were removed at 0, 30, and 90 minutes to measure rADAMTS13 degradation by western blot.
Neutrophil isolation
Blood (10 mL) was collected from healthy human donors into sodium citrate with 6% dextran and incubated for 60 minutes at room temperature. Plasma and buffy coat were removed and layered onto 7 mL of Histopaque 1077 (MilliporeSigma, Burlington, MA). The solution was centrifuged at 277g for 20 minutes at 21°C and the resulting cells were incubated with 5 mL of ammonium-chloride-potassium lysis buffer (MilliporeSigma, Burlington, MA) for 5 minutes. Hank balanced salt solution was added to a volume of 50 mL, then the solution was centrifuged at 277g for 5 minutes at 4°C with maximum acceleration and deceleration. The supernatant was discarded. The remaining polymorphonuclear neutrophils were resuspended into 10 mL RPMI 1640 medium. The cells were counted using 0.4% trypan blue–phosphate-buffered saline (PBS) solution and a hemocytometer. To confirm the presence of neutrophil cells, isolated cells were fixed with 2% paraformaldehyde for 15 minutes at room temperature. Samples were stained with e-fluor 780 (Invitrogen), anti-human CD16-phycoerythrin (BD Pharmingen), or anti-CD11b fluorescein isothiocyanate (EMD Millipore Corp: MABF802). Stained cells were analyzed with a BD Celestica flow cytometer.
ADAMTS13 degradation by activated neutrophils
Neutrophils (0 × 103 to 500 × 103 cells) were activated with 100 nM phorbol 12-myristate 13-acetate (PMA) for 4 hours at 37°C in ADAMTS13 reaction buffer (Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM CaCl2, 10 μM ZnCl2, 0.005% Tween 20, pH 7.4) or in the presence of 1:4 dilution of plasma. WT-ADAMTS13 or ADAMTS13 mutants (50 nM) were added to the activated neutrophils and incubated for 1 hour at 37°C. To identify the proteases in neutrophils responsible for ADAMTS13 degradation, 50 × 103 activated neutrophils were incubated with 2 mM AEBSF, 5 mM EDTA, or varying concentrations of Sivelestat (elastase inhibitor) or cathepsin G inhibitor 1 before incubation with rADAMTS13. ADAMTS13 degradation was analyzed by western blot or SYPRO Ruby total protein stain. Degradation was quantified by comparing the density of the full-length band at each time point with that at time 0 using ImageLab software version 5.2.1 (Bio-Rad).
Kinetic analysis using FRETS-VWF73 substrate
The activity of each purified ADAMTS13 mutant was compared with that of rADAMTS13 using FRETS-VWF73. The initial rate of 20 nM rADAMTS13 or mutant was measured toward 0 to 5 μM FRETS-VWF73 in ADAMTS13 reaction buffer at 37°C (λex = 340 nm, λem = 450 nm). Nonlinear regression analysis of Michaelis-Menten data was performed to obtain Vmax, KM, and kcat values using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, Boston, MA).
VWF-platelet string cleavage assay
Human umbilical vein endothelial cells (HUVECs; Lonza: C2517A, Kingston, ON, Canada) were plated on 2% gelatin-coated 10-cm dishes in endothelial cell growth medium-2 BulletKit media (Lonza, Kingston, ON, Canada) at passage number of ≤3. HUVECs were then seeded into an Ibidi μ-Slide VI0.4 (Ibidi: 80606) according to the manufacturer’s protocol. Briefly, each channel was coated at room temperature for 60 minutes using 30 μL of 75 μg/mL mouse collagen IV (R&D Systems, Minneapolis, MN) diluted in 0.05 M HCl. HUVECs were trypsinized, pelleted at 500g for 5 minutes, and resuspended in media to a concentration of ∼3 × 108 cells per mL. Excess collagen IV was washed from the channels using cold Dulbecco PBS, and 30 μL of cell suspension was added to each channel. The slide was placed in the incubator to allow for adherence, then 60 μL of media was added to each well. Media was changed every day until a confluent monolayer was observed. On the day of the experiment, media was aspirated from the channels and cells were incubated in 100 μL of 25 μM histamine in PBS for 5 minutes at room temperature to activate them.
Lyophilized platelets (Bio/Data Corporation, Horsham, PA) were reconstituted in the manufacturer-provided Tris buffer (200 000 cells per μL) and separated into one 2-mL aliquot per channel. A 20-mM stock solution of DiOC6 (Thermo Fisher Scientific Inc, Waltham, MA) was diluted to a 20-μM working stock in reduced-serum media. Thereafter, 100 μL of dye was added to each 2-mL platelet solution. To confirm the presence of VWF, 2 mL of ADAMTS13 reaction buffer was perfused over a HUVEC-lined channel, followed by 2 mL of either a 1:100 solution of polyclonal anti-VWF antibodies (Invitrogen: PA5-16634) to PBS, or PBS alone. Then, 3 mL of platelet solution was perfused over the channel, and VWF-platelet string formation was monitored.
For experimental conditions, the platelet solution was perfused over histamine-activated channels at a flow rate of 2000 μL/min to achieve shear stresses of ∼2.5 dyn/cm2, forming stable VWF-platelet strings, followed immediately by 2 mL of ADAMTS13 reaction buffer. Channels were then perfused with a 6-mL solution containing 5 nM WT ADAMTS13, 5 nM T4L/T8L-ADAMTS13, 5 nM MDTCS, or ADAMTS13 reaction buffer as a negative control. This concentration represents the approximate plasma concentration of ADAMTS13 in humans. Images were captured on a Leica Stellaris 5 inverted confocal microscope (λex = 490 nm; λem = 525 nm) using a 10× dry lens. Videos were captured at 0.77 frames per second. The total length of VWF-platelet strings within the field of view were quantified every 20 frames for 5 frames using the scale bar tool.
Total VWF-platelet string length at each time point was expressed as a percentage of the total VWF-platelet string length at time 0. Total length of strings was plotted as a function of time and linear regression was used to determine the rates of cleavage for each condition. Rates were compared using unpaired t tests, for which P < .05 was considered significant.
Results
ADAMTS13 linker regions are primary targets for proteolysis
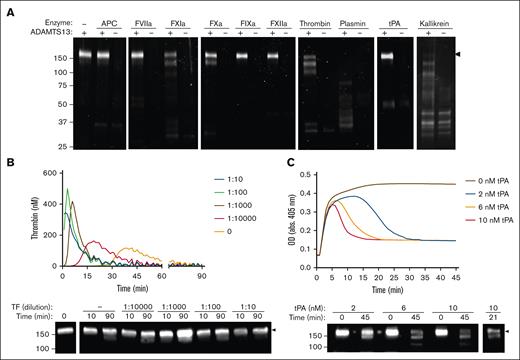
Consistent with previous reports, we found that kallikrein, plasmin, thrombin, FXIa, and FXa cleaved ADAMTS13 (Figure 1A).15-20 However, ADAMTS13 was not cleaved by activated protein C, FVIIa, FIXa, FXIIa, or tPA. Each protease produced similar ADAMTS13 fragmentation patterns with prominent bands appearing at 150 kDa and 120 kDa. In plasma-based assays, ADAMTS13 was minimally degraded after thrombin generation, regardless of tissue factor concentration (Figure 1B). In contrast, plasma fibrinolysis assays resulted in substantial degradation of ADAMTS13 at various concentrations of tPA (2, 6, or 10 nM; Figure 1C).
ADAMTS13 degradation by purified proteases and in plasma-based assays. (A) Commercial rADAMTS13 (200 nM) was incubated with various proteases (100 nM) for 2 hours at 37°C. Samples were separated under reducing conditions using SDS-PAGE, and cleavage was visualized using SYPRO Ruby. (B) rADAMTS13, varying dilutions of tissue factor (TF), and 1 mg/mL GPRP-amide were incubated in human platelet-poor plasma for 15 minutes. Thrombin generation was then initiated with calcium and quantified over time in a parallel plate by measuring fluorescence (λex = 360 nm; λem = 460) at 37°C. Curves were generated using the Technothrombin TGA Software in triplicate, and representative images are shown. (C) rADAMTS13 and varying concentrations of tPA were added to human platelet-poor plasma, followed by TF and calcium. Clot formation and lysis was quantified by measuring OD at 405 nm every 30 seconds in triplicate, and representative images are shown. Samples were separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and ADAMTS13 degradation was visualized via western blot using an anti-ADAMTS13 metalloprotease domain antibody. Molecular weight references are indicated on the left of blots (kDa), and full-length bands are indicated by the black arrows. APC, activated protein C; GPRP-amide, Gly-Pro-Arg-Pro-amide; OD, optical density.
ADAMTS13 degradation by purified proteases and in plasma-based assays. (A) Commercial rADAMTS13 (200 nM) was incubated with various proteases (100 nM) for 2 hours at 37°C. Samples were separated under reducing conditions using SDS-PAGE, and cleavage was visualized using SYPRO Ruby. (B) rADAMTS13, varying dilutions of tissue factor (TF), and 1 mg/mL GPRP-amide were incubated in human platelet-poor plasma for 15 minutes. Thrombin generation was then initiated with calcium and quantified over time in a parallel plate by measuring fluorescence (λex = 360 nm; λem = 460) at 37°C. Curves were generated using the Technothrombin TGA Software in triplicate, and representative images are shown. (C) rADAMTS13 and varying concentrations of tPA were added to human platelet-poor plasma, followed by TF and calcium. Clot formation and lysis was quantified by measuring OD at 405 nm every 30 seconds in triplicate, and representative images are shown. Samples were separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and ADAMTS13 degradation was visualized via western blot using an anti-ADAMTS13 metalloprotease domain antibody. Molecular weight references are indicated on the left of blots (kDa), and full-length bands are indicated by the black arrows. APC, activated protein C; GPRP-amide, Gly-Pro-Arg-Pro-amide; OD, optical density.
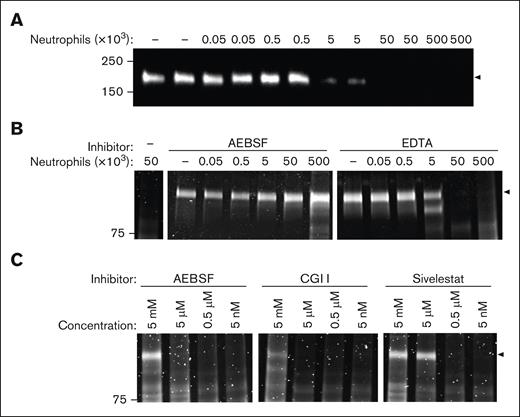
Because neutrophils are important mediators of thrombo-inflammation, we next evaluated ADAMTS13 susceptibility to degradation by activated neutrophils.31 PMA-stimulated human neutrophils dose-dependently degraded ADAMTS13 (Figure 2A). Degradation was attenuated when neutrophils were preincubated with AEBSF but not EDTA, indicating a prominent role for serine proteases rather than metalloproteases (Figure 2B). Activated neutrophils treated with an elastase inhibitor, Sivelestat, or cathepsin G inhibitor, also attenuated ADAMTS13 degradation in a concentration-dependent manner (Figure 2C). In plasma, activated neutrophils also degraded ADAMTS13, albeit at a reduced level likely due to the presence of serine protease inhibitors and competing substrates (supplemental Figure 2).
ADAMTS13 degradation by activated neutrophils. (A) ADAMTS13 was incubated with 0 × 103 to 500 × 103 PMA-stimulated neutrophils for 1 hour at 37°C, in the absence or presence of 2 mM AEBSF or 5 mM EDTA (B). (C) ADAMTS13 was incubated with 5 × 103 PMA-stimulated neutrophils with increasing concentrations of AEBSF, cathepsinG inhibitor I (CGI I), or Sivelestat for 1 hour. Samples were separated via SDS-PAGE and analyzed by (A) western blot using an anti-ADAMTS13 metalloprotease domain antibody, or (B-C) SYPRO Ruby. Black arrows indicate the level of full-length ADAMTS13 (∼180 kDa). AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride; EDTA, ethylenediaminetetraacetic acid.
ADAMTS13 degradation by activated neutrophils. (A) ADAMTS13 was incubated with 0 × 103 to 500 × 103 PMA-stimulated neutrophils for 1 hour at 37°C, in the absence or presence of 2 mM AEBSF or 5 mM EDTA (B). (C) ADAMTS13 was incubated with 5 × 103 PMA-stimulated neutrophils with increasing concentrations of AEBSF, cathepsinG inhibitor I (CGI I), or Sivelestat for 1 hour. Samples were separated via SDS-PAGE and analyzed by (A) western blot using an anti-ADAMTS13 metalloprotease domain antibody, or (B-C) SYPRO Ruby. Black arrows indicate the level of full-length ADAMTS13 (∼180 kDa). AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride; EDTA, ethylenediaminetetraacetic acid.
To further analyze potential cleavage sites for coagulation and neutrophil-mediated degradation of ADAMTS13, we compared the products of our degradation assays with known ADAMTS13 domain fragment sizes (supplemental Figure 3). SYPRO Ruby total protein stain and western blot fragmentation patterns are consistent with cleavage sites in the T4- and T8-linker domains. These results are supported by in silico analysis of predicted cleavage sites in these domains (supplemental Figure 4).
Mutating prominent cleavage sites protects ADAMTS13 from degradation
To develop protease-resistant ADAMTS13, the T4- and T8-linker were mutated to glycine-serine linkers of equivalent length to abolish all cleavage sites predicted from the in vitro assays (Figure 3A). Three mutants were developed: T4L-ADAMTS13, T8L-ADAMTS13, and T4L/T8L-ADAMTS13. These 3 mutants demonstrated varying degrees of resistance to coagulation proteases in comparison with WT-ADAMTS13. As expected, T4L-ADAMTS13 and T8L-ADAMTS13 were each cleaved at 1 site, with bands appearing at 150 kDa and 120 kDa, respectively. In contrast, the T4L/T8L-ADAMTS13 mutant exhibited near-complete resistance to cleavage by plasmin, thrombin, FXIa, and kallikrein over 180 minutes (Figure 3B). These data indicate that coagulation proteases target primarily the T4- and T8-linker regions, and mutating both cleavage sites provides the greatest protection against degradation. Consistent with these observations, the T4L/T8L-ADAMTS13 mutant showed complete resistance to degradation in a plasma-based fibrinolysis assay (Figure 3C), confirming that T4L/T8L mutations can protect ADAMTS13 from plasmin degradation when exposed to thrombolytic therapies.
Characterizing the resistance of the ADAMTS13 mutants against proteolysis by purified proteases and in plasma fibrinolysis. (A) Four mutants of ADAMTS13 were developed: 2 with 1 of each C-terminal linker region mutated (T4L and T8L mutants), 1 with both linker regions mutated (T4L/T8L mutant), and a fourth with both linker regions and an additional elastase cleavage site mutation (T4L/T8L[I380G] mutant). (B) Using commercial recombinant ADAMTS13 (WT) as a control, each linker mutant (T4L, T8L, and/or T4L/T8L) was incubated at 50 nM with purified coagulation and fibrinolytic proteases at 50 nM (plasmin, thrombin, and kallikrein) or 100 nM (FXIa) for 0 to 180 minutes at 37°C. (C) The T4L/T8L mutant and 10 nM tPA were added to human platelet-poor plasma, followed by tissue factor and calcium at 37°C. Aliquots were removed at 0 and 45 minutes. (D) Mutants were incubated with 50 nM purified neutrophil-derived proteases (elastase, proteinase 3, and cathepsin G) at 37°C. The T4L/T8L(I380G) mutant was also incubated with 50 nM elastase. Western blotting was performed using an anti-ADAMTS13 metalloprotease domain antibody. The band corresponding to full-length protein (∼180 kDa) is indicated by black arrows.
Characterizing the resistance of the ADAMTS13 mutants against proteolysis by purified proteases and in plasma fibrinolysis. (A) Four mutants of ADAMTS13 were developed: 2 with 1 of each C-terminal linker region mutated (T4L and T8L mutants), 1 with both linker regions mutated (T4L/T8L mutant), and a fourth with both linker regions and an additional elastase cleavage site mutation (T4L/T8L[I380G] mutant). (B) Using commercial recombinant ADAMTS13 (WT) as a control, each linker mutant (T4L, T8L, and/or T4L/T8L) was incubated at 50 nM with purified coagulation and fibrinolytic proteases at 50 nM (plasmin, thrombin, and kallikrein) or 100 nM (FXIa) for 0 to 180 minutes at 37°C. (C) The T4L/T8L mutant and 10 nM tPA were added to human platelet-poor plasma, followed by tissue factor and calcium at 37°C. Aliquots were removed at 0 and 45 minutes. (D) Mutants were incubated with 50 nM purified neutrophil-derived proteases (elastase, proteinase 3, and cathepsin G) at 37°C. The T4L/T8L(I380G) mutant was also incubated with 50 nM elastase. Western blotting was performed using an anti-ADAMTS13 metalloprotease domain antibody. The band corresponding to full-length protein (∼180 kDa) is indicated by black arrows.
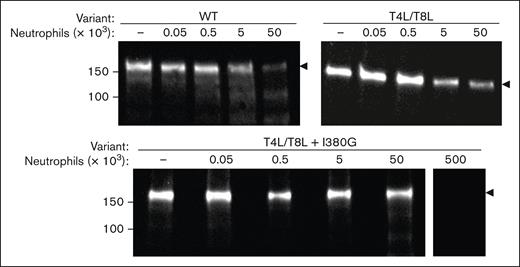
T4L-ADAMTS13, T8L-ADAMTS13, and T4L/T8L-ADAMTS13 are fully degraded after incubation with neutrophil elastase, cathepsin G, and proteinase 3 for 180 minutes (Figure 3D). However, T4L/T8L-ADAMTS13 exhibited partial resistance to degradation, as demonstrated by the persisting full-length band at 30 minutes. To enhance the protection of T4L/T8L-ADAMTS13 to neutrophil-mediated degradation, we mutated an additional elastase cleavage site in the D domain (I380G). This mutation resulted in complete resistance to elastase over 180 minutes (Figure 3D).
Next, the degradation of WT-ADAMTS13 was compared with T4L/T8L-ADAMTS13 and T4L/T8L(I380G)-ADAMTS13 in the presence of PMA-stimulated human neutrophils (Figure 4). WT-ADAMTS13 was found to be 90% degraded after 1 hour of incubation with 50 × 103 activated neutrophils. T4L/T8L-ADAMTS13 was partially protected from degradation by activated neutrophils, exhibiting 75% degradation after a 1-hour incubation. T4L/T8L-ADAMTS13(I380G) was protected the most from degradation by activated neutrophils, with only 30% degradation. However, this triple mutant was still fully degraded by 500 × 103 activated neutrophils, suggesting the presence of additional low-efficiency cleavage sites (Figure 4).
Resistance of ADAMTS13 mutants to activated neutrophils. 50 nM WT-, T4L/T8L-, or T4L/T8L(I380G)-ADAMTS13 was incubated with 0 × 103 to 50 × 103 PMA-activated neutrophils, or 500 × 103 neutrophils, as indicated. Reactions took place for 1 hour at 37°C, then samples were removed and separated via SDS-PAGE under reducing conditions. Cleavage was visualized by western blot using an anti-ADAMTS13 metalloprotease domain antibody. Molecular weight references are indicated to the left (kDa). The level of full-length protein (∼180 kDa) is indicated by black arrows.
Resistance of ADAMTS13 mutants to activated neutrophils. 50 nM WT-, T4L/T8L-, or T4L/T8L(I380G)-ADAMTS13 was incubated with 0 × 103 to 50 × 103 PMA-activated neutrophils, or 500 × 103 neutrophils, as indicated. Reactions took place for 1 hour at 37°C, then samples were removed and separated via SDS-PAGE under reducing conditions. Cleavage was visualized by western blot using an anti-ADAMTS13 metalloprotease domain antibody. Molecular weight references are indicated to the left (kDa). The level of full-length protein (∼180 kDa) is indicated by black arrows.
ADAMTS13 mutants retain their functional activity against VWF
Next, the activity of each ADAMTS13 mutant was characterized relative to WT-ADAMTS13 to determine whether any of the mutations affect their VWF-cleaving capacity. The proteolytic activity of WT-ADAMTS13 and each mutant (20 nM) was quantified toward increasing concentrations of minimal substrate FRETS-VWF73 (0-5 μM) to derive kinetic parameters (Table 1). The KM (4.2 ± 1.3 μM) and kcat (217.8 ± 38.6 min−1) values derived for WT-ADAMTS13 were not significantly different from the 4 mutants (P > .05), with similar independently derived kcat/KM values across all variants. These kinetic parameters are comparable with those of previous reports.50-52 These data suggest that the mutations do not have a meaningful effect on the VWF-cleaving activity of ADAMTS13.
Kinetic characterization of ADAMTS13 mutants
| Variant . | KM (μM) . | kcat (min−1) . | kcat/KM (μM−1 × min−1) . |
|---|---|---|---|
| WT | 4.2 ± 1.3 | 217.8 ± 38.6 | 51.9 ± 18.5 |
| T4L | 4.7 ± 1.8 | 193.7 ± 45.0 | 41.2 ± 18.5 |
| T8L | 3.5 ± 1.8 | 190.2 ± 51.7 | 54.3 ± 31.6 |
| T4L/T8L | 2.7 ± 0.8 | 206.2 ± 28.8 | 76.4 ± 25.1 |
| T4L/T8L(I380G) | 3.9 ± 1.4 | 218.4 ± 42.5 | 56.0 ± 23.0 |
| Variant . | KM (μM) . | kcat (min−1) . | kcat/KM (μM−1 × min−1) . |
|---|---|---|---|
| WT | 4.2 ± 1.3 | 217.8 ± 38.6 | 51.9 ± 18.5 |
| T4L | 4.7 ± 1.8 | 193.7 ± 45.0 | 41.2 ± 18.5 |
| T8L | 3.5 ± 1.8 | 190.2 ± 51.7 | 54.3 ± 31.6 |
| T4L/T8L | 2.7 ± 0.8 | 206.2 ± 28.8 | 76.4 ± 25.1 |
| T4L/T8L(I380G) | 3.9 ± 1.4 | 218.4 ± 42.5 | 56.0 ± 23.0 |
Values expressed as mean ± standard error.
Protease-resistant ADAMTS13 cleaves VWF-platelet complexes under conditions of flow
Next, we sought to characterize the activity of T4L/T8L-ADAMTS13 toward multimeric VWF using a microfluidic flow assay. DiOC6-stained platelets were perfused over HUVECs stimulated with histamine to form stable VWF-platelet strings (Figure 5A). As expected, a polyclonal anti-VWF antibody completely prevented VWF-platelet string formation (supplemental Figure 5). Channels perfused with 5 nM WT-ADAMTS13 exhibited time-dependent reduction in the total length of VWF-platelet strings (Figure 5B). The relative rate of string cleavage was quantified, which demonstrated that WT-ADAMTS13 cleaved VWF-platelet strings at a rate of 41.8% ± 2.3% per minute (Table 2). MDTCS cleaved VWF strings at a rate of 14.3% ± 2.5% per min, which is significantly slower than WT-ADAMTS13 (P < .05), consistent with previous studies.12,17 By comparison, T4L/T8L-ADAMTS13 cleaved VWF-platelet strings at a rate of 48.7% ± 4.6% per minute, which is comparable with WT-ADAMTS13 (P = .3) but significantly faster than MDTCS (P < .05; Figure 5B). Therefore, both WT-ADAMTS13 and T4L/T8L-ADAMTS13 cleared VWF strings at a rate approximately threefold faster than MDTCS, suggesting that the T4- and T8-linker mutations do not affect the VWF-cleaving activity of ADAMTS13 under conditions of flow.
Activity of ADAMTS13, MDTCS, and T4L/T8L-ADAMTS13 in a microfluidic flow assay. DiOC6-stained platelets were perfused over a microfluidic flow chamber lined with histamine-stimulated HUVECs, forming stable VWF-platelet strings. Channels were then perfused with 5 mL of 5 nM MDTCS, 5 nM WT-ADAMTS13, 5 nM T4L/T8L-ADAMTS13, or ADAMTS13 reaction buffer (n = 3). Using a Leica Stellaris 5 inverted confocal microscope (10× dry lens; λex = 490 nm; λem = 525 nm), videos were captured at 0.77 frames per second; (A) photographic representation of experimental replicate. Experiments occurred at room temperature. The total length of strings within the frame was quantified every 20 frames for 5 frames. Rates were determined using linear regression (B), and differences between rates were assessed using unpaired t tests (Table 2). Error bars represent standard deviation, and symbols represent 4 experimental conditions: buffer (▲), MDTCS (■), WT-ADAMTS13 (●), T4L/T8L-ADAMTS13 (▼). ∗P < .05, vs MDTCS.
Activity of ADAMTS13, MDTCS, and T4L/T8L-ADAMTS13 in a microfluidic flow assay. DiOC6-stained platelets were perfused over a microfluidic flow chamber lined with histamine-stimulated HUVECs, forming stable VWF-platelet strings. Channels were then perfused with 5 mL of 5 nM MDTCS, 5 nM WT-ADAMTS13, 5 nM T4L/T8L-ADAMTS13, or ADAMTS13 reaction buffer (n = 3). Using a Leica Stellaris 5 inverted confocal microscope (10× dry lens; λex = 490 nm; λem = 525 nm), videos were captured at 0.77 frames per second; (A) photographic representation of experimental replicate. Experiments occurred at room temperature. The total length of strings within the frame was quantified every 20 frames for 5 frames. Rates were determined using linear regression (B), and differences between rates were assessed using unpaired t tests (Table 2). Error bars represent standard deviation, and symbols represent 4 experimental conditions: buffer (▲), MDTCS (■), WT-ADAMTS13 (●), T4L/T8L-ADAMTS13 (▼). ∗P < .05, vs MDTCS.
Rate of VWF-platelet string clearance by ADAMTS13 variants
| Condition . | Cleavage rate (relative length [%] × min−1) . |
|---|---|
| Buffer | 2.5 ± 1.2 |
| MDTCS | 14.3 ± 2.5 |
| WT | 41.8 ± 2.3∗ |
| T4L/T8L | 48.7 ± 4.6∗ |
| Condition . | Cleavage rate (relative length [%] × min−1) . |
|---|---|
| Buffer | 2.5 ± 1.2 |
| MDTCS | 14.3 ± 2.5 |
| WT | 41.8 ± 2.3∗ |
| T4L/T8L | 48.7 ± 4.6∗ |
Values expressed as mean ± standard error.
P < .05, relative to MDTCS.
Discussion
Recombinant ADAMTS13 was recently approved by the US Food and Drug Administration for the treatment of heritable TTP, and trials are on-going to examine its efficacy in treating acquired TTP and vaso-occlusive crisis in patients with sickle cell anemia. ADAMTS13 is an attractive thrombolytic agent that targets the platelet component of blood clots, seemingly without increased risk of bleeding,53-55 a limitation of other antithrombotic therapies.26,48,56 However, it has been shown to be susceptible to degradation by plasmin and other proteases present in circulation that could reduce its therapeutic efficacy. This work characterized protease-resistant ADAMTS13 mutants to develop ADAMTS13 variants with improved stability to treat thrombosis. Replacing the T4- and T8-linker regions with GGGS linkers conferred resistance to cleavage by plasmin, thrombin, FXIa, and kallikrein, and partial resistance to neutrophil-derived proteases elastase, cathepsin G, and proteinase 3. Other cleavage sites were identified, including an elastase cleavage site in the disintegrin-like domain.18 Neutrophil-derived proteases, which exhibit high promiscuity,57 may be important contributors to ADAMTS13 degradation in thrombo-inflammatory conditions. T4L/T8L-ADAMTS13(I380G) exhibited near complete resistance to purified elastase, and improved resistance to activated neutrophils compared to T4L/T8L-ADAMTS13.21 These ADAMTS13 variants efficiently cleaved the minimal biochemical substrate VWF73, and the linker mutations do not negatively affect cleavage of VWF-platelet strings under flow. Therefore, protease-resistant ADAMTS13 holds potential as an advanced antithrombotic agent.
ADAMTS13 degradation has been previously identified in critical illness such as sepsis21 and TTP.16 Although purified coagulation proteases cleave ADAMTS13 in vitro, minimal degradation was observed in plasma stimulated with various concentrations of tissue factor, despite peak thrombin concentrations of ∼500 nM. Although a previous study found significant ADAMTS13 cleavage in a plasma coagulation assay,15 work by Lam et al suggest that the presence of competing thrombin substrates in plasma, such as fibrinogen, protects ADAMTS13 from degradation.19 In contrast, WT-ADAMTS13 was degraded in our plasma fibrinolysis assays, consistent with previous observations that ADAMTS13 can be rapidly degraded by plasmin.15,16,18,20,58 The contribution of neutrophil-derived proteases to ADAMTS13 degradation in these settings is not known, but may be relevant in sepsis and other thromboinflammatory conditions. Although partially degraded forms of ADAMTS13 appear to exhibit increased activity when measured by VWF73-related biochemical substrates,2,59 several lines of evidence indicate that truncated ADAMTS13 is less effective at cleaving VWF-platelet strings under flow, both in vitro9,17 and in vivo.13,14 This supports the importance of the C-terminal domains for localizing to VWF under conditions of flow, facilitating its antithrombotic activity. Thus, pathological upregulation of plasmin and other circulating proteases (eg, TTP, DIC) may diminish the efficacy of ADAMTS13.
Although ADAMTS13 therapy is beneficial for the treatment of heritable TTP, experience with plasma exchange therapy show that some patients relapse and require more aggressive forms of treatment. Elevated levels of plasmin-antiplasmin complex have been observed in patients during acute episodes of TTP, suggesting a co-incidence of plasmin activity and the onset of TTP symptoms.23 It is possible that upregulated plasmin activity during acute episodes of TTP contribute to relapse in patients with TTP by degrading administered ADAMTS13. In this scenario, protease-resistant ADAMTS13 may provide better protection against relapse in some patients with TTP.
Beyond TTP, preclinical studies have demonstrated the therapeutic benefit of ADAMTS13 infusion without causing major bleeding in models of myocardial infarction,39 thrombotic microangiopathy,41 trauma-induced organ failure,22 skin allograft,43 renal disease,44 graft-versus-host disease,47 arthritis,46 and colitis.45 Many of these conditions can be associated with VWF-dependent inflammation or microvascular thrombosis. Notably, combined ADAMTS13 and tPA therapy may represent an advanced thrombolytic option that can target both VWF and fibrin while reducing neurotoxicity associated with tPA.24 Although tPA is the gold-standard thrombolytic for the treatment of acute ischemic stroke, it carries a major bleeding risk and fails to establish recanalization in more than half of patients who receive it.60-62 Recent studies show that platelet-rich thrombi are associated with tPA resistance,63,64 and targeting nonfibrin components (ie, VWF and DNA) can overcome tPA resistance.65 Indeed, studies demonstrate the benefit of ADAMTS13 and tPA coadministration in models of acute ischemic stroke.24-27 However, ADAMTS13 degradation by plasmin in this setting may attenuate its therapeutic benefit. Therefore, the benefit of protease-resistant ADAMTS13 may be most pronounced in thrombolysis therapy when administered in combination with tPA-based therapies.
This work validates the location of 2 protease-sensitive regions within ADAMTS13 and characterizes the resistance of novel ADAMTS13 mutants to proteolysis by activated neutrophils, purified proteases, and plasma-generated proteases in vitro. Future work will compare the mutants’ therapeutic efficacy with that of the WT in vivo using mouse models of TTP and stroke.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research, the National Sciences and Engineering Research Council, and the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL172780 (C.A.K). V.D. and R.H. received funding from the CanVECTOR Network and the Canadian Institutes of Health Research-Master's (CGS-M). H.M. and P.A. received funding from the CanVECTOR Network.
Authorship
Contribution: V.D. and R.H. performed experiments, analyzed data, and wrote the manuscript; H.M. conceived the project, performed experiments, and analyzed data; R.M., P.A. and C.T. performed experiments and analyzed data; and C.A.K. conceived the project, performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: V.D., H.M., and C.A.K. have received patent protection for protease-resistant ADAMTS13 variants. The remaining authors declare no competing financial interests.
Correspondence: Colin A. Kretz, Department of Medicine, Thrombosis and Atherosclerosis Research Institute, McMaster University, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; email: colin.kretz@taari.ca.
References
Author notes
V.D. and R.H. contributed equally to this study.
Data are available on reasonable request from the corresponding author, Colin A. Kretz (colin.kretz@taari.ca).
The full-text version of this article contains a data supplement.

![Characterizing the resistance of the ADAMTS13 mutants against proteolysis by purified proteases and in plasma fibrinolysis. (A) Four mutants of ADAMTS13 were developed: 2 with 1 of each C-terminal linker region mutated (T4L and T8L mutants), 1 with both linker regions mutated (T4L/T8L mutant), and a fourth with both linker regions and an additional elastase cleavage site mutation (T4L/T8L[I380G] mutant). (B) Using commercial recombinant ADAMTS13 (WT) as a control, each linker mutant (T4L, T8L, and/or T4L/T8L) was incubated at 50 nM with purified coagulation and fibrinolytic proteases at 50 nM (plasmin, thrombin, and kallikrein) or 100 nM (FXIa) for 0 to 180 minutes at 37°C. (C) The T4L/T8L mutant and 10 nM tPA were added to human platelet-poor plasma, followed by tissue factor and calcium at 37°C. Aliquots were removed at 0 and 45 minutes. (D) Mutants were incubated with 50 nM purified neutrophil-derived proteases (elastase, proteinase 3, and cathepsin G) at 37°C. The T4L/T8L(I380G) mutant was also incubated with 50 nM elastase. Western blotting was performed using an anti-ADAMTS13 metalloprotease domain antibody. The band corresponding to full-length protein (∼180 kDa) is indicated by black arrows.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/11/10.1182_bloodadvances.2024015212/2/m_blooda_adv-2024-015212-gr3.jpeg?Expires=1765022162&Signature=zyOmm8V8TOJr1BJof6rFYCOSmqMosGDFzlgVIGPGonJbZdmOkTrgcOiFSeTtmH5HZ7oCe4IdEWrM4EL4TFQG-1Fv-E7OuVSVuve8iJRoV-9cJBjJHdDbT0jWCZJYWupJ86JTZ9v7ptrRFREcLddhlCHnfTurDUA8ThYJOnY~Fhp4g-xCFnUEM8lOSN6TRF0A6coLF1d5e5lQNa6xLmree-xwcsj7oopJPOtNQpgrU983dQzL85H0Zbmde1Lbh~0OgxW46SqDX10nqqMbl8BhW~uvh0EZlT3xUpgjTKNRi768aq3cD89wvnqsM4ooAJ4Cac6W7UGCLcVPlIIlHVR6og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)