Key Points
Recombinant Erwinia asparaginase JZP458 maintains therapeutic serum asparaginase activity levels via multiple IM and IV dosing schedules.
The safety profile of JZP458 is consistent with other asparaginases, with no new adverse safety signals identified at study completion.
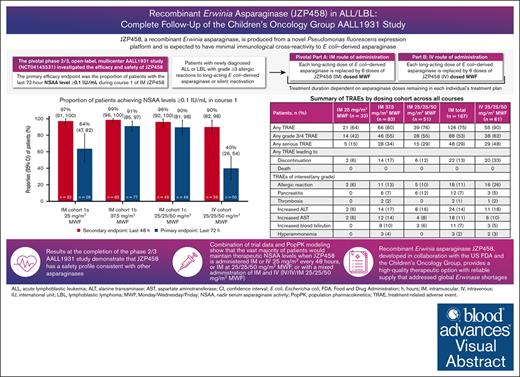
Visual Abstract
Children’s Oncology Group study AALL1931 investigated the efficacy and safety of recombinant Erwinia asparaginase (JZP458) in patients with acute lymphoblastic leukemia/lymphoblastic lymphoma and hypersensitivity reactions/silent inactivation to Escherichia coli–derived asparaginases. Each pegylated Escherichia coli asparaginase dose remaining in a patient’s treatment plan was replaced by intramuscular (IM) or IV JZP458 (6 doses) administered Monday/Wednesday/Friday (MWF). Three IM cohorts (1a [25 mg/m2 MWF], n = 33; 1b [37.5 mg/m2 MWF], n = 83; 1c [25/25/50 mg/m2 MWF], n = 51) and 1 IV cohort (25/25/50 mg/m2 MWF, n = 62) were evaluated. The proportion (95% confidence interval [CI]) of patients maintaining nadir serum asparaginase activity (NSAA) levels of ≥0.1 IU/mL at the last 72 (primary end point) and 48 hours during course 1 was 90% (95% CI, 81-98) and 96% (95% CI, 90-100) in IM cohort 1c, respectively, and 40% (95% CI, 26-54) and 90% (95% CI, 82-98) in the IV cohort. Population pharmacokinetic modeling results were comparable with observed data, predicting the vast majority of patients would maintain therapeutic NSAA levels when JZP458 is administered IM or IV 25 mg/m2 every 48 hours, or IM 25/25/50 mg/m2 MWF, or with mixed IM and IV administration (IV/IV/IM 25/25/50 mg/m2 MWF). Drug discontinuation occurred in 23% and 56% of patients in the IM and IV cohorts, respectively; 13% and 33% because of treatment-related adverse events (mainly allergic reactions and pancreatitis). JZP458 achieves therapeutic NSAA levels via multiple IM and IV dosing schedules based on combined observed and modeled data with a safety profile consistent with other asparaginases. This trial was registered at www.ClinicalTrials.gov as #NCT04145531.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer among children and the second most common acute leukemia in adults.1,2 Asparaginase is an important component of multiagent regimens for patients with ALL and lymphoblastic lymphoma (LBL).3,4 Asparaginase catalyzes the deamination of asparagine to ammonia and aspartic acid, thus leading to reduced plasma asparagine concentration.3 Unlike conventional chemotherapy, asparaginase specifically targets leukemic cells because they express limited amounts of asparagine synthetase and rely on circulating asparagine for protein synthesis and, therefore, survival.1,3 In clinical practice, serum asparaginase activity (SAA) levels are used as a surrogate marker for asparagine depletion, with nadir SAA (NSAA) levels of ≥0.1 IU/mL being the commonly accepted threshold for complete asparagine depletion, which correlates with clinical activity.1,5-8 However, some studies indicated that even lower SAA thresholds were associated with asparagine depletion.5,6,8 The fact that SAA correlates with clinical activity allows model-based simulations to predict clinical efficacy for different dosing regimens.9,10
Therapeutic asparaginases are derived from bacterial sources and can elicit immunogenic reactions in humans.3,4,11 Antibody-mediated allergic or hypersensitivity reactions (HSRs) to native and pegylated forms of Escherichia coli (E coli)–derived asparaginases occur in up to 30% of patients, often resulting in treatment discontinuation, which is associated with inferior clinical outcomes.3,12-15 Subclinical hypersensitivity or silent inactivation can occur without clinical symptoms when patients develop neutralizing antidrug antibodies (ADAs), leading to decreased SAA levels and efficacy.3,15 In the case of HSRs/silent inactivation to an E coli–derived asparaginase, guidelines recommend switching to an asparaginase with a distinct immunogenic profile, such as an Erwinia chrysanthemi–derived asparaginase.5 Switching from an E coli–derived asparaginase to an Erwinia-derived preparation had been historically difficult because of manufacturing issues with native asparaginase Erwinia chrysanthemi, resulting in global shortages.16
JZP458 is a recombinant Erwinia asparaginase produced from a novel Pseudomonas fluorescens expression platform. It has an identical amino acid sequence to native Erwinia asparaginase and is expected to have minimal immunological cross-reactivity to E coli–derived asparaginase.4,16,17 The manufacturing process for JZP458 requires only 1 fermentation per batch, instead of the multiple separate fermentation steps used in the traditional manufacturing for native Erwinia asparaginase. As a result, the production of JZP458 is easily scalable, with completion in as few as 3 weeks. AALL1931 (ClinicalTrials.gov identifier: NCT04145531) was conducted in collaboration with the Children’s Oncology Group to investigate the efficacy, safety, and pharmacokinetics (PKs) of JZP458 in patients with ALL/LBL who developed hypersensitivity/silent inactivation to E coli–derived asparaginase. Based on this study, JZP458 (Rylaze, United States; Enrylaze, European Union) was approved as a component of a multiagent chemotherapeutic regimen for the treatment of ALL/LBL in adult and pediatric patients aged ≥1 month who have developed hypersensitivity (US Food and Drug Administration [FDA]), or hypersensitivity/silent inactivation (European Commission/European Medicines Agency) to E coli–derived asparaginase.4,17-21 Here, we report the efficacy and safety results of intramuscular (IM) and IV JZP458 at study completion, in addition to the development of a population pharmacokinetic (PopPK) model to provide additional information on alternative IM/IV dosing regimens.
Materials and methods
Study design and treatment
AALL1931, a pivotal phase 2/3, 2-part, open-label, multicenter, dose-confirmation, and PK study, investigated the efficacy and safety of JZP458. Part A investigated IM and part B investigated IV administration (supplemental Figure 1). Each remaining dose of a long-acting E coli–derived asparaginase on a patient’s multiagent treatment plan was replaced by 1 course of JZP458 (6 doses administered on a Monday/Wednesday/Friday [MWF] schedule over 2 weeks), with the initial dose starting on M, W, or F to align with the planned chemotherapy schedule.4 All chemotherapy agents were continued according to the original treatment protocol and guidelines of participating institutions.4 The treatment duration was dependent on asparaginase doses remaining on each individual’s treatment plan. Final safety follow-up visit was 30 days after the last dose of the last course of JZP458 for each patient.
Based on the phase 1 study of JZP458 in healthy volunteers, the recommended starting dose for the phase 2/3 study was 25 mg/m2 administered IM MWF (cohort 1a).1 The study was designed to allow additional cohorts to be enrolled at a new dose after review of preplanned interim safety and efficacy results by the study data review committee. Part A also enrolled cohort 1b (IM at 37.5 mg/m2 MWF) and cohort 1c (IM at 25/25/50 mg/m2 MWF). The study dose for IV JZP458 was 25/25/50 mg/m2 MWF based on the totality of data from the phase 1 and phase 2/3 study part A results.1,4 For IV administration, JZP458 solution was slowly injected into an IV infusion bag containing 100 mL of 0.9% sodium chloride and infused over 2 hours.19
Patients
Patients were enrolled from 27 December 2019 to 16 August 2021, from 67 sites across North America. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The protocol and all amendments were approved by the institutional review board or ethics committee at each participating institution. All patients or their guardians (for patients aged <18 years) provided written informed consent.
The full eligibility criteria have been previously described.4 Briefly, pediatric or adult patients diagnosed with ALL/LBL were eligible if they developed a grade ≥3 allergic reaction (defined per common terminology criteria for adverse events, version 5.0) or silent inactivation to a long-acting E coli–derived asparaginase and had ≥1 course of E coli–derived asparaginase remaining on their treatment plan. Patients who had previously received native Erwinia-derived asparaginase or JZP458, relapsed ALL/LBL, a history of grade ≥3 pancreatitis, or an asparaginase-associated thrombus/hemorrhagic event were ineligible.
Study end points and assessments
The primary efficacy end point was the proportion of patients with the last 72-hour NSAA level of ≥0.1 IU/mL during course 1 of IM JZP458. Safety and tolerability were assessed by the occurrence of treatment-emergent adverse events (AE; any event whether or not considered related to JZP458) and treatment-related AEs (TRAEs; those considered related to JZP458 by the treating physician).
The key secondary end point was the proportion of patients with the last 48-hour NSAA level of ≥0.1 IU/mL during course 1 of IM JZP458. Additional secondary end points included the proportion of patients with the last 48- and 72-hour NSAA levels of ≥0.4 IU/mL during course 1 of IM JZP458, characterization of IM JZP458 PKs using a PopPK approach based on SAA, and incidence of ADA formation against IM JZP458 after repeat administration. Exploratory end points included the proportion of patients with the last 48- and 72-hour NSAA level of ≥0.1 IU/mL during course 1 of IV JZP458, characterization of IV JZP458 PKs using a PopPK approach based on SAA, and safety and immunogenicity of IV JZP458. Blood samples for SAA and asparagine assessment were collected at prespecified time points and transferred on dry ice to bioanalytical laboratories; sample analysis procedures have been previously described.4
A post hoc analysis investigated AEs of allergic reactions and grade ≥2 nausea/vomiting by timing/dosage, and TRAEs of interest (including allergic reactions, pancreatitis, thrombosis, and hepatotoxicity) by median (range) number of doses on/before event onset.
PopPK modeling and simulations
A PopPK model was developed based on SAA data from the phase 1 study and study AALL1931 using nonlinear mixed effects modeling (NONMEM; version 7.3) to characterize the PKs of IM and IV JZP458. In total, 4269 SAA data points from 250 participants (phase 1, n = 24; AALL1931, n = 226) were analyzed. The effects of potential covariates were evaluated to identify covariates likely to contribute to the variability of JZP458 PK.4 Model-based simulations of SAA vs time profiles using the National Health and Nutrition Examination Survey database were performed to predict the proportions of patients achieving a therapeutic NSAA level of ≥0.1 IU/mL at 48 and 72 hours after dose with various dosing regimens.
Anti-asparaginase antibody testing
Immunogenicity blood samples were collected from all patients before the first dose in each course, before dose 6 in course 1, and at the end-of-study visit (30 ± 3 days after last dose in the last course). For patients experiencing an allergic reaction (HSR) during treatment, additional samples were collected when possible. For patients who exhibited positive (+) ADA from samples obtained before the end of the study, efforts were made to collect follow-up ADA samples up to ∼6 months after the last dose of the last course.
A multitiered approach was used to measure ADAs and neutralizing antibodies (NAbs) that bind JZP458. Samples with potentially ADA+ results in screening were retested for binding specificity confirmation. Samples confirmed as ADA+ were evaluated further for the presence of NAbs capable of reducing the activity of JZP458 (supplemental Figure 2). The ADA assay was a validated binding assay based on an electrochemiluminescence immunosorbent assay technique. The NAb assay was a validated adaptation of the enzymatic activity assay for determining the SAA level of L-asparaginase in human serum in which SAA activity was measured in ADA+ samples after pretreatment to remove excess JZP458 followed by addition of a known amount of JZP458. Both the ADA and NAb assays were validated in accordance with 2019 FDA guidance.22 A post hoc analysis was conducted to identify patients with persistent ADA, defined as either treatment-induced ADA detected at ≥2 sampling time points during treatment (including any follow-up period), the first and last ADA+ samples separated by ≥16 weeks (regardless of any negative [−] samples in between); or treatment-induced ADA occurring only once, either in the last sample during the study or within <16 weeks before an ADA− last sample.
Statistical analysis
The primary and key secondary efficacy end points were assessed by calculating the 95% Wald confidence interval (CI) around the proportion of patients with NSAA of ≥0.1 IU/mL. The efficacy analysis population included patients who received ≥1 dose of JZP458 and had ≥1 48- or 72-hour NSAA assessment within the protocol-defined sample collection window (±2 hours) in course 1. The safety analysis population included patients who received ≥1 dose of JZP458. The PK analysis set included patients who received ≥1 dose of JZP458 and had ≥1 postdose evaluable SAA.
Results
Patient demographics and disposition
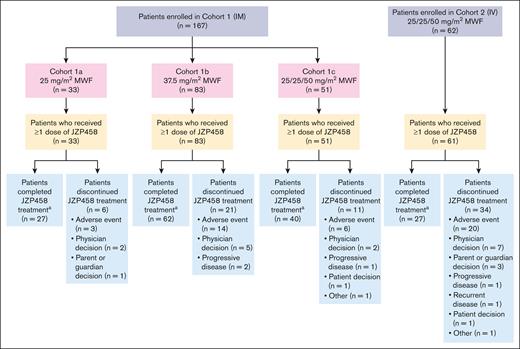
At final database lock (22 November 2022), 167 patients were enrolled in 3 IM cohorts (cohort 1a [25 mg/m2 MWF], n = 33; cohort 1b [37.5 mg/m2 MWF], n = 83; cohort 1c [25/25/50 mg/m2 MWF], n = 51) and 62 patients in the IV cohort (25/25/50 mg/m2 MWF; Figure 1). In total, 228 patients received ≥1 IM or IV JZP458 dose and were included in the safety analysis population (cohort 1a, n = 33; cohort 1b, n = 83; cohort 1c, n = 51; IV cohort, n = 61), and 224 patients (cohort 1a, n = 32; cohort 1b, n = 83; cohort 1c, n = 50; IV cohort, n = 59) were included in the efficacy analysis population. In the IM cohorts, 129 (77%) patients completed all planned JZP458 doses, and 38 (23%) patients discontinued treatment. In the IV cohort (safety analysis population), 27 (44%) completed all JZP458 planned doses, and 34 (56%) patients discontinued treatment. The most common reason for discontinuation was AEs (IM cohorts, n = 23 [14%]; IV cohort, n = 20 [33%]). The median (range) of JZP458 courses completed was 5 (0-14), 4 (0-15), 5 (1-10), and 3 (0-15) for IM cohorts 1a, 1b, 1c, and the IV cohort, respectively. For the IM cohorts, median (range) age at enrollment was 10 years (1-25) with 87% of patients aged <18 years; the majority of patients were male (62%) and White (69%), and 32% self-identified as Hispanic or Latino (Table 1). For the IV cohort, the median (range) age at enrollment was 10 years (1-24) with 84% of patients aged <18 years; the majority of patients were male (59%) and White (70%), and 34% self-identified as Hispanic or Latino.
Diagram of patient disposition by cohort.aIncludes patients who completed all planned courses of JZP458 treatment.
Diagram of patient disposition by cohort.aIncludes patients who completed all planned courses of JZP458 treatment.
Demographic and baseline characteristics (safety analysis population)
| . | IM 25 mg/m2 MWF (n = 33) . | IM 37.5 mg/m2 MWF (n = 83) . | IM 25/25/50 mg/m2 MWF (n = 51) . | IM total (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . |
|---|---|---|---|---|---|
| Median (range) age, y | 11 (1-24) | 8 (1-20) | 12 (3-25) | 10 (1-25) | 10 (1-24) |
| <6 | 9 (27) | 24 (29) | 11 (22) | 44 (26) | 20 (33) |
| ≥6 to <12 | 9 (27) | 34 (41) | 14 (27) | 57 (34) | 14 (23) |
| ≥12 to <18 | 7 (21) | 20 (24) | 18 (35) | 45 (27) | 17 (28) |
| ≥18 | 8 (24) | 5 (6) | 8 (16) | 21 (13) | 10 (16) |
| Sex | |||||
| Female | 16 (48) | 28 (34) | 20 (39) | 64 (38) | 25 (41) |
| Male | 17 (52) | 55 (66) | 31 (61) | 103 (62) | 36 (59) |
| Ethnicity∗ | |||||
| Hispanic or Latino | 13 (39) | 23 (28) | 17 (33) | 53 (32) | 21 (34) |
| Not Hispanic or Latino | 18 (55) | 56 (67) | 32 (63) | 106 (63) | 34 (56) |
| Not reported | 2 (6) | 4 (5) | 2 (4) | 8 (5) | 6 (10) |
| Race∗ | |||||
| American Indian or Alaska Native | 0 | 0 | 3 (6) | 3 (2) | 2 (3) |
| Asian | 1 (3) | 5 (6) | 1 (2) | 7 (4) | 3 (5) |
| Black or African American | 3 (9) | 11 (13) | 8 (16) | 22 (13) | 2 (3) |
| White | 24 (73) | 58 (70) | 33 (65) | 115 (69) | 43 (70) |
| Multiple | 1 (3) | 0 | 0 | 1 (1) | 1 (2) |
| Not reported | 4 (12) | 9 (11) | 6 (12) | 19 (11) | 10 (16) |
| Median (range) BMI, kg/m2 | 19.9 (13.4-42.6) | 17.9 (13.7-30.7)† | 18.4 (13.8-42.0) | 18.4 (13.4-42.6)‡ | 19.6 (13.2-43.8) |
| Median (range) BSA, m2 | 1.3 (0.4-2.5) | 1.0 (0.6-2.3)† | 1.3 (0.5-2.4) | 1.2 (0.4-2.5)‡ | 1.2 (0.5-2.4) |
| Primary disease | |||||
| ALL | |||||
| B-ALL | 27 (82) | 60 (72) | 37 (73) | 124 (74) | 51 (84) |
| T-ALL | 4 (12) | 13 (16) | 9 (18) | 26 (16) | 7 (11) |
| LBL | |||||
| B-LBL | 0 | 0 | 1 (2) | 1 (1) | 2 (3) |
| T-LBL | 2 (6) | 10 (12) | 4 (8) | 16 (10) | 1 (2) |
| Eligibility criteria met | |||||
| Grade ≥3 allergic reaction to an E coli–derived asparaginase§ | 27 (82) | 75 (90) | 44 (86) | 146 (87) | 44 (72) |
| Silent inactivation‖ | 3 (9) | 3 (4) | 1 (2) | 7 (4) | 8 (13) |
| Allergic reaction with inactivation | 3 (9) | 5 (6) | 6 (12) | 14 (8) | 9 (15) |
| . | IM 25 mg/m2 MWF (n = 33) . | IM 37.5 mg/m2 MWF (n = 83) . | IM 25/25/50 mg/m2 MWF (n = 51) . | IM total (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . |
|---|---|---|---|---|---|
| Median (range) age, y | 11 (1-24) | 8 (1-20) | 12 (3-25) | 10 (1-25) | 10 (1-24) |
| <6 | 9 (27) | 24 (29) | 11 (22) | 44 (26) | 20 (33) |
| ≥6 to <12 | 9 (27) | 34 (41) | 14 (27) | 57 (34) | 14 (23) |
| ≥12 to <18 | 7 (21) | 20 (24) | 18 (35) | 45 (27) | 17 (28) |
| ≥18 | 8 (24) | 5 (6) | 8 (16) | 21 (13) | 10 (16) |
| Sex | |||||
| Female | 16 (48) | 28 (34) | 20 (39) | 64 (38) | 25 (41) |
| Male | 17 (52) | 55 (66) | 31 (61) | 103 (62) | 36 (59) |
| Ethnicity∗ | |||||
| Hispanic or Latino | 13 (39) | 23 (28) | 17 (33) | 53 (32) | 21 (34) |
| Not Hispanic or Latino | 18 (55) | 56 (67) | 32 (63) | 106 (63) | 34 (56) |
| Not reported | 2 (6) | 4 (5) | 2 (4) | 8 (5) | 6 (10) |
| Race∗ | |||||
| American Indian or Alaska Native | 0 | 0 | 3 (6) | 3 (2) | 2 (3) |
| Asian | 1 (3) | 5 (6) | 1 (2) | 7 (4) | 3 (5) |
| Black or African American | 3 (9) | 11 (13) | 8 (16) | 22 (13) | 2 (3) |
| White | 24 (73) | 58 (70) | 33 (65) | 115 (69) | 43 (70) |
| Multiple | 1 (3) | 0 | 0 | 1 (1) | 1 (2) |
| Not reported | 4 (12) | 9 (11) | 6 (12) | 19 (11) | 10 (16) |
| Median (range) BMI, kg/m2 | 19.9 (13.4-42.6) | 17.9 (13.7-30.7)† | 18.4 (13.8-42.0) | 18.4 (13.4-42.6)‡ | 19.6 (13.2-43.8) |
| Median (range) BSA, m2 | 1.3 (0.4-2.5) | 1.0 (0.6-2.3)† | 1.3 (0.5-2.4) | 1.2 (0.4-2.5)‡ | 1.2 (0.5-2.4) |
| Primary disease | |||||
| ALL | |||||
| B-ALL | 27 (82) | 60 (72) | 37 (73) | 124 (74) | 51 (84) |
| T-ALL | 4 (12) | 13 (16) | 9 (18) | 26 (16) | 7 (11) |
| LBL | |||||
| B-LBL | 0 | 0 | 1 (2) | 1 (1) | 2 (3) |
| T-LBL | 2 (6) | 10 (12) | 4 (8) | 16 (10) | 1 (2) |
| Eligibility criteria met | |||||
| Grade ≥3 allergic reaction to an E coli–derived asparaginase§ | 27 (82) | 75 (90) | 44 (86) | 146 (87) | 44 (72) |
| Silent inactivation‖ | 3 (9) | 3 (4) | 1 (2) | 7 (4) | 8 (13) |
| Allergic reaction with inactivation | 3 (9) | 5 (6) | 6 (12) | 14 (8) | 9 (15) |
Data are n (%) unless stated otherwise. Patients received various ALL/LBL treatment protocols, including (but not limited to) Children’s Oncology Group AALL0232, AALL0434, AALL0932, AALL1131, AALL1231, AALL1631, AALL1731, and AALL1732, as well as DFC16-001 and TOT17. The prior pegylated asparaginase doses received ranged from 1-6 in this study.
B-ALL, B-cell ALL; B-LBL, B-cell LBL; BMI, body mass index; T-ALL, T-cell ALL; T-LBL, T-cell LBL.
Self-reported.
Based on 82 patients.
Based on 166 patients.
Prior long-acting E coli–derived asparaginase treatment was pegaspargase for all patients apart from 1 who received another type of E coli–derived asparaginase.
Silent inactivation was defined as NSAA of <0.5 IU/mL within 1 hour to 1 day, or <0.3 IU/mL within 7 days, or <0.1 IU/mL within 14 days of completing a long-acting E coli–derived asparaginase infusion without clinical signs/symptoms of hypersensitivity or allergy.
Efficacy
When JZP458 was administered IM at doses of 25 mg/m2, 37.5 mg/m2, and 25/25/50 mg/m2 MWF, 64% (95% CI, 46-82), 91% (95% CI, 84-97), and 90% (95% CI, 81-98) of patients, respectively, achieved NSAA levels of ≥0.1 IU/mL at the last 72-hour assessment in course 1 (Figure 2A). The proportion of patients who achieved NSAA levels of ≥0.1 IU/mL at the last 48-hour assessment in course 1 were 97% (95% CI, 91-100), 99% (95% CI, 96-100), and 96% (95% CI, 90-100), respectively. In the IV cohort (25/25/50 mg/m2 MWF), 40% (95% CI, 26-54) and 90% (95% CI, 82-98) of patients achieved NSAA levels of ≥0.1 IU/mL at the last 72-hour and 48-hour assessments in course 1, respectively. Reported as a secondary end point, the proportion of patients maintaining NSAA levels of ≥0.4 IU/mL at the last 72- and 48-hour assessment during course 1 was 4% (95% CI, 0-10) and 50% (95% CI, 33-67) in IM cohort 1a, 26% (95% CI, 16-36) and 78% (95% CI, 69-87) in IM cohort 1b, 47% (95% CI, 33-61) and 65% (95% CI, 52-79) in IM cohort 1c, respectively, and 0% (95% CI, not reported) and 17% (95% CI, 7-26) in the IV cohort. Figure 2B shows the mean NSAA levels at the last 72- and 48-hours after dose in course 1 by cohort. Mean NSAA levels of ≥0.1 IU/mL were maintained in subsequent courses for IM and IV 25/25/50 mg/m2 MWF dosing upon repeated JZP458 administration (supplemental Figure 3).
NSAA levels by cohort. (A) Proportion of patients achieving NSAA levels of ≥0.1 IU/mL in course 1a. (B) Mean NSAA levels 48 and 72 hours after dose during course 1 by cohorta (efficacy analysis population). aError bars represent 95% CI calculated by the Wald method. Total number of patients for each course is represented by n; percentages were calculated with the number of patients for each course and schedule as the denominator.
NSAA levels by cohort. (A) Proportion of patients achieving NSAA levels of ≥0.1 IU/mL in course 1a. (B) Mean NSAA levels 48 and 72 hours after dose during course 1 by cohorta (efficacy analysis population). aError bars represent 95% CI calculated by the Wald method. Total number of patients for each course is represented by n; percentages were calculated with the number of patients for each course and schedule as the denominator.
PopPK modeling and simulations
A 1-compartment model with a flexible change point absorption function for IM and zero-order input for IV described the SAA vs time data well. Evaluated model covariates included (but were not limited to) age; weight; body surface area (BSA); creatinine clearance; and levels of albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin. The model included the effects of patient status, BSA, and race on clearance; patient status and BSA on volume of distribution; and age on absorption rate constant. PopPK model-based simulations predicted that therapeutic NSAA levels (ie, ≥0.1 IU/mL) are maintained for 14 days in the vast majority of patients when JZP458 is administered IM at 25 mg/m2 every 48 hours (7 doses) or 25/25/50 mg/m2 MWF (6 doses), and when JZP458 is administered IV at 25 mg/m2 every 48 hours (7 doses), or with a mixed administration of IM and IV doses at 25/25/50 mg/m2 MWF (IV/IV/IM; 6 doses; Table 2). With IM 25/25/50 mg/m2 MWF, 88% (95% CI, 87-90) and 95% (95% CI, 94-96) of patients were expected to achieve the last 72 and 48 hours NSAA levels of ≥0.1 IU/mL, respectively. With 25 mg/m2 every 48 hours IM or IV (7 doses), 97% (95% CI, 96-97) and 83% (95% CI, 82-85) of patients were expected to achieve the last 48-hour NSAA level of ≥0.1 IU/mL, respectively. Subgroup analyses suggested that, after the proposed BSA-based dosing regimens, no clinically significant difference is expected in the probability of achieving a therapeutic NSAA of ≥0.1 IU/mL based on race, age (age of 1 month-39 years), or BSA.
Predicted response rate based on PopPK modeling and simulations
| Trough sampling time . | 25/25/50 mg/m2 MWF (6 doses) % (95% CI) . | 25 mg/m2 q48h (7 doses) % (95% CI) . | |||
|---|---|---|---|---|---|
| IM . | IV . | IV/IV/IM . | IM . | IV . | |
| 48 h | 95 (94-96) | 84 (82-86) | 90 (88-91) | 97 (96-97) | 83 (82-85) |
| 72 h | 88 (87-90) | 38 (36-40) | 89 (87-90) | N/A | N/A |
| Trough sampling time . | 25/25/50 mg/m2 MWF (6 doses) % (95% CI) . | 25 mg/m2 q48h (7 doses) % (95% CI) . | |||
|---|---|---|---|---|---|
| IM . | IV . | IV/IV/IM . | IM . | IV . | |
| 48 h | 95 (94-96) | 84 (82-86) | 90 (88-91) | 97 (96-97) | 83 (82-85) |
| 72 h | 88 (87-90) | 38 (36-40) | 89 (87-90) | N/A | N/A |
Predicted response rate is calculated from the proportion of participants achieving NSAA of ≥0.1 IU/mL. For MWF dosing regimens, data are shown for 48 hours from the last 25 mg/m2 dose and 72 hours from the last 50 mg/m2 dose. For q48h regimens, data are shown for 48 hours after the seventh dose of 25 mg/m2.
N/A, not available; q48h, every 48 hours.
Asparagine and glutamine depletion
After JZP458 administration in all cohorts, rapid depletion of plasma L-asparagine was observed in course 1 (supplemental Figure 4) and subsequent courses. Asparagine depletion lasted throughout course 1 up to before dose 6, when the last sample was collected.
Mean L-glutamine levels declined after JZP458 administration in all cohorts. In the IM cohorts, mean L-glutamine levels were moderately affected. In the IV cohort, L-glutamine levels were completely depleted based on mean glutamine profiles immediately after dose at the end of infusion and moderately affected at other time points.
Safety
A total of 126 (75%) patients in the IM cohorts and 55 (90%) in the IV cohort experienced ≥1 TRAE (Table 3). Overall, 48 (29%) patients in the IM cohorts and 29 (48%) in the IV cohort experienced a serious TRAE. In the IM cohorts, the most commonly reported (≥10% of patients) nonhematologic TRAEs were nausea (24%); vomiting (23%); decreased appetite, fatigue, and increased ALT (14% each); allergic reactions and increased AST (11% each); and febrile neutropenia and abdominal pain (10% each). In the IV cohort, vomiting (59%), nausea (44%), allergic reactions (26%), increased ALT (18%), fatigue (13%), hyperglycemia (11%), and increased AST (10%) were the most commonly reported nonhematologic TRAEs (Table 4). In the IM cohorts, the most common (≥5% of patients) nonhematologic grade 3/4 TRAEs included febrile neutropenia (10%), increased ALT (8%), pancreatitis (6%), allergic reactions (6%), and nausea (5%). In the IV cohort, the most common grade 3/4 TRAEs included allergic reactions (18%), vomiting (13%), nausea and increased ALT (11% each), hyperglycemia (10%), and febrile neutropenia and increased AST (5% each).
Summary of TRAEs by dosing cohort across all courses (safety analysis population)
| Patients, n (%) . | IM 25 mg/m2 MWF (n = 33) . | IM 37.5 mg/m2 MWF (n = 83) . | IM 25/25/50 mg/m2 MWF (n = 51) . | IM total (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . |
|---|---|---|---|---|---|
| Any TRAE | 21 (64) | 66 (80) | 39 (76) | 126 (75) | 55 (90) |
| Any grade 3/4 TRAE | 14 (42) | 46 (55) | 28 (55) | 88 (53) | 38 (62) |
| Any serious TRAE∗ | 5 (15) | 28 (34) | 15 (29) | 48 (29) | 29 (48) |
| Any TRAE leading to study drug discontinuation | 2 (6) | 14 (17) | 6 (12) | 22 (13) | 20 (33) |
| Pancreatitis† | 0 | 6 (7) | 4 (8) | 10 (6) | 2 (3) |
| Allergic reactions‡ | 2 (6) | 6 (7) | 2 (4) | 10 (6) | 13 (21) |
| Increased ALT | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Hyperammonemia | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Nausea | 0 | 0 | 0 | 0 | 1 (2) |
| Vomiting | 0 | 0 | 0 | 0 | 2 (3) |
| Hyperammonemic encephalopathy | 0 | 0 | 0 | 0 | 1 (2) |
| Deep vein thrombosis | 0 | 0 | 0 | 0 | 1 (2) |
| Any TRAE leading to death | 0 | 0 | 0 | 0 | 0 |
| Patients, n (%) . | IM 25 mg/m2 MWF (n = 33) . | IM 37.5 mg/m2 MWF (n = 83) . | IM 25/25/50 mg/m2 MWF (n = 51) . | IM total (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . |
|---|---|---|---|---|---|
| Any TRAE | 21 (64) | 66 (80) | 39 (76) | 126 (75) | 55 (90) |
| Any grade 3/4 TRAE | 14 (42) | 46 (55) | 28 (55) | 88 (53) | 38 (62) |
| Any serious TRAE∗ | 5 (15) | 28 (34) | 15 (29) | 48 (29) | 29 (48) |
| Any TRAE leading to study drug discontinuation | 2 (6) | 14 (17) | 6 (12) | 22 (13) | 20 (33) |
| Pancreatitis† | 0 | 6 (7) | 4 (8) | 10 (6) | 2 (3) |
| Allergic reactions‡ | 2 (6) | 6 (7) | 2 (4) | 10 (6) | 13 (21) |
| Increased ALT | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Hyperammonemia | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Nausea | 0 | 0 | 0 | 0 | 1 (2) |
| Vomiting | 0 | 0 | 0 | 0 | 2 (3) |
| Hyperammonemic encephalopathy | 0 | 0 | 0 | 0 | 1 (2) |
| Deep vein thrombosis | 0 | 0 | 0 | 0 | 1 (2) |
| Any TRAE leading to death | 0 | 0 | 0 | 0 | 0 |
TRAEs were reported as MedDRA (version 22.1) preferred terms. The severity of TRAEs was recorded using CTCAE 5.0. AEs were considered related to JZP458 if the event followed a reasonable temporal sequence from administration and fulfilled ≥1 instance of clinical evidence: the event followed a known or suspected response pattern to the study drug; the event disappeared after stopping the study drug; or the event reappeared after the study drug was restarted.
CTCAE, common terminology criteria for adverse events; MedDRA, Medical Dictionary for Regulatory Activities.
Serious TRAEs were events that led to death or were life threatening, or caused hospitalization, disability, permanent damage, or congenital anomalies/birth effects or required intervention to prevent permanent impairment, or other important medical events in which the event may jeopardize the patient and may require intervention to prevent 1 of the other outcomes.
Includes the preferred terms of pancreatitis and acute pancreatitis.
Includes the preferred terms of hypersensitivity, drug hypersensitivity, anaphylactic reactions, and infusion-related reactions.
TRAEs of interest and other commonly reported nonhematologic TRAEs
| Patients∗, n (%) . | IM 25 mg/m2 MWF (n = 33) . | IM 37.5 mg/m2 MWF (n = 83) . | IM 25/25/50 mg/m2 MWF (n = 51) . | IM total (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| TRAEs of interest | ||||||||||
| Allergic reactions | 2 (6) | 2 (6) | 11 (13) | 6 (7) | 5 (10) | 2 (4) | 18 (11) | 10 (6) | 16 (26) | 11 (18) |
| Hypersensitivity† | 2 (6) | 2 (6) | 6 (7) | 3 (4) | 2 (4) | 2 (4) | 10 (6) | 7 (4) | 11 (18) | 7 (11) |
| Anaphylactic reaction | 0 | 0 | 3 (4) | 3 (4) | 0 | 0 | 3 (2) | 3 (2) | 1 (2) | 1 (2) |
| Rash‡ | 0 | 0 | 3 (4) | 0 | 3 (6) | 0 | 6 (4) | 0 | 0 | 0 |
| Urticaria | 0 | 0 | 0 | 0 | 1 (2) | 0 | 1 (1) | 0 | 1 (2) | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (7) | 2 (3) |
| Pancreatitis§ | 0 | 0 | 6 (7) | 6 (7) | 6 (12) | 4 (8) | 12 (7) | 10 (6) | 3 (5) | 2 (3) |
| Thrombosis | 0 | 0 | 2 (2) | 2 (2) | 0 | 0 | 2 (1) | 2 (1) | 1 (2) | 0 |
| SSS thrombosis | 0 | 0 | 1 (1) | 1 (1) | 0 | 0 | 1 (1) | 1 (1) | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 2 (2) | 2 (2) | 0 | 0 | 2 (1) | 2 (1) | 0 | 0 |
| Jugular vein thrombosis | 0 | 0 | 1 (1) | 0 | 0 | 0 | 1 (1) | 0 | 0 | 0 |
| Deep vein thrombosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 |
| Increased ALT | 2 (6) | 2 (6) | 14 (17) | 8 (10) | 8 (16) | 3 (6) | 24 (14) | 13 (8) | 11 (18) | 7 (11) |
| Increased AST | 2 (6) | 0 | 12 (14) | 4 (5) | 4 (8) | 0 | 18 (11) | 4 (2) | 6 (10) | 3 (5) |
| Increased blood bilirubin‖ | 0 | 0 | 8 (10) | 1 (1) | 3 (6) | 2 (4) | 11 (7) | 3 (2) | 3 (5) | 1 (2) |
| Hyperammonemia | 0 | 0 | 3 (4) | 2 (2) | 0 | 0 | 3 (2) | 2 (1) | 2 (3) | 1 (2) |
| Other commonly reported nonhematologic TRAEs | ||||||||||
| Nausea | 7 (21) | 1 (3) | 20 (24) | 6 (7) | 13 (25) | 2 (4) | 40 (24) | 9 (5) | 27 (44) | 7 (11) |
| Vomiting | 7 (21) | 0 | 22 (27) | 3 (4) | 10 (20) | 1 (2) | 39 (23) | 4 (2) | 36 (59) | 8 (13) |
| Decreased appetite | 3 (9) | 1 (3) | 12 (14) | 3 (4) | 9 (18) | 1 (2) | 24 (14) | 5 (3) | 5 (8) | 0 |
| Fatigue | 3 (9) | 1 (3) | 17 (20) | 0 | 3 (6) | 0 | 23 (14) | 1 (1) | 8 (13) | 0 |
| Febrile neutropenia | 2 (6) | 2 (6) | 11 (13) | 11 (13) | 4 (8) | 4 (8) | 17 (10) | 17 (10) | 3 (5) | 3 (5) |
| Abdominal pain | 2 (6) | 0 | 9 (11) | 2 (2) | 6 (12) | 0 | 17 (10) | 2 (1) | 3 (5) | 1 (2) |
| Hyperglycemia | 3 (9) | 1 (3) | 5 (6) | 2 (2) | 7 (14) | 3 (6) | 15 (9) | 6 (4) | 7 (11) | 6 (10) |
| Patients∗, n (%) . | IM 25 mg/m2 MWF (n = 33) . | IM 37.5 mg/m2 MWF (n = 83) . | IM 25/25/50 mg/m2 MWF (n = 51) . | IM total (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| TRAEs of interest | ||||||||||
| Allergic reactions | 2 (6) | 2 (6) | 11 (13) | 6 (7) | 5 (10) | 2 (4) | 18 (11) | 10 (6) | 16 (26) | 11 (18) |
| Hypersensitivity† | 2 (6) | 2 (6) | 6 (7) | 3 (4) | 2 (4) | 2 (4) | 10 (6) | 7 (4) | 11 (18) | 7 (11) |
| Anaphylactic reaction | 0 | 0 | 3 (4) | 3 (4) | 0 | 0 | 3 (2) | 3 (2) | 1 (2) | 1 (2) |
| Rash‡ | 0 | 0 | 3 (4) | 0 | 3 (6) | 0 | 6 (4) | 0 | 0 | 0 |
| Urticaria | 0 | 0 | 0 | 0 | 1 (2) | 0 | 1 (1) | 0 | 1 (2) | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (7) | 2 (3) |
| Pancreatitis§ | 0 | 0 | 6 (7) | 6 (7) | 6 (12) | 4 (8) | 12 (7) | 10 (6) | 3 (5) | 2 (3) |
| Thrombosis | 0 | 0 | 2 (2) | 2 (2) | 0 | 0 | 2 (1) | 2 (1) | 1 (2) | 0 |
| SSS thrombosis | 0 | 0 | 1 (1) | 1 (1) | 0 | 0 | 1 (1) | 1 (1) | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 2 (2) | 2 (2) | 0 | 0 | 2 (1) | 2 (1) | 0 | 0 |
| Jugular vein thrombosis | 0 | 0 | 1 (1) | 0 | 0 | 0 | 1 (1) | 0 | 0 | 0 |
| Deep vein thrombosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 |
| Increased ALT | 2 (6) | 2 (6) | 14 (17) | 8 (10) | 8 (16) | 3 (6) | 24 (14) | 13 (8) | 11 (18) | 7 (11) |
| Increased AST | 2 (6) | 0 | 12 (14) | 4 (5) | 4 (8) | 0 | 18 (11) | 4 (2) | 6 (10) | 3 (5) |
| Increased blood bilirubin‖ | 0 | 0 | 8 (10) | 1 (1) | 3 (6) | 2 (4) | 11 (7) | 3 (2) | 3 (5) | 1 (2) |
| Hyperammonemia | 0 | 0 | 3 (4) | 2 (2) | 0 | 0 | 3 (2) | 2 (1) | 2 (3) | 1 (2) |
| Other commonly reported nonhematologic TRAEs | ||||||||||
| Nausea | 7 (21) | 1 (3) | 20 (24) | 6 (7) | 13 (25) | 2 (4) | 40 (24) | 9 (5) | 27 (44) | 7 (11) |
| Vomiting | 7 (21) | 0 | 22 (27) | 3 (4) | 10 (20) | 1 (2) | 39 (23) | 4 (2) | 36 (59) | 8 (13) |
| Decreased appetite | 3 (9) | 1 (3) | 12 (14) | 3 (4) | 9 (18) | 1 (2) | 24 (14) | 5 (3) | 5 (8) | 0 |
| Fatigue | 3 (9) | 1 (3) | 17 (20) | 0 | 3 (6) | 0 | 23 (14) | 1 (1) | 8 (13) | 0 |
| Febrile neutropenia | 2 (6) | 2 (6) | 11 (13) | 11 (13) | 4 (8) | 4 (8) | 17 (10) | 17 (10) | 3 (5) | 3 (5) |
| Abdominal pain | 2 (6) | 0 | 9 (11) | 2 (2) | 6 (12) | 0 | 17 (10) | 2 (1) | 3 (5) | 1 (2) |
| Hyperglycemia | 3 (9) | 1 (3) | 5 (6) | 2 (2) | 7 (14) | 3 (6) | 15 (9) | 6 (4) | 7 (11) | 6 (10) |
TRAEs were reported as MedDRA (version 22.1) preferred terms. The severity of TRAEs was recorded using CTCAE 5.0. AEs were considered related to JZP458 if the event followed a reasonable temporal sequence from administration and fulfilled ≥1 instance of clinical evidence: the event followed a known or suspected response pattern to the study drug; the event disappeared after stopping the study drug; or the event reappeared after the study drug was restarted. Patients could have experienced multiple events listed as individual preferred terms in the table. TRAEs of interest and other commonly reported (≥10% in IM total or IV cohort) nonhematologic TRAEs (safety analysis population).
CTCAE, Common Terminology Criteria for Adverse Events; MedDRA, Medical Dictionary for Regulatory Activities; SSS, superior sagittal sinus.
Some patients had ≥1 TRAE.
Includes the preferred terms hypersensitivity and drug hypersensitivity.
Includes the preferred terms of rash maculo-papular, rash, and rash erythematous.
Includes the preferred terms of pancreatitis and acute pancreatitis.
Includes the preferred terms blood bilirubin increased and conjugated bilirubin increased.
In total, 22 (13%) patients in the IM cohorts and 20 (33%) in the IV cohort experienced TRAEs leading to drug discontinuation (mainly allergic reactions and pancreatitis; Table 3). An additional patient in cohort 1a discontinued because of an AE unrelated to JZP458 (necrotizing fasciitis), which was attributed by the treating physician to the IM injection procedure. In 1 patient experiencing deep vein thrombosis, grade 2 subclavian vein thrombosis developed in course 4 of IV JZP458, and the event resolved with enoxaparin after drug discontinuation. Three (2%) patients in the IM cohorts had fatal events: sepsis (cohort 1a, n = 1), aspiration pneumonia (cohort 1b, n = 1), and multiorgan failure (cohort 1b, n = 1). None of these events were deemed related to JZP458 by the treating physicians.
Results of a post hoc analysis reporting frequency of allergic reactions and grade ≥2 nausea/vomiting by timing/dosage along with TRAEs of interest by number of doses on/before event onset are presented in supplemental Table 1. In patients who received JZP458 IM or IV 25/25/50 mg/m2 MWF, a broadly similar number of allergic reactions or grade ≥2 nausea/vomiting occurred after 25 mg and 50 mg doses. Among patients who developed treatment-related allergic reactions, the events occurred after a median of 13 and 6 doses in the total IM and IV cohort, respectively.
Immunogenicity
In the IM cohorts, ADA data were available from a median (range) of 5 (1-14) courses from 33 patients in cohort 1a, 4 (1-15) courses from 83 patients in cohort 1b, and 4 (1-11) courses from 51 patients in cohort 1c; in the IV cohort, ADA data were available from a median (range) of 3 (1-15) courses from 61 patients.
In the IM cohorts, 82 of 167 (49%) patients had confirmed ADAs toward JZP458 (Table 5); 75 patients developed ADAs toward JZP458 after treatment, 55 (73%) of whom subsequently became ADA− at least once during the study. The proportion of patients with persistent ADAs was 35%. Overall, 18 of 167 (11%) patients in the IM cohort developed treatment-related HSRs. Among patients with ADA+ status, 11 of 82 (13%) experienced a treatment-related HSR, and 4 (5%) had NAbs. One patient with NAbs discontinued because of HSRs. Notably, 2 patients with NAbs did not experience HSRs and their NSAA levels were maintained at ≥0.1 IU/mL throughout treatment. Another patient tested positive for NAbs in course 2 and experienced a grade 2 HSR; nevertheless, this patient received additional courses of JZP458 and maintained therapeutic SAA levels in subsequent courses. Of 85 (51%) patients in the IM cohort who were ADA− toward JZP458, 7 (8%) experienced a treatment-related HSR during the study.
Summary of immunogenicity results (safety analysis population)
| Patients . | IM total∗ (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . |
|---|---|---|
| Patients with available ADA samples, n | 167 | 61 |
| Confirmed ADA+† | 82 (49) | 34 (56) |
| NAb+ | 4 (2) | 2 (3) |
| HSR‡ | 11 (7) | 12 (20) |
| ADA−§ | 85 (51) | 27 (44) |
| HSR‡ | 7 (4) | 4 (7) |
| Patients . | IM total∗ (n = 167) . | IV 25/25/50 mg/m2 MWF (n = 61) . |
|---|---|---|
| Patients with available ADA samples, n | 167 | 61 |
| Confirmed ADA+† | 82 (49) | 34 (56) |
| NAb+ | 4 (2) | 2 (3) |
| HSR‡ | 11 (7) | 12 (20) |
| ADA−§ | 85 (51) | 27 (44) |
| HSR‡ | 7 (4) | 4 (7) |
Data are n (%) unless otherwise specified. ADAs refer to anti-JZP458 antibodies.
The IM cohorts consisted of cohort 1a (25 mg/m2 MWF), cohort 1b (37.5 mg/m2 MWF), and cohort 1c (25/25/50 mg/m2 MWF).
Defined as patients with a positive result on the first test and a positive result on the confirmatory test.
HSR was defined as at least 1 TRAE of special interest for allergic reactions (including hypersensitivity and anaphylaxis).
Defined as patients with a negative result on the first test.
Analysis of the IV cohort showed that 34 of 61 (56%) patients had confirmed ADAs toward JZP458. A total of 33 patients (54%) developed ADAs toward JZP458 after treatment, 18 (55%) of whom subsequently became ADA− at least once during the study. The proportion of patients with persistent ADAs was 46%. Overall, 16 of 61 (26%) patients in the IV cohort developed treatment-related HSRs. Among patients with ADA+ status, 12 of 34 (35%) experienced treatment-related HSRs and 2 (6%) had NAbs. One patient developed HSRs and NAbs in course 1 and discontinued JZP458. The other patient developed grade 2 HSRs and NAbs in course 1 and subsequently discontinued JZP458 in course 2 because of grade 3 HSRs; this patient had inadequate NSAA levels in course 1. Of 27 patients who were ADA−, 4 (15%) experienced treatment-related HSRs.
Overall, there were 2 patients who did not experience treatment-related HSRs but had a 48-hour postdose SAA below the lower limit of quantification on ≥2 measurements, raising concerns for potential silent inactivation. However, in both patients, undetectable SAA was followed by therapeutic SAA levels in subsequent courses.
Overall, baseline demographics and characteristics were generally similar between patients with ADA+ and ADA− status (supplemental Table 2). Mean SAA levels were similar between patients with ADA+ and ADA− status (data not shown). ADA status was evaluated as a potential covariate for the PKs of IM or IV administration of JZP458 in the PopPK analysis, and it was determined not to be a significant covariate.
Discussion
To address global Erwinaze shortages, recombinant Erwinia asparaginase JZP458 was developed in collaboration with the US FDA and the Children’s Oncology Group to provide a high-quality therapeutic option with reliable supply.23 In this pivotal phase 2/3 study, observed data and model-based simulations demonstrate that JZP458 achieves therapeutic NSAA levels via multiple dosing regimens, providing flexibility to patients and physicians. The IM dose of 25/25/50 mg/m2 MWF (cohort 1c), which used a higher Friday dose to improve the 72-hour SAA coverage, is efficacious and tolerable based on both observed and modeled data.4 Asparaginase PKs differ greatly between routes of administration and according to formulation.24 Because of the shorter half-life of IV JZP458 compared with IM, 40% of patients receiving IV JZP458 25/25/50 mg/m2 MWF maintained NSAA levels of ≥0.1 IU/mL at the last 72-hour assessment during course 1. In comparison, studies of native Erwinia asparaginase (administered at 25 000 IU/m2 MWF) showed that 43% of patients receiving the IV formulation maintained NSAA levels of ≥0.1 IU/ml at 72 hours after dose 6, in contrast to 88% of patients receiving the IM formulation.25,26 However, modeling data show that the vast majority of patients would maintain therapeutic SAA levels with JZP458 IV 25 mg/m2 every 48 hours or IV/IV/IM 25/25/50 mg/m2 MWF. Administering IV JZP458 25/25/50 mg/m2 MWF is feasible but should include therapeutic drug monitoring to enable assessment of adequate asparaginase activity.19 It is worth noting that the recommended dosing regimens differ in the United States vs the European Union: the European Commission/European Medicines Agency approved IM and IV administration of JZP458 at 25 mg/m2 every 48 hours, IM and IV 25/25/50 mg/m2 MWF, or a mixed regimen of IV/IV/IM 25/25/50 mg/m2 MWF, whereas the US FDA approved JZP458 administered IM 25 mg/m2 every 48 hours or 25/25/50 mg/m2 MWF.17,27 Because of the differences in labels, we recommend the reader check the respective package insert for the number of doses to replace a long-acting asparaginase dose.
Mean NSAA levels were maintained after repeated JZP458 administration, indicating a sustained therapeutic benefit beyond course 1. Plasma L-asparagine levels were also evaluated as a direct measure of asparaginase activity, with the caveat that accurate measurement of asparagine levels is technically challenging and not practical in the clinical setting.4 We observed rapid depletion of plasma L-asparagine after 1 dose of IM or IV administration of JZP458 and asparagine levels remained low in course 1 for all IM and IV cohorts.
The combined PopPKs modeling/simulation and observed data supported the regulatory approval of JZP458 including dosing schedules not directly tested in the study, that is, 25 mg/m2 IM or IV every 48 hours or 25/25/50 mg/m2 IV/IV/IM MWF.21 Advantages of PopPKs modeling include use of pooled data from all study participants, all dose levels, all time points, and across all courses, resulting in a more accurate estimation of NSAA levels.4 The use of simulations in a large virtual population can predict efficacy in a broader patient population compared with that of study AALL1931.4
The safety profile of JZP458 was consistent with other asparaginases, with no new adverse safety signals identified.3,11,28-30 The study was not designed to draw comparison across cohorts but it was observed that IM cohorts had lower rates of TRAEs than the IV cohort, particularly TRAEs previously associated with IV asparaginases such as infusion-related reactions, nausea, and vomiting.1,25,30 Similarly, treatment-related discontinuation rates with IV JZP458 were higher than with IM JZP458 (primarily because of increased incidence of HSRs) but overall consistent with previous reports.14,25,26,31-33 Notably, allergic reactions/HSRs were the most common reason for discontinuation in this study because the study drug was discontinued, per protocol, in patients with grade ≥3 allergic reactions (including anyone requiring IV intervention). However, the study did not assess asparaginase inactivation after discontinuations because of allergic reactions, thus allergic-like reactions could not be excluded. Nonetheless, the median (range) courses of JZP458 completed (5 [0-14], 4 [0-15], 5 [1-10], and 3 [0-15] for IM cohorts 1a, 1b, 1c, and the IV cohort, respectively) were similar to the planned courses of 5 (1-14), 5 (1-15), 5 (1-15), and 5 (1-15). Physician decision accounted for 9 (5%) and 7 (11%) discontinuations in the IM cohorts and IV cohort, respectively. The main reasons based on available data were availability of commercial JZP458, change of planned therapy (which did not include asparaginase), or physician assessment of what was in the patients’ best interest.
Nausea and vomiting were common TRAEs in this study, but they infrequently led to JZP458 discontinuation (IM, 0%; IV, 2% and 3%, respectively). Additionally, a post hoc analysis determined that a broadly similar number of allergic reactions or grade ≥2 nausea/vomiting occurred after 25 mg/m2 and 50 mg/m2 doses in patients treated with IM or IV 25/25/50 mg/m2 MWF.34 Rates of treatment-related pancreatitis, thrombosis, and hepatotoxicity were similar for the IM and IV cohorts and aligned with, or were lower than, reported rates for other asparaginases.25,35-39 Although monitoring of serum ammonia levels was not mandated by protocol, 2 patients discontinued treatment because of elevation of ammonia.
Although 49% and 56% of patients receiving IM or IV JZP458, respectively, in this study tested ADA+ after JZP458 administration, most tested ADA+ transiently and became ADA− by the end of study (data not shown). Most of the patients with confirmed ADAs against JZP458 did not experience treatment-related HSRs. The overall rate of treatment-related HSRs associated with JZP458 was similar to those reported for asparaginases.11,25,26,29,30,33,40-45 In the IM cohorts, HSR rates were similar between patients with ADA+ and ADA− status (13% vs 8%). In the IV cohort, the HSR rate was numerically higher in patients with ADA+ vs ADA− status (35% vs 15%). However, because of the small sample size, we are unable to determine whether a statistically significant difference exists. This study was not designed to evaluate the association between ADA status and HSRs, and although some previous research may support this, results across studies are inconsistent.13,46 In addition, ADA status was not identified as a significant covariate for the PKs of JZP458. Thus, ADA status did not reliably predict HSR nor impact SAA levels in this study. The number of patients who developed NAbs was low overall (IM, 2%; IV, 3%), so no definitive conclusions regarding their potential impact on SAA or HSRs can be made.
As in this and other studies, immunogenicity assays may have generated inconclusive results. SAA levels are the most reliable predictors of NAbs.13,46 Expert-consensus guidelines suggest that a 48-hour NSAA level below the lower limit of quantification after a dose of Erwinia asparaginase should raise concerns for silent inactivation.5 Interestingly, 2 patients in our study met these criteria at 2 separate 48-hour time points, although all subsequent SAA levels were therapeutic. This highlights the challenge of defining silent inactivation in patients receiving a short-acting asparaginase preparation.
Limitations of this study include the single-arm design and the small number of adolescent and young adult patients. Nonetheless, there were more patients aged ≥18 years in this study (IM cohorts, 13%; IV cohort, 16%) than previous studies of native Erwinia asparaginase and pegaspargase.14,25 The use of PopPK modeling and simulations in a large virtual population provided us with additional information in this patient population. Nonetheless, given literature reports of different toxicity profiles of asparaginases in adults vs children, ongoing evaluations of toxicities among adolescents and young adults are warranted.47 This study was also limited by the evaluation of efficacy outcomes for cohorts 25 mg/m2 IV every 48 hours and 25/25/50 mg/m2 IV/IV/IM through the PopPK model, future real-world evidence data may further support efficacy and safety of these dosing regimens in clinical practice.
Results at AALL1931 completion in patients with ALL/LBL who developed allergic reactions or silent inactivation to E coli–derived asparaginases show a JZP458 safety profile that is consistent with other asparaginases.3,24 Both observed and modeled data demonstrate that JZP458 achieves therapeutic NSAA levels via multiple IM and IV dosing schedules, therefore providing flexibility to patients and physicians. Although the proposed dosing schedule for 25 mg/m2 IV every 48 hours and 25/25/50 mg/m2 IV/IV/IM were not directly studied in the trial, the individual doses (25 mg/m2 IV at 48-hour intervals and 50 mg/m2 IM at 72-hour intervals) were tested in the trial and were recommended based on interpolation from PKs and response rates from the tested regimens. No specific immunological concerns were identified with JZP458 beyond the known immunogenicity risks associated with other asparaginases and no specific clinical decisions are recommended based on immunogenicity testing. These data demonstrate that JZP458 is a reliable option for patients with ALL/LBL who develop HSRs to, or silent inactivation of, E coli–derived asparaginases.
Acknowledgments
The authors thank the study patients and their families, the investigators, the study data review committee, and the study staff from all the participating Children’s Oncology Group institutions, and those involved in data collection and analysis. Medical writing support, under the direction of the authors, was provided by Katie Groschwitz and Alice Christakou of CMC Affinity, a division of IPG Health Medical Communications, funded by Jazz Pharmaceuticals, in accordance with good publication practice (2022) guidelines.
This study was supported by Jazz Pharmaceuticals.
The study was designed by the sponsor and the study investigators. Study data were collected by the investigators and analyzed by the sponsor. Reporting of the study, including the interpretation of results, was the responsibility of the sponsor.
Authorship
Contribution: L.M., M.L.L., M.R.C., R.I., T.L., E.A.R., and R.E.R. conceived and designed the study; L.M., M.L.L., M.R.C., T.L., E.A., S.A., L.B.S., E.A.R., and R.E.R. collected and assembled data; and all authors performed data analysis and interpretation, wrote the manuscript, and provided final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: L.M. served on an advisory board for Servier Pharmaceuticals, and serves as a consultant and on an advisory board and speaker's bureau for Jazz Pharmaceuticals. M.R.C., S.A., E.A., Y.L., T.L., S.G., C.C., S.R., V.C., and R.I. are employees of and hold stock ownership and/or stock options in Jazz Pharmaceuticals. L.B.S. served on scientific advisory boards for Jazz Pharmaceuticals and Servier Pharmaceuticals. E.A.R. received institutional research funding from Pfizer and serves on a data and safety monitoring board for Bristol Myers Squibb. R.E.R. served on advisory boards for Jazz Pharmaceuticals and Servier Pharmaceuticals. M.L.L. declares no competing financial interests.
Correspondence: Luke Maese, Department of Pediatrics Division of Hematology-Oncology University of Utah, Huntsman Cancer Institute, Primary Children’s Hospital, 100 N Mario Capecchi Dr, Salt Lake City, UT 84113; email: luke.maese@hsc.utah.edu.
References
Author notes
Presented in abstract and poster form at the 65th American Society of Hematology annual meeting and exposition, San Diego, CA, 9 to 12 December 2023.
All relevant data are provided with the manuscript and supporting files. Jazz Pharmaceuticals has established a process to review requests from qualified external researchers for data from Jazz-sponsored clinical trials in a responsible manner that includes protecting patient privacy, assurance of data security and integrity, and furthering scientific and medical innovation. Additional details on Jazz Pharmaceuticals data sharing criteria and process for requesting access can be found at: https://www.jazzpharma.com/science/clinical-trial-data-sharing/.
The full-text version of this article contains a data supplement.