Key Points
LY500307, a selective ERβ agonist, reduces CTCL cell viability with minimal impact on normal skin cells, offering targeted therapy potential.
LY500307 induces apoptosis and G2/M cell cycle arrest in CTCL cells, reducing tumor growth in xenograft models without toxicity.
Visual Abstract
Cutaneous T-cell lymphomas (CTCLs), including mycosis fungoides (MF) and Sézary syndrome (SS), are rare hematological malignancies with limited curative treatment options. Despite early-stage responsiveness, these malignancies often relapse in advanced stages, highlighting the need for novel, durable therapies. Similar to other non-Hodgkin lymphomas (NHLs), MF and SS have a greater incidence rate in males than females. The endocrine contribution to this sex difference is unknown. Although several studies could show a potential role of estrogen receptor β (ERβ) on NHL lymphomagenesis, its impact on CTCL development is unknown. In this study, we investigated LY500307, a selective ERβ agonist, as a potential treatment for CTCL. Our results show that LY500307 selectively reduced the viability of CTCL cells, sparing noncancerous skin cells. Liquid chromatography with tandem mass spectrometry analysis revealed that CTCL cells accumulated significantly higher concentrations of LY500307 than normal skin cells, likely contributing to its selective cytotoxicity. Mechanistically, LY500307 induced apoptosis, G2/M cell cycle arrest, and increased sensitivity to chemotherapeutic agents, particularly Monomethyl auristatin E. Furthermore, LY500307 treatment significantly reduced tumor growth in a CTCL xenograft mouse model without notable toxicity. These findings suggest LY500307 as a promising therapeutic agent for CTCL, warranting further clinical investigation, including the potential for topical applications.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a group of extranodal non-Hodgkin T-cell lymphomas that comprise a heterogeneous group of rare malignancies characterized by monoclonal proliferations of T lymphocytes. The most frequent clinicopathological forms of CTCLs are mycosis fungoides (MF), presenting with primarily skin involvement, and Sézary syndrome (SS), in which malignant T cells expand to maintain a clonal population in the peripheral blood. Even if indolent at early stages, advanced stages are characterized by aggressive clinical behavior.1-3 Common therapies for advanced CTCLs comprise immune modulators, targeted systemic therapies, antibodies, and single-agent or combination chemotherapy.4-6 However, aside from limited therapeutic success with peripheral blood stem cell transplantation, no curative therapy is available to date, and despite initial therapy response, disease usually relapses. Moreover, treatment-related side effects further complicate therapeutic management.5 Thus, there remains an unmet medical need for novel, tolerable, and durable treatment approaches.
Similar to most non-Hodgkin lymphomas (NHLs), MF and SS have a greater incidence rate in males than females.7-9 Furthermore, male sex has been identified as a negative prognostic factor.10,11 The endocrine contribution to this sex difference is yet unknown.12 In general, it is hypothesized that the reduced rate of NHL among females might be based on a protective role of estrogens in lymphomagenesis.13 Basically, estrogens exert their effects through estrogen receptor α (Erα; ESR1) and ERβ (ESR2), which are ligand-regulated transcription factors.14 Both ER subtypes, when bound to estrogens, either stimulate or repress target gene transcription and have distinctly unique biological functions. This is mediated by binding directly to the DNA at estrogen responsive elements (EREs) by target genes or indirectly by interacting with other transcription factors. In addition, estrogens may act through the 7-transmembrane G-protein–coupled ER1, which mediates both rapid nongenomic signaling and transcriptional regulation. The predominantly expressed receptors in normal lymphocytes and lymphomas are ESR2 and G-protein–coupled ER1.15-17 ERβ is considered as a possible tumor suppressor, reducing cell proliferation and enhancing apoptosis in several cancer in vitro and in vivo models.18-20 Notably, the activation of ERβ by agonists has been demonstrated to inhibit lymphoma cell growth and reduce their spread, suggesting a valuable role in controlling lymphoma progression.21-25 Furthermore, ERβ stimulation has been associated with increased sensitivity to chemotherapy, potentially providing a dual benefit by enhancing therapeutic efficacy of chemotherapeutics and directly inhibiting tumor growth.26,27
Altogether, such evidence encourages the investigation of ERβ-selective agonists as therapeutic tools in lymphoma treatment. To date, several natural and selective synthetic ERβ agonists, which show receptor selectivity in contrast to 17β-estradiol, exist and are being assessed for therapeutic utility. One of those, LY500307, is a synthetic, highly selective, and potent ERβ agonist. The efficacy of LY500307 has been proven in several preclinical cancer models and was tolerable with no side effects in initial clinical studies.20,28-30
Here, we tested the efficacy of the ERβ agonist LY500307 as a novel therapeutic agent for treating CTCLs using in vitro and in vivo preclinical models. Our results demonstrate that LY500307 selectively kills established and primary CTCL cells with minimal toxicity on normal skin cells. In the mechanistic studies, it was observed that LY500307 alters cell cycle, apoptosis, and DNA damage response pathways, increasing the sensitivity of CTCL cells to current chemotherapeutic agents. Additionally, LY500307 demonstrated a reduction in CTCL growth in xenograft mice. This represents, to our knowledge, the first report demonstrating effectiveness of ERβ ligand LY500307 on CTCL cells and suggests that LY500307-mediated ERβ activation may be an effective strategy for targeted therapy.
Methods
Cell culture and reagents
SS cell line HuT78 was a gift from Michael U. Martin (Justus-Liebig-University Gieβen, Gieβen, Germany); SS cell line SeAx and MF cell line HH were gifts from Jan Nicolay (University Heidelberg, Heidelberg, Germany); MF cell line MyLa was a gift from Jean-Philippe Merilo and Edith Chevret (University of Bordeaux, Bordeaux, France); and HaCaT cells were donated by Fusenig (German Cancer Research Institute Heidelberg, Heidelberg, Germany). To authenticate the cell lines used in this study, short tandem repeat profiling was performed for HuT78, SeAx, HH, and MyLa cells and regular mycoplasma contamination checks were conducted for all cell lines. HuT78, SeAx, HH, and MyLa cells were grown in RPMI 1640 medium, supplemented with 1 % penicillin/streptomycin, 1% l-Glutamine (all Gibco/Thermo Fisher, Waltham, MA), and 7.5% fetal calf serum (FCS). HaCaT cells were grown in Dulbecco modified Eagle medium, high glucose (Gibco/Thermo Fisher) with 1 % penicillin/streptomycin, 1% l-Glutamine, and 5% FCS. Normal primary human keratinocytes and fibroblasts were isolated from infantile foreskins of donors. Keratinocytes were seeded in DermaLife K cell medium (CellSystems, Troisdorf, Germany). Fibroblast cultures were propagated in Dulbecco (or Dulbecco’s) modified Eagle medium, high glucose (Gibco/Thermo Fisher) with 1 % penicillin/streptomycin, 1-mM ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO), and 5% FCS. All cells were incubated at 37°C in a humidified 5% CO2 incubator. LY500307 was purchased from Cayman Chemical (Ann Arbor, MI).
Blood samples
Blood samples were obtained from patients with CTCL at the University Hospital Frankfurt and the Municipal Hospital Karlsruhe. All participants provided written informed consent in compliance with the Ethics Committee of the Faculty of Medicine at the University Hospital Frankfurt and the Medical Faculty Mannheim. Peripheral blood samples from consenting patients and healthy donors were collected in lithium heparin bags. Peripheral blood mononuclear cells were isolated from whole blood using Ficoll density gradient centrifugation. CD4+ T cells were isolated using the magnetic-activated cell sorting CD4+ negative selection kit (Miltenyi Biotec, Bergisch Gladbach, Germany), per the manufacturer’s protocol, and supplemented with antibodies to isolate CD26– and/or CD7– cells, depending on the patient’s known aberrant phenotype. Aliquots of purified samples were obtained to verify expression of phenotypic markers. After isolation, cells were activated with TransAct (1:100; Miltenyi Biotech) for 48 hours according to the manufacturer’s protocol and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL), as well as interleukin-2 (IL-2; 10 ng/mL), IL-7 (5 ng/mL), IL-13 (10 ng/mL), and IL-15 (10 ng/mL; all cytokines from Miltenyi Biotec) at 37°C, 5% CO2, and 95% humidity.
Cell viability
To measure cell viability, we used the CellTiter 96 AQueous One Solution Cell Proliferation kit (Promega, Madison, WI). A total of 2 × 104 CTCL cells, primary human keratinocytes and fibroblasts and 1 × 105 HaCaT cells per well were seeded in 96-well plates in a 100-μL volume. After overnight incubation, cells were treated with either vehicle or varying concentrations of LY500307 for 48 hours. Then, 10-μL MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium) was added to each well and incubated at 37°C for 1 to 5 hours. The absorbance at 490 nm was recorded using the microplate reader (Asys Expert 96; Deelux Labortechnik GmbH, Gödenstorf, Germany). For drug combination studies after an overnight incubation, the cells were treated with either vehicle or LY500307 and chemotherapeutics at different concentrations for 48 hours. Synergy for drug pairs was calculated by using the Chou-Talalay method,31,32 with a combination index (CI) <1 indicating a synergistic interaction.
Annexin V apoptosis assay
A total of 1.5 × 106 CTCL cells were seeded in 6-well plates in 3-mL medium and treated with vehicle or LY500307 (10 μM) for 48 hours. Cells were harvested in annexin V binding buffer, and 100 μL of cell suspension was incubated with annexin V fluorescein isothiocyanate (Miltenyi Biotech) and 7-aminoactinomycin (Miltenyi Biotech) for 20 minutes at room temperature in the dark. Then, 200 μL of annexin V binding buffer was added to each sample, and stained cells were analyzed using flow cytometry.
Reporter gene assays
The day before transfection, MyLa and SeAx cells (4 × 105 cells per well in 24-well plates) were seeded in phenol red–free RPMI with 5 % charcoal-stripped FCS without antibiotics. The next day, cells were transfected with 1.5-μg ERE firefly luciferase construct and 0.5-μg thymidine kinase promoter- Renilla vector per well (Promega) using the Lipofectamine 2000 transfection reagent (Invitrogen, Waltham, MA) in Opti-MEM without phenol red (Thermo Fisher). The plasmid 3× ERE TATA luciferase was a gift from Donald McDonnell (Addgene plasmid catalog no. 11354; RRID:Addgene_11354). Six hours after transfection, cells were treated with vehicle (0.1% dimethyl sulfoxide) or LY500307 (10 μM) for 24 hours. Luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega) in the Lumat LB 9507 (Berthold, Bad Wildbad, Germany).
Cell cycle analysis
CTCL cells were seeded at 2.5 × 105 cells (24-well plate) in 250 μL of medium. Cells were treated with either vehicle or LY500307 (10 μM) for additional 48 hours. Cells were then harvested in phosphate-buffered saline, followed by fixation in ice-cold 70% ethanol for 60 minutes at 4°C. Cells were washed again with phosphate-buffered saline and stained with a mixture of 50 μg/mL propidium iodide (PI, Sigma Aldrich) and 5 μg/mL ribonuclease for 30 minutes at 37°C. The PI-stained cells were subjected to flow cytometry using a fluorescence-activated cell sorter.
Western blotting
Whole-cell lysates were prepared from CTCL cells using radioimmunoprecipitation assay buffer (Sigma Aldrich) containing protease and phosphatase inhibitors. Total proteins (15-50 μg) were mixed with sodium dodecyl sulfate sample buffer and separated on polyacrylamide gel electrophoresis gels. Resolved proteins were then transferred onto nitrocellulose membranes, blocked with 5% nonfat dry milk powder for 1 hour at room temperature and incubated with respective primary antibodies over night at 4°C followed by secondary antibody incubation for 1 hour at room temperature. Blots were developed using different enhanced chemiluminescence detection systems (Thermo Fisher or Cyanagen, Bologna, Italy), according to the manufacturer's instructions.
Relative quantitative real-time RT-PCR
Relative quantitative reverse transcriptase polymerase chain reaction (RT-PCR) using SYBR green was performed on total RNA (150 ng) extracted from 1 × 106 cells grown in 6-well plates. Total cellular messenger RNA was isolated using the EXTRACTtME Total RNA Kit (Blirt, Gdansk, Poland) including deoxyribonuclease digestion. RNA concentration and purity were determined by photometrical measurement at 260/280 nm. RT-PCR analyses of ERα, ERβ, and androgen receptor (AR) were performed with the QuantiTect SYBR green RT-PCR Kit (Qiagen, Hilden, Germany) on a LightCycler 2.0 (Roche, Basel, Switzerland) with the primer pairs listed below. PCR was performed over 40 cycles at 94°C for 15 seconds, 60°C for 20 seconds, and 72°C for 20 seconds, followed by melting curve analysis. Nontemplate controls were included. Each primer pair was checked for error rate and efficiency using serial dilutions on each cell line, and specificity was confirmed through basic local alignment search tool analysis. Porphobilinogen deaminase was used as the reference gene to ensure comparable quantification cycle (Cq) values and reaction efficiency. Relative RNA expression levels were calculated using the 2–ΔΔCq method.33
Caspase-3/7 assay
Caspase-3/7 activity was detected by Caspase-Glo 3/7 Assay (Promega) according to the manufacturer's protocol. In brief, 2 × 104 cells were disseminated in a white bottom 96-well plate and cultivated for 24 hours in a 37°C humidified incubator. Cells were then treated with 10-μM LY-500307 for 48 hours. After adding a luminogenic caspase-3/7 substrate, caspase-3/7 activity was measured in a plate-reading luminometer. The detected luminogenic signal is proportional to caspase-3/7 activity within the cells and is therefore an indicator for drug-induced apoptosis.
LC-MS/MS
A total of 1 × 106 HaCaT cells were grown in 20 cm2 cell culture dishes and 1 × 106 fibroblasts in 60 cm2 dishes. SeAx and MyLa cells were grown in 6-well culture plates at a density of 2 × 106 cells in 2.5 mL medium. After treatment, the supernatants of HaCaT cells and fibroblasts were collected, and the cell pellets were washed after trypsinization and stored at –80°C. CTCL cells were centrifuged, washed, and stored at –80°C. Before storage, the amounts of healthy cells and total cells were determined. Analysis of erteberel was performed using a liquid chromatography with tandem mass spectrometry (LC-MS/MS) method originally developed for the analysis of sphingoid basis, as described in detail by Hahnefeld et al.34 Briefly, cell samples were resuspended in extraction buffer, and for supernatant samples, a total volume of 200 μL was used. Samples were mixed with internal standard solution, whereas standard and quality control samples using the extraction buffer as surrogate matrix were spiked with 20-μL internal standard and 20-μL standard working solution. The samples were vortexed, centrifuged, and extracted using liquid extraction by adding an extraction solution. The whole solution was vortexed, followed by centrifugation. Afterward, the lower organic layer was transferred into a new tube. Samples were evaporated and resuspended using methanol and formic acid solution. The resuspended samples were analyzed with an LC-MS/MS system composed of a triple quadrupole mass spectrometer QTRAP 6500+ with a Turbo Ion Spray source (both Sciex, Toronto, Canada) coupled to an Agilent 1290 Infinity II LC system with a binary high-performance liquid chromatography pump, column oven, and an auto sampler (Agilent, Santa Clara, CA). Erteberel was analyzed using gradient elution on an ultra high-performance liquid chromatography Zorbax Eclipse Plus C8 2.1 × 30 mm, 1.8 μm column. Samples were processed using Analyst software 1.7.1, and the obtained concentrations were evaluated using MultiQuant Software 3.0 (both Sciex, Toronto, Canada). For erteberel, the multiple reaction monitorin transition 283.1/106.9 was used as the quantifier, and 283.1/189.0 was used as the qualifier.
In vivo tumor models
Animal studies were performed in accordance with the German Animal Welfare Act and approved by local authorities (Landesamt für Gesundheit und Soziales, Berlin, Germany; permission number Reg 0010/19) for in vivo therapy experiments.
Twelve 6- to 8-week-old NOG-F (Taconic, Leverkusen, Germany) mice were each injected subcutaneously with 3 × 105 MyLa cells mixed with Matrigel (Corning, Corning, NY) after the animals had been on daily treatment for 7 days. Group A mice received oral vehicle control consisting of 30% (weight-to-volume ratio) capitsol in 0.9% (weight-to-volume ratio) NaCl solution. Group B received orally 10 mg/kg body weight LY500307 dissolved in the control vehicle solution. Treatment was continued until the completion of the experimental groups (∼4 weeks). Tumor volume and body weight of the mice were measured 3 times per week initially and 5 times during the acute growth phase of the tumors.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 10 software (GraphPad Software, San Diego, CA). Area under the curve calculations and 2-tailed unpaired t tests with Welch correction were used to assess the statistical difference between control and LY500307-treated groups. All the data represented in bar graphs are shown as means ± standard deviation. A P value < .05 was considered as statistically significant.
Results
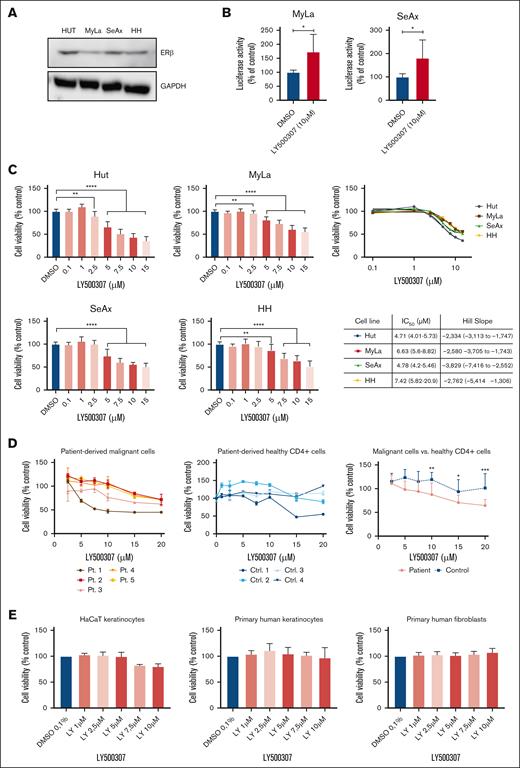
Selective ERβ agonist LY500307 enhances ERβ signaling and reduces cell viability in CTCL cells
ERβ protein expression in CTCL cells was determined by performing western blotting. Western blot analysis showed ERβ expression in all 4 different CTCL cell lines (Figure 1A). Expression of ERβ, Erα, and AR messenger RNA in CTCL cells was determined by quantitative PCR assays. ERβ was the predominant steroid hormone receptor expressed across all CTCL lines. ERα expression was inconsistent, being expressed only in some cell lines. Notably, AR shows no expression in any of the tested CTCL lines (supplemental Figure 1). To test whether LY500307 promotes activation of classical ERβ-ERE signaling, MyLa and SeAx cells were transfected with the ERE-luciferase reporter and treated with LY500307 for 24 hours. LY500307 significantly increased the ERE-luciferase activity in both cells (Figure 1B). To evaluate whether LY500307 reduces cell viability of CTCL cells, MTS cell viability assays were performed. Treatment with LY500307 significantly reduced the viability in 4 different CTCL cell lines in a dose-dependent manner (Figure 1C). To further validate these findings, we analyzed the effects of LY500307 on isolated CD4+CD7– malignant cells from samples of patients with SS (n = 5) and CD4+ T cells from healthy volunteers (n = 4). Patient characteristics are shown in supplemental Table 1. LY500307 reduced the viability of malignant cells in a dose-dependent manner across all patient samples, with some individuals exhibiting stronger responses to lower concentrations of LY500307 (Figure 1D, left panel). In contrast, most CD4+ T cells from healthy donors were unaffected by LY500307 treatment (Figure 1D, middle panel). However, 1 donor from the healthy group showed a reduction in cell viability at higher doses of LY500307. A comparison of malignant cells and healthy CD4+ T cells revealed a significant distinction in sensitivity to LY500307, demonstrating a more selective cytotoxicity toward malignant cells (Figure 1D, right panel). Additionally, the viability of HaCaT cells, primary human keratinocytes, and fibroblasts was not affected at the tested doses, suggesting that LY500307 has tumor cell–specific activity (Figure 1D).
Selective ERβ agonist LY500307 enhances ERβ signaling and reduces cell viability in CTCL cells, sparing noncancerous skin cells. (A) ERβ protein expression in CTCL cells was determined by performing western blots; 50 μg of protein was loaded for each sample, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a loading control (Ctrl). The data shown are from 3 independent experiments. (B) CTCL cells were transfected with ERE-luc plasmid. After 6 hours, the cells were treated with either vehicle or LY500307 for additional 24 hours, and then reporter activity was measured. (C) Antiproliferative effect of LY500307 compared with placebo in CTCL cells, determined by performing MTS assays. Cells were treated for 48 hours with increasing compound concentrations, from which 50% inhibitory concentration (IC50) values and Hill slopes were calculated. Cell growth was normalized to cells treated with dimethyl sulfoxide (DMSO; untreated Ctrl). Mean values of minimum 3 independent experiments with standard deviation (SD) are plotted. (D) Isolated malignant cells from samples of patients (Pts) with SS (n = 5; left) and CD4+ T cells isolated from peripheral blood of healthy volunteers (n = 4; middle) were incubated with increasing concentrations of LY500307 for 72 hours, and cell viability was assessed using MTS assays. Cell growth was normalized to cells treated with DMSO (untreated Ctrl). Mean values of at least 3 replicates with SD are plotted. A comparison is presented of the average cell viability between malignant and healthy cells across the tested LY500307 concentrations (right). (E) Antiproliferative effect of LY500307 compared with placebo in HaCaT cells, primary human keratinocytes, and fibroblasts, determined by performing MTS assays. Cells were treated for 48 hours with increasing compound concentrations. Cell growth was normalized to cells treated with DMSO (untreated Ctrl). Mean values of a minimum of 3 independent experiments with SD are plotted. ∗P < .5; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Selective ERβ agonist LY500307 enhances ERβ signaling and reduces cell viability in CTCL cells, sparing noncancerous skin cells. (A) ERβ protein expression in CTCL cells was determined by performing western blots; 50 μg of protein was loaded for each sample, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a loading control (Ctrl). The data shown are from 3 independent experiments. (B) CTCL cells were transfected with ERE-luc plasmid. After 6 hours, the cells were treated with either vehicle or LY500307 for additional 24 hours, and then reporter activity was measured. (C) Antiproliferative effect of LY500307 compared with placebo in CTCL cells, determined by performing MTS assays. Cells were treated for 48 hours with increasing compound concentrations, from which 50% inhibitory concentration (IC50) values and Hill slopes were calculated. Cell growth was normalized to cells treated with dimethyl sulfoxide (DMSO; untreated Ctrl). Mean values of minimum 3 independent experiments with standard deviation (SD) are plotted. (D) Isolated malignant cells from samples of patients (Pts) with SS (n = 5; left) and CD4+ T cells isolated from peripheral blood of healthy volunteers (n = 4; middle) were incubated with increasing concentrations of LY500307 for 72 hours, and cell viability was assessed using MTS assays. Cell growth was normalized to cells treated with DMSO (untreated Ctrl). Mean values of at least 3 replicates with SD are plotted. A comparison is presented of the average cell viability between malignant and healthy cells across the tested LY500307 concentrations (right). (E) Antiproliferative effect of LY500307 compared with placebo in HaCaT cells, primary human keratinocytes, and fibroblasts, determined by performing MTS assays. Cells were treated for 48 hours with increasing compound concentrations. Cell growth was normalized to cells treated with DMSO (untreated Ctrl). Mean values of a minimum of 3 independent experiments with SD are plotted. ∗P < .5; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
LY500307 induces apoptosis and G2/M cell cycle arrest in CTCL cells
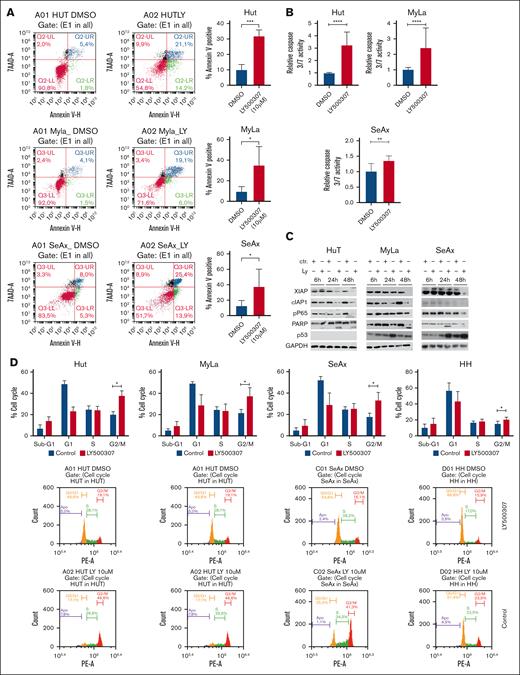
To determine whether LY500307 induces apoptosis in CTCL cells, we performed an annexin V assay. LY500307 treatment significantly increased the number of annexin V–positive apoptotic cells in the MyLa, Hut, and SeAx cell lines (Figure 2A). Additionally, a 48-hour treatment with LY500307 significantly induced caspase-3/7 activity in CTCL cells (Figure 2B). Western blot analysis revealed that LY500307 significantly increased p53 expression in MyLa and SeAx cells, whereas p53 was not detected in Hut cells (Figure 2C). LY500307 also led to a marked increase in poly (ADP-ribose) polymerase cleavage in all 3 cell lines, indicating enhanced apoptosis (Figure 2C). Furthermore, LY500307 inhibited the expression of the apoptosis inhibitors cellular inhibitor of apoptosis protein 1 (cIAP1) and X-linked inhibitor of apoptosis (XIAP). Although XIAP inhibition was observed across all 3 cell lines, the reduction of cIAP1 was most pronounced in SeAx and Hut cells, with only a very weak overall signal in MyLa cells (Figure 2C). Flow cytometry analysis of PI-stained cells demonstrated that LY500307 treatment significantly increased the percentage of cells in the G2/M phase compared with the vehicle control (Figure 2D). This suggests that LY500307 induces a G2/M-phase cell cycle arrest.
LY500307 induces apoptosis and G2/M cell cycle arrest in CTCL cells. (A) CTCL cells were treated with 10-μM LY500307 or DMSO for 48 hours. Cells were then stained for 20 minutes with annexin V fluorescein isothiocyanate and 7AAD. Annexin V–positive cells were determined by flow cytometry. Representative data shown from 3 independent experiments (left) and mean value of 3 independent experiments with SD (right). Mean value of 3 independent experiments. (B) LY500307 activates caspase 3/7, which was determined using Caspase-Glo 3/7 assay. Cells were treated for 48 hours with 10 μM of LY500307. Activity was normalized to cells treated with DMSO (untreated Ctrl). Mean values of 3 independent experiments with SD are plotted. (C) Protein expression in CTCL cells, determined by performing western blots; 30 μg of protein was loaded for each sample, and GAPDH served as a loading Ctrl. The data shown are from 3 independent experiments. (D) LY500307 induces G2/M arrest of CTCL cells. Hut, MyLa, SeAx, and HH cells were either treated with vehicle or LY500307 for 24 hours. Cells were then fixed with 70% ethanol, and PI staining was performed for 30 minutes. Cell cycle distribution was analyzed using flow cytometry. Mean value of 3 independent experiments with SD is plotted. ∗P < .5. 7AAD, 7-aminoactinomycin; Ly, LY500307; PE-A, phycoerythrin-A.
LY500307 induces apoptosis and G2/M cell cycle arrest in CTCL cells. (A) CTCL cells were treated with 10-μM LY500307 or DMSO for 48 hours. Cells were then stained for 20 minutes with annexin V fluorescein isothiocyanate and 7AAD. Annexin V–positive cells were determined by flow cytometry. Representative data shown from 3 independent experiments (left) and mean value of 3 independent experiments with SD (right). Mean value of 3 independent experiments. (B) LY500307 activates caspase 3/7, which was determined using Caspase-Glo 3/7 assay. Cells were treated for 48 hours with 10 μM of LY500307. Activity was normalized to cells treated with DMSO (untreated Ctrl). Mean values of 3 independent experiments with SD are plotted. (C) Protein expression in CTCL cells, determined by performing western blots; 30 μg of protein was loaded for each sample, and GAPDH served as a loading Ctrl. The data shown are from 3 independent experiments. (D) LY500307 induces G2/M arrest of CTCL cells. Hut, MyLa, SeAx, and HH cells were either treated with vehicle or LY500307 for 24 hours. Cells were then fixed with 70% ethanol, and PI staining was performed for 30 minutes. Cell cycle distribution was analyzed using flow cytometry. Mean value of 3 independent experiments with SD is plotted. ∗P < .5. 7AAD, 7-aminoactinomycin; Ly, LY500307; PE-A, phycoerythrin-A.
LY500307 sensitizes CTCL cells to chemotherapeutic agents
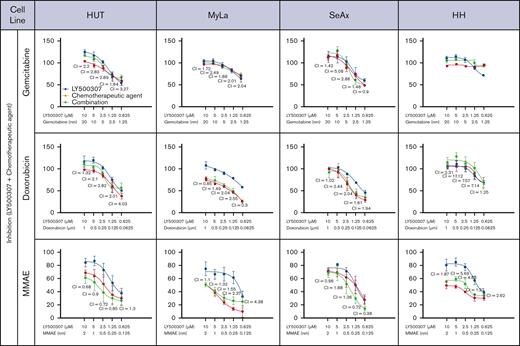
Clinical data provide evidence that using a multitargeted approach is advantageous compared with using a single agent for lymphoma therapy, and sensitizer drugs that potentiate the effect of chemotherapeutics are advantageous. We performed an in vitro screen of commonly used chemotherapeutics (gemcitabine, doxorubicin, and monomethyl auristatin E (MMAE) in combination with LY500307. The degree of synergy was quantified as CI using the Chou-Talalay method, from dose-response curves with constant ratios of agents tested (CI of 1 indicates a purely additive effect, whereas CI <1 reveals synergy).31,32 Our results demonstrated synergy when LY500307 was combined with MMAE in the SS cell lines SeAx and Hut. Further synergism at high doses of LY500307 in combination with doxorubicin in MyLa cells and gemcitabine in SeAx cells was indicated by the CI values at the highest investigated concentrations. However, this could not be confirmed by the dose-response effect curves, because the observed effects did not display clear additive or synergistic patterns in the graphical representation (Figure 3).
LY500307 sensitizes CTCL cells to chemotherapeutic agents. Antiproliferative effect of LY500307 and/or chemotherapeutic agent compared with placebo in CTCL cells, determined by performing MTS assays. Cells were treated with either vehicle or LY500307 and chemotherapeutics at different concentrations for 48 hours. The degree of synergy was quantified as CI using the Chou-Talalay method, from dose-response curves with constant ratios of agents tested (CI = 1 indicates a purely additive effect; CI < 1 reveals synergy). Mean value of 3 independent experiments with SD is plotted.
LY500307 sensitizes CTCL cells to chemotherapeutic agents. Antiproliferative effect of LY500307 and/or chemotherapeutic agent compared with placebo in CTCL cells, determined by performing MTS assays. Cells were treated with either vehicle or LY500307 and chemotherapeutics at different concentrations for 48 hours. The degree of synergy was quantified as CI using the Chou-Talalay method, from dose-response curves with constant ratios of agents tested (CI = 1 indicates a purely additive effect; CI < 1 reveals synergy). Mean value of 3 independent experiments with SD is plotted.
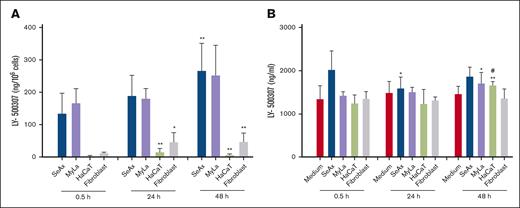
Metabolism of LY500307 in fibroblasts, HaCaT keratinocytes, and CTCL cell lines. CTCL cells accumulate significantly higher concentrations of LY500307 than normal skin cells. Fibroblasts and HaCaT keratinocytes catabolize intracellular LY-500307 faster than CTCL cell lines. SeAx cells, MyLa cells, HaCaT keratinocytes, and fibroblasts were treated with 10-μM LY-500307 for 0.5, 24, and 48 hours. The concentrations of LY-500307 were measured by LC-MS/MS in the cell pellet (A) and supernatant (B). Data are shown as means with SD (n = 3-4). ∗P < .05; ∗∗P < .01; # indicates significant difference compared with 24 hours.
Metabolism of LY500307 in fibroblasts, HaCaT keratinocytes, and CTCL cell lines. CTCL cells accumulate significantly higher concentrations of LY500307 than normal skin cells. Fibroblasts and HaCaT keratinocytes catabolize intracellular LY-500307 faster than CTCL cell lines. SeAx cells, MyLa cells, HaCaT keratinocytes, and fibroblasts were treated with 10-μM LY-500307 for 0.5, 24, and 48 hours. The concentrations of LY-500307 were measured by LC-MS/MS in the cell pellet (A) and supernatant (B). Data are shown as means with SD (n = 3-4). ∗P < .05; ∗∗P < .01; # indicates significant difference compared with 24 hours.
Metabolism of LY500307 in fibroblasts, HaCaT keratinocytes, and CTCL cell lines
Because HaCaT keratinocytes and fibroblasts are more resistant to LY500307 than the malignant SeAx and MyLa cell lines, we looked for differences in the rapidity of LY500307 depletion. Therefore, all cell lines were incubated with 10-μM LY500307 for 0.5, 24, and 48 hours, and we detected already after 0.5 hour a large gap between the LY500307 levels in SeAx and MyLa cells compared with fibroblasts and HaCaT keratinocytes. An increase in LY500307 concentrations was measured in both CTCL cell lines after 24 hours and 48 hours. We also detected an increase in fibroblasts after 24 hours. Nevertheless, after 48 hours, the LY500307 level did not continue to rise (Figure 4A). Interestingly, the LY500307 concentration in HaCaT cells increased in the first 24 hours and decreased after 48 hours. To prove the stability of LY500307, cell culture medium was treated with 10-μM LY500307. We detected no significant differences between 0.5 hour and 48 hours (Figure 4B).
Significantly, less LY500307 was detected after 24 hours but not after 48 hours compared with 0.5 hour in the supernatant of SeAx cells. We measured in the supernatant of MyLa cells significantly more LY500307 after 48 hours but not after 24 hours compared with 0.5 hour. We also detected in HaCaT keratinocytes significantly more LY500307 after 48 hours but not after 24 hours than at 0.5 hour. There were no significant differences in the LY500307 concentrations in the supernatant after 48 hours or 24 hours compared with 0.5 hour in fibroblasts (Figure 4B).
LY500307 inhibits tumor growth progression in a CTCL xenograft mouse model
To confirm our findings in vivo, MyLa cells were injected subcutaneously into NSG mice. One week before tumors were established, mice were randomized into a control group, which received vehicle, and a treatment group. LY500307 was received through oral gavage. On day 17 of tumor induction, all animals in the placebo group had to be euthanized. None of the animals experienced weight loss or other severe side effects or died from the medication (data not shown). Figure 5A-B depicts the course of tumor growth in both groups, showing slower growth and smaller tumor volumes in the treatment group already on day 13. On day 17, the analysis of the final tumor volumes revealed a significant difference between the 2 groups (Figure 5C). The comparison of tumor growth over time represented by area under the curve values for each individual animal also showed significantly impaired growth in LY500307-treated mice (Figure 5D).
LY500307 inhibits tumor growth progression in a CTCL xenograft mouse model. (A) NSG mice were xenografted with MyLa cells subcutaneously and treated once daily with either 10 mg/kg body weight of LY500307 per os or placebo (n = 12 each); mean tumor volume ± SD over time of subcutaneous MyLa xenografts. (B) Tumor volume growth curves for individual mice in each treatment group. (C) Final tumor volumes for individual mice on day 17. Data are shown both as individual values and as mean ± SD. (D) Area under the curve (AUC) values reflecting the entire tumor growth curve for individual mice in each treatment group. Data are shown both as individual AUC values and as mean ± SD. ∗∗∗P < .001; ∗∗∗∗P < .0001.
LY500307 inhibits tumor growth progression in a CTCL xenograft mouse model. (A) NSG mice were xenografted with MyLa cells subcutaneously and treated once daily with either 10 mg/kg body weight of LY500307 per os or placebo (n = 12 each); mean tumor volume ± SD over time of subcutaneous MyLa xenografts. (B) Tumor volume growth curves for individual mice in each treatment group. (C) Final tumor volumes for individual mice on day 17. Data are shown both as individual values and as mean ± SD. (D) Area under the curve (AUC) values reflecting the entire tumor growth curve for individual mice in each treatment group. Data are shown both as individual AUC values and as mean ± SD. ∗∗∗P < .001; ∗∗∗∗P < .0001.
Discussion
In recent years, there has been intensive research on estrogen and its receptors as potential targets for treating different cancers including hematological malignancies.18-25 Several studies demonstrated that ERβ expression was downregulated during tumor progression, and ligands that increase ERβ expression or activity suppress cancer growth and promote apoptosis.35-37 Although the above studies could show a potential role of ERβ on cancerogenesis and NHL lymphomagenesis in particular, its impact on CTCL is yet unknown. To the best of our knowledge, our study is the first to examine the effect of LY500307 on CTCL cancer models in vitro and in vivo. Our work provides evidence that LY500307, a synthetic ERβ agonist, has the potential to efficiently impair the proliferation of different CTCL cells, induce apoptosis, promote G2/M cell cycle arrest, and sensitize CTCLs cells to selected chemotherapeutic agents. Furthermore, our results also demonstrated that LY500307 has the ability to reduce CTCL progression in vivo.
Previous studies demonstrated that activation with ligands or overexpression of ERβ results in an inhibition of cell proliferation and induction of apoptosis of cancer cells. Depending on the tumor and cell type, activation of ERβ was shown to promote either G2 or G1 arrest.19,38 Our results show that LY500307 treatment causes cell cycle arrest of CTCL cells in G2/M phase in CTCL cells and thereby confirm earlier observations in other cancer models.
Mechanistic investigations found that LY500307 reduced nuclear factor κB (NF-κB) activity. CTCL cells show constitutive activation of the transcription factor NF-κB.39-41 NF-κB is also known to act as a prosurvival factor and as a protective factor against apoptosis in various hematological malignancies.42-44 For these reasons, NF-κB remains an attractive therapeutic target in CTCL; however, its pharmacological manipulation is still a difficult challenge. Compounds that directly target NF-κB have recently shown highly effective cell death induction in CTCL in vitro, but their toxicity was too high to be used in humans.39,41 NF-κB has been shown to induce the expression of antiapoptotic inhibitor of apoptosis proteins (IAPs) such as cIAP1, cIAP2, and xIAP.45,46 We demonstrated that c-IAP1 and XIAP were downregulated upon LY500307 treatment in CTCL cells. CIAP1 and XIAP negatively regulate the main apoptotic effector, caspase-3, and thus contribute to apoptotic resistance in CTCL.47,48 This is consistent with our results showing increased caspase-3/7 activity in LY500307-treated CTCL cells.
A critical point for successful chemotherapy is the selective killing of cancer cells without affecting normal cells. In our studies, the selective agonist LY500307 significantly reduced the cell viability of CTCLs with no effect on noncancerous skin cells, such as HaCaT cells, primary human keratinocytes, and fibroblasts. In this context, our LC-MS/MS data suggest that CTCL cells accumulate higher concentrations of LY500307 than normal skin cells, such as HaCaT keratinocytes and fibroblasts, potentially enhancing its therapeutic effect in cancer cells while sparing healthy tissues. The differing rates of LY500307 metabolism between CTCL and noncancerous cells likely contribute to this selective cytotoxicity, because noncancerous cells may catabolize the compound more efficiently or absorb it less readily, making them less sensitive to LY500307-induced cell death. Given that ERβ agonists can penetrate cells and skin, topical LY500307 treatment for CTCL could be worth exploring. Currently, topical options for CTCL, such as glucocorticoids and mechlorethamine, are limited and not curative, with CTCL often relapsing despite initial therapy. Therefore, further investigation into LY500307's potential as a topical therapy is warranted, particularly because studies on other ERβ agonists have shown promise in skin applications.49-51 Our findings on patient-derived cells from peripheral blood of patients with SS and healthy individuals demonstrate selective action of LY500307 against CTCL cells while sparing the majority of healthy CD4+ T cells. However, the observation of 1 healthy donor showing reduced viability at higher doses, as well as variability in susceptibility among malignant cells, highlights the need for further studies to identify patient-specific factors that influence therapeutic response and investigate potential off-target effects in specific individuals.
Our in vivo data support the promising therapeutic effects observed of LY500307 in CTCL cell lines. We showed that oral LY500307 treatment led to reduced tumor growth in our CTCL xenograft mouse model. These results underscore the findings from other studies.20 None of the mice experienced severe side effects by LY500307 treatment, which underlines the excellent tolerability of the drug that has already been proven in clinical trials.28,52
Recent studies have demonstrated that combination therapies are superior in SS treatment.53 In our experiments, LY500307 showed promising synergistic effects with MMAE. Notably, MMAE, which is a key component of the established CTCL drug brentuximab vedotin, has already been identified as a promising combination partner in CTCL therapy, with recent findings showing that its combination with topical treatments can significantly extend response duration.54 Although LY500307 demonstrated synergy with MMAE, this synergy was not observed consistently across all cell lines. This variability likely reflects differences in the molecular mechanisms of each CTCL subtype, indicating that patient-specific factors could influence the efficacy of combination therapies. Further studies exploring a broader range of drug combinations and resistance mechanisms are needed. Nonetheless, our results suggest that LY500307 holds promise in combination therapies for CTCL and warrants further investigation. Our findings indicate that LY500307 appears to be a compelling novel treatment option for CTCL, showing selective activity against cancer cells and might have potential synergy with established therapies such as brentuximab vedotin. Because LY500307 has demonstrated good tolerability in clinical trials, it could be easily transferred to clinical testing in CTCL. We recommend further clinical trials, including the exploration of topical formulations of LY500307, as a potential treatment for CTCL.
Acknowledgment
This work was supported by the Dr. Rolf M. Schwiete Stiftung, the August Scheidel-Stiftung, and the Prof. Dr. Harry und Rosa-Neumann-Stiftung.
Authorship
Contribution: D.Ö., M.M., and J.K. conceptualized and designed the study; D.Ö., R.W., G.R., M.D., K.B., P.S., T.S., and L.W. acquired data; D.Ö., R.W., T.S., and L.W. analyzed and interpreted data; D.Ö., M.M., and J.K. drafted the manuscript; R.K., B.S., S.K., J.K., and M.M. critically revised the manuscript for important intellectual content; D.Ö., R.W., and T.S. performed statistical analysis; D.Ö. obtained funding; R.W., N.Z., F.S., A. König, J.P.N., J.P., M.J., and A. Koch provided administrative, technical, or material support; and S.K., J.K., and M.M. provided supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deniz Özistanbullu, Department of Dermatology, University Hospital Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; email: oezistanbullu@med.uni-frankfurt.de.
References
Author notes
J.K. and M.M. contributed equally to this study.
Original data are available on request from the corresponding author, Deniz Özistanbullu (oezistanbullu@med.uni-frankfurt.de).
The full-text version of this article contains a data supplement.