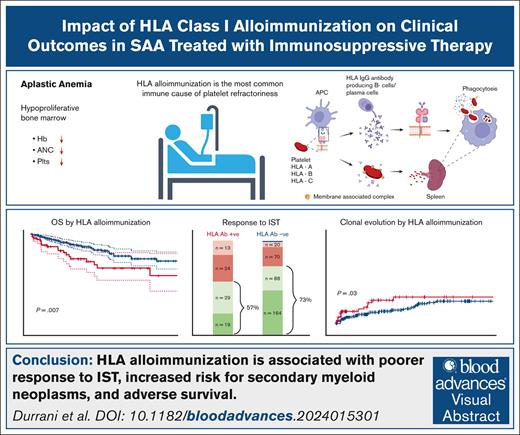
Key Points
HLA class I alloimmunization, older age, and low ANC are adverse risk factors for survival in SAA patients treated with immunosuppression.
HLA class I alloimmunization is a negative predictor for response to IST and is associated with higher risk of secondary myeloid malignancies.
Visual Abstract
Immune aplastic anemia (iAA) frequently results in transfusion dependence on platelets and packed red blood cells, increasing the risk for complications. The most common immune-mediated cause for platelet-transfusion refractoriness is alloimmunization with HLA antibody (Ab) to nonself class I antigens. The clinical impact of the HLA alloimmunization has not been well studied in patients with iAA. We investigated the clinical relevance of HLA alloimmunization in our large cohort of patients with iAA from 5 prospective trials and correlated with disease outcomes. Of 444 patients with severe AA treated with immunosuppressive therapy (IST), 99 (22%) had HLA alloimmunization. The presence of HLA Ab was associated with shorter overall survival, reduced responses to IST and higher risk of clonal evolution. Our data suggest that HLA alloimmunization is a marker of disease outcome. Furthermore, using single-cell RNA sequencing, we show enhanced activation of both complement-mediated pathways and the adaptive immune system in alloimmunized patients, indicating an interconnection between immune compartments.
Introduction
Immune aplastic anemia (iAA) presents as pancytopenia and a hypocellular marrow; the underlying etiology is immune dysregulation secondary to an autoimmune T-cell mediated destruction of hematopoietic and progenitor stem cells.1,2 Treatment depends on age, fitness, and donor availability, and includes hematopoietic stem cell transplant (HSCT) or immunosuppressive therapy (IST).
Patients with severe AA (SAA) are often platelet-transfusion dependent until therapeutic intervention results in response; transfusion requirement can extend for months to years in those without a response. Older literature suggests that patients with AA may have higher risk of alloimmunization compared to those with hematologic malignancies, potentially due to the disease’s underlying immune etiology.3 Traditionally, platelet transfusion is recommended to keep counts above 10 × 103/μL with a higher minimum transfusion threshold in the case of bleeding, fevers, and/or during antithymocyte globulin (ATG) administration.4-6
In the era before leukocyte reduction, platelet refractoriness in bone marrow failure syndromes (BMFs) was reported in 30% to 70% of cases.7,8 Leukoreductive techniques have substantially decreased platelet refractoriness, now ranging from 4% to 14% in the setting of HSCT and acute leukemia, respectively, but specific data are lacking in BMF.9 Although platelet refractoriness is commonly caused by nonimmune factors such as disseminated intravascular coagulation, sepsis and splenomegaly, the primary immune cause is attributed to HLA class I antibodies (Abs).4,5,10 Platelets express HLA class I (HLA-A, -B and -C) antigens (Ag)10-12; alloimmunization to class I Ag is responsible for platelet clearance (supplemental Figure 1). Platelet transfusion is the leading risk factor for development of HLA Abs; nevertheless, some chronically transfused patients do not develop alloimmunization, and conversely others may rapidly become alloimmunized after a single platelet transfusion.13-16 Other reported risk factors for HLA alloimmunization include history of pregnancy, renal impairment, and the presence of red cell Abs.17-19 Platelets carry between 50 000 to 120 000 HLA molecules per cell over a smaller surface area (approximate size 2-4 μL), compared with red cells (approximate size 7-8 μL) presenting only 90 HLA molecules per cell (range, 40-550), conferring a much higher risk of alloimmunization when transfused.20-22 One study of 54 patients with AA from Switzerland showed 80% alloimmunization in those who had HLA Ab testing, but no differences were reported in bleeding events, response to IST, transplant outcomes, and overall survival (OS) at 1 year between those with and without HLA alloimmunization.23
In BMF, most of the reported data are in patients with SAA treated with HSCT.24 We aimed to elucidate the incidence of HLA alloimmunization in our large cohort of patients with SAA treated with IST from 2000 to 2022 and assessed for predisposing factors for the development of alloimmunization. We then correlated presence or absence of HLA alloimmunization with clinical outcomes and survival.
Study methods
Study design and patient population
This study was a retrospective review of 444 SAA patients (Table 1) treated on consecutive prospective clinical trials with horse ATG (hATG) based regimens at the National Institutes of Health (NIH; supplemental Table 1). Patients were enrolled on clinical trials (ClinicalTrials.gov identifiers: NCT04304820, NCT01623167, NCT00260689, NCT00061360 and NCT00001964) between 2000 to 2022. Patients treated with rabbit ATG on (ClinicalTrials.gov identifier NCT00260689) were excluded. Data collected included age, sex, platelet refractoriness, HLA class I Ab status, baseline complete blood count, therapy received, hematologic responses at 3 and 6 months, relapse, presence of paroxysmal nocturnal hemoglobinuria (PNH) clone, malignant clonal evolution, and survival (Table 1). OS was censored for patients treated with HSCT. Survival data at last follow-up and cause of death were collected for patients who received HSCT. Number of live births (not pregnancies) in females above 13 years of age were extracted since gravidity and parity status were not consistently available.
Patient characteristics (HLA class I Ab-positive vs HLA class I Ab-negative)
| . | All patients (N = 444) . | HLA Class 1 Ab-positive (n = 99) . | HLA Class 1 Ab-negative or NT (n = 345) . | P value . |
|---|---|---|---|---|
| Age at first IST, median (range) | 31 (2-82) | 31 (6-73) | 30 (2-82) | .75 |
| Sex, n (%) | ||||
| Male | 242 (55) | 43 (43) | 199 (58) | .01 |
| Female | 202 (45) | 56 (57) | 146 (42) | |
| Disease severity, n (%) | ||||
| VSAA | 176 (40) | 58 (59) | 118 (34) | <.0001 |
| SAA | 268 (60) | 41 (41) | 227 (66) | |
| Baseline blood counts (× 103/uL), median (range) | ||||
| ANC | 0.3 (0-20.0) | 0.2 (0-1.7) | 0.3 (0-20.0) | .0002 |
| ARC | 16.1 (0-119.4) | 10.8 (0-106) | 18.9 (0-119.4) | .0002 |
| PLT | 8.5 (0-254.0) | 6.0 (0-46.0) | 9.0 (0-254.0) | <.0001 |
| First IST ATG, n (%) | 444 (100) | 99 (100) | 345 (100) | |
| >1 IST, n (%) | ||||
| Yes | 79 (18) | 18 (18) | 61 (18) | .91 |
| No | 365 (82) | 81 (82) | 284 (82) | |
| PNH clone, n (%) | ||||
| Yes | 133 (30) | 24 (24) | 109 (32) | .25 |
| No | 206 (46) | 48 (48) | 158 (46) | |
| Eltrombopag exposure, n (%) | ||||
| Yes | 220 (50) | 53 (54) | 167 (48) | .38 |
| No | 223 (50) | 46 (46) | 177 (51) | |
| BM transplant recipient, n (%) | ||||
| Yes | 85 (19) | 27 (27) | 58 (17) | .02 |
| No | 359 (81) | 72 (73) | 287 (83) | |
| Live births | ||||
| Median (range) | 2 (0-8) | 2 (0-8) | 2 (0-6) | .85 |
| Yes, n (%) | 71 (16) | 22 (22) | 49 (14) | 1 |
| No, n (%) | 20 (5) | 6 (6) | 14 (4) |
| . | All patients (N = 444) . | HLA Class 1 Ab-positive (n = 99) . | HLA Class 1 Ab-negative or NT (n = 345) . | P value . |
|---|---|---|---|---|
| Age at first IST, median (range) | 31 (2-82) | 31 (6-73) | 30 (2-82) | .75 |
| Sex, n (%) | ||||
| Male | 242 (55) | 43 (43) | 199 (58) | .01 |
| Female | 202 (45) | 56 (57) | 146 (42) | |
| Disease severity, n (%) | ||||
| VSAA | 176 (40) | 58 (59) | 118 (34) | <.0001 |
| SAA | 268 (60) | 41 (41) | 227 (66) | |
| Baseline blood counts (× 103/uL), median (range) | ||||
| ANC | 0.3 (0-20.0) | 0.2 (0-1.7) | 0.3 (0-20.0) | .0002 |
| ARC | 16.1 (0-119.4) | 10.8 (0-106) | 18.9 (0-119.4) | .0002 |
| PLT | 8.5 (0-254.0) | 6.0 (0-46.0) | 9.0 (0-254.0) | <.0001 |
| First IST ATG, n (%) | 444 (100) | 99 (100) | 345 (100) | |
| >1 IST, n (%) | ||||
| Yes | 79 (18) | 18 (18) | 61 (18) | .91 |
| No | 365 (82) | 81 (82) | 284 (82) | |
| PNH clone, n (%) | ||||
| Yes | 133 (30) | 24 (24) | 109 (32) | .25 |
| No | 206 (46) | 48 (48) | 158 (46) | |
| Eltrombopag exposure, n (%) | ||||
| Yes | 220 (50) | 53 (54) | 167 (48) | .38 |
| No | 223 (50) | 46 (46) | 177 (51) | |
| BM transplant recipient, n (%) | ||||
| Yes | 85 (19) | 27 (27) | 58 (17) | .02 |
| No | 359 (81) | 72 (73) | 287 (83) | |
| Live births | ||||
| Median (range) | 2 (0-8) | 2 (0-8) | 2 (0-6) | .85 |
| Yes, n (%) | 71 (16) | 22 (22) | 49 (14) | 1 |
| No, n (%) | 20 (5) | 6 (6) | 14 (4) |
PLT, platelet.
Primary objectives were to determine the rate of HLA alloimmunization, assess for outcome differences, and long-term complications for SAA patients treated with IST at our institution. Survival and rates of alloimmunization analyses included all 99 patients who became alloimmunized at any time point. Fourteen patients were excluded from clinical outcomes analysis due to testing positive for alloimmunization >6 months after receiving hATG. Two HLA nonalloimmunized patients were also excluded from survival analysis; 1 lacked HSCT date and the second was lost to follow-up. Secondary objectives included evaluation of predictors for HLA alloimmunization, HSCT outcomes, and rates of clinically significant bleeding. All bleeding adverse events were captured if occurring within 30 days before or anytime after confirmation of alloimmunization; these were assigned to HLA alloimmunized group for analysis.
Definitions
SAA was defined as per the modified Camitta criteria with those having neutrophil counts (≤0.2 × 103/μL) meeting criteria for very severe AA (VSAA).25 Complete response (CR) for AA was defined as meeting (1) absolute neutrophil count (ANC) ≥ 1 × 103/μL; (2) platelet count ≥100 × 103/μL; and (3) hemoglobin ≥10 g/dL independent of transfusion. Partial response (PR) was defined as blood counts that did not meet Camitta criteria but not sufficient for a CR. A weak PR was defined as platelet recovery levels <50 × 103/μL.
Relapse was defined within protocols as a decline in blood counts requiring reinitiation of therapy, not explained by another clinical process, and either (1) a substantial decline in 1 or more blood counts or (2) a progressive decline in 1 or more blood counts on at least 2 consecutive blood draws. Secondary myeloid neoplastic progression was categorized as clonal evolution in all included studies and classified as high risk or low risk as previously described.26
Severe adverse bleeding events were categorized as resulting in death, life threatening, requiring hospitalization, causing persistent or significant disability, or in the judgment of the investigators represented a significant hazard. All other bleeding incidents were recorded as adverse events.
Platelet refractoriness
Clinical platelet-transfusion refractoriness was defined as a lack of platelet increment of ≥5 × 103/μL or a CCI of ≥5 × 103/μL post ≥2 consecutive platelet transfusions.5,27,28 HLA Abs were tested only when patients met ≥1 clinical criteria: (1) previous history of HLA Abs, (2) history or clinical evidence of current platelet-transfusion refractoriness, or (3) evaluation for BM transplantation (supplemental Figure 2).
A traditional enzyme-linked immunosorbent assay method was used for screening for HLA class I Ab before 2008. For samples tested after 2008, a bead-based solid phase assay with multiple Ag specificities on each bead was utilized.29 Details of each testing method are provided in supplemental Methods; supplemental Table 2. Patients lacking clinical evidence of refractoriness were presumed HLA Ab-negative and not tested. Those that tested or were presumed negative received unmatched platelets for any HLA Ag (supplemental Figure 2).5
HLA Ag avoidance was employed by providing compatible platelets after reviewing patient’s class I HLA Ab and generating permissible HLA alleles for platelet selection and donor recruitment. This is based on HLAMatchmaker with modification.30 Posttransfusion platelet counts were obtained to measure response. Undetected HLA incompatibility can result in new Abs and screening was periodically updated for permitted HLA alleles.
Statistical analysis
Data were analyzed using R (v 4.2.0), with P < .05 being considered statistically significant. Univariable logistic regression was performed to determine predictors of HLA alloimmunization. Survival was analyzed for the whole cohort using Kaplan-Meier curves and the Cox proportional-hazards model with HSCT-censoring. A correlation matrix was used to identify and eliminate redundant features. Multivariable Cox proportional-hazards model for survival was used to reduce confounding, and a stepwise procedure was used to select the final model. Time to relapse and clonal evolution were analyzed among evaluable patients using Kaplan-Meier curves.
scRNA-seq and data analysis
Single-cell RNA-sequencing (scRNA-seq) with BM mononuclear cells (BMMNCs) was performed with the 10x Genomics System using the 10x Genomics Single Cell Immune Profiling Solution v 1.1 (the Chromium Single Cell 5’ Reagent Kit v1 and v1.1, Cat# 1000165, 10x Genomics), following the manufacturer’s protocol.31 Library preparation, sequencing, data processing, and quality control were done following previously published pipelines.32 Differentially expressed genes were defined with the FindMarkers function in Seurat, by comparing gene expression in 1 cell subset with expression in all others. Gene Set Enrichment Analysis was done based on fold changes of all detected genes; analysis was done and plots were generated on the website (http://software.broadinstitute.org/gsea). Gene network was generated using the merged interactions of BioGrid (https://thebiogrid.org/) and STRING (http://string-db.org/).33 Comparison between 2 groups was performed using the Prism software (v.7.02; GraphPad), and results were shown as mean ± standard derivation. Statistical analysis was performed using 2-sided unpaired Mann-Whitney test for 2 groups. P < .05 was considered statistically significant.
Approval was obtained from the NIH Central Institutional Review Board.
Results
Demographics
The median age at treatment was 31 (range, 2-82) years and 55% of patients were male. About one-third of the patients had a PNH clone >1%, and 40% had VSAA (Table 1). At the NIH, clinical trials enroll both domestic (from the United States) and international patients. Among our cohort of 444 patients, 62% (275) of patients were domestic (supplemental Table 3A). Of the domestic patients, most identified as White (n = 207, 75%) and the remaining were African American (n = 43) or other ethnicities (n = 25; supplemental Table 3B). Within the international group (37%, n = 157), the largest patient enrollment was from the West Indies and Caribbean islands (Afro-Caribbean; 73/164, 45%), frequently from Jamaica (37, 23% of the international patients), followed by Central and South America (supplemental Table 2A,C). International patients were younger in comparison to those from the United States; 26 years vs 36 years, respectively (P < .0001; supplemental Table 3) at diagnosis. There were no other clinically significant baseline differences between the domestic and international cohorts. All patients included in this study received hATG-based IST as first treatment (supplemental Table 1).
Frequency of HLA class I Abs, predictors of HLA alloimmunization, and response to HLA-matched products
HLA Ab testing was conducted on 155 (35%) patients, whereas 289 (65%) patients did not meet the standard prespecified criteria for testing at our institution (supplemental Figure 2). Thus, the detection rate was high (99/155, 64%). Overall, HLA alloimmunization occurred in 22% (99/444) of our cohort; the majority were female (n = 56; 57% of those alloimmunized). The most common trigger for testing was platelet refractoriness to transfusions; 65 patients were confirmed to be alloimmunized among 78 (83%) of suspected cases (supplemental Table 4).
When we compared disease characteristics between HLA class I Ab-positive and -negative groups, those with alloimmunization had more severe disease (VSAA 59% vs 34%, P < .0001); all blood counts were significantly lower in those with HLA class I Ab (Table 1).
In terms of Ab specificities, HLA-A (n = 61) and HLA-B (n = 60) were most frequent, followed by HLA-C (n = 22; supplemental Table 5A). Specific HLA Ab data were lacking for 27 of the alloimmunized patients (supplemental Table 5B) because they were tested before 2008 and therefore not using a multispecific enzyme-linked immunosorbent assay method for HLA. None of the patients were alloimmunized to HLA-C independently of HLA-A and HLA-B (supplemental Figure 3). HLA-A24 (33 patients) and HLA-B76 (32 patients) were the most frequently observed Abs. The median number of HLA class I Ab per alloimmunized patient was 51 (range, 5-73). The median panel reactive antibody for our cohort was 65.5% (range, 2%-100%, interquartile range, 26.5%-93.5%); 5 patients had panel reactive antibody <10% and 10 were not available/not reported. (supplemental Table 6A-B). HLA-A and HLA-A and HLA-B Abs were found in 62% (61/99) and 51% (50/99), respectively; rates were higher in patients from Jamaica (78% with HLA-A and 74% with HLA-A and -B; supplemental Table 3A,C), and lower rates at 50% and 40%, respectively, were observed in patients from the United States.
Using logistic regression female sex (odds ratio [OR], 2.05; P = .004), disease severity (OR, 3.03; P < .0001), and Black ethnicity (OR, 3.63; P < .0001) predicted for development of HLA alloimmunization. Other minority ethnicities including Asian and other (Mixed, Native American) also had a numerically higher rates of HLA class I Ab development (supplemental Table 7A). Among 91 female patients over the age of 13 years with available live birth data, HLA alloimmunization occurred in 22% of women with reported live births compared to 14% in those without (Table 1). Due to our referral pattern, we analyzed data by country of origin and found that international patients had twofold higher rate of alloimmunization compared to domestic (14%, 38/275 vs 36%, 61/169; OR 3.67, P < .0001; supplemental Table 7A). Alloimmunization rates in Afro-Caribbean population were highest (50%; 38/76 patients), particularly in those from Jamaica at 73% (27/37 patients, supplemental Table 3C). The US African American population did not exhibit an increased incidence of alloimmunization, being present in 5 of 43 patients (12%) compared to 29 of 208 (14%) of the White patients. Most patients who were alloimmunized (76/99; 76%) had a positive measurable response to HLA-matched platelets; 11 patients (11%) were unresponsive, and data were not available for 12 patients.
Clinical outcomes and survival
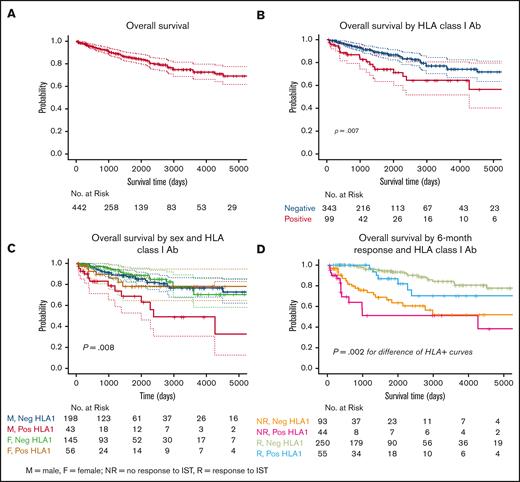
At median time of follow-up of 3.6 years (1320 days), OS censored for HSCT was 88% for the entire cohort (Figure 1A,). Patients without HLA class I Abs had a significantly improved survival at median follow-up compared to those with HLA alloimmunization (91% vs 79%, P = .007; Figure 1B). Correlation matrix identified high negative correlation between disease severity and ANC (r = −0.686, P < .001; supplemental Table 7B) and absolute reticulocyte count (ARC) (r = −0.552, P < .001; supplemental Table 7B). Due to this correlation, VSAA was excluded from the multivariable Cox proportional hazard models for survival, and we performed forward stepwise selection on the remaining covariates. A very low but statistically significant correlation was found between severity of disease and HLA alloimmunization (r = 0.207, P < .001; supplemental Table 7B). Using stepwise multivariable model, older age (hazard ratio [HR], 1.04; P < .0001) and positive HLA class I Ab (HR, 2.17; P= .006) predicted worse survival whereas female sex (HR, 0.57; P= .03) and higher ANC (HR, 0.35; P = .03) predicted for better outcomes. When we assessed for survival according to sex and HLA alloimmunization status, male patients with HLA class I Abs had the worst OS compared to other groups (74% at 3.6 years, P= .008; Figure 1C). Reduced survival was also seen in the non-US cohort compared to the US cohort (supplemental Figure 4; P = .03). Of 81 patients who died, causes of death was known for 64 patients (supplemental Figure 5). Cytopenia related complications (30/64) including infection (n = 26) and bleeding (n = 4) were common, followed by post-HSCT complications (10/64) and evolution to secondary myeloid malignancy (10/64).
Overall survival. (A) OS curve for the combined cohort of 442 patients censored for HSCT, 88.4% at a median time to follow-up of 3.6 years (1320 days). (B) Kaplan-Meier survival curve depicting worse OS in HLA alloimmunized vs nonimmunized patients, P = .007. (C) Kaplan-Meier survival curves of patients segregated by sex and HLA alloimmunization status. Alloimmunized male patients had the worst reported OS, P = .004. (D) Kaplan-Meier survival curves at median survival time (1320 days): HLA class I Ab-negative nonresponders = 71.3%; HLA class I Ab-positive nonresponders = 51.4%; HLA class I Ab-negative responders = 96.3%; HLA class I Ab-positive responders = 93.5%. Statistically significant difference in OS between nonresponders to IST with HLA class 1 Ab-positive (pink curve) compared to responders with HLA class I Ab (blue curve), 51.4% vs 93.5%; P= .002. No difference in the OS between nonresponders regardless of HLA alloimmunization; P = .1. Pos, positive; Neg, negative.
Overall survival. (A) OS curve for the combined cohort of 442 patients censored for HSCT, 88.4% at a median time to follow-up of 3.6 years (1320 days). (B) Kaplan-Meier survival curve depicting worse OS in HLA alloimmunized vs nonimmunized patients, P = .007. (C) Kaplan-Meier survival curves of patients segregated by sex and HLA alloimmunization status. Alloimmunized male patients had the worst reported OS, P = .004. (D) Kaplan-Meier survival curves at median survival time (1320 days): HLA class I Ab-negative nonresponders = 71.3%; HLA class I Ab-positive nonresponders = 51.4%; HLA class I Ab-negative responders = 96.3%; HLA class I Ab-positive responders = 93.5%. Statistically significant difference in OS between nonresponders to IST with HLA class 1 Ab-positive (pink curve) compared to responders with HLA class I Ab (blue curve), 51.4% vs 93.5%; P= .002. No difference in the OS between nonresponders regardless of HLA alloimmunization; P = .1. Pos, positive; Neg, negative.
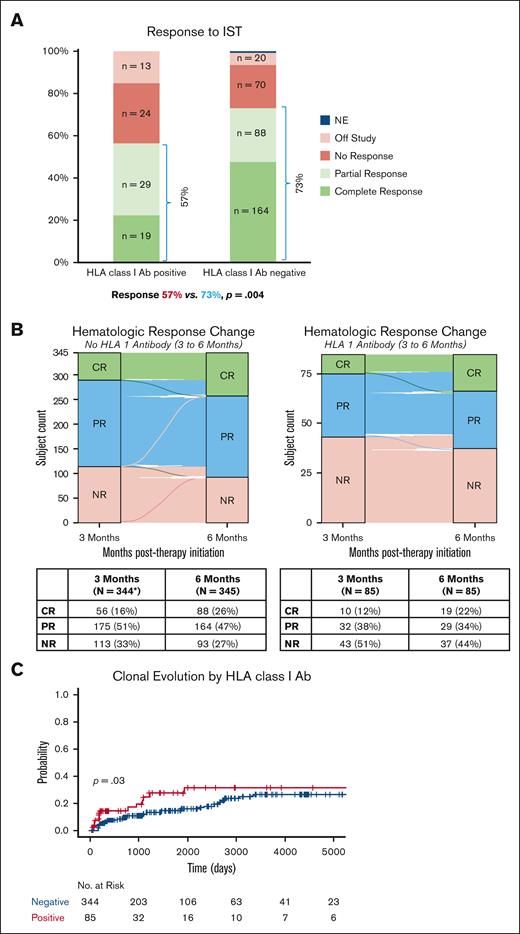
Response to IST and long-term complications, including relapse and clonal evolution, were assessed in 85 HLA alloimmunized patients who were tested within the first 6 months of treatment. As shown previously,34 patients with refractory disease (no response at 6 months) also had poorer OS, worst in those with HLA alloimmunization (Figure 1D). IST responses were significantly lower in those with HLA class I Abs (48/85, 57%) compared to those without alloimmunization (252/345, 74%, P= .004; Figure 2A). Half of the HLA alloimmunized patients were refractory to treatment at 3 months (42/85); of these only 17% (7/42) achieved a PR at 6 months, all of whom had a weak PR (defined as platelets <50 × 103/μL). Further improvement in blood counts was noted only in 2 of 7 subjects at 12 months (1 met criteria for CR and another robust PR). In contrast, of the 111 (32%) nonalloimmunized patients categorized as nonresponders at 3 months, 89 (80%) achieved a response at 6 months (Figure 2B). In patients with VSAA, hematologic response was not different between those with and without alloimmunization (52% vs 58%, P= .53). Once adjusted to exclude the 14 patients with late acquisition of HLA Abs, there was no difference between the number of transplants (18/85, 21% vs 54/345,16%, P = .28; supplemental Table 8), but alloimmunized patients were at a higher risk of clonal evolution to secondary malignancy (rates at median time to follow-up of 3.6 years, 27% vs 13%, P= .03; Figure 2C). Relapse rates were no different between the groups (HLA Ab-positive 42% vs HLA Ab-negative 35%, P= .5; supplemental Figure 6). In patients undergoing HSCT, there was no difference in survival in those with alloimmunization vs those without; in both groups the most common cause of death was transplant-related complications (supplemental Figure 7; supplemental Table 8). Alloimmunization was not associated with an increased risk of bleeding-related severe adverse events (alloimmunized 4% vs nonalloimmunized 4.3%, P = .8). The most common bleeding site was gastrointestinal (supplemental Figure 8). The incidence of death from bleeding was higher in alloimmunized (2/24, 8% vs 2/60, 3%, P = .6).
Response and clonal evolution. (A) Response rates to IST regimens in HLA alloimmunized patients compared to nonalloimmunized patients separated out by the degree of response. Total number of patients n = 430. Response to IST in HLA alloimmunized (positive) patients (48/85; 57%) and HLA nonalloimmunized (negative) patients (252/345; 73%); P = .004. (B) Alluvial plots of the improvement in response between 3-month and 6-month time points in HLA alloimmunized vs nonalloimmunized patients. (C) Kaplan-Meier curves depicting statistically significant difference in the risk of clonal evolution based on HLA alloimmunization status; P= .03. NE, not evaluable; NR, no response.
Response and clonal evolution. (A) Response rates to IST regimens in HLA alloimmunized patients compared to nonalloimmunized patients separated out by the degree of response. Total number of patients n = 430. Response to IST in HLA alloimmunized (positive) patients (48/85; 57%) and HLA nonalloimmunized (negative) patients (252/345; 73%); P = .004. (B) Alluvial plots of the improvement in response between 3-month and 6-month time points in HLA alloimmunized vs nonalloimmunized patients. (C) Kaplan-Meier curves depicting statistically significant difference in the risk of clonal evolution based on HLA alloimmunization status; P= .03. NE, not evaluable; NR, no response.
Single-cell sequencing comparing HLA alloimmunized to nonalloimmunized patients with SAA
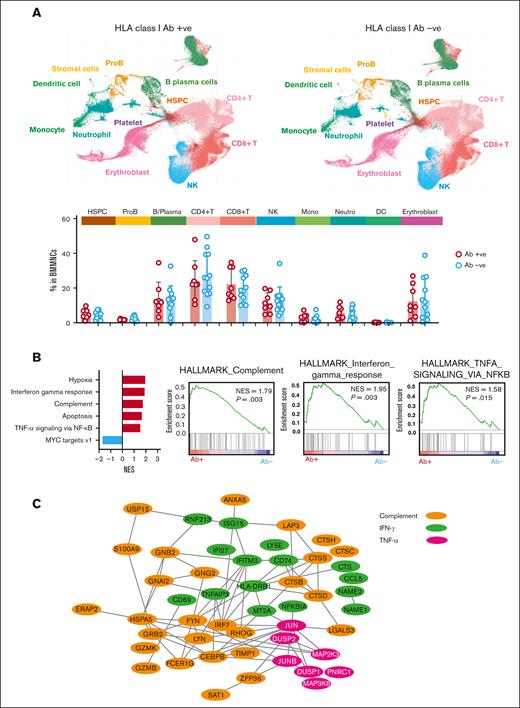
To investigate why outcomes were poorer in HLA alloimmunized patients, we performed subanalysis of an existing scRNA-seq data set on BMMNCs of 20 patients. In this cohort, 8 had HLA class I alloimmunization and 12 were nonalloimmunized (supplemental Table 9). When disease severity (SAA and VSAA) and baseline blood counts (ANC, ARC, and platelets) were compared between these 2 groups in scRNA-seq cohort, there were no significant differences (supplemental Table 9). Cell population abundance was similar between the 2 groups of patients (Figure 3A). BMMNCs from alloimmunized patients exhibited upregulated gene expression associated with interferon gamma response, tumor necrosis factor alpha (TNF-α) via nuclear factor-κB (NF-κB), and complement (Figure 3B). TNF-α via NF-κB associated pathways was also upregulated in CD4+, CD8+ T and natural killer cells. Among these upregulated genes TNAIP3, NFKBIA, CCL5, JUN, IRF7, CD69, and CD74 pathways have been associated with chemotaxis, recruiting a variety of leukocytes to inflammatory sites, and T-cell maturation, consequently modulating immune responses.35-38 Similarly, upregulated genes in alloimmunized patients were also enriched in inflammatory and complement activation pathways in myeloid cell subpopulations. Proteins encoded by upregulated genes in HLA alloimmunized SAA involved in interferon gamma response, TNF-α signaling via NF-κB , and complement pathway were functionally interactive (Figure 3C).
Single cell RNA sequencing. (A) UMAP plot of single-cell gene expression in BMMNCs of HLA alloimmunized (n = 8) and nonalloimmunized (n = 12) patients with SAA. Cells are colored by type (HSPC; ProB; erythroblast, neutrophil at different differentiation stages, monocyte at different differentiation stages, CD8+ T cell, CD4+ T cell, NK cell, B/plasma, DC, stromal cell, and platelet). Frequency (% in BMMNCs) of cell populations in HLA alloimmunized SAA (n = 8) and nonalloimmunized (n = 12) patients with SAA, by 2-sided unpaired Mann-Whitney test. No statistical significance is observed. (B) A bar chart showing enriched gene sets by Gene Set Enrichment Analysis (GSEA). Enriched plots of differentially expressed genes for Hallmark complement, Hallmark interferon gamma response, Hallmark TNF-α signaling via NFkB, and Hallmark complement in BMMNCs of HLA alloimmunized compared with nonalloimmunized patients with SAA. GSEA based on the Kolmogorov-Smirnov test. (C) A network of upregulated genes involved in interferon gamma response, TNF-α signaling via NFkB, and complement pathway in those with postive HLA class I Ab (n = 8) compared to nonalloimmunized (n = 12) patients with SAA. B/Plasma, B cell and plasma cell; DC, dendritic cell; HSPC, hematopoietic stem and progenitor cells; IFN-γ, interferon gamma; NES, normalized enrichment score; NK, natural killer; ProB, B-cell progenitors.
Single cell RNA sequencing. (A) UMAP plot of single-cell gene expression in BMMNCs of HLA alloimmunized (n = 8) and nonalloimmunized (n = 12) patients with SAA. Cells are colored by type (HSPC; ProB; erythroblast, neutrophil at different differentiation stages, monocyte at different differentiation stages, CD8+ T cell, CD4+ T cell, NK cell, B/plasma, DC, stromal cell, and platelet). Frequency (% in BMMNCs) of cell populations in HLA alloimmunized SAA (n = 8) and nonalloimmunized (n = 12) patients with SAA, by 2-sided unpaired Mann-Whitney test. No statistical significance is observed. (B) A bar chart showing enriched gene sets by Gene Set Enrichment Analysis (GSEA). Enriched plots of differentially expressed genes for Hallmark complement, Hallmark interferon gamma response, Hallmark TNF-α signaling via NFkB, and Hallmark complement in BMMNCs of HLA alloimmunized compared with nonalloimmunized patients with SAA. GSEA based on the Kolmogorov-Smirnov test. (C) A network of upregulated genes involved in interferon gamma response, TNF-α signaling via NFkB, and complement pathway in those with postive HLA class I Ab (n = 8) compared to nonalloimmunized (n = 12) patients with SAA. B/Plasma, B cell and plasma cell; DC, dendritic cell; HSPC, hematopoietic stem and progenitor cells; IFN-γ, interferon gamma; NES, normalized enrichment score; NK, natural killer; ProB, B-cell progenitors.
Discussion
Patients with SAA are often heavily platelet-transfusion dependent and are at an increased risk for HLA alloimmunization. Higher number of required platelet transfusion with disease severity adds to risk of alloimmunization. In this large, retrospective, single-center study, we determined the impact of HLA alloimmunization on outcomes in patients with SAA treated with frontline IST. A higher than expected rate of HLA alloimmunization (22%) was detected in our cohort compared to recent reports in other diseases with chronic transfusion needs (in acute myeloid leukemia and after HSCT).9 This difference might be explained by our patient population which includes both those from the United States and international patients, often from resource-limited countries. Indeed, as observed by subgroup analysis, the HLA alloimmunization rate was double in international patients; in those from Afro-Caribbean countries, HLA Abs were positive in 50% cases. The Jamaican population had the highest rates within this subgroup, but this intergroup comparison may have been biased due to very small comparator groups from other countries. Higher rates of HLA alloimmunization in countries with limited medical resources are unsurprising and possibly due to suboptimal transfusion practices such as absence of leukocyte reduction techniques.14,39 Although HLA matching may not be feasible in resource poor settings, use of lower platelet-transfusion thresholds, early testing for alloimmunization, and prompt initiation of definitive therapies to avoid prolonged transfusion dependence should be applied. Family directed donations should be avoided, as feasible, to prevent alloimmunization and loss of a potential HSCT donor. This is particularly relevant for ethnicities that lack a rich donor pool in registries.
Other risk factors for HLA alloimmunization included female sex and SAA disease severity. As previously reported, pregnant women are likely at an increased risk of HLA alloimmunization by their fetuses,40 whereas the association with severity of disease may be due to frequent transfusion requirements. The true HLA alloimmunization rates may be higher than reported in our study because it is possible that among the 289 untested patients, subclinical alloimmunization occurred. Platelet-transfusion refractoriness increases hospital length of stay41 and leads to higher treatment costs, excessive use of labile blood products, increased risk of death, and higher rates of graft-versus-host disease (GVHD) after HSCT.42-44 Multicenter data shows that cross-reactive group matching can lead to a reduced frequency of sensitization to HLA.45 Lower platelet-transfusion thresholds of 5 × 103/μL as a trigger for preemptive platelet transfusions in patients with SAA without bleeding may be considered, as suggested by the British Society of Haematology to reduce exposure to Ags.46 Alternatively, in higher risk patients (greater transfusion needs, female patients, and those with very severe disease), lower threshold to perform preemptive testing for HLA Abs and HLA matching could be considered.
In our study, HLA alloimmunization was a negative prognostic marker, in contrast to Julen et al.23 Our study population was larger, ethnically more diverse, followed longer, and included patients from resource poor health systems. Multivariate analysis to adjust for potential confounders maintained this significance (supplemental Table 6C), indicating a true correlation. Most deaths were directly related to cytopenias. Infections were the leading cause of death in nearly 30% of patients consistent with our prior reports.47 Alloimmunized patients had both poorer response to IST and higher risk of clonal evolution, explaining lower survival.26,34 One possible explanation for this association could be that the HLA class I Abs constitute a part of broadly exaggerated and also nonspecific immune response. The underlying pathophysiology driving higher risk of clonal evolution in alloimmunized patients is not clear, but selection of maladaptive clones in a chronic inflammation may be contributory.48,49 Lower ANC resulting in shorter OS is consistent with previous reports.50
Treatment response to ATG-based IST in SAA has been associated with baseline ARC,50-52 ANC,50,53-56 and absolute lymphocyte count.50,57-59 Other known prognostic factors include subpopulations of regulatory T cell, 60 B cells, 61 normal telomere length of lymphocytes,62,63 elevated thrombopoietin levels,53 specific somatic mutations,64 and presence of a PNH clone.51,56,62,65,66 Our study shows that HLA alloimmunization status is both predictive, of response to IST, and prognostic in patients with SAA. Indeed, HLA alloimmunization is a potentially modifiable risk factor that may be ameliorated by the simple strategies highlighted above. Unlike nonalloimmunized patients, those with HLA Abs have very low rates of improvement in blood counts beyond 3 months.
A greater percentage of patients with HLA alloimmunization proceeded to undergo HSCT with equivalent survival compared to nonalloimmunized patients. In contrast to our report of a limited HSCT cohort, a larger study of 244 allogeneic transplants for patients with SAA (82 patients with HLA alloimmunization) by Wei et al24 reported alloimmunization to be detrimental for platelet recovery, with increased graft failure and reduced OS. Notably, GVHD, like iAA, is an immune-mediated disease, and the presence of HLA class I Abs has been linked to increased acute GVHD in pediatric HLA-matched HSCT for sickle cell disease.44 Hence, an earlier second line salvage treatment (IST or BM transplant) may improve overall outcomes in these patients. We lacked the availability of data to analyze for risk of GVHD after transplant because two-thirds of the HSCTs were at other institutes.
We hypothesized that HLA Abs might modulate autoimmunity through complement-mediated immune activation. Indeed, single-cell data revealed a complex interconnected upregulation of inflammation and complement pathways. Transfused platelets are cleared via immunoglobulin G alloantibody (HLA class I Ab)–mediated opsonization, C1q-associated membrane attack complex, deglycosylation, and activation of CD32a/Fc-gamma-IIa receptor pathways.10,67-69 The HLA complex presents nonself HLA class I Ags via Fc-gamma-IIa receptor pathways to effector cells (including cytotoxic CD8+ cells) and engages cellular and adaptive immune responses (supplemental Figure 1). As a result of complement cleavage, for opsonization (C3b) and membrane attack complex (C5b-9) complexes, residual small molecules of C3a and C5a, which themselves are phlogogenic anaphylotoxins recruiting inflammatory cells, activating phagocytotic cells, releasing granule-based enzymes and generating oxidants. Clinically worse marrow failure disease with poor response to IST and single-cell data showing increased expression of TNF-α, IFN, and complement associated gene pathways may indicate persistent immune dysregulation. While it is possible that the increased immune activation observed in our single-cell data may be a measure of more severe disease or increased transfusion requirement in those with HLA alloimmunization, it provides possible evidence for an enhanced and interlinked immune attack in some patients with SAA.
There are inherent limitations to retrospective studies limiting analysis, especially missing data, but in our study all patients were treated as part of 5 prospective clinical trials. We lacked detailed platelet-transfusion records, particularly for international patients before their arrival at NIH, limiting our analysis. Adherence to processing techniques and barriers to acquisition of blood product was assumed based on prevalent regional practices.70 Genetic predisposition risks for alloimmunization were not explored in this work but we acknowledge genetic ethnic variation in the Afro-Caribbean population.
In summary, HLA class I alloimmunization, older age and lower ANC are risk factors adversely impacting OS. Alloimmunized patients show poorer responses to standard immunosuppressive therapies for iAA and higher rates of clonal evolution. Alloimmunization is a preventable prognostic factor, and the risk of HLA Ab development is higher among women, patients with more severe disease and those from countries not practicing the accepted standard blood product techniques. Lowering platelet-transfusion threshold to 5 × 103/μL for stable patients and use of leukoreduced platelets are some strategies to reduce the antigenic exposure and optimize blood product utilization.9,46 Periodic HLA Ab testing in alloimmunized patient is an additional practice followed at the NIH Clinical Center. Complement immune-mediated synergy possibly worsening autoimmunity deserves further exploration in AA and across autoimmune diseases.
Acknowledgments
This study was funded by the National Heart, Lung, and Blood Institute Intramural Research Program and the NIH Clinical Center (ZIC CL 002128).
Authorship
Contribution: J.D., L.N.C., B.A.P., and E.M.G. designed the study, analyzed data, and wrote and edited the manuscript; J.D., V.B., and J.G. collected the data; N.R.C. and W.A.F. performed and analyzed the HLA antigen and antibody tests; Z.W and S.G perfromed single cell sequencing and data analysis, X.M., R.N.S. and C.O.W. performed statistical analysis; B.A.P., E.M.G., N.S.Y., J.L., and O.R. provided patient care; and E.M.G., B.A.P., W.A.F., and N.S.Y. provided expert review of the manuscript.
Conflict-of-interest disclosure: N.S.Y. has a Cooperative Research and Development Agreement with Novartis. The remaining authors declare no competing financial interests.
Correspondence: Bhavisha A. Patel, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Drive, Room 3-5140, Bethesda, MD 20892; email: bhavisha.patel@nih.gov.
References
Author notes
J.D. and L.N.C. contributed equally to this study.
E.M.G. and B.A.P. are joint last authors.
The raw and analyzed sequencing data in this study have been deposited in the NCBI Gene Expression Omnibus (under series accession code GSE247531).
The full-text version of this article contains a data supplement.