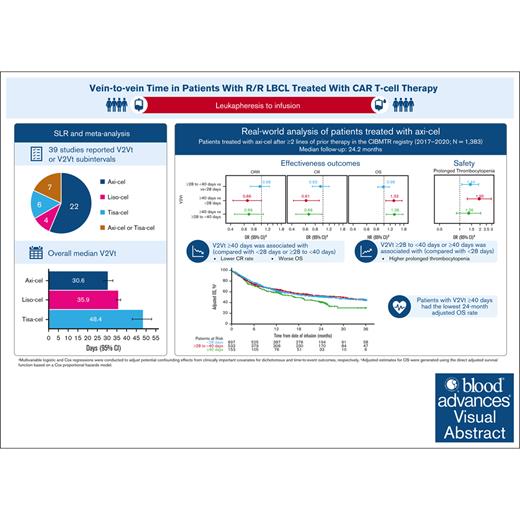
Key Points
Patients with R/R LBCL had the shortest median V2Vt with axi-cel, 30.6 days, compared with tisa-cel, 48.4 days, or liso-cel, 35.9 days.
Complete response rate and OS were better in patients with axi-cel V2Vt <28 days or ≥28 to <40 days compared with V2Vt ≥40 days.
Visual Abstract
Chimeric antigen receptor (CAR) T-cell products axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel) are approved for relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Emerging evidence indicates that delayed CAR T-cell infusion, including prolonged time from leukapheresis to infusion, known as vein-to-vein time (V2Vt), may adversely impact clinical outcomes. We conducted a systematic literature review (SLR) and meta-analysis to identify differences in V2Vt in patients with R/R LBCL treated with axi-cel, tisa-cel, or liso-cel. The impact of V2Vt (<28 days vs ≥28 to <40 days vs ≥40 days) on effectiveness and safety outcomes was evaluated in patients treated with axi-cel enrolled in a post-authorization safety study using the Center for International Blood and Marrow Transplant Research data. SLR and meta-analysis showed that patients treated with axi-cel had the shortest median V2Vt (30.6 days) compared with tisa-cel (48.4 days) or liso-cel (35.9 days). Real-world analysis of patients treated with axi-cel demonstrated that V2Vt ≥40 days was associated with significantly lower complete response rate than V2Vt <28 days (odds ratio [OR], 0.61) or ≥28 to <40 days (OR, 0.66) and significantly worse overall survival than V2Vt <28 days (hazard ratio [HR], 1.33) or ≥28 to <40 days (HR, 1.36). Higher prolonged thrombocytopenia rates were observed in patients with axi-cel V2Vt ≥28 to <40 days or ≥40 days compared with <28 days (OR, 1.44 or 1.95, respectively). Together, these results show the impact of V2Vt on patient outcomes with axi-cel therapy and that earlier infusion with CD19-CAR therapies may be beneficial.
Introduction
Autologous chimeric antigen receptor (CAR) T-cell therapy has demonstrated significant clinical benefit in patients with chemorefractory aggressive B-cell lymphomas, including those with relapsed or refractory (R/R) large B-cell lymphoma (LBCL).1-5 Three CAR T-cell products, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel), have been approved by the US Food and Drug Administration and the European Medicines Agency for use in adult patients with R/R LBCL after ≥2 lines of systemic therapy.6-11 Axi-cel and liso-cel have since been approved for use in patients with LBCL refractory to first-line chemoimmunotherapy or in certain patients who experience relapse after first-line chemoimmunotherapy.6,7,10,11 The ZUMA-7 and TRANSFORM trials demonstrated significant improvements in median event-free survival in patients treated with second-line axi-cel or liso-cel compared with standard-of-care therapies (8.3 vs 2.0 months and 10.1 vs 2.3 months, respectively).2,5
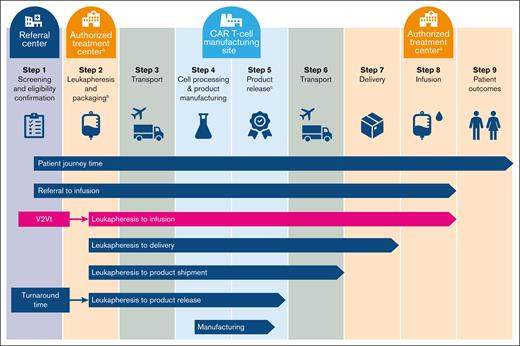
Despite significant improvements in survival outcomes for patients, large-scale deployment may pose a challenge in implementing CAR T-cell therapy in routine clinical practice.12-14 CAR T-cell production requires an individualized manufacturing process that demands high-quality coordination between commercial manufacturing sites and authorized treatment centers (ATCs).12-14 The overall manufacturing process is generally similar across approved CAR T-cell products and begins with the collection of peripheral blood mononuclear cells from the patient by leukapheresis at an ATC (Figure 1).16-18 Leukapheresis materials are then transported to a central manufacturing site, where T cells are transduced with a replication-incompetent viral vector containing a CAR transgene, expanded, and harvested. Expanded CAR T cells, which must pass stringent quality requirements, are then cryopreserved and delivered to the ATC for infusion into the patient.16-18 Definitions for stages of CAR T-cell production vary, but the time period from leukapheresis to CAR T-cell infusion is widely known as the vein-to-vein time (V2Vt).12,19
Overview of the patient journey with CAR T-cell therapy.aATCs are also referred to as qualified treatment centers. bTisa-cel leukapheresis products are frozen before transport to the manufacturing facility.4,15cProduct release includes QC testing and QA review. QA, quality assurance; QC, quality control.
Overview of the patient journey with CAR T-cell therapy.aATCs are also referred to as qualified treatment centers. bTisa-cel leukapheresis products are frozen before transport to the manufacturing facility.4,15cProduct release includes QC testing and QA review. QA, quality assurance; QC, quality control.
Recent analysis of patients treated with tisa-cel in the JULIET trial (patients with R/R diffuse LBCL who received ≥2 prior therapies) demonstrated that shorter overall wait times from enrollment to infusion were associated with increased tisa-cel efficacy, indicating that timely treatment may be crucial for patients with R/R LBCL and that delayed access to treatment may impact survival outcomes.4,20 Although the steps comprising the V2Vt interval are similar among CAR T-cell products, recent studies in patients with R/R LBCL have reported shorter median V2Vt for axi-cel compared with tisa-cel or liso-cel.1,5,21-25 In the real-world setting, axi-cel had a median V2Vt of 27 days, and tisa-cel had a median V2Vt of 45 days.23,24 In clinical trials, axi-cel, tisa-cel, and liso-cel had median V2Vts of 23 days, 52 days, and 36 to 37 days, respectively.1,5,21,22,25
Given that V2Vt may be associated with clinical outcomes after CAR T-cell therapy, we conducted an analysis in patients with R/R LBCL to further characterize V2Vt using available real-world evidence. Here, we describe V2Vt and V2Vt subintervals (eg, leukapheresis-to-product release, leukapheresis-to-delivery, and leukapheresis-to-start of lymphodepleting chemotherapy) and identify differences in the duration of these intervals in patients with R/R LBCL treated with axi-cel, tisa-cel, or liso-cel. We also evaluated the impact of V2Vt on real-world outcomes (effectiveness and safety) in patients with R/R LBCL treated with axi-cel using data from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry.
Methods
Systematic literature review (SLR) and meta-analysis
Systematic searches of the Medical Literature Analysis and Retrieval System Online, Excerpta Medica Database, and Cochrane Central Register of Controlled Trials online database were conducted on 5 October 2022. The Medical Literature Analysis and Retrieval System Online and Excerpta Medica databases were searched for records up to 4 October 2022, and the Cochrane Central Register of Controlled Trials database was searched for records up to August 2022. Studies were eligible if they described V2Vt or V2Vt subintervals for ≥10 adult patients with R/R LBCL who had received ≥1 prior line of therapy before treatment with axi-cel, tisa-cel, or liso-cel. Clinical trials or observational studies, including prospective and retrospective studies, were included. Studies with pooled estimates for different CAR T-cell therapies (analyses that were not run separately for axi-cel, tisa-cel, or liso-cel) and studies that presented clinical outcomes only without V2Vt or at least 1 V2Vt subinterval were excluded. Additional details regarding screening processes and statistical analysis can be found in the supplemental Methods.
Patient eligibility for CIBMTR study
A noninterventional postauthorization safety study (PASS) was facilitated using the CIBMTR registry, which prospectively enrolled patients treated with commercial axi-cel in the United States after ≥2 lines of prior therapy for R/R LBCL between October 2017 and August 2020. Eligible patients were identified from the PASS cohort, and patients who were enrolled in clinical trials or received axi-cel in noncommercial settings were excluded (see the supplemental Methods for additional criteria).
End points and assessments for CIBMTR study
Effectiveness outcomes of interest included objective response rate (ORR), complete response (CR) rate, duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Safety outcomes of interest included incidence and severity of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), prolonged cytopenias (including neutropenia and thrombocytopenia), clinically significant infections, and subsequent neoplasms. Response was assessed via positron emission tomography or computed tomography scans obtained after the first dose of axi-cel and before the initiation of any subsequent therapy. For safety outcomes, CRS was graded according to the Lee 2014 criteria,26 and ICANS was graded based on the American Society of Transplant and Cellular Therapy consensus criteria.27 For patients alive at day 30, prolonged neutropenia was defined as failure to recover absolute neutrophil count ≥500/mm3 and/or sustain 3 laboratory assessments ≥500/mm3 within the first 30 days after infusion, and prolonged thrombocytopenia was defined as failure to recover platelet count ≥20 × 109/L within the first 30 days after infusion. Additional definitions for effectiveness and safety outcomes are described in the supplemental Methods.
Statistical analysis
No formal hypothesis was tested, and descriptive statistics were used to summarize baseline demographics, clinical/disease characteristics, and study end points, which were analyzed by V2Vt subgroups (<28 days vs ≥28 to <40 days vs ≥40 days; supplemental Methods). Sensitivity analyses were conducted to identify the impact of V2Vt in patients who did not receive bridging therapy (as bridging therapies may affect V2Vt) and to assess the validity of the primary analysis using V2Vt subgroups of <36 days vs ≥36 days.
Proportions and 95% Clopper-Pearson confidence intervals (CIs) were calculated for dichotomous outcomes. The Kaplan–Meier estimator and cumulative incidence function were used to describe time-to-event outcomes with and without competing risks, respectively. Multivariable logistic and Cox regressions were conducted to adjust potential confounding effects from clinically important covariates for dichotomous and time-to-event outcomes, respectively. The proportionality assumption for Cox regression was tested using an interaction term between the main variable of interest, V2Vt, and the logarithm of time-to-event.
For multivariate analysis, potential covariates were preselected based on clinical and biological relevance. A stepwise procedure was further used for variable selection using a P value cutoff of ≤0.2 for variables to enter the model and ≤0.05 for variables to stay in the model. The main variable of interest, V2Vt, was forced in the model. Key prognostic factors subject to stepwise selection were age, sex, race, ethnicity, Eastern Cooperative Oncology Group performance status (ECOG PS) before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal) before infusion, histologic transformation, disease characteristics at initial diagnosis (double/triple-hit status,28 Ann Arbor disease stage, elevated lactate dehydrogenase [LDH], and >1 extranodal involvement site), chemosensitivity before infusion, number of lines of prior therapy, prior hematopoietic cell transplantation (HCT), year of infusion, time from initial diagnosis to infusion, and use of bridging therapy (see supplemental Tables 3-5 for the list of significant covariates included in each analysis). Adjusted estimates for PFS, OS, and DOR (including 95% CIs) were generated using the direct-adjusted survival function based on a Cox proportional hazards model, stratified by V2Vt.29-31 All analyses were conducted using SAS 9.4 M1.
This is a noninterventional study with no prespecified interventions and no interaction with patients, and therefore, no potential physical or psychological risks to patients exist. The CIBMTR uses standard processes for ensuring the protection of human participants for patients whose cellular therapy data are reported to the CIBMTR Research Database. The NMDP (formerly known as the National Marrow Donor Program and Be The Match) institutional review board has primary oversight for the CIBMTR Research Database Protocol (www.clinicaltrials.gov identifier: NCT01166009), which is an umbrella protocol governing this study. All US centers are required to have a Federal Wide Assurance with the Office for Human Research Protection and, as part of their Data Transmission Agreement with the CIBMTR, agree to obtain local institutional review board approval for the CIBMTR Research Database Protocol.
Results
SLR and meta-analysis of V2Vt and V2Vt subintervals
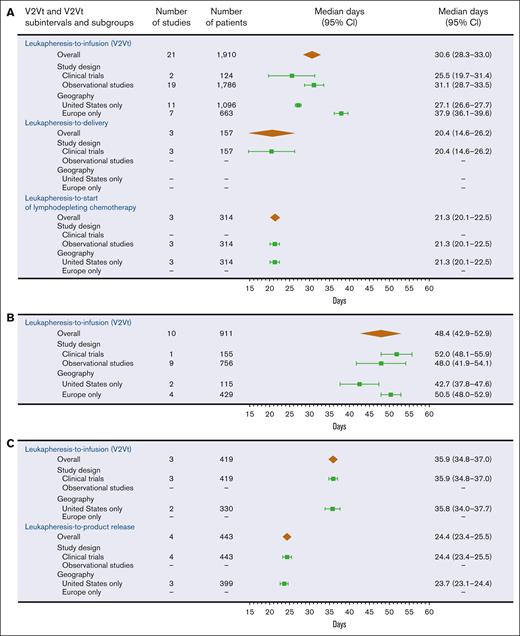
A total of 5031 records were identified in an SLR, of which 894 were evaluated at the full-text level, and 65 records met the eligibility criteria (supplemental Figure 1). Manual searches identified an additional 12 publications that also met the eligibility criteria, for a total of 77 publications describing 44 studies. Of the 44 studies, 39 studies reported V2Vt or V2Vt subintervals, and 7 studies evaluated V2Vt and V2Vt subintervals as prognostic factors (2 reported medians and evaluated V2Vt and V2Vt subintervals as prognostic factors). Of the 39 studies, 28 were observational (retrospective, n = 20; prospective, n = 8), and 11 were clinical trials (single arm, n = 8; randomized, n = 3). The 39 studies included patients treated with either axi-cel or tisa-cel (n = 7), axi-cel (n = 22), tisa-cel (n = 6), or liso-cel (n = 4).
Overall, V2Vt was the most described interval in the CAR T-cell process up to infusion, reported in 29 studies (clinical trials, n = 6; observational studies, n = 23). Patients treated with axi-cel had a significantly shorter median V2Vt (30.6 days; 95% CI, 28.3-33.0) when compared with tisa-cel (48.4 days; 95% CI, 42.9-52.9) or liso-cel (35.9 days; 95% CI, 34.8-37.0), irrespective of geography or study design (Figure 2A-C). At study sites in the United States only, patients treated with axi-cel also had a shorter median V2Vt (27.1 days; 95% CI, 26.6-27.7) compared with tisa-cel (42.7 days; 95% CI, 37.8-47.6) or liso-cel (35.8 days; 95% CI, 34.0-37.7; Figure 2A-C).
Meta-analysis for V2Vt and V2Vt subintervals. V2Vt and V2Vt subintervals for axi-cel (A), tisa-cel (B), and liso-cel (C). The median days of V2Vt and V2Vt subintervals and 95% CIs are represented by brown diamonds for all studies and by green squares with error bars for subgroups. One clinical trial in patients treated with axi-cel that reported a median of 13.0 days for leukapheresis-to-product release (eg, when the product passed quality control testing and was made available to the investigator) interval was not presented because the study did not report a 95% CI.
Meta-analysis for V2Vt and V2Vt subintervals. V2Vt and V2Vt subintervals for axi-cel (A), tisa-cel (B), and liso-cel (C). The median days of V2Vt and V2Vt subintervals and 95% CIs are represented by brown diamonds for all studies and by green squares with error bars for subgroups. One clinical trial in patients treated with axi-cel that reported a median of 13.0 days for leukapheresis-to-product release (eg, when the product passed quality control testing and was made available to the investigator) interval was not presented because the study did not report a 95% CI.
Patients who received axi-cel or tisa-cel had a significantly shorter median V2Vt at study sites in the United States, 27.1 days (axi-cel; 95% CI, 26.6-27.7) or 42.7 days (tisa-cel; 95% CI, 37.8-47.6), versus study sites in Europe, 37.9 days (axi-cel; 95% CI, 36.1-39.6) or 50.5 days (tisa-cel; 95% CI, 48.0-52.9; Figure 2A-B). Patients who received axi-cel had a shorter median V2Vt in clinical trials (25.5 days) versus observational studies (31.1 days), whereas patients who received tisa-cel had a shorter median V2Vt in observational studies (48.0 days) versus a clinical trial (52.0 days; Figure 2A-B). Median V2Vt in patients who received liso-cel was reported only at study sites in the United States (35.8 days) and in clinical trials (35.9 days; Figure 2C).
Median duration of V2Vt subintervals was reported in multiple studies with patients treated with axi-cel or liso-cel. Meta-analysis was performed for both the median leukapheresis-to-delivery and median leukapheresis-to-start of lymphodepleting chemotherapy subintervals in patients treated with axi-cel (20.4 and 21.3 days, respectively; Figure 2A) and for the leukapheresis-to-product release category in patients treated with liso-cel (24.4 days; Figure 2C). The prognostic value of V2Vt for clinical outcomes with CAR T-cell therapy was evaluated in 7 studies, but the parameterization of the data, by which most studies analyzed V2Vt and V2Vt subintervals as continuous variables instead of categorical variables, did not allow for meta-analyses.
Analysis of patients treated with axi-cel in the CIBMTR registry
Demographics and baseline characteristics
A total of 1497 patients with R/R LBCL who were treated with commercial axi-cel at 79 ATCs were identified from the CIBMTR PASS, and 1383 patients (from 78 ATCs) were included in the analysis, with a data cutoff date of 4 May 2022 (Table 1). The median follow-up time (from axi-cel infusion to data cutoff date) for all patients was 24.2 months (95% CI, 24.1-24.4). Excluded from the analysis were 114 patients, based on the following criteria: prior nontransplant cellular therapy (n = 30), primary central nervous system lymphoma (n = 1) or other B-cell lymphoma (n = 22), missing data on comorbidities (n = 43), unknown or outlying date of leukapheresis (n = 13), and lack of follow-up (n = 5).
Baseline patient and disease characteristics of patients treated with axi-cel
| Characteristic . | V2Vt . | Overall N = 1383 . | ||
|---|---|---|---|---|
| <28 d, n = 697 . | ≥28 to <40 d, n = 533 . | ≥40 d, n = 153 . | ||
| Median age at infusion (range), y | 61.5 (19.6-86.0) | 62.5 (19.6-90.8) | 63.1 (28.2-84.4) | 62.1 (19.6-90.8) |
| ≥65, n (%) | 239 (34) | 217 (41) | 65 (42) | 521 (38) |
| <65, n (%) | 458 (66) | 316 (59) | 88 (58) | 862 (62) |
| Male, n (%) | 455 (65) | 348 (65) | 91 (59) | 894 (65) |
| Female, n (%) | 242 (35) | 185 (35) | 62 (41) | 489 (35) |
| Race, n (%) | ||||
| White | 573 (82) | 428 (80) | 124 (81) | 1125 (81) |
| Black | 28 (4) | 34 (6) | 9 (6) | 71 (5) |
| Asian | 43 (6) | 27 (5) | 9 (6) | 79 (6) |
| Other or unknown | 53 (8) | 44 (8) | 11 (7) | 108 (8) |
| Ethnicity, n (%) | ||||
| Non-Hispanic | 583 (84) | 450 (84) | 128 (84) | 1161 (84) |
| Hispanic or Latino | 76 (11) | 56 (11) | 18 (12) | 150 (11) |
| Unknown | 38 (5) | 27 (5) | 7 (5) | 72 (5) |
| ECOG PS before infusion, n (%) | ||||
| 0-1 | 613 (88) | 460 (86) | 132 (86) | 1205 (87) |
| ≥2 | 35 (5) | 20 (4) | 9 (6) | 64 (5) |
| Unknown | 49 (7) | 53 (10) | 12 (8) | 114 (8) |
| Histologic transformation, n (%) | 202 (29) | 159 (30) | 41 (27) | 402 (29) |
| Double/triple hit, n (%)∗ | 106 (26) | 87 (29) | 18 (20) | 211 (26) |
| Chemoresistant† before infusion, n (%) | 469 (67) | 355 (67) | 101 (66) | 925 (67) |
| >1 extranodal sites at initial diagnosis, n (%)∗ | 164 (27) | 144 (32) | 49 (36) | 357 (30) |
| Disease stage, n (%)∗ | ||||
| I or II | 168 (29) | 89 (21) | 22 (18) | 279 (25) |
| III or IV | 414 (71) | 341 (79) | 101 (82) | 856 (75) |
| HCT-CI score before infusion, n (%) | ||||
| 0 | 243 (35) | 163 (31) | 32 (21) | 438 (32) |
| 1 | 129 (19) | 97 (18) | 32 (21) | 258 (19) |
| 2 | 84 (12) | 71 (13) | 23 (15) | 178 (13) |
| ≥3 | 241 (35) | 202 (38) | 66 (43) | 509 (37) |
| Any comorbidities, n (%)‡ | 479 (69) | 382 (72) | 125 (82) | 986 (71) |
| Disease histology, n (%) | ||||
| DLBCL | 556 (80) | 424 (80) | 128 (84) | 1108 (80) |
| PMBCL | 26 (4) | 13 (2) | 5 (3) | 44 (3) |
| HGBCL§ | 115 (16) | 96 (18) | 20 (13) | 231 (17) |
| Number of prior lines of systemic therapy, n (%)∗,|| | ||||
| 1–2 | 197 (29) | 153 (30) | 26 (18) | 376 (28) |
| ≥3 | 485 (71) | 361 (70) | 118 (82) | 964 (72) |
| Use of bridging therapy, n (%)∗ | 132 (20) | 109 (22) | 65 (46) | 306 (23) |
| Elevated LDH at initial diagnosis, n (%)∗ | 210 (67) | 156 (74) | 43 (75) | 409 (70) |
| Median time from initial diagnosis to infusion (range), mo | 14.1 (1.2-281.9) | 14.5 (1.2-405.8) | 14.6 (2.6-264.3) | 14.3 (1.2-405.8) |
| <12, n (%) | 300 (43) | 217 (41) | 63 (41) | 580 (42) |
| ≥12, n (%) | 397 (57) | 316 (59) | 90 (59) | 803 (58) |
| Year of axi-cel infusion, n (%) | ||||
| ≤2018 | 210 (30) | 155 (29) | 30 (20) | 395 (29) |
| 2019 | 324 (46) | 252 (47) | 69 (45) | 645 (47) |
| 2020 | 163 (23) | 126 (24) | 54 (35) | 343 (25) |
| Characteristic . | V2Vt . | Overall N = 1383 . | ||
|---|---|---|---|---|
| <28 d, n = 697 . | ≥28 to <40 d, n = 533 . | ≥40 d, n = 153 . | ||
| Median age at infusion (range), y | 61.5 (19.6-86.0) | 62.5 (19.6-90.8) | 63.1 (28.2-84.4) | 62.1 (19.6-90.8) |
| ≥65, n (%) | 239 (34) | 217 (41) | 65 (42) | 521 (38) |
| <65, n (%) | 458 (66) | 316 (59) | 88 (58) | 862 (62) |
| Male, n (%) | 455 (65) | 348 (65) | 91 (59) | 894 (65) |
| Female, n (%) | 242 (35) | 185 (35) | 62 (41) | 489 (35) |
| Race, n (%) | ||||
| White | 573 (82) | 428 (80) | 124 (81) | 1125 (81) |
| Black | 28 (4) | 34 (6) | 9 (6) | 71 (5) |
| Asian | 43 (6) | 27 (5) | 9 (6) | 79 (6) |
| Other or unknown | 53 (8) | 44 (8) | 11 (7) | 108 (8) |
| Ethnicity, n (%) | ||||
| Non-Hispanic | 583 (84) | 450 (84) | 128 (84) | 1161 (84) |
| Hispanic or Latino | 76 (11) | 56 (11) | 18 (12) | 150 (11) |
| Unknown | 38 (5) | 27 (5) | 7 (5) | 72 (5) |
| ECOG PS before infusion, n (%) | ||||
| 0-1 | 613 (88) | 460 (86) | 132 (86) | 1205 (87) |
| ≥2 | 35 (5) | 20 (4) | 9 (6) | 64 (5) |
| Unknown | 49 (7) | 53 (10) | 12 (8) | 114 (8) |
| Histologic transformation, n (%) | 202 (29) | 159 (30) | 41 (27) | 402 (29) |
| Double/triple hit, n (%)∗ | 106 (26) | 87 (29) | 18 (20) | 211 (26) |
| Chemoresistant† before infusion, n (%) | 469 (67) | 355 (67) | 101 (66) | 925 (67) |
| >1 extranodal sites at initial diagnosis, n (%)∗ | 164 (27) | 144 (32) | 49 (36) | 357 (30) |
| Disease stage, n (%)∗ | ||||
| I or II | 168 (29) | 89 (21) | 22 (18) | 279 (25) |
| III or IV | 414 (71) | 341 (79) | 101 (82) | 856 (75) |
| HCT-CI score before infusion, n (%) | ||||
| 0 | 243 (35) | 163 (31) | 32 (21) | 438 (32) |
| 1 | 129 (19) | 97 (18) | 32 (21) | 258 (19) |
| 2 | 84 (12) | 71 (13) | 23 (15) | 178 (13) |
| ≥3 | 241 (35) | 202 (38) | 66 (43) | 509 (37) |
| Any comorbidities, n (%)‡ | 479 (69) | 382 (72) | 125 (82) | 986 (71) |
| Disease histology, n (%) | ||||
| DLBCL | 556 (80) | 424 (80) | 128 (84) | 1108 (80) |
| PMBCL | 26 (4) | 13 (2) | 5 (3) | 44 (3) |
| HGBCL§ | 115 (16) | 96 (18) | 20 (13) | 231 (17) |
| Number of prior lines of systemic therapy, n (%)∗,|| | ||||
| 1–2 | 197 (29) | 153 (30) | 26 (18) | 376 (28) |
| ≥3 | 485 (71) | 361 (70) | 118 (82) | 964 (72) |
| Use of bridging therapy, n (%)∗ | 132 (20) | 109 (22) | 65 (46) | 306 (23) |
| Elevated LDH at initial diagnosis, n (%)∗ | 210 (67) | 156 (74) | 43 (75) | 409 (70) |
| Median time from initial diagnosis to infusion (range), mo | 14.1 (1.2-281.9) | 14.5 (1.2-405.8) | 14.6 (2.6-264.3) | 14.3 (1.2-405.8) |
| <12, n (%) | 300 (43) | 217 (41) | 63 (41) | 580 (42) |
| ≥12, n (%) | 397 (57) | 316 (59) | 90 (59) | 803 (58) |
| Year of axi-cel infusion, n (%) | ||||
| ≤2018 | 210 (30) | 155 (29) | 30 (20) | 395 (29) |
| 2019 | 324 (46) | 252 (47) | 69 (45) | 645 (47) |
| 2020 | 163 (23) | 126 (24) | 54 (35) | 343 (25) |
Patients are from the CIBMTR registry PASS Cohort.
DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation–specific comorbidity index; HGBCL, high grade B-cell lymphoma; LDH, lactate dehydrogenase; NOS, not otherwise specified; PD, progressive disease; PMBCL, primary mediastinal large B-cell lymphoma; SD, stable disease.
Percentages were based on nonmissing cases.
Patients with SD or PD after chemotherapy.
Defined based on the HCT-CI and includes pulmonary (moderate/serve), cardiac/cerebrovascular/heart valve disease, hepatic (moderate/severe), renal (moderate severe), obesity, and/or prior malignancy.
Includes patients with HGBCL NOS and HGBCL with MYC and BCL2 and/or BCL6 rearrangements.
Not including prior HCT.
Most patients either had V2Vt <28 days (n = 697 [50%]) or ≥28 to <40 days (n = 533 [39%]) compared with ≥40 days (n = 153 [11%]; Table 1). Across all V2Vt subgroups, median age at infusion (61.5-63.1 years), percentage of patients with ECOG PS 0 to 1 (86%-88%), and percentage of patients with chemoresistant tumors before infusion (66%-67%) were similar. Most patients were male (59%-65%), White (80%-82%), and non-Hispanic (84%) and presented with stage III or IV disease (71%-82%). Patients with V2Vt ≥40 days had a higher rate of comorbidities (82%) compared with V2Vt <28 days (69%) or ≥28 to <40 days (72%). A higher percentage of patients with V2Vt ≥40 days had ≥3 prior lines of systemic therapy (82%) than those who had V2Vt <28 days (71%) or ≥28 to <40 days (70%). Additionally, patients with V2Vt ≥40 days were more likely to have had bridging therapy between leukapheresis and infusion (46%) than those who had V2Vt <28 days (20%) or ≥28 to <40 days (22%).
Effectiveness outcomes
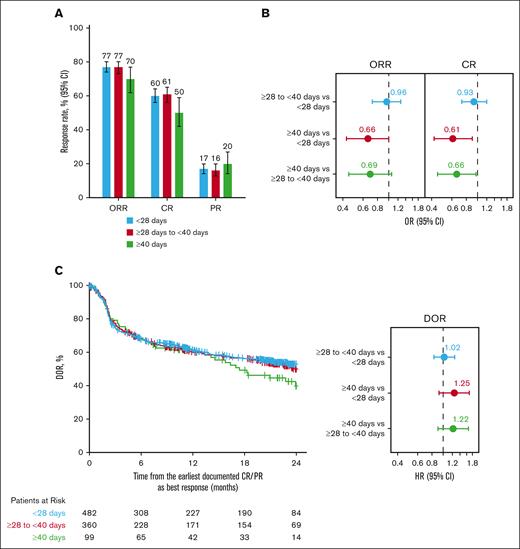
ORRs of 77% were reported in patients with axi-cel V2Vt <28 days (95% CI, 74-80) or ≥28 to <40 days (95% CI, 73-80), whereas an ORR of 70% (95% CI, 62-77) was reported in patients with V2Vt ≥40 days (Figure 3A). CR rates were 60% (95% CI, 56-64), 61% (95% CI, 56-65), and 50% (95% CI, 42-59) in patients with V2Vt <28 days, ≥28 to <40 days, and ≥40 days, respectively. In a multivariate analysis of ORRs that adjusted for key prognostic factors, no significant differences between V2Vt subgroups were found (Figure 3B; supplemental Table 3). However, patients with V2Vt ≥40 days had a significantly lower CR rate than those who had V2Vt <28 days (odds ratio [OR], 0.61; 95% CI, 0.42-0.90) or ≥28 to <40 days (OR, 0.66; 95% CI, 0.45-0.97). CR rates in patients with V2Vt <28 days or ≥28 to <40 days were comparable (OR, 0.93; 95% CI, 0.73-1.20; supplemental Table 3).
Axi-cel response rates by V2Vt. Response rate (A), adjusted ORs of ORR and CR (B), and adjusted DOR (C) by V2Vt in patients treated with axi-cel. Patients were from the CIBMTR registry PASS Cohort. Covariates for stepwise selection and multivariable adjustment were age, sex, race, ethnicity, ECOG PS before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal), histologic transformation, disease characteristics at initial diagnosis (double/triple hit, disease stage, elevated LDH, and >1 extranodal involvement), chemosensitivity before infusion, number of lines of prior therapy, prior HCT, year of infusion, time from initial diagnosis to infusion, and use of bridging therapy. PR, partial response.
Axi-cel response rates by V2Vt. Response rate (A), adjusted ORs of ORR and CR (B), and adjusted DOR (C) by V2Vt in patients treated with axi-cel. Patients were from the CIBMTR registry PASS Cohort. Covariates for stepwise selection and multivariable adjustment were age, sex, race, ethnicity, ECOG PS before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal), histologic transformation, disease characteristics at initial diagnosis (double/triple hit, disease stage, elevated LDH, and >1 extranodal involvement), chemosensitivity before infusion, number of lines of prior therapy, prior HCT, year of infusion, time from initial diagnosis to infusion, and use of bridging therapy. PR, partial response.
No statistically significant differences were observed in the DOR between patients with V2Vt ≥40 days compared with <28 days (hazard ratio [HR], 1.25; 95% CI, 0.92-1.69) or ≥28 to <40 days (HR, 1.22; 95% CI, 0.90-1.66; Figure 3C; supplemental Table 3). Among patients who achieved CR or partial response, the adjusted proportion of patients remaining in response 12 months after achieving CR or partial response was comparable across V2Vt subgroups (Figure 3C): 61% in patients with V2Vt <28 days (95% CI, 57-65) or ≥40 days (95% CI, 52-70) and 60% (95% CI, 55-65) in those with V2Vt ≥28 to <40 days.
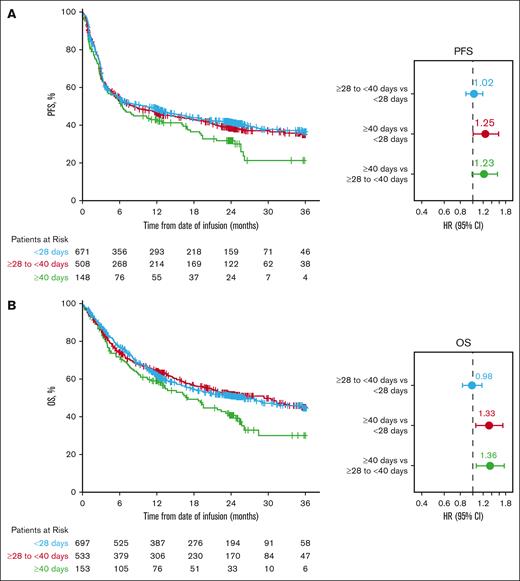
Multivariate analysis of adjusted PFS showed that V2Vt ≥40 days was associated with worse PFS than in patients with V2Vt <28 days (HR, 1.25; 95% CI, 1.00-1.57; Figure 4A; supplemental Table 3). Patients with V2Vt ≥40 days also appeared to have worse PFS than patients with V2Vt ≥28 to <40 days (HR, 1.23; 95% CI, 0.98-1.54). The 24-month adjusted PFS rate was 42% (95% CI, 38-45) in patients with V2Vt <28 days, 39% (95% CI, 35-43) in those with V2Vt ≥28 to <40 days, and 32% (95% CI, 25-40) in those with V2Vt ≥40 days (Figure 4A).
Axi-cel adjusted PFS and OS by V2Vt. Adjusted PFS (A) and adjusted OS (B) by V2Vt in patients treated with axi-cel. Patients were from the CIBMTR registry PASS cohort. For PFS, subsequent cellular therapy and HCT were censored. Covariates for stepwise selection and multivariable adjustment were age, sex, race, ethnicity, ECOG PS before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal), histologic transformation, disease characteristics at initial diagnosis (double/triple hit, disease stage, elevated LDH and >1 extranodal involvement), chemosensitivity before infusion, number of prior lines of therapy, prior HCT, year of infusion, time from initial diagnosis to infusion, and use of bridging therapy.
Axi-cel adjusted PFS and OS by V2Vt. Adjusted PFS (A) and adjusted OS (B) by V2Vt in patients treated with axi-cel. Patients were from the CIBMTR registry PASS cohort. For PFS, subsequent cellular therapy and HCT were censored. Covariates for stepwise selection and multivariable adjustment were age, sex, race, ethnicity, ECOG PS before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal), histologic transformation, disease characteristics at initial diagnosis (double/triple hit, disease stage, elevated LDH and >1 extranodal involvement), chemosensitivity before infusion, number of prior lines of therapy, prior HCT, year of infusion, time from initial diagnosis to infusion, and use of bridging therapy.
Multivariate analysis of adjusted OS demonstrated that V2Vt ≥40 days was associated with significantly worse OS than V2Vt <28 days (HR, 1.33; 95% CI, 1.05-1.70) or ≥28 to <40 days (HR, 1.36; 95% CI, 1.06-1.74; Figure 4B; supplemental Table 3). The 24-month adjusted OS rates were 51% (95% CI, 47-55), 52% (48-56), and 41% (33-49) in patients with V2Vt <28 days, ≥28 to <40 days, and ≥40 days, respectively (Figure 4B).
Safety
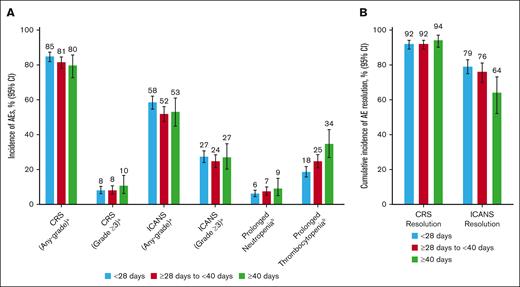
Any-grade CRS or ICANS were reported at comparable rates in patients treated with axi-cel across V2Vt subgroups (Figure 5A; supplemental Table 2). Grade ≥3 CRS was reported in 8% of patients with V2Vt <28 days (95% CI, 6-10) or ≥28 to <40 days (95% CI, 6-11) and in 10% of patients with V2Vt ≥40 days (95% CI, 6-16). Grade ≥3 ICANS was reported in 27% of patients with V2Vt <28 days (95% CI, 24-31) or ≥40 days (95% CI, 20-35) and in 24% of patients with V2Vt ≥28 to <40 days (95% CI, 21-28). Most occurrences of CRS and ICANS were resolved within 21 days from onset, regardless of V2Vt.
Incidence of axi-cel AEs by V2Vt. Incidence of AEs (A) and cumulative incidence of CRS and ICANS resolved within 21 days of onset (B) by V2Vt in patients treated with axi-cel. aEvaluated based on events reported on the 100-day follow-up case-report form in patients from the CIBMTR registry PASS Cohort. bEvaluated among patients alive at day 30 after infusion in patients from the CIBMTR registry PASS cohort. AE, adverse event.
Incidence of axi-cel AEs by V2Vt. Incidence of AEs (A) and cumulative incidence of CRS and ICANS resolved within 21 days of onset (B) by V2Vt in patients treated with axi-cel. aEvaluated based on events reported on the 100-day follow-up case-report form in patients from the CIBMTR registry PASS Cohort. bEvaluated among patients alive at day 30 after infusion in patients from the CIBMTR registry PASS cohort. AE, adverse event.
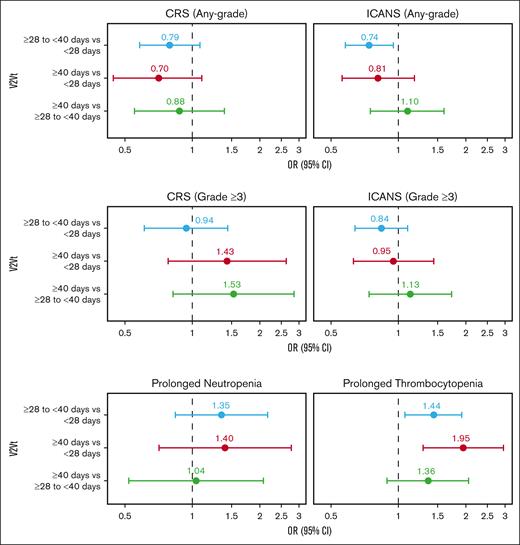
In multivariate analyses adjusted for key prognostic factors, the rate of any-grade CRS was comparable between V2Vt subgroups (Figure 6; supplemental Table 3). However, the rate of any-grade ICANS was significantly higher in patients with V2Vt <28 days than in those with V2Vt ≥28 to <40 days (OR, 1.34; 95% CI, 1.06-1.71). Rates of grade ≥3 CRS or grade ≥3 ICANS were comparable between V2Vt subgroups.
Multivariate analyses of axi-cel safety outcomes by V2Vt. Multivariate analyses of CRS (any-grade or grade ≥3), ICANS (any-grade or grade ≥3), prolonged neutropenia, and prolonged thrombocytopenia in patients treated with axi-cel. Patients were from the CIBMTR registry PASS cohort. CRS and ICANS were evaluated based on events reported on the 100-day follow-up case-report form, and prolonged neutropenia and thrombocytopenia were evaluated among patients alive at day 30 after infusion. Covariates for stepwise selection and multivariable adjustment were age, sex, race, ethnicity, ECOG PS before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal), histologic transformation, disease characteristics at initial diagnosis (double/triple hit, disease stage, elevated LDH and >1 extranodal involvement), chemosensitivity before infusion, number of prior lines of therapy, prior HCT, year of infusion, time from initial diagnosis to infusion, and use of bridging therapy.
Multivariate analyses of axi-cel safety outcomes by V2Vt. Multivariate analyses of CRS (any-grade or grade ≥3), ICANS (any-grade or grade ≥3), prolonged neutropenia, and prolonged thrombocytopenia in patients treated with axi-cel. Patients were from the CIBMTR registry PASS cohort. CRS and ICANS were evaluated based on events reported on the 100-day follow-up case-report form, and prolonged neutropenia and thrombocytopenia were evaluated among patients alive at day 30 after infusion. Covariates for stepwise selection and multivariable adjustment were age, sex, race, ethnicity, ECOG PS before infusion, comorbidities (pulmonary, cardiac/cerebrovascular/heart valve disease, hepatic, and renal), histologic transformation, disease characteristics at initial diagnosis (double/triple hit, disease stage, elevated LDH and >1 extranodal involvement), chemosensitivity before infusion, number of prior lines of therapy, prior HCT, year of infusion, time from initial diagnosis to infusion, and use of bridging therapy.
Prolonged (beyond Day 30) neutropenia was reported in 6% of patients with V2Vt <28 days (95% CI, 4-8), 7% of patients with V2Vt ≥28 to <40 days (95% CI, 5-10), and 9% of patients with V2Vt ≥40 days (95% CI, 5-15; Figure 5A; supplemental Table 2). Incidence of prolonged thrombocytopenia was lower in patients with V2Vt <28 days (18%; 95% CI, 16-22) compared with V2Vt ≥28 to <40 days (25%; 95% CI, 21-28) or ≥40 days (34%; 95% CI, 27-43). In multivariate adjusted analysis, the rates of prolonged neutropenia were not significantly different between V2Vt subgroups (Figure 6; supplemental Table 3). Higher rates of prolonged thrombocytopenia were observed in patients with V2Vt ≥28 to <40 days (OR 1.44; 95% CI, 1.07–1.92) or ≥40 days (OR 1.95; 95% CI, 1.29–2.95) compared with V2Vt <28 days.
Sensitivity analysis in patients who did not receive bridging therapy
The multivariate analysis among patients who did not receive bridging therapy (n = 1005 [of 1311]; 72 patients had no reported status for the use of bridging therapy) was largely consistent with the primary analysis, because no significant differences were found in ORR, DOR, grade ≥3 CRS, grade ≥3 ICANS, or prolonged neutropenia end points between the defined V2Vt subgroups (supplemental Table 4; supplemental Figures 2-4). Similar to the primary analysis, OS was significantly worse in patients who did not have bridging therapy and had V2Vt ≥40 days compared with V2Vt <28 days (HR, 1.54). In patients without bridging therapy, the rate of any-grade ICANS was significantly higher in those who had V2Vt <28 days compared with ≥28 to <40 days (OR, 1.45), and higher rates of prolonged thrombocytopenia were observed in patients with V2Vt ≥28 to <40 days compared with <28 days (OR, 1.43). Some outcomes in patients who did not have bridging therapy differed from the primary analysis: no significant differences were found in CR rates between V2Vt subgroups; no significant differences in PFS were found in patients with V2Vt ≥40 days compared with V2Vt <28 days (although patients with V2Vt ≥40 days did appear to have worse PFS [HR, 1.18]); the rate of any-grade CRS was significantly higher in patients with V2Vt <28 days than those with ≥40 days (OR, 1.99); and the rate of any-grade ICANS was significantly higher in those who had V2Vt <28 days than those with ≥40 days (OR, 2.45). Additional sensitivity analyses using a 36-day cutoff for V2Vt are presented in the supplemental Results.
Discussion
Timely treatment is crucial for patients with R/R LBCL, given that rapid disease progression and clinical deterioration may occur over time.32 For patients who decide to receive CAR T-cell therapy, delays in the treatment process (from referral to infusion) may adversely affect clinical outcomes. To further describe and evaluate steps in the journey to CAR T-cell infusion for patients with R/R LBCL, we compared V2Vt and V2Vt subintervals in patients treated with approved CAR T-cell therapies (axi-cel, tisa-cel, and liso-cel) and analyzed the real-world impact of V2Vt on effectiveness and safety outcomes in patients treated with axi-cel.
In our SLR of studies that reported V2Vt, we found that patients with R/R LBCL treated with axi-cel consistently had the shortest V2Vt compared with those treated with tisa-cel or liso-cel in clinical trials or real-world/commercial settings. Analysis of V2Vt across the included studies was limited by the data available at the time of the SLR. Many studies did not report V2Vt, and no real-world data from observational studies in patients treated with liso-cel were available. The association between V2Vt and effectiveness and/or other clinical outcomes was evaluated only in a small number of studies, most of which analyzed V2Vt and V2Vt subintervals as continuous variables (eg, impact of single-day differences in V2Vt on clinical outcomes) instead of categorical variables, making meta-analysis impractical.
Our real-world analysis of V2Vt in patients treated with axi-cel demonstrated that a longer V2Vt was associated with a significantly worse CR rate and OS, after adjustment for key prognostic factors. Our results were consistent with recent analysis of time from enrollment to infusion in patients treated with tisa-cel in the JULIET trial, further supporting that shorter times to infusion are associated with improved clinical outcomes.20 A previously published analysis in patients with R/R LBCL who were eligible for treatment with axi-cel identified disease progression as a common factor for failure to receive CAR T-cell infusion.33 Thus, the association of longer V2Vt and poorer clinical outcomes may be due to rapid disease progression during the waiting period, which may also be affected by events before leukapheresis, such as delayed referrals and/or insurance approvals.20
Bridging therapies, which are administered to patients between leukapheresis and lymphodepletion to debulk disease and/or prevent disease progression, may have contributed to longer V2Vt for certain patients.34,35 The use of bridging therapies (immunotherapy, chemotherapy, targeted therapy, or radiotherapy) may vary by clinician preference and/or patient-specific characteristics such as disease burden; they can potentially delay CAR T-cell infusion and/or lead to organ-specific toxicities that limit a patient’s subsequent ability to tolerate CAR T-cell therapy.34,36-38 In patients treated with axi-cel in this analysis, longer V2Vt was significantly associated with higher rates of prolonged thrombocytopenia. Cytopenias are more common in patients treated with bridging chemotherapy or radiotherapy,39 which could explain this association between prolonged thrombocytopenia and longer V2Vt, because patients with V2Vt ≥40 days had the highest proportion treated with bridging therapies. Although the association between V2Vt and clinical outcomes in patients who did not receive bridging therapy was largely consistent with the primary analysis, a causal relationship could not be determined due to the limited information on why bridging therapy was prescribed.
Infections must be controlled before CAR T-cell infusion to reduce the risk of increased morbidity and mortality.40 Several factors, including immunosuppression from prior therapies, can increase the probability of a patient acquiring an infection that may delay CAR T-cell infusion.34,41 Although infections may delay infusion and contribute to a prolonged V2Vt, the impact of infections on V2Vt could not be assessed using CIBMTR data.
Patients in the CIBMTR PASS study were treated with axi-cel from 2017 to 2020, and for those treated in 2020, the severe acute respiratory syndrome coronavirus 2 (COVID-19) pandemic may have affected V2Vt. COVID-19 disrupted routine operations of medical systems (limiting resources such as hospital beds), constrained travel, and extended delivery times of commercial CAR T-cell products.14 Tocilizumab, a key CRS treatment, was also used to treat COVID-19, and its shortage during the pandemic may have extended V2Vt, given that at least 1 dose was required to be available before CAR T-cell infusion.14,37 Recent analysis of axi-cel manufacturing in Europe found a reduction in the median turnaround time (time from leukapheresis to qualified person’s product release) from 2020 to 2022, compared with the first 2 years (2018-2020) of postmarketing manufacturing experience (19 days and 25 days, respectively).42 Analysis of axi-cel manufacturing in the United States from 2017 to 2023 found high overall manufacturing (96%) and delivery success rates (97%) and a first-pass manufacturing success rate of 93%.43 Maintaining a high first-pass manufacturing success rate is a critical factor in reducing V2Vt, because it eliminates the need for remanufacturing or releukapheresis that may arise if the product does not meet specifications in the first round of manufacturing. Reducing manufacturing times remains important, but several components of V2Vt are outside the manufacturers’ control. Delays can also be related to routine procedures in medical institutions and/or shipping logistics.
Our study did have limitations; for example, the meta-analysis did not account for potential further improvements in V2Vt in the real-world setting or patients who required leukapheresis more than once. The real-world CIBMTR data included only patients who received axi-cel and did not represent an intention-to-treat population (eg, patients who became ineligible to receive CAR T cells after leukapheresis, had out-of-specification products, or did not survive to infusion, possibly because of infections or progressive disease, were not observable in the CIBMTR data). We used multivariable regression models to adjust for potential confounding effects caused by other prognostic factors (including those related to prior treatment history and disease burden); however, due to the nature of observational studies, there may be residual confounding variables (not fully adjustable in the statistical models). Therefore, the impact of prolonged V2Vt could be only partially ascertained. The significance of V2Vt on the rate of successful CAR T-cell infusion warrants further study.
In summary, the SLR and meta-analysis demonstrated that patients treated with axi-cel had the shortest V2Vt compared with patients treated with tisa-cel or liso-cel. Further evaluation of real-world data in patients who received axi-cel showed that a shorter V2Vt was associated with a more favorable CR rate, OS, and reduced risk of prolonged thrombocytopenia, even after adjustment for other prognostic factors, although any-grade ICANS may be higher among patients with V2Vt <28 days. Together, these results show the impact of V2Vt on patient outcomes with axi-cel therapy and that earlier infusion with CD19-directed CAR T-cell therapies may be beneficial. Further studies of CAR T-cell therapies in patients with R/R LBCL are warranted to identify and address potential factors in the patient journey that may delay infusion (eg, patient selection and timing) or prolong V2Vt (eg, bridging therapy).
Acknowledgments
The authors thank all investigators, coordinators, study-site personnel, and the patients and their families for participating in this study. Editorial and writing assistance was provided by Andrea Angstadt of Avalere Health, with funding provided by Kite, a Gilead Company.
This study was supported by Kite, a Gilead Company. Kite was involved in the development of the study protocol and in data collection, analysis, and interpretation. CIBMTR is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; Cellular Immunotherapy Data Resource (CIDR- NCI, U24CA233032); 75R60222C00011 from the Health Resources and Services Administration; N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research; and the Medical College of Wisconsin, NMDP, Gateway for Cancer Research, and Pediatric Transplantation and Cellular Therapy Consortium. CIBMTR received support from AbbVie, Actinium Pharmaceuticals, Adaptive Biotechnologies Corporation, ADC Therapeutics, Adienne SA, Alexion, AlloVir, Amgen, Astellas Pharma US, AstraZeneca, Atara Biotherapeutics, BeiGene, BioLineRx, Blue Spark Technologies, Bluebird bio, Blueprint Medicines, Bristol Myers Squibb, CareDx, CSL Behring, CytoSen Therapeutics, DKMS, Elevance Health, Eurofins Viracor, DBA Eurofins Transplant Diagnostics, Gamida Cell, Gift of Life Biologics, Gift of Life Marrow Registry, GlaxoSmithKline, HistoGenetics, Incyte Corporation, Iovance, Janssen Research & Development, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals, Karius, Kashi Clinical Laboratories, Kiadis Pharma, Kite, a Gilead Company, Kyowa Kirin, Labcorp, Legend Biotech, Mallinckrodt Pharmaceuticals, Med Learning Group, Medac GmbH, Merck, Mesoblast, Millennium, Takeda Oncology, Miller Pharmacal Group, Miltenyi Biotec, MorphoSys, MSA-EDITLife, Neovii Pharmaceuticals, Novartis Pharmaceuticals Corporation, Omeros Corporation, OptumHealth, Orca Biosystems, OriGen BioMedical, Ossium Health, Pfizer, Pharmacyclics, PPD Development, REGiMMUNE, Registry Partners, Rigel Pharmaceuticals, Sanofi, Sarah Cannon, Seagen, Sobi, Stemcell Technologies, Stemline Technologies, STEMSOFT, Takeda Pharmaceuticals, Talaris Therapeutics, Vertex Pharmaceuticals, Vor Biopharma Inc, and Xenikos BV.
F.L.L. is a Scholar in Clinical Research of the Leukemia & Lymphoma Society.
Authorship
Contribution: Z-H.H. and H.X. conceptualized the study design; M.C.P., Z-H.H., M.T.H., M.J.Z., and H.X. curated the data; and all authors were involved in the interpretation of the data and writing of the manuscript, and they provided final approval to submit for publication.
Conflict-of-interest disclosure: F.L.L. has served in a consultancy or advisory role for Allogene, Amgen, Bluebird Bio, Bristol Myers Squibb/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; has received research funding from Kite/Gilead, Allogene, CERo Therapeutics, Novartis, Bluebird Bio, Bristol Myers Squibb, National Cancer Institute, and Leukemia & Lymphoma Society; has several patents held by the institution (unlicensed) in the field of cellular immunotherapy; has received travel, accommodations, or expenses from A2 Bio; and has served in an education or editorial role for Aptitude Health, American Society of Hematology, BioPharma Communications, CARE Education, Clinical Care Options Oncology, Imedex, and Society for Immunotherapy of Cancer. T.S. has served in a consultancy or advisory role for AstraZeneca, Pharmacyclics, Celgene, Juno, Kite/Gilead, and BeiGene; has been paid to participate in a speakers’ bureau by AstraZeneca, Bristol Myers Squibb, and BeiGene; and has received research funding from Bristol Myers Squibb, Pharmacyclics, Juno Therapeutics, Kite, AstraZeneca, BeiGene, Oncternal, TG Therapeutics, Ascentage Pharma, and Celgene. C.A.J. has been paid honoraria previously by Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, Bluebird Bio, Epizyme, Ipsen, Daiichi-Sankyo, Instill Bio, ImmPACT Bio, Caribou Bio, and Abintus Bio; has served in a consultancy or advisory role for Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, Bluebird Bio, Ipsen, Daiichi-Sankyo, Instill Bio, ImmPACT Bio, Caribou Bio, and Abintus Bio; and has received research funding from Kite/Gilead and Pfizer, and personal fees from Bristol Myers Squibb/Celgene. S.N. has served in a consultancy or advisory role for ad hoc advisory board for Iovance, Kite/Gilead, SmartImmune, and Legend; and has received travel, accommodations, and expenses from A2 Bio. S.A. has been paid honoraria previously by Kite, ADCT, Novartis, and Nektar; has served in a consultancy or advisory role for Myeloid, Nektar, and Tessa; and has received research funding from Xencor, Tessa, Nektar, Merck, and Bristol Myers Squibb. D.B.M. has been paid honoraria previously by Janssen; has served in a consultancy or advisory role for Janssen, Adaptive Biotechnologies, Kite, Bristol Myers Squibb, and Miltenyi Biotec; has received research funding from Kite, Adicet, Allogene, 2Seventy Bio, Fate Therapeutics, and Miltenyi Biotec; is a chronic graft-versus-host disease patent holder for ibrutinib as chronic graft-versus-host disease therapy, with no compensation; and has received personal fees from Janssen. Y.L. has served in a consultancy or advisory role for Kite/Gilead, Celgene/Bristol Myers Squibb, Juno/Bristol Myers Squibb, Bluebird Bio, Janssen, Legend BioTech, Gamida Cell, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer; and has received research funding from Kite/Gilead, Celgene/Bristol Myers Squibb, Bluebird Bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. M.A.L. has been paid honoraria and/or has served in a consultancy or advisory role for AbbVie, Acrotech, ADC Therapeutics, AstraZeneca, Astellas, Bristol Myers Squibb, Caribou, CRISPR, Daiichi Sankyo, EUSA, Fate Therapeutics, Genentech, GenMab, InstilBio, Ipsen, Janssen, Kite, Loxo, Miltenyi, MorphoSys, Novartis, Nurix, Pharmacyclics, Regeneron, Sanofi, Seagen, Takeda, and TG Therapeutics; and has received research funding from Bristol Myers Squibb, Curis, FATE Therapeutics, and Sana Therapeutics. B.T.H. has been paid an honorarium and has served in a consulting or advisory role for Kite; and has received research funding and travel, accommodations, and expenses from Kite. A.G. has been paid an honorarium from Kite; has served in a consulting or advisory role for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc; and has received research funding from Amgen, Genentech, and Kite. Z-H.H. owns stock in Gilead and is an employee of Kite, a Gilead Company. M.T.H. has been paid an honorarium and has served in a consulting or advisory role for Pfizer; and is an employee of Kite, a Gilead Company. M.J.Z. reported being employed by RainCity Analytics. S.V. owns stock in Gilead; received travel, accommodation, and expenses from Kite; and is an employee of Kite, a Gilead Company. J.T. owns stock in Biomarin, Bristol Myers Squibb, Bayer, and Gilead; received travel, accommodation, and expenses from Kite; and is an employee of Kite, a Gilead Company. C.S. has received research support from Delta Hat Limited, and is an employee of Kite, a Gilead Company. H.S. owns stock in Gilead; and is an employee of Kite, a Gilead Company. C.F. has an immediate family member who has patents, royalties, or other intellectual property from Cellares; owns stock in Amgen; and is an employee of Kite, a Gilead Company. A.P. is an employee of Kite, a Gilead Company. H.M. is an employee of Kite, a Gilead Company. S.A.S. has been a paid speaker for Amgen, and is an employee of Kite, a Gilead Company. D.L.M. owns stock in Gilead, and is an employee of Kite, a Gilead Company. H.X. is an employee of Kite, a Gilead Company. M.C.P. has received fees for consulting or an advisory role from Bristol Myers Squibb; and has received research funding from Kite, Novartis, Bristol Myers Squibb, Janssen, and GlaxoSmithKline.
Correspondence: Frederick L. Locke, Department of Blood and Marrow Transplant and Cellular Immunotherapy, Moffitt Cancer Center, FOB 3, 12902 USF Magnolia Dr, Tampa, FL 33612; email: frederick.locke@moffitt.org.
References
Author notes
Kite is committed to sharing clinical data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The full-text version of this article contains a data supplement.