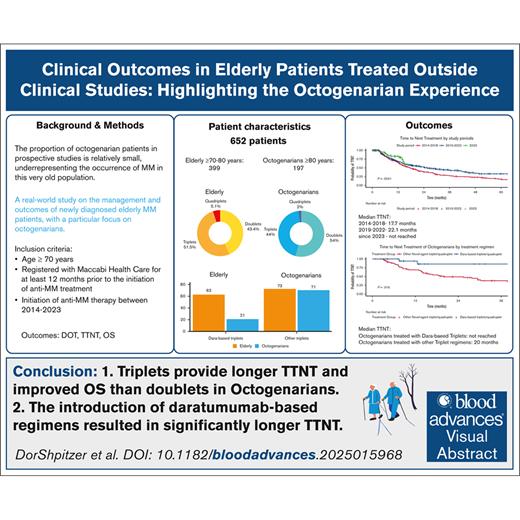
Key Points
Triplet regimens provide longer TTNT and improved OS than doublets in Octos.
The introduction of daratumumab-based regimens resulted in significantly longer TTNT.
Visual Abstract
With an aging population, multiple myeloma (MM) increasingly affects octogenarians (Octos; age of ≥80 years), yet data on their management and outcomes, particularly if treated outside clinical trials, remain limited. This retrospective study analyzed 652 patients aged ≥70 years, diagnosed with active MM between 2014 and 2023, identified in the Maccabi Healthcare Services medical records. Patient characteristics, treatment, time to next treatment (TTNT), overall survival (OS), and factors influencing outcomes, were compared between Octo and older adults (OL) aged 70 to <80 years. Octo patients (median age, 83 years) had more comorbidities and higher Charlson Comorbidity Index (CCI) scores than OL patients (≥6; 53% vs 23%), leading to lower rates of anti-MM therapy administration and reduced triplet/quadruplet regimen use. Over a median follow-up of 25 months, Octo patients had significantly shorter median OS; 25.9 vs 71.3 months, and 33 vs 76.9 months among treated patients only. TTNT was similar (17.8 vs 22.1 months). Multivariate analysis showed that triplet/quadruplet regimen was associated with improved TTNT (hazard ratio [HR], 0.61; P < .001) and OS (HR, 0.63; P < .001) compared with doublets, whereas higher CCI scores and age of ≥80 years predicted worse OS (HR, 1.5; P = .003; and HR, 2.27; P < .001; respectively). Daratumumab-based therapies enhanced TTNT in both age groups (HR, 0.54; P = .017). In conclusion, higher comorbidities in Octo patients adversely affected their management and survival. However, daratumumab positively influenced outcomes, underscoring the need for tailored approaches to optimize treatment in older patients with MM.
Introduction
Patients with myeloma aged >70 years are usually considered as nontransplant eligible and their management relies on prospective clinical trials that focused on this group of patients. The proportion of octogenarian (Octo) patients in these prospective studies is relatively small, underrepresenting the occurrence of multiple myeloma (MM) in this very old population.1-5 This proportion is likely to grow, considering the improvement in overall survival (OS) in the Western world and the higher risk for developing MM with aging. Moreover, patients included in clinical prospective studies are often fitter than their age group, having a lower number of comorbidities, especially, a lower frequency of dementia and renal impairment.1-3,6-8 Data on Octo patients treated with novel agents outside of clinical trials are limited and primarily derived from retrospective studies, which often include only a few hundred patients.9-11 Indeed, the outcome of these patients and, particularly, their median OS, appear to be inferior to those reported in younger counterparts treated during the same time period, approaching only ∼2 years.9-11 Lastly, most of these retrospective studies, including the largest one that included 1140 patients,12 evaluated patients that were treated before introduction of daratumumab (Dara) to the up-front setting, highlighting the need for updated analyses on treatment outcomes and survival benefits in Octo patients with the current Dara-inclusive therapies. We investigated the characteristics and outcome of patients with MM aged ≥70 but <80 years (termed older adults [OL]) vs those aged ≥80 years (Octo patients), defining treatment approaches, duration of treatment (DOT), time to next treatment (TTNT), number of subsequent therapies and, OS, assuming that Octo patients who are treated outside clinical studies, would have a greater number of comorbidities, leading to worse outcomes.
Methods
Study population
The current retrospective study, approved by the Maccabi Healthcare Services (MHS) ethical committee (approval number MHS-0075-23), included patients registered with the MHS, the second-largest health care organization in Israel. According to the infrastructure of the health care system in Israel, all patients with MM receive treatment in specialized hospital-based hemato-oncological day care units with expertise in MM, either in secondary or tertiary institutions. All health care providers (including the MHS) are responsible for coordinating patient care and reimbursing treatment costs.
Inclusion criteria required individuals to be aged ≥70 years with a diagnosis of active MM requiring therapy between January 2014 and December 2023. Patients needed to present with high calcium level, renal failure, anemia or bone lesions (CRAB) or sixty percent bone marrow plasma cells, high light chain ratio or MRI lesions (SLIM) CRAB features13 at diagnosis and be enrolled in the MHS for at least 12 months before the official diagnosis or initiation of anti-MM treatment. Patients diagnosed with plasma cell leukemia14 or smoldering MM15 were excluded from the analysis.
Comparisons were made between Octo and EL patients, as well as between the “treated cohort” and those who did not receive anti-MM therapy and were instead managed with palliative care. The analysis assessed OS, DOT, TTNT, and factors influencing these outcomes, including patient-related, MM-related, and treatment-related factors.
Study variables and definitions
Patient characteristics, including demographics and preexisting comorbidities such as non–insulin-dependent diabetes mellitus, chronic kidney disease, dementia, cerebrovascular accident, congestive heart disease, hypertension, osteoporosis, and prior cancer (excluding basal or squamous cell carcinoma of the skin) were recorded from MHS registries and patient electronic medical records.16-18 All patients were included in the registry before their diagnosis of MM. Charlson Comorbidity Index (CCI) scores were calculated using International Classification of Diseases (ninth revision) coding system.19
Laboratory results at the time of treatment initiation or official MM diagnosis, including hemoglobin (Hb), creatinine, calcium, immunoglobulins, and free light chains, were recorded from the MHS laboratory database. Anemia was defined as Hb of <10 g/dL or a decrease in Hb level of ≥2 g/dL from baseline13; acute renal failure, as elevated creatinine of >1.3 mg/dL within the last 6 month; and hypoalbuminemia as albumin of <3.5 g/dL.
First-line (1L) treatment was administered in accordance with the Israeli reimbursement policy in effect at the time of diagnosis. From 2014 to 2018, patients received bortezomib (Velcade [V]) combined with low-dose dexamethasone (d), with or without cyclophosphamide or melphalan (Vd/V + cyclophosphamide + d or V + melphalan + d). From 2019 to 2022, regimens included Vd or Vd combined with lenalidomide (R) or Rd. Starting in 2023, Dara combined with Rd or Vd was introduced. These timeframes are collectively referred to as “treatment periods” and outline the available regimens during the study period. DOT was defined as the time from the first purchase of anti-MM therapy (index date) to the last purchase of agents applied in 1L treatment. OS was measured from the index date until death or last follow-up (FU) date, and TTNT, defined as the time from index date until the purchase of a second-line anti-MM therapy.
Statistical analyses
Descriptive statistics were reported as absolute numbers and percentages for categorical variables, and as medians with interquartile ranges (IQRs) for continuous variables. The χ2 test was used to compare proportions across cohorts, whereas the Mann-Whitney U test was applied to compare medians.
Survival curves for DOT, OS, and TTNT were generated using the Kaplan-Meier method, with differences between cohorts assessed via the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and adjusted HRs, respectively, for DOT, OS, and TTNT. All tests were 2-tailed, and a P value <.05 was considered statistically significant. All analyses were conducted using R statistical software.
Results
Patient characteristics
A total of 652 consecutive patients were included in the analysis, with a median age of 77 years (range, 70-96); 64% of patients (n = 415) were aged ≥70 to <80 years and 36% patients (n = 237) were aged ≥80 years. The mean number of comorbidities in the entire Octo cohort was 3 (range, 0-6), compared with 2 in the entire OL patient cohort (range, 0-6; P = .001). In line with that, the proportion of patients that presented with a CCI score of ≥6 was also significantly higher in the Octo (53% [n = 125] vs the OL cohort, 22% [n = 93]; P < .001). Specific comorbidities present in Octo vs OL patients before MM diagnosis included dementia (13% [n = 31] vs 4.6% [n = 19]), osteoporosis (43% [n = 101]) vs 30% [n = 125]; P = .001], chronic kidney disease (30% [n = 72] vs 16% [n = 68]; P < .001), and hypertension (85% [n = 201] vs 73% [n = 305]; P < .001). Additionally, 83% (n = 197) of Octo and 96% (n = 399) of OL patients were treated with an anti-MM therapy, whereas 17% (n = 40) of Octo and 4% (n = 16) of OL patients received palliative therapy. Patient characteristics for the entire cohort and dependent on age group are presented in Table1 (treated cohort) and supplemental Table 1 (treated vs palliative cohorts).
Patient characteristics of the treated cohort
| Variable . | The entire population N = 596 . | OL Age, 70-80 y n = 399 . | Octo Age, >80 y n = 197 . | P value∗ . |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis, median (IQR), y | 77.0 (73.0-81.0) | 74.0 (72.0-77.0) | 83.0 (81.0-85.0) | <.001 |
| Male, n (%) | 355 (60) | 248 (62) | 107 (54) | .067 |
| Comorbidities, n (%) | ||||
| Diabetes | 215 (36) | 141 (35) | 74 (38) | .6 |
| Osteoporosis | 205 (34) | 123 (31) | 82 (42) | .009 |
| CKD | 108 (18) | 62 (16) | 46 (23) | .020 |
| CVA | 26 (4.4) | 14 (3.5) | 12 (6.1) | .15 |
| Hypertension | 454 (76) | 293 (73) | 161 (82) | .025 |
| Dementia | 39 (6.5) | 18 (4.5) | 21 (11) | .004 |
| CHF | 33 (5.5) | 17 (4.3) | 16 (8.1) | .053 |
| Prior malignancy | 58 (9.7) | 43 (11) | 15 (7.6) | .2 |
| CCI score, n (%) | <.001 | |||
| 3-5 | 426 (71) | 317 (79) | 109 (55) | |
| 6-7 | 130 (22) | 66 (17) | 64 (32) | |
| 8-12 | 40 (7) | 16 (4) | 24 (13) | |
| CCI score of ≥6, n (%) | 170 (29) | 82 (21) | 88 (45) | <.001 |
| MM-related laboratory results | ||||
| Anemia, n (%) | 122 (22) | 70 (18) | 52 (28) | .007 |
| Creatinine, median (IQR), mg/dL | 1.00 (0.80-1.40) | 0.90 (0.80-1.30) | 1.00 (0.80-1.40) | .026 |
| Acute renal failure, n (%) | 6 (1.1) | 5 (1.3) | 1 (0.5) | .7 |
| Calcium, median (IQR), mg/dL | 9.10 (8.70-9.50) | 9.10 (8.70-9.50) | 9.20 (8.80-9.50) | .5 |
| Albumin, median (IQR), g/dL | 3.80 (3.50-4.10) | 3.90 (3.60-4.10) | 3.70 (3.40-4.10) | .002 |
| Albumin <3.5 g/dL, n (%) | 126 (24) | 70 (20) | 56 (32) | .003 |
| Abnormal FLCR, n (%) | 223 (76) | 134 (68) | 89 (90) | <.001 |
| Treatment regimens | ||||
| Treatment period, n (%) | .5 | |||
| 2014-2018 | 241 (40) | 167 (42) | 74 (38) | |
| 2019-2022 | 275 (46) | 178 (45) | 97 (49) | |
| 2023 | 80 (13) | 54 (14) | 26 (13) | |
| Triplets/quadruplets, n (%) | 321 (54) | 229 (57) | 92 (47) | .014 |
| Regimen, n (%) | .002 | |||
| Vd | 215 (36) | 136 (34) | 79 (40) | |
| Rd | 41 (6.9) | 24 (6.0) | 17 (8.6) | |
| VRd | 167 (28) | 118 (30) | 49 (25) | |
| VCd/VMd/RMd | 70 (11.7) | 48 (12) | 22 (11.1) | |
| Dara-d | 19 (3.2) | 10 (2.5) | 9 (4.6) | |
| Dara-based triplets/quadruplets: Dara-Vd/Dara-Rd/Dara-VRd | 84 (14.1) | 63 (15.8) | 21 (10.7) | |
| Dara-based regimen, n (%) | 103 (17) | 73 (1) | 30 (15) | .4 |
| Variable . | The entire population N = 596 . | OL Age, 70-80 y n = 399 . | Octo Age, >80 y n = 197 . | P value∗ . |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis, median (IQR), y | 77.0 (73.0-81.0) | 74.0 (72.0-77.0) | 83.0 (81.0-85.0) | <.001 |
| Male, n (%) | 355 (60) | 248 (62) | 107 (54) | .067 |
| Comorbidities, n (%) | ||||
| Diabetes | 215 (36) | 141 (35) | 74 (38) | .6 |
| Osteoporosis | 205 (34) | 123 (31) | 82 (42) | .009 |
| CKD | 108 (18) | 62 (16) | 46 (23) | .020 |
| CVA | 26 (4.4) | 14 (3.5) | 12 (6.1) | .15 |
| Hypertension | 454 (76) | 293 (73) | 161 (82) | .025 |
| Dementia | 39 (6.5) | 18 (4.5) | 21 (11) | .004 |
| CHF | 33 (5.5) | 17 (4.3) | 16 (8.1) | .053 |
| Prior malignancy | 58 (9.7) | 43 (11) | 15 (7.6) | .2 |
| CCI score, n (%) | <.001 | |||
| 3-5 | 426 (71) | 317 (79) | 109 (55) | |
| 6-7 | 130 (22) | 66 (17) | 64 (32) | |
| 8-12 | 40 (7) | 16 (4) | 24 (13) | |
| CCI score of ≥6, n (%) | 170 (29) | 82 (21) | 88 (45) | <.001 |
| MM-related laboratory results | ||||
| Anemia, n (%) | 122 (22) | 70 (18) | 52 (28) | .007 |
| Creatinine, median (IQR), mg/dL | 1.00 (0.80-1.40) | 0.90 (0.80-1.30) | 1.00 (0.80-1.40) | .026 |
| Acute renal failure, n (%) | 6 (1.1) | 5 (1.3) | 1 (0.5) | .7 |
| Calcium, median (IQR), mg/dL | 9.10 (8.70-9.50) | 9.10 (8.70-9.50) | 9.20 (8.80-9.50) | .5 |
| Albumin, median (IQR), g/dL | 3.80 (3.50-4.10) | 3.90 (3.60-4.10) | 3.70 (3.40-4.10) | .002 |
| Albumin <3.5 g/dL, n (%) | 126 (24) | 70 (20) | 56 (32) | .003 |
| Abnormal FLCR, n (%) | 223 (76) | 134 (68) | 89 (90) | <.001 |
| Treatment regimens | ||||
| Treatment period, n (%) | .5 | |||
| 2014-2018 | 241 (40) | 167 (42) | 74 (38) | |
| 2019-2022 | 275 (46) | 178 (45) | 97 (49) | |
| 2023 | 80 (13) | 54 (14) | 26 (13) | |
| Triplets/quadruplets, n (%) | 321 (54) | 229 (57) | 92 (47) | .014 |
| Regimen, n (%) | .002 | |||
| Vd | 215 (36) | 136 (34) | 79 (40) | |
| Rd | 41 (6.9) | 24 (6.0) | 17 (8.6) | |
| VRd | 167 (28) | 118 (30) | 49 (25) | |
| VCd/VMd/RMd | 70 (11.7) | 48 (12) | 22 (11.1) | |
| Dara-d | 19 (3.2) | 10 (2.5) | 9 (4.6) | |
| Dara-based triplets/quadruplets: Dara-Vd/Dara-Rd/Dara-VRd | 84 (14.1) | 63 (15.8) | 21 (10.7) | |
| Dara-based regimen, n (%) | 103 (17) | 73 (1) | 30 (15) | .4 |
CHF, congestive heart failure; CKD, chronic kidney disease; CVA, cerebrovascular accident; RMd, lenalidomide + melphalan + dexamethasone; VCD, bortezomib + cyclophosphamide + dexamethasone; VMD, bortezomib + melphalan + dexamethasone.
Wilcoxon rank-sum test; Pearson χ2 test; Fisher exact test.
There were no significant differences in the proportions of Octo vs OL patients treated during the different treatment periods. Of those who received an anti-MM therapy, V-based regimens were used in 48% (n = 285) of patients, Rd in 7% (n = 41), RVd in 28% (n = 167), and Dara-containing regimens in 17% (n = 103; supplemental Table 2). Novel agent–based triplet/quadruplet regimens were less frequently used in Octo patients (47%, 87/5) compared with OL patients (57%, 205/24; P = .01). Nevertheless, since the reimbursement of Dara in the up-front setting in January 2023, the use of triplet/quadruplet regimens in Octo patients increased and Dara-containing triplets were used in 42% (n = 11) vs 68% (n = 37) in OL patients (P = .046).
Of note, a subgroup analysis comparing the characteristics of patients treated with Dara-based triplets/quadruplets with the characteristics of patients treated with other triplet/quadruplet regimens (proteasome inhibitor [PI]–immunomodulatory drug [IMiD] based) revealed no significant differences in terms of age, comorbidity burden, and laboratory results (supplemental Table 3).
Outcomes
The median FU period for the entire cohort was 25 months (IQR, 12-50); 29 months (IQR, 14-53) for treated and 7 months (IQR, 4-11) for palliated patients. Of the treated cohort, 55% (n = 326) received subsequent lines of treatment; 49% (n = 97) in the Octo cohort and 57% (n = 229) in the OL cohort (P = .07).
At the last FU date of the treated cohort, 24.3% of patients (n = 145) who started 1L therapy were still receiving this 1L treatment, with 17% (n = 35) in the Octo cohort and 27.5% (n = 110) in the OL cohort (P = .01). A total of 27% (n = 162) patients had progressed to subsequent lines of treatment and remained alive: 17% (n = 34) in the Octo cohort and 32% (n = 128) in the OL cohort (P < .001). Among those who received additional lines of therapy, 49% (n = 161) ultimately died, including 65% (n = 63) in the Octo cohort and 43% (n = 98) in the OL cohort (P < .001). Additionally, 21% of patients (n = 123) died before receiving any subsequent lines of therapy, with significantly more deaths in the Octo cohort (33% [n = 65] vs 14.5% [n = 58] in the OL cohort; P < .001). Among 57 patients in the palliative cohort, 57% (n = 32) had died at last FU date.
Duration of therapy
The median duration of 1L treatment for all treated patients in the cohort, was 10.2 months (IQR, 4.3-18.6), shorter in the Octo cohort; 7 months (IQR, 3.5-13.6), compared with 11.8 months (IQR, 5.3-20.7) in the OL cohort (P < .001). Patients treated with triplet/quadruplet regimens experienced longer treatment duration irrespective of age, with a median DOT of 11.5 months; 6.6 months in the Octo cohort, and 17.9 months in the OL cohort (P < .001). Notably, Dara-containing triplets provided the longest median DOT, approaching 23.7 months for the entire cohort; 7.2 months in the Octo and 23.7 months in the EL cohorts (P = .23).
Multivariate Cox regression analyses (Table 2), based on univariate analysis (supplemental Table 4), showed that older age (HR, 1.43; 95% confidence interval [CI], 1.18-1.72; P < .001) and dementia (HR, 1.46; 95% CI, 1.03-2.09; P = .036) were associated with shorter DOT. Additionally, a high CCI score (≥6) was linked to shorter DOT (HR, 1.26; 95% CI, 1.04-1.53; P = .019). In contrast, triplet/quadruplet therapies were significantly associated with longer DOT than doublet regimens (HR, 0.52; 95% CI, 0.43-0.62; P < .001).
Multivariate analysis for factors associated with DOT
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Age, ≥80 y | 1.43 | 1.18-1.72 | <.001 |
| Dementia | 1.46 | 1.03-2.09 | .036 |
| Triplet/quadruplet regimens | 0.52 | 0.43-0.62 | <.001 |
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Age, ≥80 y | 1.43 | 1.18-1.72 | <.001 |
| Dementia | 1.46 | 1.03-2.09 | .036 |
| Triplet/quadruplet regimens | 0.52 | 0.43-0.62 | <.001 |
Time to second-line treatment
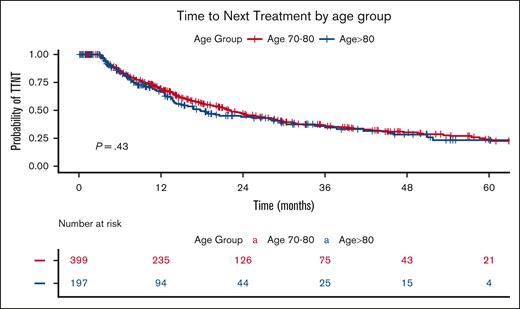
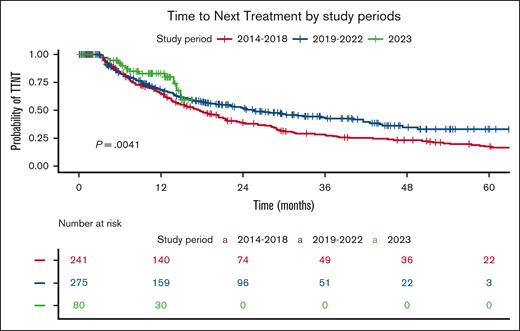
Based on Kaplan-Meier curves, the median TTNT for the entire cohort of treated patients was 21.4 months, with the Octo cohort having a median TTNT of 17.8 months compared with 22.1 months in the OL cohort (P = .43; Figure 1). Stratification by treatment periods (Figure 2) revealed a significantly longer TTNT during the Dara era (2019-2023), with a median TTNT of 17.7 months in 2014 to 2018, 25.6 months in 2019 to 2022, and not reached for patients treated in 2023 (P = .004). During the 2014-to-2018 period, the Octo cohort had a median TTNT of 16.8 months, compared with 18.8 months in the Ol cohort (P = .7). The difference widened in 2019 to 2023, with a median TTNT of 18.4 months for the Octo cohort vs 29.7 months for the OL cohort (P = .25).
TTNT by age group. The Kaplan-Meier plot illustrates TTNT stratified by age group. The blue curve represents the Octo cohort (aged ≥80 years), whereas the red curve represents the OL cohort (aged 70 to <80 years). The table below displays the number of patients at risk (individuals who have not yet progressed to second-line treatment and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
TTNT by age group. The Kaplan-Meier plot illustrates TTNT stratified by age group. The blue curve represents the Octo cohort (aged ≥80 years), whereas the red curve represents the OL cohort (aged 70 to <80 years). The table below displays the number of patients at risk (individuals who have not yet progressed to second-line treatment and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
TTNT by study period. The Kaplan-Meier plot illustrates TTNT, stratified by the study period during which 1L treatment was administered, reflecting the Israeli reimbursement policy in effect at the time of diagnosis. The red curve represents patients diagnosed between 2014 and 2018, the blue curve represents those diagnosed between 2019 and 2022, and the green curve represents patients diagnosed since 2023. The table below shows the number of patients at risk (individuals who have not yet progressed to second-line treatment and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
TTNT by study period. The Kaplan-Meier plot illustrates TTNT, stratified by the study period during which 1L treatment was administered, reflecting the Israeli reimbursement policy in effect at the time of diagnosis. The red curve represents patients diagnosed between 2014 and 2018, the blue curve represents those diagnosed between 2019 and 2022, and the green curve represents patients diagnosed since 2023. The table below shows the number of patients at risk (individuals who have not yet progressed to second-line treatment and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
The median TTNT in patients receiving any triplet/quadruplet therapy, approached 29.4 months; being 29.4 months in Octo patients and 29.7 months in OL patients (P = .67). Of note, triplets/quadruplets containing Dara provided longer TTNT than non-Dara triplets; being not reached vs 28.3 months (P = .05). When stratifying Dara-based triplets to age, there was no significant difference in TTNT between Octo and OL adults (not reached in Octo vs 24.4 months in OL adults; P = .11).
Multivariate Cox regression analyses (Table 3), based on univariate analysis (supplemental Table 5), showed that patients on triplet or quadruplet therapy had a 40% lower risk of progressing to a second-line treatment than those on doublet therapy (HR, 0.61; 95% CI, 0.49-0.75; P < .001). Age group (70-80 vs >80 years) or higher CCI score did not affect the risk of next treatment (HR, 1.1; 95% CI, 0.86-1.41; P = .5; and HR, 0.9; 95% CI, 0.69-1.16; P = .4; respectively). In a separate multivariate model, Dara-based triplets or quadruplets were associated with a significantly longer TTNT than other triplet regimens with HR = 0.54 (95% CI, 0.32-0.90; P = .017; supplemental Table 6).
Multivariate analysis for factors associated with TTNT
| Characteristic . | HR∗ . | 95% CI . | P value . |
|---|---|---|---|
| Age, ≥80 y | 1.10 | 0.86-1.41 | .5 |
| CCI score of ≥6 | 0.90 | 0.69-1.16 | .4 |
| Triplet/quadruplet regimens | 0.61 | 0.49-0.75 | <.001 |
| Characteristic . | HR∗ . | 95% CI . | P value . |
|---|---|---|---|
| Age, ≥80 y | 1.10 | 0.86-1.41 | .5 |
| CCI score of ≥6 | 0.90 | 0.69-1.16 | .4 |
| Triplet/quadruplet regimens | 0.61 | 0.49-0.75 | <.001 |
OS
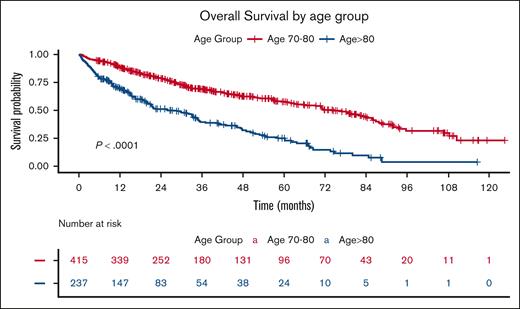
Based on Kaplan-Meier curves, the median OS for the entire cohort was 51.3 months, with significantly shorter survival in the Octo cohort (25.9 months) than the OL cohort (71.3 months; P < .001; Figure 3).
OS by age group. The Kaplan-Meier plot illustrates OS stratified by age group. The blue curve represents the Octo cohort (age of ≥80 years), whereas the red curve represents the OL cohort (aged 70 to <80 years). The table below displays the number of patients at risk (individuals who have not yet died and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
OS by age group. The Kaplan-Meier plot illustrates OS stratified by age group. The blue curve represents the Octo cohort (age of ≥80 years), whereas the red curve represents the OL cohort (aged 70 to <80 years). The table below displays the number of patients at risk (individuals who have not yet died and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
Within the treated cohort, median OS was 56.5 months, significantly shorter for Octo patients than the OL cohort, at 33 months vs 76.9 months, respectively (P < .001). In contrast, in the palliated cohort, the median OS was 7.9 months, with no statistically significant difference between the Octo and the OL cohorts (9.7 months vs 7.9 months [P = .44]).
Multivariate Cox regression analyses (Table 4), derived from the results of univariate analysis (supplemental Table 7), identified several factors associated with shorter OS within the entire treated cohort: age of ≥80 years (HR, 2.27; 95% CI, 1.76-2.94; P < .001), CCI score of ≥6 (HR. 1.48; 95% CI, 1.15-1.92; P = .003), and anemia (HR, 1.57; 95% CI, 1.20-2.04; P < .001). Conversely, treatment with novel agent–based triplets/or quadruplets provided longer OS than doublets (HR, 0.64; 95% CI, 0.5-0.81; P < .001).
Multivariate analysis for factors associated with OS
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Age, ≥80 y | 2.27 | 1.76-2.94 | <.001 |
| CCI score of ≥6 | 1.48 | 1.15-1.92 | .003 |
| Anemia | 1.57 | 1.20-2.04 | <.001 |
| Triplet/quadruplet regimens | 0.63 | 0.49-0.81 | <.001 |
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Age, ≥80 y | 2.27 | 1.76-2.94 | <.001 |
| CCI score of ≥6 | 1.48 | 1.15-1.92 | .003 |
| Anemia | 1.57 | 1.20-2.04 | <.001 |
| Triplet/quadruplet regimens | 0.63 | 0.49-0.81 | <.001 |
In a separate multivariate model (supplemental Table 8), additional factors linked to shorter OS included congestive heart failure (HR, 2.41; 95% CI, 1.57-3.70; P < .001) and dementia (HR, 1.74; 95% CI, 1.14-2.64; P = .01). Dara-based triplet regimens showed a trend toward improved OS but it did not reach statistical significance (HR, 0.90; 95% CI, 0.54-1.51; P = .7; supplemental Table 9).
Analysis of factors affecting OS of Octo patients only
The adoption of triplet/quadruplet therapies for Octo patients increased significantly over the study periods; from 27% (n = 20) in 2014 to 2018 to 56% (n = 54) and 50% (n = 13) in 2019 to 2022, and in 2023, respectively. Stratifying the Octo cohort into fitter patients (CCI score of 4-5) and frailer (CCI score of ≥6) revealed no difference in the proportion receiving triplet/quadruplet regimens, with 47% (n = 51) of the fitter cohort and 47% (n = 41) of the frailer cohort receiving such treatments.
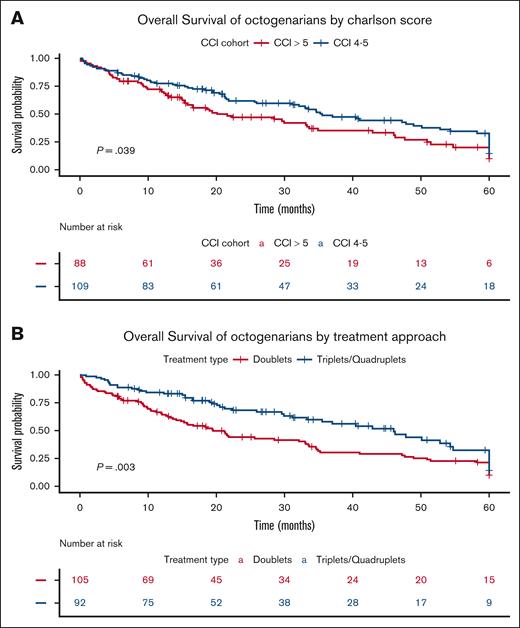
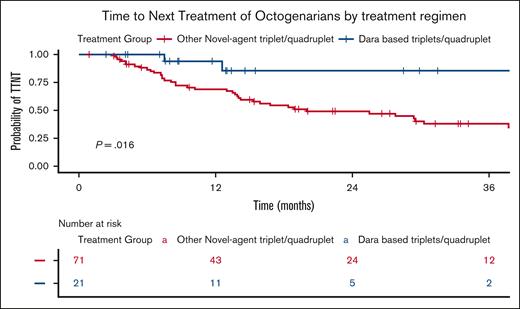
Based on Kaplan-Meier curves, the median OS for treated Octo patients was 33 months, significantly longer for fitter Octo (35.5 months vs 21.6 months; P = .04; Figure 4A) and for those treated with triplets compared with doublets (46 months vs 21 months; P = .003; Figure 4B). Additionally, TTNT was significantly longer for Octo patients treated with Dara-based triplets/quadruplets regimens (median not reached) than with other novel agent–based triplets/quadruplets (median, 20 months; P = .016; Figure 5).
OS of Octos. (A) by CCI score. The Kaplan-Meier plot illustrates OS in the Octo cohort, stratified by CCI score. The blue curve represents patients with a CCI score of 4 to 5 at the index date, whereas the red curve represents patients with a CCI score of ≥6. (B) by treatment approach. This Kaplan-Meier plot illustrates OS in the Octo cohort, stratified by 1L treatment approach. The blue curve represents patients who received triplet or quadruplet regimens, whereas the red curve represents those who received doublets. The table below the figures displays the number of patients at risk (individuals who have not yet died and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
OS of Octos. (A) by CCI score. The Kaplan-Meier plot illustrates OS in the Octo cohort, stratified by CCI score. The blue curve represents patients with a CCI score of 4 to 5 at the index date, whereas the red curve represents patients with a CCI score of ≥6. (B) by treatment approach. This Kaplan-Meier plot illustrates OS in the Octo cohort, stratified by 1L treatment approach. The blue curve represents patients who received triplet or quadruplet regimens, whereas the red curve represents those who received doublets. The table below the figures displays the number of patients at risk (individuals who have not yet died and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
TTNT of Octos by treatment regimen. The Kaplan-Meier plot illustrates TTNT in Octos treated with triplet or quadruplet regimens, stratified by the inclusion of Dara. The blue curve represents patients who received Dara-based triplet or quadruplet regimens, whereas the red curve represents those treated with other triplet or quadruplet regimens. The table below displays the number of patients at risk (individuals who have not yet progressed to second-line treatment and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
TTNT of Octos by treatment regimen. The Kaplan-Meier plot illustrates TTNT in Octos treated with triplet or quadruplet regimens, stratified by the inclusion of Dara. The blue curve represents patients who received Dara-based triplet or quadruplet regimens, whereas the red curve represents those treated with other triplet or quadruplet regimens. The table below displays the number of patients at risk (individuals who have not yet progressed to second-line treatment and are still under observation) at each time point. The P value represents the result of the log-rank test comparing the survival curves.
Multivariate analysis for factors affecting OS in Octo patients indicated that treatment with novel agent–based triplet or quadruplet regimens was associated with longer OS (HR, 0.59; 95% CI, 0.41-0.86; P = .006). In contrast, congestive heart failure (HR, 2.22; 95% CI, 1.22-4.03; P = .009) and dementia (HR, 1.84; 95% CI, 1.06-3.19; P = .029) were associated with shorter OS (Table 5). In a separate model, a CCI score of ≥6 was also associated with shorter OS (HR, 1.45; 95% CI, 1.0-2.11; P = .048).
Multivariate analysis for factors associated OS in Octo patients
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Dementia | 1.84 | 1.06-3.19 | .029 |
| CHF | 2.22 | 1.22-4.03 | .009 |
| Anemia | 1.39 | 0.94-2.05 | .10 |
| Triplet/quadruplet regimens | 0.59 | 0.41-0.86 | .006 |
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Dementia | 1.84 | 1.06-3.19 | .029 |
| CHF | 2.22 | 1.22-4.03 | .009 |
| Anemia | 1.39 | 0.94-2.05 | .10 |
| Triplet/quadruplet regimens | 0.59 | 0.41-0.86 | .006 |
CHF, congestive heart failure.
Discussion
Our study examined the outcomes of a comprehensive, unselected cohort of OL patients, newly diagnosed with MM, aged ≥70 years, with a particular focus on Octo patients (aged ≥80 years). It compared their outcomes with those of younger OL patients (aged 70-79 years) and evaluated the influence of treatment regimens and comorbidity burden on clinical outcomes.
As expected, Octo patients exhibited a higher comorbidity burden and elevated CCI scores, which, in some cases, precluded the initiation of active anti-MM therapy. Consequently, 16% of Octo patients received supportive care alone as up-front management, compared with only 4% of their younger counterparts, who were more frequently treated with active regimens. Correspondingly, a significantly smaller proportion of Octo patients received triplet or quadruplet therapies compared with the OL cohort. Notably, the adoption of triplet or quadruplet regimens among Octo patients increased significantly over time, particularly after the introduction of Dara, suggesting that advancements in therapeutic options and improved tolerability may have enabled the use of more intensive regimens even in older patients with higher comorbidity burdens.
The shorter DOT observed in the Octo cohort, likely reflects the challenges of maintaining intensive therapy (especially PI-IMiD based) in older patients, who often present with greater comorbidities, frailty, and reduced tolerance to extended treatment courses.7,11 The inferior DOT compared with other studies may be attributed to the inclusion of a broader, nonselected patient population in this analysis, as well as differences in DOT definitions across studies.1 Despite these limitations, the consistent association of Dara-based triplet or quadruplet regimens with longer DOT across age groups highlights their efficacy and tolerability, even among older, frailer populations, when selected appropriately. Despite the short DOT, TTNT was relatively encouraging, approaching ∼2 years, with no significant differences in TTNT between Octo and OL cohorts, reflecting the broader adoption of effective therapies across age groups. Interestingly, the use of Dara-based triplet therapy appeared to extend TTNT compared with other triplets, with the median TTNT not yet reached within the 24-month FU period. This benefit was particularly pronounced in the Octo group, reflecting the superior efficacy of these regimens, as well as the improved toxicity profile and the higher tolerability of Dara-containing triplets compared with PI-IMiD–based triplets.11
A restricted analysis of the Octo cohort revealed that TTNT was approximately twice as long for patients receiving triplet or quadruplet therapies, approaching 2.5 years. This likely reflects the selection of “fitter” patients for these regimens and/or the higher impact of introducing Dara-based triplets in this cohort, reflecting their greater tolerability compared with previously existing triplets (eg, PI-IMiDs, etc)
As expected, median OS referring to all patients was significantly shorter for Octo patients; slightly >2 years compared with ∼6 years in their younger counterparts aged 70 to 80 years. Nevertheless, it appeared to be higher than reported in some prospective5,20 studies that refer to these population of patients. Consistent with findings from the MAIA trial,21 fitter Octo patients demonstrated better OS regardless of the 1L regimen received. This survival should be considered as relatively encouraging considering that these patients, as opposed to patients who are recruited into clinical studies, have significant comorbidities, precluding many from participation in prospective studies.
Moreover, the 2-year OS refers to the entire population of Octo patients, whereas those that were eventually treated, accounting for 84% of Octo patients, achieved a median OS of almost 3 years, significantly longer than previously reported in treated Octo patients.9,11,12,22 This improved OS might be because of the relatively high proportion of patients that received subsequent lines of treatment, similar to that reported in other prospective and real-world studies.23
This study has several limitations. First, as a retrospective analysis of data from the MHS, it relies on the accuracy and completeness of electronic health records. Misclassification or incomplete capture of comorbidities, treatments, or outcomes may affect the results, especially because some comorbidities may be underreported. Second, selection bias is possible, because patients managed exclusively in other health care systems or those who did not meet the MHS inclusion criteria were not included, potentially limiting generalizability to the broader Israeli or international population. Furthermore, because of the structure of the health care system in Israel, all patients in this study received treatment in hematology day care units at secondary or tertiary care hospitals. This precludes our ability to assess the potential influence of treatment in community settings vs MM-specialized centers on patient management and outcomes.
Moreover, treatment regimens were influenced by changes in the Israeli health basket guidelines over the study period, meaning some patients in earlier cohorts may not have had access to newer therapies, particularly Dara. This variability could contribute to observed differences in outcomes across treatment periods rather than differences solely attributable to age. Similarly, although this study suggests that triplet and quadruplet therapies provide a survival advantage, it does not directly address whether patients with frailty were inherently less likely to receive these regimens, potentially leading to residual confounding despite adjustments for CCI scores. Choice of treatment may reflect a patient’s frailty, performance status, and comorbidities at presentation, which may have contributed to the poorer outcomes observed in those receiving palliative care. The study also lacks data on quality-of-life metrics and treatment-related toxicity profiles, limiting insight into tolerability, especially for Octo patients who might experience substantial side effects. Lastly, the median FU time of 26 months may be inadequate to capture long-term survival, particularly in patients who received Dara-based therapies, which may show prolonged benefits beyond the FU window.
In conclusion, our study highlights the challenges in managing MM in very OL patients, because Octo patients face higher comorbidity burdens, are less likely to tolerate intensive regimens, and generally have shorter TTNT and OS than younger OL patients. However, the introduction of Dara appears beneficial in extending TTNT even in the Octo cohort. The findings suggest a need for tailored treatment strategies that consider both age and comorbidity, to optimize outcomes in this increasingly prevalent patient population. Future prospective studies will be required to confirm these findings and provide more robust evidence for optimizing MM management in older and frail populations.
Authorship
Contribution: D.S. and I.A. contributed to the study design; I.A. supervised the study; A.R.B. was responsible for statistical methodology; D.S. and I.A. were involved in data retrieval; D.S., Y.C.C., A.R.B., and I.A. participated in data analysis; D.S., Y.C.C., T.S., O.G., D.A., and I.A. collectively contributed to writing the manuscript; and all authors read and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dor Shpitzer, Tel Aviv Sourasky Medical Center, Osishkin 66/2, Tel Aviv 624911, Israel; email: shpitzerd@gmail.com.
References
Author notes
Individual participant data will not be shared.
The full-text version of this article contains a data supplement.