Addition of emapalumab improves laboratory parameters and overall survival in patients with pHLH in real-world clinical practice.
Addition of emapalumab provides an effective bridge to HSCT in evaluable patients with pHLH in real-world clinical practice.
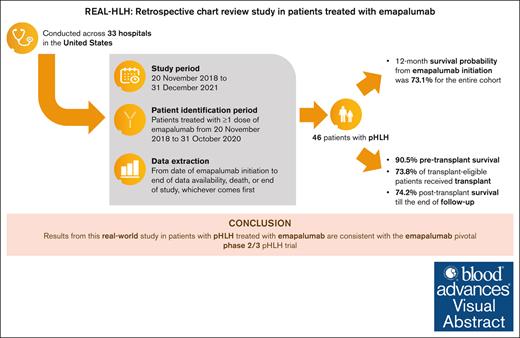
Visual Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening, hyperinflammatory syndrome. Emapalumab, a fully human monoclonal antibody that neutralizes the proinflammatory cytokine interferon gamma, is approved in the United States to treat primary HLH (pHLH) in patients with refractory, recurrent, or progressive disease, or intolerance with conventional HLH treatments. REAL-HLH, a retrospective study, conducted across 33 US hospitals, evaluated real-world treatment patterns and outcomes in patients treated with ≥1 dose of emapalumab between 20 November 2018 and 31 October 2021. In total, 46 patients met the pHLH classification criteria. Median age at diagnosis was 1.0 year (range, 0.3-21.0). Emapalumab was initiated for treating refractory (19/46), recurrent (14/46), or progressive (7/46) pHLH. At initiation, 15 of 46 patients were in the intensive care unit, and 35 of 46 had received prior HLH-related therapies. Emapalumab treatment resulted in normalization of key laboratory parameters, including chemokine ligand 9 (24/33, 72.7%), ferritin (20/45, 44.4%), fibrinogen (37/38, 97.4%), platelets (39/46, 84.8%), and absolute neutrophil count (40/45, 88.9%). Forty-two (91.3%) patients were considered eligible for transplant. Pretransplant survival was 38 of 42 (90.5%). Thirty-one (73.8%) transplant-eligible patients proceeded to transplant, and 23 of 31 (74.2%) of those who received transplant were alive at the end of the follow-up period. Twelve-month survival probability from emapalumab initiation for the entire cohort (N = 46) was 73.1%. There were no discontinuations because of adverse events. In conclusion, results from the REAL-HLH study, which describes treatment patterns, effectiveness, and outcomes in patients with pHLH treated with emapalumab in real-world settings, are consistent with the emapalumab pivotal phase 2/3 pHLH trial.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare, potentially life-threatening, hyperinflammatory syndrome of which primary features are pathologic immune activation and hyperinflammation accompanied by the release of proinflammatory cytokines, such as interferon gamma (IFN-γ).1-5 Common symptoms include high fever, hepatosplenomegaly, cytopenia, coagulopathy, seizures, and multiorgan failure.1,2,6-8 The hyperinflammatory response in HLH generally manifests in response to an immunogenic trigger, such as an infection.1,4 Primary HLH (pHLH), which tends to present during infancy, is a heterogenous disorder commonly associated with inherited mutations, predominantly in genes responsible for lymphocyte-mediated cytotoxicity.1,7,9,10 Diagnosis of HLH is commonly based on the Histiocyte Society HLH 2004 criteria, which comprise a combination of symptoms and laboratory findings that reflect intrinsic immune dysregulation and resulting pathologic inflammation.6,10
In mouse models of HLH, neutralization of tissue IFN-γ, but not of other cytokines, reduced HLH-like symptoms and prolonged survival.11,12 These findings suggest that IFN-γ plays a key role in HLH pathogenesis.11-15 Moreover, serum IFN-γ levels are substantially elevated in hyperinflammatory diseases, including HLH, and correlate with disease severity.16-21 Targeting IFN-γ activity may therefore help control immune activation and hyperinflammation in HLH.
Currently, the only potentially curative treatment for pHLH is allogeneic hematopoietic stem cell transplantation (HSCT).6,7,22,23 Without HSCT, patients remain at risk of relapse or refractory disease, multiorgan failure, and death.6,24 Conventional treatments generally include chemotherapy and immunosuppressive agents, such as etoposide and high-dose dexamethasone, to suppress hyperinflammation until HSCT can be performed.6,24 However, prolonged therapy with these agents is associated with drug toxicities, severe myelotoxicity, opportunistic infections, and high risk of morbidity.25 Moreover, in 20% to 30% of patients, HLH may be refractory to conventional immunochemotherapeutic agents.26 The 5-year probability of survival in children treated with immunochemotherapy remains at ∼60%, with ∼20% mortality before proceeding to HSCT.25 Thus, there is an urgent need for more effective therapies with lower toxicities.25
Emapalumab (Gamifant; Sobi Inc, Waltham, MA) is a fully humanized immunoglobulin G1 monoclonal antibody that neutralizes IFN-γ activity in vitro.27 In the pivotal phase 2/3 study, >60% of children 18 years of age and younger with pHLH refractory to, or intolerant of, standard HLH therapies showed improved clinical and laboratory parameters when treated for up to 8 weeks with emapalumab.28 Of the patients in the study, >65% proceeded to HSCT. The estimated probability of survival at 12 months after transplant was 90.2% (95% confidence interval [CI], 66.2-97.5), and emapalumab was not associated with significant organ toxicity.28 Emapalumab was first approved in the United States on 20 November 2018, for treating adult and pediatric patients with pHLH with refractory (patients who did not respond to treatment), recurrent, or progressive disease (patients who exhibited initial response but the disease progressed, nonetheless), or intolerance with conventional HLH therapy, and remains the only approved biologic treatment for pHLH. However, there is an important knowledge gap in the literature regarding the use of emapalumab in the treatment of patients with pHLH in real-world clinical practice. Being aware of the general use and efficacy of emapalumab is essential to understanding its utility in the clinic. In this study, we report on the results of patients with pHLH from the REAL-HLH study, which investigated real-world treatment patterns and outcomes among patients in the United States with HLH treated with emapalumab in addition to other HLH-related treatments.
Methods
Study design
This was a retrospective, noninterventional medical chart review study conducted across 33 hospitals in the United States. Charts of patients treated with emapalumab in clinical practice between 20 November 2018 and 31 October 2021 (patient identification period) were abstracted. Patient records were evaluated from the time of emapalumab initiation (index date) to either the end of the study (31 December 2021), the end of data availability for a patient, or the death of a patient. The protocol and data collection forms were reviewed by the Western Institutional Review Copernicus Group institutional review board and local institutional review boards, as required. There was no direct patient involvement in the study and anonymized data collection was conducted in compliance with US Health Insurance Portability and Accountability Act (HIPAA) policies.
Patients
At all institutions, patients were identified through a retrospective review of medical records via unbiased data extraction using the keyword “emapalumab.” Patients of any age treated with at least 1 dose of emapalumab in addition to other HLH-related treatments in a routine clinical practice setting were eligible for inclusion. Given the complexity of the disease and risk for misclassification, a consistent rule was applied across all study patients with HLH. Patients were classified as diagnosed with pHLH if they met at least 1 of 3 following criteria without evidence of an underlying malignancy, rheumatologic, or metabolic disease: (1) identification of a known genetic mutation associated with pHLH (biallelic mutation for PRF1, UNC13D, STX11, STXBP2, RAB27A, LYST, or AP3B1, or a monoallelic mutation for SH2D1A, or XIAP); (2) fulfillment of at least 5 of the 8 HLH-2004 criteria; or (3) family history of HLH.6,10 A multispecialty panel of experts adjudicated the classification of patients who were diagnosed with pHLH by their physician but did not meet the classification criteria and those with a physician diagnosis of secondary HLH but who met the pHLH classification criteria. Thus, the cohort of patients classified as diagnosed with pHLH for this study includes those with verified and unverified familial HLH.
Study outcomes and evaluations
The primary study objective was to describe patient demographics, clinical characteristics, and treatment patterns in patients with pHLH treated with emapalumab. In addition, HLH-related therapies given before emapalumab initiation, the reasons for initiating emapalumab, time to initiation of emapalumab from HLH diagnosis, emapalumab dosing patterns, and treatment duration, HLH-related therapies concurrently administered with emapalumab, and corticosteroid use in the first 8 weeks of emapalumab treatment were evaluated and summarized.
Secondary outcomes assessed were (1) proportion of eligible patients who proceeded to HSCT; (2) survival (overall and at 12 months from emapalumab initiation); (3) proportion of patients who achieved normalization of predefined laboratory parameters, as determined by the physician, at any time during treatment; and (4) time to first normalization of laboratory parameters (determined only for parameters for which data from ≥50% of patients were available). Normalization of laboratory parameters was based on the treating physician’s report. Normalization of ferritin was based on the patient achieving a value of <2000 ng/mL or at least an 80% reduction from the index date. In addition, reasons for patients not receiving transplant, survival before and after transplant, and causes of death were evaluated. Exploratory analyses included investigating the relationship between treatment-related parameters and overall survival.
Statistical analysis
Data were analyzed for all patients with pHLH and separately for patients stratified by age into those aged <12 years (children) and those aged at least 12 years (adolescents and adults) at initiation of emapalumab. Continuous variables were described using means, standard deviations, medians, and interquartile ranges. Categorical variables were summarized using counts and percentages. Kaplan-Meier curves were used to evaluate time-to-event analyses, with a 2-sided 95% CI for survival estimates. χ2 goodness of fit test was used to evaluate the statistical significance of the relationship between treatment-related parameters and overall survival (statistical significance at P < .05). Data analyses were conducted using Statistical Analysis Software version 9.4 (SAS Institute Inc, Cary, NC) and R Software version 4.1.1. (R Core Team, Vienna, Austria).
Results
Patient demographics
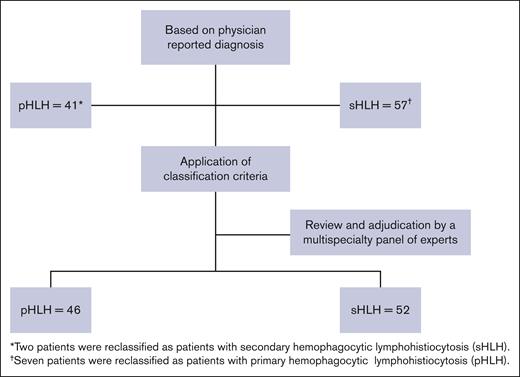
A total of 105 patients across 33 hospitals in the United States were included in this retrospective chart review study. The median number of patients per site was 2 (range, 1-15). Of these, 98 patients were reportedly treated for HLH-related conditions, and 7 were treated for non-HLH-related conditions. After the application of pHLH classification criteria and adjudication by the multispecialty study panel, 46 of 98 (46.9%) patients with HLH were determined to have met the pHLH classification criteria used in this report (Figure 1). The median age at pHLH diagnosis was 1.0 year (range, 0.3-21.0); the majority were children aged <2 years (n = 39) with a median age of 1.0 year (range, 0.3-10.0) at diagnosis, and the remainder (n = 7) were adolescents and adults aged ≥12 years with a median age at diagnosis of 14 years (range, 12.0-21.0; Table 1). In addition, most patients were male (n = 28; 60.9%) and an equal number were non-White (n = 28; 60.9%), similar to data reported in literature.29
Demographic and clinical characteristics of patients with pHLH
| Parameter . | Overall (N = 46) . | Children (aged <12 y) (n = 39)∗ . | Adolescents and adults (aged ≥12 y) (n = 7)∗ . |
|---|---|---|---|
| Age at pHLH diagnosis, median (range), y | 1.0 (0.3-21.0) | 1.0 (0.3-10) | 14.0 (12.0-21.0) |
| Male, n (%) | 28 (60.9) | 23 (59.0) | 5 (71.4) |
| Female, n (%) | 18 (39.1) | 16 (41.0) | 2 (28.6) |
| Race, n (%) | |||
| White | 18 (39.1) | 15 (38.5) | 3 (42.9) |
| Black | 7 (15.2) | 7 (18.0) | 0 |
| Hispanic | 9 (19.6) | 8 (20.5) | 1 (14.3) |
| Asian | 5 (10.9) | 2 (5.1) | 3 (42.9) |
| ≥2 | 2 (4.4) | 2 (5.1) | 0 |
| Other | 2 (4.4) | 2 (5.1) | 0 |
| Unknown | 3 (6.5) | 3 (7.7) | 0 |
| Genetic testing, n (%) | 44/46 (95.7) | 37/39 (94.9) | 7/7 (100.0) |
| PRF1 | 15/40 (37.5)† | 13/33 (39.4) | 2/7 (28.6) |
| UNC13D | 10/40 (25.0)† | 8/33 (24.2) | 2/7 (28.6) |
| STX11 | 2/40 (5.0)† | 1/33 (3.0) | 1/7 (14.3) |
| STXBP2 | 3/40 (7.5)† | 3/33 (9.1) | 0 |
| RAB27A | 4/40 (10.0)† | 3/33 (9.1) | 1/7 (14.3) |
| LYST | 5/40 (12.5)† | 5/33 15.2 | 0 |
| AP3B1 | 3/40 (7.5)† | 2/33 (6.1) | 1/7 (14.3) |
| SH2D1A | 3/40 (7.5)† | 2/33 (6.1) | 1/7 (14.3) |
| XIAP/BIRC4 | 3/40 (7.5)† | 3/33 (9.1) | 0 |
| Patients with ≥5 of 8 HLH-2004 criteria,‡ n (%) | 27/30 (90.0) | 23/25 (92.0) | 4/5 (80.0) |
| Patients with infection at diagnosis,§ n (%) | 25/46 (54.3) | 20/39 (51.3) | 5/7 (71.4) |
| Patients with CNS involvement at diagnosis, n (%) | 10/46 (21.7) | 9/39 (23.1) | 1/7 (14.3) |
| Age, y, at emapalumab initiation, median (range) | 1.0 (0.5-22.0) | 1 (0.5-11) | 15.0 (12.0-22.0) |
| Patients initiated emapalumab in an ICU, n (%) | 15/46 (32.6) | 13/39 (33.3) | 2/7 (28.6) |
| Patients receiving supportive care,||n (%) | 11/46 (23.9) | 9/39 (23.1) | 2/7 (28.6) |
| Ventilator only | 6/11 (54.5) | 5/9 (55.6) | 1/2 (50.0) |
| Dialysis only | 2/11 (18.2) | 1/9 (11.1) | 1/2 (50.0) |
| Multiple (≥1 of the following: mechanical ventilation, dialysis, vasopressors, ECMO, MARS) | 3/11 (27.3) | 3/9 (33.3) | 0 |
| Patients with abnormal laboratory markers or cytokine levels at emapalumab initiation, n (%) | |||
| Platelet count (<100 × 109/L) | 25/46 (54.4) | 22/39 (56.4) | 3/7 (42.9) |
| Absolute neutrophil count (<1.0 × 109/L) | 21/44 (47.7) | 19/37 (51.4) | 2/7 (28.6) |
| Fibrinogen (≤1.5 g/L) | 13/40 (32.5) | 11/34 (32.4) | 2/6 (33.3) |
| Ferritin (>500 μg/L) | 33/41 (80.9) | 27/34 (79.4) | 6/7 (85.7) |
| sCD25 (≥2400 U/mL) | 21/31 (67.7) | 19/26 (73.1) | 2/5 (40.0) |
| Absolute lymphocyte count¶ | 28/42 (66.7) | 23/36 (63.9) | 5/6 (83.3) |
| Alanine transaminase¶ | 35/45 (77.8) | 29/38 (76.3) | 6/7 (85.7) |
| CXCL9¶ | 19/20 (95.0) | 15/16 (93.8) | 4/4 (100.0) |
| Parameter . | Overall (N = 46) . | Children (aged <12 y) (n = 39)∗ . | Adolescents and adults (aged ≥12 y) (n = 7)∗ . |
|---|---|---|---|
| Age at pHLH diagnosis, median (range), y | 1.0 (0.3-21.0) | 1.0 (0.3-10) | 14.0 (12.0-21.0) |
| Male, n (%) | 28 (60.9) | 23 (59.0) | 5 (71.4) |
| Female, n (%) | 18 (39.1) | 16 (41.0) | 2 (28.6) |
| Race, n (%) | |||
| White | 18 (39.1) | 15 (38.5) | 3 (42.9) |
| Black | 7 (15.2) | 7 (18.0) | 0 |
| Hispanic | 9 (19.6) | 8 (20.5) | 1 (14.3) |
| Asian | 5 (10.9) | 2 (5.1) | 3 (42.9) |
| ≥2 | 2 (4.4) | 2 (5.1) | 0 |
| Other | 2 (4.4) | 2 (5.1) | 0 |
| Unknown | 3 (6.5) | 3 (7.7) | 0 |
| Genetic testing, n (%) | 44/46 (95.7) | 37/39 (94.9) | 7/7 (100.0) |
| PRF1 | 15/40 (37.5)† | 13/33 (39.4) | 2/7 (28.6) |
| UNC13D | 10/40 (25.0)† | 8/33 (24.2) | 2/7 (28.6) |
| STX11 | 2/40 (5.0)† | 1/33 (3.0) | 1/7 (14.3) |
| STXBP2 | 3/40 (7.5)† | 3/33 (9.1) | 0 |
| RAB27A | 4/40 (10.0)† | 3/33 (9.1) | 1/7 (14.3) |
| LYST | 5/40 (12.5)† | 5/33 15.2 | 0 |
| AP3B1 | 3/40 (7.5)† | 2/33 (6.1) | 1/7 (14.3) |
| SH2D1A | 3/40 (7.5)† | 2/33 (6.1) | 1/7 (14.3) |
| XIAP/BIRC4 | 3/40 (7.5)† | 3/33 (9.1) | 0 |
| Patients with ≥5 of 8 HLH-2004 criteria,‡ n (%) | 27/30 (90.0) | 23/25 (92.0) | 4/5 (80.0) |
| Patients with infection at diagnosis,§ n (%) | 25/46 (54.3) | 20/39 (51.3) | 5/7 (71.4) |
| Patients with CNS involvement at diagnosis, n (%) | 10/46 (21.7) | 9/39 (23.1) | 1/7 (14.3) |
| Age, y, at emapalumab initiation, median (range) | 1.0 (0.5-22.0) | 1 (0.5-11) | 15.0 (12.0-22.0) |
| Patients initiated emapalumab in an ICU, n (%) | 15/46 (32.6) | 13/39 (33.3) | 2/7 (28.6) |
| Patients receiving supportive care,||n (%) | 11/46 (23.9) | 9/39 (23.1) | 2/7 (28.6) |
| Ventilator only | 6/11 (54.5) | 5/9 (55.6) | 1/2 (50.0) |
| Dialysis only | 2/11 (18.2) | 1/9 (11.1) | 1/2 (50.0) |
| Multiple (≥1 of the following: mechanical ventilation, dialysis, vasopressors, ECMO, MARS) | 3/11 (27.3) | 3/9 (33.3) | 0 |
| Patients with abnormal laboratory markers or cytokine levels at emapalumab initiation, n (%) | |||
| Platelet count (<100 × 109/L) | 25/46 (54.4) | 22/39 (56.4) | 3/7 (42.9) |
| Absolute neutrophil count (<1.0 × 109/L) | 21/44 (47.7) | 19/37 (51.4) | 2/7 (28.6) |
| Fibrinogen (≤1.5 g/L) | 13/40 (32.5) | 11/34 (32.4) | 2/6 (33.3) |
| Ferritin (>500 μg/L) | 33/41 (80.9) | 27/34 (79.4) | 6/7 (85.7) |
| sCD25 (≥2400 U/mL) | 21/31 (67.7) | 19/26 (73.1) | 2/5 (40.0) |
| Absolute lymphocyte count¶ | 28/42 (66.7) | 23/36 (63.9) | 5/6 (83.3) |
| Alanine transaminase¶ | 35/45 (77.8) | 29/38 (76.3) | 6/7 (85.7) |
| CXCL9¶ | 19/20 (95.0) | 15/16 (93.8) | 4/4 (100.0) |
ECMO, extracorporeal membrane oxygenation; MARS, molecular adsorbent recirculating system; sCD25, soluble interleukin-2 receptor.
Patients were classified as children (aged <12 years) and adolescents and adults (aged ≥12 years) based on age at time of emapalumab initiation.
The denominator (n = 40) is based on number of patients with genetic mutations known to cause pHLH.
As reported by treating physician. Data on at least 5 of 8 parameters were available for 30 patients. Data on <5 parameters were available for the remaining 16 patients and therefore not included in the evaluation.
Viral infections were the most common (18/25, 72%).
At emapalumab initiation.
As reported by treating physician. Because these parameters are not part of the HLH-2004 criteria, we have no criteria or cut off point for them.
Of 46 patients with pHLH, 44 (95.7%) patients had genetic testing data available, including 37 of 39 (94.9%) children and 7 of 7 (100.0%) adolescents and adults (Table 1). At least 1 genetic mutation known to cause pHLH was found in 40 of 44 (90.9%) patients tested. Among these, the most commonly occurring mutations were PRF1 (n = 15; 37.5%), UNC13D (n = 10; 25%), and LYST (n = 5; 12.5%). Biallelic mutations were found in 13 of 40 (32.5%) patients. The details of patients with genetic mutations are given in supplemental Table 1. Of 30 patients for whom data were available, 27 (90.0%) patients fulfilled ≥5 of the 8 HLH-2004 criteria at diagnosis. At diagnosis, infection was recorded in 20 children and 5 adolescents/adults (25/46, 54.3%), the details of which are given in supplemental Table 2; and central nervous system (CNS) involvement was recorded in 9 children and 1 adolescent/adult (10/46, 21.7%).
The median age at emapalumab initiation was 1.0 year (range, 0.5-22 years). At time of emapalumab initiation, 15 (32.6%) patients were being treated in the intensive care unit (ICU) and 11 (23.9%) were receiving supportive care, which included mechanical ventilation only (n = 6; 54.5%), dialysis only (n = 2; 18.2%), or multiple measures (n = 3; 27.3%).
Treatment patterns
Based on physician reporting, emapalumab was initiated for treating refractory disease (n = 9; 41.3%), recurrent disease (n = 14; 30.4%), progressive disease (n = 7; 15.2%), and for other reasons (n = 6; 13.0%), which included maintenance therapy, initial treatment of HLH, primary CNS disease, pretransplant management of HLH, conditioning drug for stem cell transplant, and need for transplant and access to better care (all n = 1 each). Overall, 35 (76.1%) patients had received other HLH-related therapies before emapalumab (Table 2), and emapalumab was used concurrently with other HLH-related therapies in 44 (95.7%) patients (Table 2; supplemental Table 3). The most common HLH-related therapies used before, or concurrently with, emapalumab were corticosteroids and etoposide (Table 2). During the week preceding emapalumab initiation, the median average daily dose was 2.4 mg/kg prednisone equivalent; 2.0 mg/kg during week 2; 1.4 mg/kg during week 4, and 1.1 mg/kg during week 8. No discontinuations were reported because of emapalumab–related adverse events.
HLH-related therapies used before and concurrently with emapalumab treatment among patients with pHLH
| Parameter . | Overall (N = 46) . | Children (aged <12 y) (n = 39)∗ . | Adolescents and adults (aged ≥12 y) (n = 7)∗ . |
|---|---|---|---|
| Therapies before emapalumab treatment | |||
| Patients who received other HLH therapies, n (%) | 35 (76.1) | 30 (76.9) | 5 (71.4) |
| Corticosteroids | 35 (100.0) | 30 (100.0) | 5 (100.0) |
| Etoposide | 20 (57.1) | 18 (60.0) | 2 (40.0) |
| Anakinra | 4 (11.4) | 3 (10.0) | 1 (20.0) |
| Methotrexate + hydrocortisone | 2 (5.7) | 1 (3.3) | 1 (20.0) |
| Cyclosporine | 1 (2.9) | 1 (3.3) | 0 |
| Alemtuzumab | 1 (2.9) | 1 (3.3) | 0 |
| Rituximab | 1 (2.9) | 0 | 1 (20.0) |
| Basiliximab | 1 (2.9) | 1 (3.3) | 0 |
| Therapies concurrent with emapalumab treatment | |||
| Patients who received other HLH-related therapies, n (%) | 44/46 (95.7) | 38/39 (97.4) | 6/7 (85.7) |
| Corticosteroids | 44/44 (100.0) | 38/38 (100.0) | 6/6 (100.0) |
| Etoposide | 22/44 (50.0) | 20/38 (52.6) | 2/6 (33.3) |
| Anakinra | 6/44 (13.6) | 6/38 (15.8) | 0 |
| Methotrexate + hydrocortisone | 5/44 (11.4) | 4/38 (10.5) | 1/6 (16.7) |
| Ruxolitinib | 4/44 (9.1) | 4/38 (10.5) | 0 |
| Alemtuzumab | 3/44 (6.8) | 3/38 (7.9) | 0 |
| Cyclophosphamide | 3/44 (6.8) | 1/38 (2.6) | 2/6 (33.3) |
| Cyclosporine | 3/44 (6.8) | 3/38 (7.9) | 0 |
| Rituximab | 2/44 (4.5) | 1/38 (2.6) | 1/6 (16.7) |
| Doxorubicin | 1/44 (2.3) | 0 | 1/6 (16.7) |
| Tocilizumab | 1/44 (2.3) | 1/38 (2.6) | 0 |
| Nivolumab | 1/44 (2.3) | 1/38 (2.6) | 0 |
| Abatacept | 1/44 (2.3) | 1/38 (2.6) | 0 |
| Parameter . | Overall (N = 46) . | Children (aged <12 y) (n = 39)∗ . | Adolescents and adults (aged ≥12 y) (n = 7)∗ . |
|---|---|---|---|
| Therapies before emapalumab treatment | |||
| Patients who received other HLH therapies, n (%) | 35 (76.1) | 30 (76.9) | 5 (71.4) |
| Corticosteroids | 35 (100.0) | 30 (100.0) | 5 (100.0) |
| Etoposide | 20 (57.1) | 18 (60.0) | 2 (40.0) |
| Anakinra | 4 (11.4) | 3 (10.0) | 1 (20.0) |
| Methotrexate + hydrocortisone | 2 (5.7) | 1 (3.3) | 1 (20.0) |
| Cyclosporine | 1 (2.9) | 1 (3.3) | 0 |
| Alemtuzumab | 1 (2.9) | 1 (3.3) | 0 |
| Rituximab | 1 (2.9) | 0 | 1 (20.0) |
| Basiliximab | 1 (2.9) | 1 (3.3) | 0 |
| Therapies concurrent with emapalumab treatment | |||
| Patients who received other HLH-related therapies, n (%) | 44/46 (95.7) | 38/39 (97.4) | 6/7 (85.7) |
| Corticosteroids | 44/44 (100.0) | 38/38 (100.0) | 6/6 (100.0) |
| Etoposide | 22/44 (50.0) | 20/38 (52.6) | 2/6 (33.3) |
| Anakinra | 6/44 (13.6) | 6/38 (15.8) | 0 |
| Methotrexate + hydrocortisone | 5/44 (11.4) | 4/38 (10.5) | 1/6 (16.7) |
| Ruxolitinib | 4/44 (9.1) | 4/38 (10.5) | 0 |
| Alemtuzumab | 3/44 (6.8) | 3/38 (7.9) | 0 |
| Cyclophosphamide | 3/44 (6.8) | 1/38 (2.6) | 2/6 (33.3) |
| Cyclosporine | 3/44 (6.8) | 3/38 (7.9) | 0 |
| Rituximab | 2/44 (4.5) | 1/38 (2.6) | 1/6 (16.7) |
| Doxorubicin | 1/44 (2.3) | 0 | 1/6 (16.7) |
| Tocilizumab | 1/44 (2.3) | 1/38 (2.6) | 0 |
| Nivolumab | 1/44 (2.3) | 1/38 (2.6) | 0 |
| Abatacept | 1/44 (2.3) | 1/38 (2.6) | 0 |
Patients were classified as children (aged <12 years) and adolescents and adults (aged ≥12 years) based on age at time of emapalumab initiation.
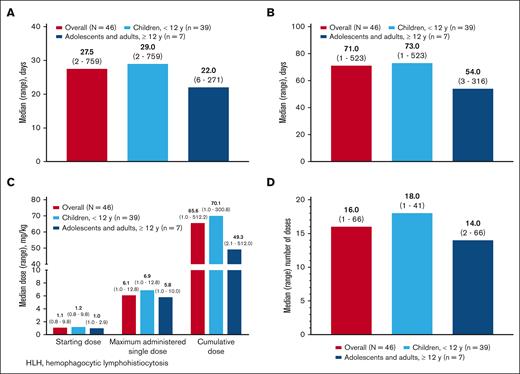
The median time from HLH diagnosis to emapalumab initiation was 27.5 days (range, 2-758); Figure 2A). The overall median treatment duration was 71 days (range, 1-523; Figure 2B). The median starting dose was 1.1 mg/kg (range, 0.8-9.8) and was similar in children and adolescents/adults, although the maximum dose tended to be slightly higher in children than in adolescents/adults (median, 6.9 mg/kg and 5.8 mg/kg, respectively; Figure 2C). The starting dose was >1 mg/kg for 30 of 46 (65.2%) patients, and ≤1 mg/kg for 16 (34.8%) patients. The cumulative treatment dose was 65.6 mg/kg (range, 1.0-512.2; Figure 2C). The median number of doses was 16 (range, 1-66.0), with 40 of 46 (87.0%) patients receiving ≥3 doses, and 6 (13.0%) patients receiving <3 doses (Figure 2D). The cumulative treatment dose and number of doses tended to be higher in children than in adolescents/adults. The distribution of starting dose, maximum dose, maximum number of doses, and total dose in the ICU and non-ICU settings is shown in supplemental Figure 1. At emapalumab initiation, 17 patients had fever that resolved for 15 patients during treatment with emapalumab within an average of 7.1 days (standard deviation, 4.0) and median of 7 days (range, 1-16).
Emapalumab dosing. (A) Timing of emapalumab treatment initiation from HLH diagnosis. (B) Duration of emapalumab treatment. (C) Median (range) emapalumab dosing. (D) Number of emapalumab doses.
Emapalumab dosing. (A) Timing of emapalumab treatment initiation from HLH diagnosis. (B) Duration of emapalumab treatment. (C) Median (range) emapalumab dosing. (D) Number of emapalumab doses.
Treatment outcomes
Normalization of laboratory parameters
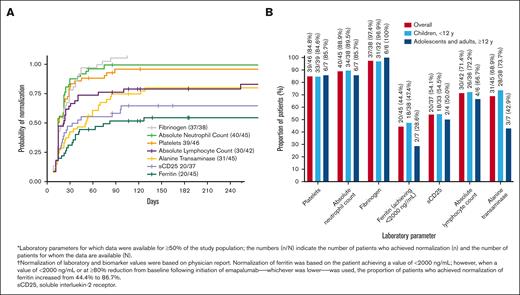
The levels for various laboratory parameters at the time of emapalumab initiation are given in supplemental Table 3. Results for normalization of laboratory parameters and biomarkers for which data were available for at least 50% of patients are presented in Figure 3A-B. The median time to first normalization of laboratory parameters ranged from 7 days for absolute lymphocyte counts, to 26 days for alanine transaminase (Figure 3A). Laboratory parameters for which most of the patients achieved normalization were fibrinogen (37/38, 97.4%), absolute neutrophil count (40/45, 88.9%), platelets (39/46, 84.8%), absolute lymphocyte count (30/42, 71.4%), and alanine transaminase (31/45, 68.9%; Figure 3B). Normalization was also observed among 20 of 45 (44.4%) and 20 of 37 (54.1%) patients for ferritin and soluble CD25, respectively. When a value of <2000 ng/mL or at least an 80% reduction from index date after initiation of emapalumab, whichever was lower, was used, the proportion of patients who achieved normalization of ferritin was 39 of 45 (86.7%). In addition, normalization of chemokine ligand 9 (CXCL9) was reported for 24 of 33 (72.7%) patients. The median time to first normalization of CXCL9 was 28.5 days (range, 4-84).
Normalization of laboratory parameters in response to treatment with emapalumab. (A) Time to first normalization of laboratory parameters from emapalumab initiation to the end of study period. (B) Proportion of patients exhibiting normalization of laboratory parameters.
Normalization of laboratory parameters in response to treatment with emapalumab. (A) Time to first normalization of laboratory parameters from emapalumab initiation to the end of study period. (B) Proportion of patients exhibiting normalization of laboratory parameters.
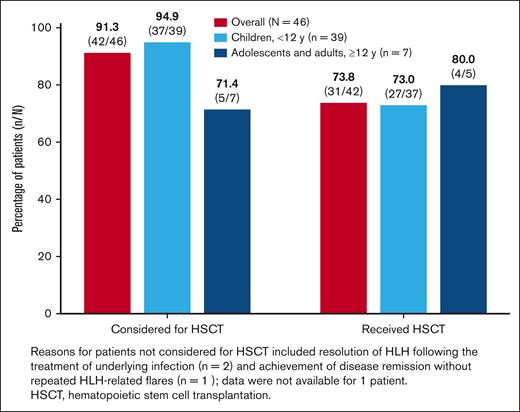
Emapalumab as a bridge to HSCT
Of 46 patients, 42 (91.3%) were considered eligible for transplant, of whom 31 (73.8%) received HSCT (Figure 4). The reasons reported for 3 of 4 patients not considered eligible for transplant include resolution of HLH (n = 1), remission without repeated HLH flares (n = 1), and resolution upon treatment of infection without recurrence of symptoms (n = 1). No information was provided for the fourth patient, including whether the patient received transplantation or not. The reasons provided for why the remaining eligible patients did not receive transplant included death (n = 4); family refusal against medical advice (n = 2); end-stage renal disease and awaiting kidney transplant (n = 1); end-stage renal disease and irreversible restrictive lung disease (n = 1); irreversible neurologic damage (n = 1); awaiting HSCT (n = 1); and complete response to treatment (n = 1).
Survival
PRETRANSPLANT SURVIVAL
Pretransplant survival rate among the 42 patients considered eligible for transplant was 38 of 42 (90.5%). The cause of death in 3 of 4 patients was reported to be related to pHLH, whereas the fourth patient was reported to have died during the conditioning regimen for stem cell transplant.
POSTTRANSPLANT SURVIVAL
Posttransplant survival rate among the 31 patients who received transplant was 74.2% (23/31). Five of 8 deaths were considered related to pHLH, 2 were related to HSCT, and 1 was due to multiple organ failure. The 12-month probability of survival after transplant was 73.7%.
OVERALL SURVIVAL
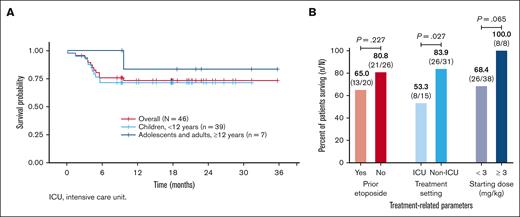
Overall survival for the entire pHLH cohort was 73.9% (34/46), and the 12-month survival probability from emapalumab initiation was 73.1% (95% CI, 61.2-87.4; Figure 5A). Patients were followed-up for a median of 534 days (range, 3-1075) from the initiation of emapalumab.
Survival in patients treated with emapalumab. (A) Overall survival from emapalumab initiation to the end of study period. (B) Relationship between the emapalumab treatment parameters and survival.
Survival in patients treated with emapalumab. (A) Overall survival from emapalumab initiation to the end of study period. (B) Relationship between the emapalumab treatment parameters and survival.
Exploratory analysis
Overall survival rate was significantly higher (P = .027; Figure 5B) among patients for whom emapalumab treatment was initiated in a non-ICU setting (26/31, 83.9%) than those for whom emapalumab treatment was initiated within an ICU setting (8/15, 53.3%). Whereas the relationship between overall survival and achievement of normalization of CXCL9 levels was not statistically significant (P = .297), overall survival rate was numerically higher among patients who achieved normalization of CXCL9 levels (20/24, 83.3%) than among those who did not (6/9, 66.7%). Similarly, overall survival rate was numerically higher but not statistically significant (P = .0645) among patients who received an emapalumab starting dose of at least 3 mg/kg (8/8, 100%) than among those who received a starting dose of <3 mg/kg (26/38, 68.4%). In addition, a higher overall survival rate was observed among patients without prior etoposide treatment (21/26, 80.8%) than among those who received prior etoposide treatment (13/20, 65.0%); however, it was not statistically significant (P = .227). Finally, there was no statistically significant difference in overall survival rate (P = .620) from emapalumab initiation between patients with and without CNS disease at diagnosis (8/10 [80%] and 26/36 [72.2%], respectively).
Discussion
The REAL-HLH study was conducted to address the limited availability of data on emapalumab use in real-world clinical settings. This retrospective chart review included patients who had received at least 1 dose of emapalumab in addition to other HLH-related treatments. In this report, we present the findings for patients with pHLH. The patient population was diverse with a substantial proportion (>60%) of non-White patients. Most patients were diagnosed with pHLH in infancy, although a small number were diagnosed in adolescence or later.6
The current US Food and Drug Administration-approved indication for emapalumab treatment is for adult and pediatric (newborn and older) patients with pHLH with refractory, recurrent, or progressive disease, or intolerance with conventional HLH therapy.30 Despite identification of a distinctive constellation of signs and symptoms characteristic of HLH, diagnosis remains a challenge.10 The disease presentations can substantially vary and numerous triggering factors make accurate application of the pHLH classification criteria difficult in the real world.10 A multispecialty expert study panel adjudicated application of consistent criteria across all patients.
Of patients in this study, >76% had received other HLH-related treatments before emapalumab initiation. The most frequent reasons for starting emapalumab in this pHLH study cohort were refractory, recurrent, or progressive disease, and the majority of these patients (35/46) were previously treated with other HLH-related therapies, most commonly corticosteroids and etoposide, both in keeping with current treatment guidelines.6 Based on the retrospective chart review, it was difficult to accurately determine whether the remaining 11 patients also received HLH-related therapies before initiating emapalumab or whether emapalumab was used as part of first-line therapy.
Several HLH-related laboratory parameters rapidly normalized with emapalumab treatment, indicating response to emapalumab.6 In the phase 2/3 study (www.clinicaltrials.gov identifier: #NCT01818492), the probability of a treatment response correlated with the decrease in serum CXCL9 levels. This chemokine, which is exclusively induced by IFN-γ, and thus serves as a biomarker for IFN-γ activity, was decreased with emapalumab treatment, normalizing in almost 75% of the pHLH cohort for whom data were available.31
Of patients treated with emapalumab, >90% were considered eligible for HSCT, with a 90% pretransplant survival rate, and almost 74% of these transplant-eligible patients subsequently undergoing HSCT. Twelve-month survival probability following HSCT was 73.7%, and survival at the end of the study period for patients who received transplant was 74.2%, comparable with other reports.23,32 These outcomes are consistent with the results of the phase 2/3 trial, and are higher than historic controls.28,33,34 Because most patients in this cohort also received other HLH-related treatments before or concurrent with emapalumab, these results cannot be entirely attributable to emapalumab treatment. Nevertheless, because most of the patients presented with refractory, recurrent, or progressive disease at emapalumab initiation, our data suggest that the prior HLH-related treatments did not elicit sufficient response and that addition of emapalumab played an important role in progressing the patients to transplant.
Results from the exploratory analysis suggest a relationship between overall survival and the treatment setting in which emapalumab was initiated (ie, higher survival in the non-ICU setting vs the ICU setting). Although there are many confounding factors, these results may support potential benefit of early initiation of emapalumab before development of organ damage requiring critical care. Also, the proportion of patients who survived when starting treatment with at least 3 mg/kg of emapalumab was greater than those starting treatment at <3 mg/kg, suggesting a potential benefit of higher initial dose for these patients. This requires further evaluation in future studies to establish if a true relationship exists.
Most deaths in the pHLH cohort in this study were attributed to the disease. Importantly, none of the deaths were attributed to emapalumab treatment, further confirming the safety observations of the phase 2/3 trial.28
Studies in real-world settings enable comparison of findings in clinical trials with those of similar interventions in clinical practice in which populations are likely to be more heterogeneous in terms of resources, demographic characteristics, medical histories, prior treatments, and treatment patterns. Furthermore, data on the use and utility of emapalumab in a real-world setting may enable physicians to make decisions that may improve treatment outcomes and highlight the benefits of early and accurate diagnosis of HLH to patients.
As with other retrospective chart review studies, however, limitations of the REAL-HLH study included the risk of missing or incomplete information, because data may not have been uniformly available or collected or available across all treatment centers. The limited availability and lack of consistent uniformity in the timing of assessment of laboratory values also contributed to the inability to comprehensively evaluate treatment “response.” Safety-related data were not collected or evaluated given that the study has no safety-related end points. There may also have been a risk of bias toward patients with poor prognoses, because emapalumab is currently indicated for previously treated patients with pHLH. Because patients also received concomitant HLH-related therapies, these results cannot be attributed exclusively to emapalumab treatment and may not be generalizable beyond the study population. Finally, the rarity of pHLH limited the size of the population in this study and the types of analyses that could be performed, particularly when comparing outcomes between children and adolescents/adults.
In conclusion, the REAL-HLH study describes the clinical characteristics, treatment patterns, and outcomes of emapalumab treatment across a large and diverse patient population with pHLH treated in real-world settings. Of patients treated with emapalumab, >90% were considered eligible for transplant, with a 90% pretransplant survival rate. Of these transplant-eligible patients, ∼74% received transplant. Overall survival and 12-month survival probability from emapalumab initiation for the entire cohort of patients with pHLH were 74% and 73%, respectively. Outcomes were consistent across patients aged <12 years and at least 12 years of age at start of emapalumab treatment. These findings from a diverse patient population with pHLH treated with emapalumab are consistent with the results from the emapalumab pivotal phase 2/3 clinical trial in pHLH.28 Results from a real-world study that are comparable with those from a prospective trial are particularly notable for a high-acuity population, in which no patient meeting diagnostic eligibility requirements was excluded because of clinical state or comorbidities.
Acknowledgments
The authors thank all investigators and the research team for their contributions (investigators and sites are listed below). The authors extend a special thanks to Corey Best (Sobi Inc), Nicole Bariahtaris, Katie Everson, and Sajjad Raza (PRECISIONheor) for their assistance in this study.
This study was supported by Sobi Inc. Medical writing assistance was provided by Mukund Nori, of rareLife solutions and funded by Sobi Inc.
Authorship
Contribution: C.E.A., S.C., M.B.J., J.W.L., A.O., K.J.W., and J.D.Y. contributed to study design; C.E.A., S.C., M.B.J., A.O., and P.P. contributed to data analysis; and all authors contributed to the data and critical review of the manuscript, and provided final approval of the manuscript for submission.
Conflict-of-interest disclosure: C.E.A. reports advisory board membership with Sobi Inc and Opna, and research support from Genentech and Opna. M.M.H. serves on a speaker/advisory board for Sobi Inc and is a speaker for ClearPoint. M.B.J. reports advisory board membership with Sobi Inc and received research support from Sobi Inc and Bristol Myers Squibb. J.W.L. serves on a speaker/advisory board for Sobi Inc; serves on the advisory board of Horizon; is an employee of bluebird bio; and holds stock in bluebird bio. A.O. is an employee of Sobi Inc and holds stock in Sobi Inc. P.P. is an employee of PRECISIONheor and served as consultant for Sobi Inc at the time of study. S.A.P. serves on a speaker/advisory board for Sobi Inc. A.K.R. is a speaker for BTC International Inc and Sobi Inc. J.D.Y. was an employee, at time of study, of Sobi Inc. A.Z.-L. serves as consultant for Sobi Inc. The remaining authors declare no competing financial interests.
A complete list of the members of the REAL-HLH Investigators appears in “Appendix.”
Correspondence: Carl E. Allen, Texas Children's Hospital, Feigin Center, 1102 Bates St, Houston, TX 77030; email: ceallen@texaschildrens.org.
Appendix:
Joanna Weinstein, Ann & Robert H. Lurie Children’s Hospital.
Ashley P. Hinson, Atrium Health, Levine Children’s Hospital.
Carl E. Allen, Olive S. Eckstein, and Nitya Gulati, Baylor College of Medicine in the Texas Children’s Cancer and Hematology Centers.
Shanmuganathan Chandrakasan, Children’s Healthcare of Atlanta, Egleston Hospital.
Hilary Haines, Children’s Hospital of Alabama at University of Alabama at Birmingham.
Taizo Nakano, Children’s Hospital Colorado.
Sachit A. Patel, Children’s Hospital & Medical Center at University of Nebraska Medical Center.
Edward M Behrens, Children’s Hospital of Philadelphia.
Stefanos Intzes, Children’s Hospital at Providence Sacred Heart Medical Center.
Allyson Hays, Children’s Mercy Hospital.
Blachy Dávila Saldaña, Children’s National Medical Center.
Michael B. Jordan and Adi Zoref-Lorenz, Cincinnati Children’s Hospital Medical Center.
Rabi Hanna, Cleveland Clinic Children’s.
Michael Isakoff, Connecticut Children’s Medical Center.
Anish K. Ray, Cook Children’s Medical Center.
Jennifer Rothman, Duke University Medical Center.
Anand Tandra, Franciscan Health Hospital.
Robert Cooper, Kaiser Permanente Los Angeles Medical Center.
Jennifer W. Leiding, Johns Hopkins University.
May Chien, Lucile Packard Children’s Hospital Stanford.
Susmita Sarangi, Medstar Georgetown University Hospital.
Diaz Gidvani, Methodist Hospital at University of Texas Health San Antonio.
Prakesh Satwani, New York Presbyterian Hospital.
John Carter, Oregon Health and Science University.
Michael Henry, Phoenix Children’s.
Nicholas Gloude, Rady Children’s Hospital at University of California San Diego.
Sima Bhatt, Saint Louis Children’s Hospital, Washington University.
Deepika Bhatla and Lauren Draper, Saint Louis University School of Medicine.
Arun Panigrahi, University of California, Davis.
Michelle Hermiston, University of California, San Francisco Medical Center.
Renee Modica, University of Florida Health Shand’s Hospital.
Mona Riskalla, University of Minnesota Medical Center.
Ashley Baker, University of Oklahoma Health Sciences Center.
Brant Ward, Virginia Commonwealth University Medical Center.
References
Author notes
Investigators requesting original deidentified data may submit their proposal to the corresponding author, Carl E. Allen (ceallen@texaschildrens.org); requests will be reviewed to meet regulatory requirements and approved by the study team.
The full-text version of this article contains a data supplement.