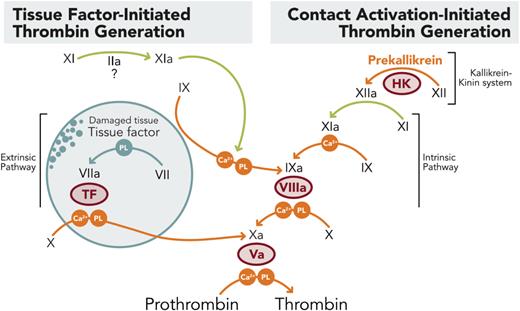
FXI/FXIa inhibition may allow for inhibition of contact pathway activation of thrombin generation depicted on the right side of the coagulation cascade, while allowing for tissue factor mediated activation of thrombin as seen on the left side, thus preventing thrombosis mediated by pathologic activation of thrombin by devices and inflammatory activators of coagulation while preserving hemostasis in the setting of trauma.
FXI/FXIa inhibition may allow for inhibition of contact pathway activation of thrombin generation depicted on the right side of the coagulation cascade, while allowing for tissue factor mediated activation of thrombin as seen on the left side, thus preventing thrombosis mediated by pathologic activation of thrombin by devices and inflammatory activators of coagulation while preserving hemostasis in the setting of trauma.
Concept
Inhibiting the activation of factor XI (FXI) or its active form (FXIa) may offer similar protection as current anticoagulant therapies but with less bleeding. Blocking the contact pathway of thrombin generation by inhibiting FXI/FXIa, typically activated by blood contact with artificial surfaces or inflammatory mediators (contact activation initiated), may allow for sufficient generation of thrombin by the tissue factor/FVII pathway, usually activated by trauma, to prevent bleeding (tissue factor initiated; figure).
Evolution
Patients with severe congenital FXI deficiency rarely have spontaneous bleeding, often coming to attention only after bleeding provoked by surgery. This has led to the concept of targeting FXI/FXIa as an anticoagulant strategy. Proof of concept is supported by 20 years of animal model data. Strategies include parenteral monoclonal antibodies to decrease FXI and FXIa levels, antisense oligonucleotides, as well as oral small molecule inhibitors of FXIa.1
Application
Inhibitors of FXI, FXIa, or both have been studied in phase 2 trials for thrombosis prophylaxis in patients undergoing knee replacement with similar or better efficacy than enoxaparin; and in phase 2 trials for patients on hemodialysis with similar incidence of bleeding as placebo but with lower numbers of circuit thromboses and fewer major thrombotic events.
Future steps
Phase 3 randomized controlled trials of FXI/FXIa inhibitors are underway in patients with a variety of prothrombotic conditions to determine whether inhibition of pathologic thrombosis while allowing for necessary hemostasis can be achieved in clinical practice.
Conflict-of-interest disclosure: J.M.C. receives honoraria for being a member of scientific advisory boards for Abbott, Anthos, Bristol Myers Squibb, Janssen, Perosphere Technologies, Pfizer, and Werfen.
Reference
Author notes
Authorship contribution: J.M.C. wrote the manuscript and worked with the Blood Advances graphics staff to design the figure.