Tumor mutational load is a strong prognostic factor for progression to therapy among individuals with HCMBL, independent of CLL-IPI.
Accounting for both CLL-IPI and tumor mutational load, we identified individuals with HCMBL who have a worse prognosis than patients at low risk with CLL.
Visual Abstract
High-count monoclonal B-cell lymphocytosis (HCMBL) is a precursor condition to chronic lymphocytic leukemia (CLL). We have shown that among individuals with HCMBL, the CLL-International Prognostic Index (CLL-IPI) is prognostic for time-to-first therapy (TTFT). Little is known about the prognostic impact of somatically mutated genes among individuals with HCMBL. We sequenced DNA from 371 individuals with HCMBL using a targeted sequencing panel of 59 recurrently mutated genes in CLL to identify high-impact mutations. We compared the sequencing results with that of our treatment-naïve CLL cohort (N = 855) and used Cox regression to estimate hazard ratios and 95% confidence intervals (CIs) for associations with TTFT. The frequencies of any mutated genes were lower in HCMBL (52%) than CLL (70%). At 10 years, 37% of individuals with HCMBL with any mutated gene had progressed requiring treatment compared with 10% among individuals with HCMBL with no mutations; this led to 5.4-fold shorter TTFT (95% CI, 2.6-11.0) among HCMBL with any mutated gene vs none, independent of CLL-IPI. When considering individuals with low risk of progression according to CLL-IPI, those with HCMBL with any mutations had 4.3-fold shorter TTFT (95% CI, 1.6-11.8) vs those with none. Finally, when considering both CLL-IPI and any mutated gene status, we observed individuals with HCMBL who were high risk for both prognostic factors had worse prognosis than patients with low-risk CLL (ie, 5-year progression rate of 32% vs 21%, respectively). Among HCMBL, the frequency of somatically mutated genes at diagnosis is lower than that of CLL. Accounting for both the number of mutated genes and CLL-IPI can identify individuals with HCMBL with more aggressive clinical course.
Introduction
Monoclonal B-cell lymphocytosis (MBL) is a precursor state to chronic lymphocytic leukemia (CLL)1-3 and is characterized by a circulating population of clonal B cells (<5 × 109/L) in the peripheral blood and in the absence of lymphadenopathy, cytopenias, or organomegaly.4,5 MBL can be subclassified based on immunophenotype (the most common being the immunophenotype that is similar to that of CLL) and the size of the MBL clone, with high-count MBL (HCMBL) defined as the absolute clonal B-cell count between 0.5 × 109/L and 4.9 × 109/L.6,7
On average, progression to CLL requiring therapy among individuals with HCMBL occurs at a rate of ∼1% to 2% per year.8-10 A number of investigators have evaluated individual biomarkers to predict MBL progression to CLL requiring therapy and overall survival (OS), including mutation status of the immunoglobulin heavy chain variable region (IgHV), high-risk cytogenetic aberrations, high serum β2 microglobulin, CD49d expression, and absolute B-cell count.8-11 More recently, investigators have integrated various markers into a single prognostic score, called the CLL-International Prognostic Index (CLL-IPI). The CLL-IPI is composed of 5 individual clinical and leukemic prognostic factors and was found to be highly prognostic for OS and time to first therapy (TTFT) among individuals with CLL,12-14 even early-stage CLL.15 The CLL-IPI has recently been found to stratify TTFT among individuals with HCMBL.11
Large sequencing studies of treatment-naïve patients with CLL have identified recurrently mutated genes,16-18 mostly affecting cell cycle, DNA damage response, and NOTCH and NF-kB signaling pathways. Investigative teams have also evaluated patients with CLL at later stages of disease, including symptomatic patients with CLL and patients with relapsed CLL, and found, in general, higher gene mutation frequencies at these later disease stages, supporting the evidence that these genes affect disease progression.19-22 Related, researchers have also reported on the clinical impact of individual mutations, individual driver genes, and total number of mutated genes with TTFT and OS among patients with CLL.16-25 We have previously shown that the total number of mutated genes (or the tumor mutational load [TML]) is a robust and an independent prognostic marker for progression to therapy among treatment-naïve patients with CLL, beyond CLL-IPI.25
The effect of recurrently mutated CLL driver genes among individuals with HCMBL has been understudied.16,23,25-28 To our knowledge, in the largest study to date of 112 individuals with HCMBL, we reported generally lower gene mutation frequency across the 59 CLL driver genes among individuals with HCMBL than that of CLL.25 We also reported that individuals with ≥2 mutated CLL driver genes had a 4.1-fold increased risk of progression requiring CLL therapy, adjusting for CLL-IPI. These data indicated that the number of genes with high-impact or hot spot mutations may be a prognostic biomarker to identify individuals with HCMBL who are at high risk for progression. Herein, we significantly expanded our cohort with more than triple the number of individuals with sequenced HCMBL (N = 371, including the previous 112 individuals with HCMBL) and longer follow-up to further evaluate the prognostic impact of these CLL driver genes or their aggregate on disease progression relative to a cohort of 855 patients with CLL, also expanded from our earlier TML study of 445 patients with CLL.
Methods
Study participants
From the Mayo Clinic CLL Resource,11,25,29 individuals with HCMBL or CLL who were clinically seen in the Division of Hematology, Mayo Clinic, with available treatment-naïve peripheral blood mononuclear cells (PBMCs) were selected. Additional patients with CLL with treatment-naïve PBMC were also obtained from the CLL clinics at Duke University (n = 57), Weill Cornell Medical College (n = 18), and from the CLL Research Consortium (n = 44).30 PBMCs for sequencing studies were collected within a median 0.03 years (range, 0-26) from diagnosis. Clinical characteristics were obtained at the time of diagnosis or at time of sample collection and included age, sex, Rai stage, serum β2 microglobulin levels, IgHV mutation status, and genetic abnormalities detectable by fluorescence in situ hybridization (FISH), when available. We also included 54 individuals with HCMBL from the Mayo Clinic MBL Biobank Cohort31; these individuals were identified to have HCMBL through MBL screening using 8-color flow cytometry and had the immunophenotype of CLL-like MBL (n = 346), atypical MBL (n = 6), and non–CLL-like MBL (n = 19). Abstracted clinical characteristics included age and sex, whereas del(17p) genetic abnormality was obtained through sequencing.
All individuals provided written informed consent for this research whose protocol was approved by the respective institutional review boards.
DNA sequencing
DNA was extracted from PBMCs that had a tumor purity >80% or otherwise from PBMCs enriched for CD5+/CD19+ clonal B cells. We sequenced the entire coding regions of 59 somatically recurring mutated CLL genes as previously described25 (supplemental Table 1). In brief, DNA samples were sequenced using Illumina HiSeq 4000 sequencer. The median coverage depth per individual across the 59 recurrently mutated genes was 1741×, with >80% of the individuals having a median coverage depth >1000× per nucleotide, allowing for the detection of mutations with variant allelic fraction (VAF) as low as 1%. Somatic mutations were called using MuTect2 in tumor-only mode. After filtering, high-impact mutations (frameshift, nonsense, and splicing variants) and missense mutations in previously identified CLL hot spots (supplemental Table 2) were used for statistical analyses. Raw variants were annotated using GATK Variant Annotator for variant quality, and Biological Reference Repository was used for variant annotation. To remove germline polymorphisms, common variants were eliminated based on the minor allele frequencies >0.01%, available in the following germline variant databases: 1000 Genomes Project, ExAC, and ESP6500 from NHLBI Exome Sequencing Project, unless present in known CLL/MBL mutation hot spots or in Catalog of Somatic Mutations in Cancer (COSMIC). In addition, we filtered out all somatic variants with <10 supportive reads or <1% VAF.
Statistical analyses
CLL-IPI was calculated based on clinical stage (Rai 0 vs Rai I-IV), IgHV mutation status (mutated vs unmutated), TP53 status (wild type vs del17p, TP53 mutations, or both), β2 microglobulin level (>3.5 mg/L), and age (>65 years) as previously described.12 We classified all individuals with HCMBL as Rai stage 0 when calculating the CLL-IPI score. TML was calculated by counting the number of genes with high-impact and hot spot mutations but excluding TP53, which is used in calculating the CLL-IPI. We then categorized the TML either as binary (none or any mutated gene) or as a categorical variable with 0 mutated genes serving as the reference category. We also considered TML as a continuous variable. TTFT was defined as the time from date of sample to the earliest date of first treatment or date of last follow-up. The event in the TTFT analyses was of those individuals who received CLL treatment; otherwise, individuals were censored. Any patients with CLL who were enrolled on early interventional trials were censored on the date of treatment. Deaths in untreated patients were considered a competing risk. OS was defined as the time from date of sample to the earliest date of death or last follow-up. The event in OS was those who died, regardless of cause; otherwise, individuals were censored. We used Cox regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the effect of individual genes or TML with TTFT and OS. In addition, we evaluated the TML with TTFT and OS stratified by CLL-IPI status (within the HCMBL cohort and in the combined HCMBL and CLL cohort). For the full cohort analyses, we added diagnosis status (CLL or HCMBL) as an additional covariate. OS curves were displayed using the Kaplan-Meier method and the TTFT curves using the cumulative incidence method. To evaluate model discriminative ability, we computed a c-statistic and 95% CI for the adjusted Cox regression models. Significant findings were those that had a P value <.05. Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
A total of 371 individuals with HCMBL were sequenced and compared with a cohort of 855 patients with CLL. Among the individuals with HCMBL, the median age was 67 years (range, 42-95), and 63% were male. Among patients with CLL, the median age was 61 years (range, 27-94), and 70% were male (Table 1; supplemental Table 3). The majority (n = 179 [61%]) of the individuals with HCMBL were considered low risk according to the CLL-IPI; whereas among patients with CLL, 268 (36%) were considered low risk (Table 1; supplemental Table 3). A total of 181 individuals (HCMBL, n= 76; CLL, n = 105) were missing CLL-IPI scores and not included in outcome analyses.
Demographic, clinical, and tumor characteristics
| Clinical characteristic . | Total cohort . | HCMBL . | CLL . | |||
|---|---|---|---|---|---|---|
| (N = 1226) . | (n = 371) . | (n = 855) . | ||||
| n . | % . | n . | % . | n . | % . | |
| Sex | ||||||
| Female | 327 | 31.90 | 114 | 36.80 | 213 | 29.30 |
| Male | 699 | 68.10 | 196 | 63.20 | 503 | 70.30 |
| Age, median (range) | 63 (27-95) | 67 (42-95) | 61 (27-94) | |||
| Race | ||||||
| Caucasian | 1089 | 88.80 | 364 | 98.15 | 725 | 84.80 |
| Other | 137 | 11.20 | 7 | 1.20 | 130 | 15.20 |
| Rai stage risk group | ||||||
| Rai 0 | 831 | 68.80 | 371 | 100 | 460 | 55.00 |
| Rai I-II | 326 | 27.00 | - | - | 326 | 38.90 |
| Rai III-IV | 51 | 4.20 | - | - | 51 | 6.10 |
| Missing | 18 | 18 | ||||
| Fluorescence in situ hybridization at diagnosis∗ | ||||||
| 13q deletion | 587 | 57.50 | 162 | 54.60 | 425 | 58.90 |
| Trisomy 12 | 164 | 16.10 | 49 | 16.40 | 115 | 15.90 |
| 11q deletion | 103 | 10.10 | 15 | 5.00 | 88 | 12.20 |
| 17p deletion | 55 | 5.40 | 8 | 2.60 | 47 | 6.50 |
| Normal | 233 | 22.70 | 85 | 28.10 | 148 | 20.50 |
| Other | 15 | 1.50 | 3 | 1.00 | 12 | 1.70 |
| Missing | 200 | 68 | 132 | |||
| TP53 | ||||||
| Mutated | 124 | 10.10 | 30 | 8.10 | 124 | 10.10 |
| Unmutated | 921 | 89.80 | 287 | 92.60 | 634 | 88.50 |
| Missing | 0 | 0 | 0 | |||
| IgHV mutation status | ||||||
| Mutated | 611 | 55.90 | 221 | 75.40 | 390 | 48.70 |
| Unmutated | 483 | 44.10 | 72 | 24.60 | 411 | 51.30 |
| Missing | 132 | 78 | 54 | |||
| β2 microglobulin | ||||||
| ≤3.5 mg/L | 867 | 81.60 | 278 | 89.70 | 589 | 78.30 |
| >3.5 mg/L | 195 | 18.40 | 32 | 10.30 | 163 | 21.70 |
| Missing | 164 | 61 | 103 | |||
| Median (range) | 2.4 (0.2-121.5) | 2.1 (1.0-21.5) | 2.4 (0.2-16.2) | |||
| CLL-IPI | ||||||
| Low risk (0-1) | 447 | 42.78 | 179 | 60.70 | 268 | 35.73 |
| Intermediate risk (2-3) | 313 | 29.95 | 70 | 23.70 | 243 | 32.40 |
| High/very high risk (4-10) | 285 | 27.27 | 46 | 15.60 | 239 | 31.87 |
| Missing | 181 | 76 | 105 | |||
| B-cell count, ×109/L | ||||||
| Median (range) | 6.7 (0.2-394.5) | 2.9 (0.5-4.9) | 11.8 (0.2-394.5) | |||
| Missing | 164 | 48 | 116 | |||
| Clinical characteristic . | Total cohort . | HCMBL . | CLL . | |||
|---|---|---|---|---|---|---|
| (N = 1226) . | (n = 371) . | (n = 855) . | ||||
| n . | % . | n . | % . | n . | % . | |
| Sex | ||||||
| Female | 327 | 31.90 | 114 | 36.80 | 213 | 29.30 |
| Male | 699 | 68.10 | 196 | 63.20 | 503 | 70.30 |
| Age, median (range) | 63 (27-95) | 67 (42-95) | 61 (27-94) | |||
| Race | ||||||
| Caucasian | 1089 | 88.80 | 364 | 98.15 | 725 | 84.80 |
| Other | 137 | 11.20 | 7 | 1.20 | 130 | 15.20 |
| Rai stage risk group | ||||||
| Rai 0 | 831 | 68.80 | 371 | 100 | 460 | 55.00 |
| Rai I-II | 326 | 27.00 | - | - | 326 | 38.90 |
| Rai III-IV | 51 | 4.20 | - | - | 51 | 6.10 |
| Missing | 18 | 18 | ||||
| Fluorescence in situ hybridization at diagnosis∗ | ||||||
| 13q deletion | 587 | 57.50 | 162 | 54.60 | 425 | 58.90 |
| Trisomy 12 | 164 | 16.10 | 49 | 16.40 | 115 | 15.90 |
| 11q deletion | 103 | 10.10 | 15 | 5.00 | 88 | 12.20 |
| 17p deletion | 55 | 5.40 | 8 | 2.60 | 47 | 6.50 |
| Normal | 233 | 22.70 | 85 | 28.10 | 148 | 20.50 |
| Other | 15 | 1.50 | 3 | 1.00 | 12 | 1.70 |
| Missing | 200 | 68 | 132 | |||
| TP53 | ||||||
| Mutated | 124 | 10.10 | 30 | 8.10 | 124 | 10.10 |
| Unmutated | 921 | 89.80 | 287 | 92.60 | 634 | 88.50 |
| Missing | 0 | 0 | 0 | |||
| IgHV mutation status | ||||||
| Mutated | 611 | 55.90 | 221 | 75.40 | 390 | 48.70 |
| Unmutated | 483 | 44.10 | 72 | 24.60 | 411 | 51.30 |
| Missing | 132 | 78 | 54 | |||
| β2 microglobulin | ||||||
| ≤3.5 mg/L | 867 | 81.60 | 278 | 89.70 | 589 | 78.30 |
| >3.5 mg/L | 195 | 18.40 | 32 | 10.30 | 163 | 21.70 |
| Missing | 164 | 61 | 103 | |||
| Median (range) | 2.4 (0.2-121.5) | 2.1 (1.0-21.5) | 2.4 (0.2-16.2) | |||
| CLL-IPI | ||||||
| Low risk (0-1) | 447 | 42.78 | 179 | 60.70 | 268 | 35.73 |
| Intermediate risk (2-3) | 313 | 29.95 | 70 | 23.70 | 243 | 32.40 |
| High/very high risk (4-10) | 285 | 27.27 | 46 | 15.60 | 239 | 31.87 |
| Missing | 181 | 76 | 105 | |||
| B-cell count, ×109/L | ||||||
| Median (range) | 6.7 (0.2-394.5) | 2.9 (0.5-4.9) | 11.8 (0.2-394.5) | |||
| Missing | 164 | 48 | 116 | |||
Individuals can have >1.
Characteristics of somatic variants detected in HCMBL compared with CLL
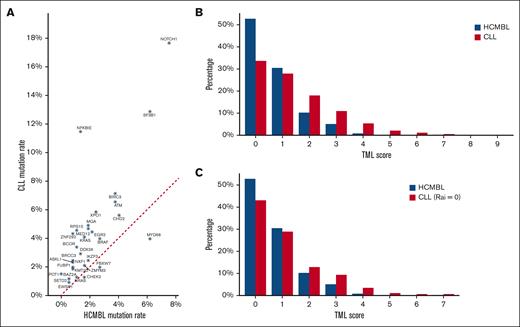
The type and distribution of high-impact or hot spot mutations among the HCMBL cohort is shown in Figure 1A. The most frequently mutated genes were TP53 (8%), NOTCH1 (8%), SF3B1 (6%), and MYD88 (6%); 49% of the individuals with HCMBL had at least 1 mutated gene (Table 2), and 17 (29%) genes had no evidence of high-impact or hot spot mutations (supplemental Table 1). The majority of the genes were frequently more mutated among individuals with HCMBL with IgHV unmutated status compared with individuals with HCMBL with IgHV mutated status; exceptions included TP53, MYD88, CHD2, ITPKB, and CHEK2 (supplemental Table 1). When comparing HCMBL with CLL, we observed similar distributions of the variant allele fraction (Figure 1B) and the type of mutation (ie, splice site, frame shift, etc; Figure 1C). However, the frequency of individual genes with high-impact or hot spot mutations were higher in CLL than HCMBL (supplemental Table 1; Figure 2A). The frequency of any mutated gene (including TP53) was 52% compared with 70% among CLL. When looking at the TML, patients with CLL had a higher total number of mutated genes (median, 1) than individuals with HCMBL (median, 0; P < .001; Figure 2B). This higher TML distribution held, even among the 460 patients with CLL Rai 0, compared with those with HCMBL (Figure 2C; P < .001).
Characteristics of somatic mutations between HCMBL and CLL. (A) Heat map representing the distribution of high-impact and hot spot mutations in 59 genes frequently mutated in CLL and HCMBL. Each column represents a single individual from our cohort. The external bar indicates individuals with HCMBL (gray) or CLL (red) diagnosis; the second bar stratifies the heat map by CLL-IPI (low risk [green], intermediate risk [yellow], and high/very high risk [red]); the third bar represents the TML distribution (ie, the number of mutated genes per individual); then the next 5 bars represent clinical components: Rai stage (0-gray; I-green; II-yellow; III-red; and IV-purple), sex, IgHV, B2M, and del(17p) by fluorescence in situ hybridization. Finally, the internal bars represent the list of mutated genes in our study color-coded by the type of mutation: missense (yellow), in frame (gray), frameshift (red), nonsense (purple), splice (blue), and >1 mutation (green). (B) Distribution of the variant allele fraction of high-impact and hot spot mutations between individuals with CLL and HCMBL. (C) Distribution of mutation type between individuals with HCMBL and CLL. B2M, Beta-2 microglobulin; FISH17p, fluorescence in situ hybridization 17p deletion.
Characteristics of somatic mutations between HCMBL and CLL. (A) Heat map representing the distribution of high-impact and hot spot mutations in 59 genes frequently mutated in CLL and HCMBL. Each column represents a single individual from our cohort. The external bar indicates individuals with HCMBL (gray) or CLL (red) diagnosis; the second bar stratifies the heat map by CLL-IPI (low risk [green], intermediate risk [yellow], and high/very high risk [red]); the third bar represents the TML distribution (ie, the number of mutated genes per individual); then the next 5 bars represent clinical components: Rai stage (0-gray; I-green; II-yellow; III-red; and IV-purple), sex, IgHV, B2M, and del(17p) by fluorescence in situ hybridization. Finally, the internal bars represent the list of mutated genes in our study color-coded by the type of mutation: missense (yellow), in frame (gray), frameshift (red), nonsense (purple), splice (blue), and >1 mutation (green). (B) Distribution of the variant allele fraction of high-impact and hot spot mutations between individuals with CLL and HCMBL. (C) Distribution of mutation type between individuals with HCMBL and CLL. B2M, Beta-2 microglobulin; FISH17p, fluorescence in situ hybridization 17p deletion.
Association between TML and time-to-first treatment among individuals with HCMBL
| CLL-IPI . | Mutated genes . | Total . | Event . | HR . | 95% CI . | P value . | |
|---|---|---|---|---|---|---|---|
| N . | col % . | N . | |||||
| Overall∗ | None | 147 | 51.0 | 9 | 1.00 | Reference | |
| (N = 288) | Any | 141 | 49.0 | 48 | 5.37 | (2.63-10.98) | <.0001 |
| 0 | 147 | 51.0 | 9 | 1.00 | Reference | ||
| 1 | 90 | 31.3 | 31 | 5.30 | (2.51-11.21) | <.0001 | |
| ≥2 | 51 | 17.7 | 17 | 5.50 | (2.44-12.36) | <.0001 | |
| Cont. | 288 | 100 | 56 | 1.59 | (1.33-1.89) | <.0001 | |
| c-statistic | 0.726 | (0.65-0.80) | |||||
| Low-risk CLL-IPI† | None | 103 | 57.5 | 5 | 1.00 | Reference | |
| (n = 179) | Any | 76 | 42.5 | 18 | 4.30 | (1.57-11.79) | .005 |
| 0 | 103 | 57.5 | 5 | 1.00 | Reference | ||
| 1 | 56 | 31.3 | 14 | 4.47 | (1.56-12.77) | .005 | |
| ≥2 | 20 | 11.2 | 4 | 3.88 | (1.04-14.47) | .043 | |
| Cont. | 179 | 100 | 23 | 1.75 | (1.15-2.68) | .009 | |
| c-statistic | 0.660 | (0.56-0.76) | |||||
| Intermediate-risk CLL-IPI† | None | 27 | 38.6 | 4 | 1.00 | Reference | |
| (n = 70) | Any | 43 | 61.4 | 20 | 3.04 | (1.02-9.01) | .045 |
| 0 | 27 | 38.6 | 4 | 1.00 | Reference | ||
| 1 | 23 | 32.9 | 11 | 3.51 | (1.11-11.14) | .033 | |
| ≥2 | 20 | 28.6 | 9 | 2.57 | (0.77-8.55) | .123 | |
| Cont. | 70 | 100 | 24 | 1.48 | (1.04-2.11) | .028 | |
| c-statistic | 0.650 | (0.54-0.76) | |||||
| High- to very high–risk CLL-IPI† | None | 24 | 52.2 | 0 | 1.00 | Reference | |
| (n = 46) | Any | 22 | 57.8 | 9 | NA | NA | NA |
| 0 | 24 | 52.2 | 0 | 1.00 | Reference | ||
| 1 | 11 | 23.9 | 5 | NA | NA | NA | |
| ≥2 | 11 | 23.9 | 4 | NA | NA | NA | |
| Cont. | 46 | 100 | 9 | 1.48 | (1.07-2.07) | .019 | |
| c-statistic | 0.803 | (0.71-0.90) | |||||
| CLL-IPI . | Mutated genes . | Total . | Event . | HR . | 95% CI . | P value . | |
|---|---|---|---|---|---|---|---|
| N . | col % . | N . | |||||
| Overall∗ | None | 147 | 51.0 | 9 | 1.00 | Reference | |
| (N = 288) | Any | 141 | 49.0 | 48 | 5.37 | (2.63-10.98) | <.0001 |
| 0 | 147 | 51.0 | 9 | 1.00 | Reference | ||
| 1 | 90 | 31.3 | 31 | 5.30 | (2.51-11.21) | <.0001 | |
| ≥2 | 51 | 17.7 | 17 | 5.50 | (2.44-12.36) | <.0001 | |
| Cont. | 288 | 100 | 56 | 1.59 | (1.33-1.89) | <.0001 | |
| c-statistic | 0.726 | (0.65-0.80) | |||||
| Low-risk CLL-IPI† | None | 103 | 57.5 | 5 | 1.00 | Reference | |
| (n = 179) | Any | 76 | 42.5 | 18 | 4.30 | (1.57-11.79) | .005 |
| 0 | 103 | 57.5 | 5 | 1.00 | Reference | ||
| 1 | 56 | 31.3 | 14 | 4.47 | (1.56-12.77) | .005 | |
| ≥2 | 20 | 11.2 | 4 | 3.88 | (1.04-14.47) | .043 | |
| Cont. | 179 | 100 | 23 | 1.75 | (1.15-2.68) | .009 | |
| c-statistic | 0.660 | (0.56-0.76) | |||||
| Intermediate-risk CLL-IPI† | None | 27 | 38.6 | 4 | 1.00 | Reference | |
| (n = 70) | Any | 43 | 61.4 | 20 | 3.04 | (1.02-9.01) | .045 |
| 0 | 27 | 38.6 | 4 | 1.00 | Reference | ||
| 1 | 23 | 32.9 | 11 | 3.51 | (1.11-11.14) | .033 | |
| ≥2 | 20 | 28.6 | 9 | 2.57 | (0.77-8.55) | .123 | |
| Cont. | 70 | 100 | 24 | 1.48 | (1.04-2.11) | .028 | |
| c-statistic | 0.650 | (0.54-0.76) | |||||
| High- to very high–risk CLL-IPI† | None | 24 | 52.2 | 0 | 1.00 | Reference | |
| (n = 46) | Any | 22 | 57.8 | 9 | NA | NA | NA |
| 0 | 24 | 52.2 | 0 | 1.00 | Reference | ||
| 1 | 11 | 23.9 | 5 | NA | NA | NA | |
| ≥2 | 11 | 23.9 | 4 | NA | NA | NA | |
| Cont. | 46 | 100 | 9 | 1.48 | (1.07-2.07) | .019 | |
| c-statistic | 0.803 | (0.71-0.90) | |||||
col, column; Cont., continuous; NA, not applicable.
Adjusted for CLL-IPI and sex.
Adjusted for sex.
Gene frequencies and TML distribution between HCMBL and CLL. (A) Scatterplot of the frequency of mutated genes found in HCMBL and CLL. Red dotted line represents equal mutated gene frequency. (B) Distribution of the TML score between individuals with CLL and HCMBL. (C) Distribution of mutation type between individuals with HCMBL and patients with CLL with Rai stage 0. TML is the number of genes out of 59 with high-impact mutations (excluding TP53).
Gene frequencies and TML distribution between HCMBL and CLL. (A) Scatterplot of the frequency of mutated genes found in HCMBL and CLL. Red dotted line represents equal mutated gene frequency. (B) Distribution of the TML score between individuals with CLL and HCMBL. (C) Distribution of mutation type between individuals with HCMBL and patients with CLL with Rai stage 0. TML is the number of genes out of 59 with high-impact mutations (excluding TP53).
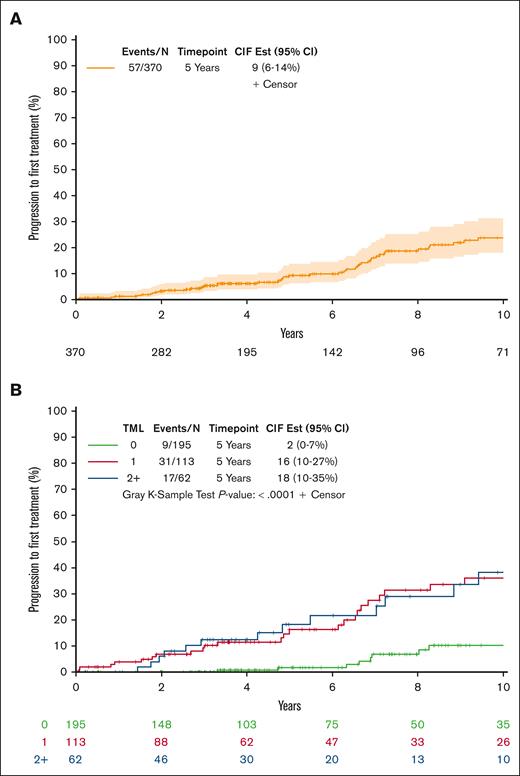
TTFT according to the TML
Among individuals with HCMBL, the median follow-up from sample collection was 6 years (range, 0-20), and 57 individuals progressed to needing therapy. Overall, the 5- and 10-year cumulative progression to CLL requiring therapy was 9% and 24%, respectively (Figure 3A). However, this cumulative risk varied according to the TML (Figure 3B; P < .0001). The estimated 5- and 10-year cumulative progression to therapy among those individuals with HCMBL with no mutated genes was of 2% and 10%, respectively. In contrast, the estimated 5- and 10-year cumulative progression to therapy among those with ≥2 mutated genes was 18% and 37%, respectively. This association between TML and TTFT held in multivariate Cox regression analyses adjusting for CLL-IPI and sex; individuals with any mutated genes had a 5.4-fold increased risk of progression to therapy (95% CI, 2.6-11.0; P < .0001; Table 2). We observed similar effect size for progression to therapy between those with just 1 mutated gene (HR = 5.3; 95% CI, 2.5-11.2; P < .0001) and those with ≥2 mutated genes (HR = 5.5; 95% CI, 2.4-12.4; P < .0001; Table 2). When considering each gene individually, 8 genes (NOTCH1, SF3B1, BRAF, FBXW7, XPO1, EGR2, MED12, and ARID1A) were found to be statistically associated with TTFT univariately and after adjusting for CLL-IPI and sex (supplemental Table 4). Although mutated TP53 had elevated effect sizes in both univariate and multivariate models, these associations were not statistically significant. However, when we stratified by IgHV mutation status, mutated TP53 had a 6.35-fold (95% CI, 1.71-23.6) shorter time to therapy than no TP53 mutations among individuals with HCMBL who were unmutated for IgHV (supplemental Table 4).
Associations of TML with TTFT among individuals with HCMBL. (A) Cumulative incidence plot of progression to first treatment among individuals with HCMBL. (B) Cumulative incidence plot of progression to first treatment among individuals with HCMBL by TML. TML is the number of genes out of 59 with high-impact mutations (excluding TP53). CIF Est, cumulative incidence function estimation.
Associations of TML with TTFT among individuals with HCMBL. (A) Cumulative incidence plot of progression to first treatment among individuals with HCMBL. (B) Cumulative incidence plot of progression to first treatment among individuals with HCMBL by TML. TML is the number of genes out of 59 with high-impact mutations (excluding TP53). CIF Est, cumulative incidence function estimation.
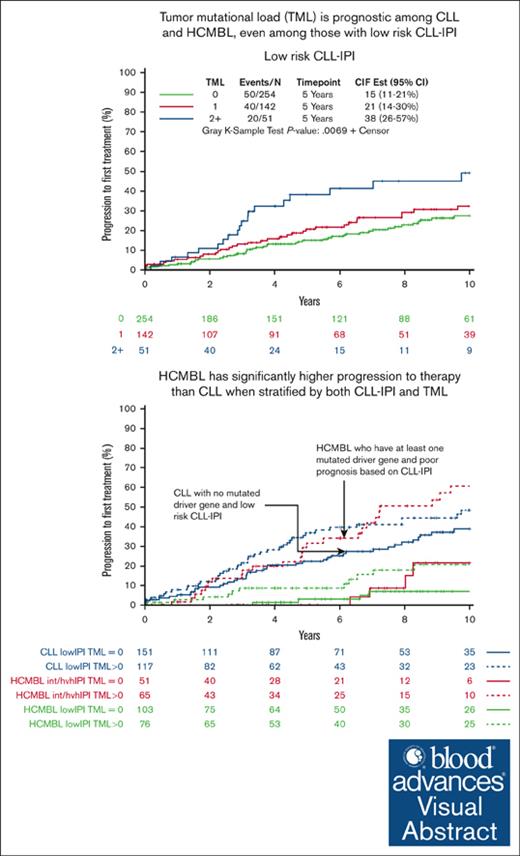
Next, we evaluated the association between TML and TTFT stratified by CLL-IPI to see whether further separation of risk among individuals with HCMBL could be achieved. We found that TML was prognostic for TTFT within each of the CLL-IPI levels (all continuous TML P < .05; Table 2). Among those individuals with HCMBL who were low risk according to CLL-IPI, the 5-year cumulative incidence to requiring therapy for those with any mutated gene was 9% compared with 3% with no mutated genes, and the 10-year cumulative incidence was 21% compared with 7%, respectively. In Cox regression analyses, this corresponds to 4.3-fold increased risk of therapy (95% CI, 1.6-11.8; P = .005; Table 2). Among those individuals with HCMBL who were intermediate CLL-IPI risk, those with any mutation had threefold increased risk of therapy (95% CI, 1.0-9.0; P = .045; Table 2). Among the high- or very high–risk CLL-IPI, 9 individuals with HCMBL were treated, and all 9 had a at least 1 gene with a high-impact or hot spot mutation. None of the individuals with HCMBL with no mutated genes were treated, precluding us from evaluating the binary effect of TML (Table 2). However, the continuous TML was associated with TTFT (HR = 1.5; 95% CI, 1.1-2.1; P = .019).
More aggressive disease among HCMBL compared with low-risk CLL
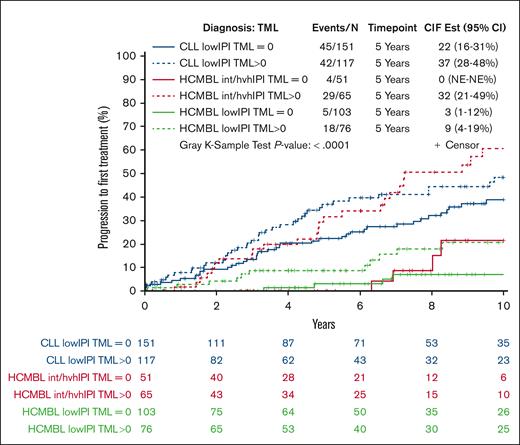
Next, we evaluated whether there were situations in which individuals with HCMBL had a rate of progression to therapy similar or worse than that of patients with low-risk CLL when considering both TML and CLL-IPI status. To answer this, we stratified the HCMBL individuals by CLL-IPI (CLL-IPI low risk vs CLL-IPI intermediate/high/very high risk) and TML status (TML = 0 vs TML > 0) and compared these individuals with HCMBL with patients with low-risk CLL (defined as those with CLL-IPI low risk and TML = 0). We found that individuals with HCMBL with TML >0 and CLL-IPI intermediate/high/very high risk had a 5-year rate of progression to therapy of 32%, higher than the 22% among patients with low-risk CLL (ie, CLL-IPI low risk and TML = 0; HR = 1.93; 95% CI, 1.2-3.1; P = .007, Figure 4).
HCMBL and CLL stratified by both TML and CLL-IPI for association with TTFT. Cumulative incidence plot of progression to first treatment among individuals with HCMBL and CLL stratified by TML (any vs no mutations) and CLL-IPI (low risk vs more than low risk). TML is the number of genes out of 59 with high-impact mutations (excluding TP53). CIF Est, cumulative incidence function estimation.
HCMBL and CLL stratified by both TML and CLL-IPI for association with TTFT. Cumulative incidence plot of progression to first treatment among individuals with HCMBL and CLL stratified by TML (any vs no mutations) and CLL-IPI (low risk vs more than low risk). TML is the number of genes out of 59 with high-impact mutations (excluding TP53). CIF Est, cumulative incidence function estimation.
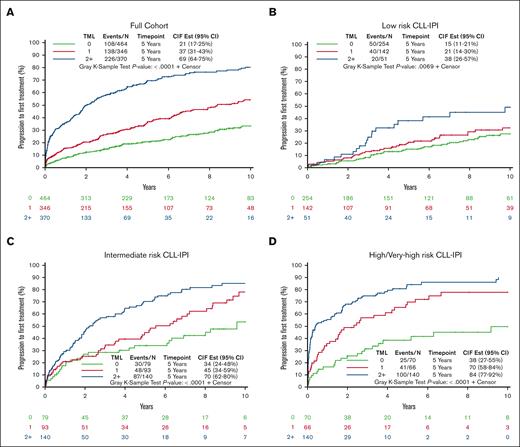
Combined analysis of HCMBL and CLL cohorts to evaluate TML and CLL-IPI with TTFT and OS
Finally, we combined the CLL and HCMBL cohorts to further evaluate the interaction of TML and CLL-IPI with TTFT and OS, while adjusting for MBL/CLL status. In the combined cohort, the median follow-up from sample collection was 7.3 years (range, 0-22.5), and 472 individuals progressed to needing therapy. At 5-years, the rate of progression requiring CLL therapy was 40%; but according to the TML, a clear and significant separation of risk of TTFT was observed (P < .0001; Figure 5A; supplemental Table 5). TML was also strongly associated with TTFT within each of the CLL-IPI levels (all continuous TML P < .0001; Figures 5B-D; supplemental Table 5). In particular, individuals with low risk of progression according to CLL-IPI and with ≥2 mutated genes had a 38% cumulative progression to therapy compared with 15% for those with no mutated genes at 5 years. Even among those with a high or very high CLL-IPI, a clear separation based on the TML was observed within 1 year; for example, estimated 1-year cumulative progression to therapy was 55% among those with ≥2 mutated genes compared with 18% among those with no mutated genes (Figure 4D), resulting in a 3.4-fold shorter TTFT (95% CI, 2.2-5.4; supplemental Table 5).
Associations of TML with TTFT among full cohort and stratified by CLL-IPI. (A) Cumulative incidence plot of progression to first treatment among individuals with either HCMBL or CLL by TML. (B) Cumulative incidence plot of progression to first treatment among individuals with HCMBL or CLL by TML among low-risk CLL-IPI. (C) Cumulative incidence plot of progression to first treatment among individuals with HCMBL or CLL by TML among intermediate-risk CLL-IPI. (D) Cumulative incidence plot of progression to first treatment among individuals with HCMBL or CLL by TML among high-risk or very high–risk CLL-IPI. TML is the number of genes out of 59 with high-impact mutations (excluding TP53). CIF Est, cumulative incidence function estimation.
Associations of TML with TTFT among full cohort and stratified by CLL-IPI. (A) Cumulative incidence plot of progression to first treatment among individuals with either HCMBL or CLL by TML. (B) Cumulative incidence plot of progression to first treatment among individuals with HCMBL or CLL by TML among low-risk CLL-IPI. (C) Cumulative incidence plot of progression to first treatment among individuals with HCMBL or CLL by TML among intermediate-risk CLL-IPI. (D) Cumulative incidence plot of progression to first treatment among individuals with HCMBL or CLL by TML among high-risk or very high–risk CLL-IPI. TML is the number of genes out of 59 with high-impact mutations (excluding TP53). CIF Est, cumulative incidence function estimation.
In the combined cohort, 351 individuals died. The 5-year OS rate was 89% (supplemental Figure 1A). When evaluating OS according to TML, we observed a significant difference in survival (P < .0001; supplemental Figure 1B); the 5-year OS rate was 92% for those with no mutated genes and 82% for those with ≥2 mutated genes. After adjusting for CLL-IPI, sex, and diagnosis (MBL/CLL), the association of TML with OS held in Cox regression analyses; individuals with ≥2 mutated genes had 1.4-fold higher mortality than those without mutated genes (95% CI, 1.1-1.9; P = .017; supplemental Table 6). TML did not provide additional information for OS within low and intermediate CLL-IPI levels. However, in the high- or very high–risk CLL-IPI, individuals with any mutated gene had twofold higher mortality than those without a mutated gene (95% CI, 1.3-3.2; supplemental Table 6).
Discussion
To our knowledge, in the largest study to date of 371 individuals with HCMBL, we analyzed the tumor mutations of 59 genes recurrently mutated in CLL and found that a higher total number of mutated genes (or the TML) was associated with a greater risk of progression to therapy, independent of CLL-IPI, extending our prior study of 112 individuals with HCMBL.25 Moreover, we found that accounting for both CLL-IPI and TML, we identified individuals with HCMBL with worse prognosis than patients with low-risk CLL.
HCMBL is a fairly common condition with a prevalence of ∼0.2% to 1% in the general population31,32 or 2% among individuals with a strong family history of CLL.2 Typically, individuals with HCMBL are clinically identified due to incidental findings with lymphocytosis or through assessments from another clinical condition. Once identified, individuals with HCMBL are often evaluated annually with a complete blood count and physical examination. The understanding is that these individuals with HCMBL have a small risk of progression to CLL requiring therapy of about 10% to 20% in 10 years (or ∼1%-2% per year).8-10 We recently showed that we could improve this estimate of progression to therapy using the CLL-IPI.11 Specifically, individuals with HCMBL with low risk according to CLL-IPI had a 15% cumulative risk of progression requiring therapy at 10 years compared with 68% risk among those with intermediate CLL-IPI and 82% progression risk among those with high-risk CLL-IPI. Herein, we demonstrated we can further improve progression estimates, particularly among those individuals in the CLL-IPI low-risk group, by also considering somatic mutations in CLL driver genes.
In our prior work of 112 individuals with HCMBL,24 we found that those with ≥2 mutated genes had a 4.1-fold increased risk of progression requiring therapy independent of CLL-IPI. Herein, with the larger sample size and longer follow-up, we reported a significant 5.5-fold increased risk, adjusting for CLL-IPI. Importantly, the TML was able to further stratify risk of progression to therapy within the risk levels of CLL-IPI. Among individuals with low risk according to CLL-IPI, individuals with HCMBL with no mutated genes had a 7% cumulative incidence of progression in 10 years compared with 21% among those with detectable mutations. This refinement of stratification compares favorably with the previously reported 15% based only on CLL-IPI (ie, ignoring TML). Knowledge of a lower risk (on average) of progression based on both CLL-IPI and TML may allow for clinicians to reassure a large segment of individuals with HCMBL about their prognosis and potentially may reduce the need for annual follow-up visits. In addition, for future clinical trials wanting to study early intervention, our results herein support that a combination of TML and CLL-IPI would be invaluable for risk stratification and the possibility of including individuals with HCMBL.
By definition, individuals with HCMBL have lower clonal B-cell counts than patients with CLL and are at a precursor stage to CLL. Our results, based on somatic sequencing, also supported that individuals with HCMBL are, in general, at an earlier pathobiological stage than that of CLL. We saw this by the lower number of high-impact mutations, lower individual gene frequencies, and lower TML distribution in HCMBL than in CLL. However, based on both CLL-IPI and TML, we observed individuals with HCMBL with a more aggressive clinical course as evidenced by shorter time to therapy than patients with CLL with low-risk disease based on CLL-IPI and TML. These results suggest that using TML and CLL-IPI, in addition to clonal B-cell count, may better detect individuals at high risk.
Finally, in the full cohort of individuals with MBL or CLL, the TML was a significant prognostic factor for OS, independent of CLL-IPI, sex, and MBL/CLL diagnosis. Interestingly, we found that individuals with any mutated gene at diagnosis will have a higher risk of dying. Even among the individuals in the high- or very high–risk group according to CLL-IPI, any mutated gene was significantly prognostic for OS.
Strengths of this study included using a large cohort of newly diagnosed individuals with HCMBL compared with a cohort of patients with CLL. All of our study participants were treatment naïve at the time of blood sample collection and had extensive annotated clinical data, allowing for us to account for the effect of CLL-IPI, a well-established CLL prognostic factor that has also been shown to have clinical utility for stratifying TTFT among individuals with HCMBL.11 Our sequencing depth was >1000× in most individuals, allowing for the detection of small clones on a comprehensive set of recurrently mutated CLL genes. This study also had several limitations. Among the individuals with HCMBL, we were not able to fully evaluate the association of TML with TTFT within the high- or very high–risk CLL-IPI category due to limited number of events. Second, our targeted sequencing panel contained 59 genes, whereas a recent publication of whole exome sequencing in >1000 patients with CLL identified 37 additional genes not included here.18 These new putative CLL driver genes had frequencies <1.5%, and thus, we would not expect them to have a significant impact on the conclusions of our study. Finally, our definition of TML assumed that all somatic mutations have equal weight and thus the same clinical impact on outcomes, regardless of type of high-impact mutation, the direction of effect the gene has on TTFT, or which gene the mutation was located. Alternative approaches could provide different weights for each mutation type or mutated gene. By adding weights, the TML score would be expected to better refine prognostication, similar to what has been observed with inherited common variants and the polygenic risk scores.29,33
In summary, our study highlighted that the TML can identify individuals with HCMBL who are at high risk of progression to CLL, requiring therapy beyond CLL-IPI. These findings are similar to and support our previous findings of the effect of TML among patients with CLL. More importantly, we observed individuals with HCMBL considered to have a low risk of progression based on the CLL-IPI could be further stratified into lower or higher risk of progression based on the TML. Although HCMBL is considered to be a precursor state to CLL, our study identified individuals with HCMBL who have a more aggressive clinical course than patients with low-risk CLL based on both TML and CLL-IPI. Our findings may help future clinical practice guidelines.
Acknowledgments
The authors thank the study participants for their generosity of time in participating in our study.
This work was supported by the National Institutes of Health grants R01AG58266, R21CA256648, R01CA235026, R01CA258465, and P50CA97274; and by the Zuckerman STEM Leadership Program.
Authorship
Contribution: G.K., S.L.S., E.B., and S.A.P. conceptualized and designed the study; S.L.S., E.B., and S.A.P. performed acquisition of data; S.L.S., C.A., and K.G.R. analyzed data; all authors interpreted data; G.K. and S.L.S. drafted the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: S.A.P. reports that research funding has been provided to the institution from Janssen, AstraZeneca, Merck, and Genentech for clinical studies in which S.A.P. is a principal investigator; and honoraria has been provided to the institution from Pharmacyclics, Merck, AstraZeneca, Janssen, Genentech, Amgen, MingSight Pharmaceuticals, TG Therapeutics, Novalgen Limited, Kite Pharma, and AbbVie for S.A.P.’s participation in consulting activities/advisory board meetings. N.E.K. reports advisory board fee from AbbVie, AstraZeneca, BeiGene, Behring, Boehringer Ingelheim Pharmaceuticals, Inc, Dava Oncology, Janssen, Juno Therapeutics, Pharmacyclics; data safety monitoring committee fee from Agios Pharm, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Dren Bio, and Janssen; and research funding from AbbVie, Acerta Pharma, BMS, Celgene, Genentech, Pharmacyclics, Sunesis, and Vincerx. The remaining authors declare no competing financial interests.
Correspondence: Susan L. Slager, Divisions of Hematology and Computational Biology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: slager.susan@mayo.edu.
References
Author notes
S.A.P., E.B., and S.L.S. jointly supervised this study.
Deidentified individual participant data are available upon request to the corresponding author, Susan L. Slager (slager.susan@mayo.edu).
The full-text version of this article contains a data supplement.

![Characteristics of somatic mutations between HCMBL and CLL. (A) Heat map representing the distribution of high-impact and hot spot mutations in 59 genes frequently mutated in CLL and HCMBL. Each column represents a single individual from our cohort. The external bar indicates individuals with HCMBL (gray) or CLL (red) diagnosis; the second bar stratifies the heat map by CLL-IPI (low risk [green], intermediate risk [yellow], and high/very high risk [red]); the third bar represents the TML distribution (ie, the number of mutated genes per individual); then the next 5 bars represent clinical components: Rai stage (0-gray; I-green; II-yellow; III-red; and IV-purple), sex, IgHV, B2M, and del(17p) by fluorescence in situ hybridization. Finally, the internal bars represent the list of mutated genes in our study color-coded by the type of mutation: missense (yellow), in frame (gray), frameshift (red), nonsense (purple), splice (blue), and >1 mutation (green). (B) Distribution of the variant allele fraction of high-impact and hot spot mutations between individuals with CLL and HCMBL. (C) Distribution of mutation type between individuals with HCMBL and CLL. B2M, Beta-2 microglobulin; FISH17p, fluorescence in situ hybridization 17p deletion.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/9/10.1182_bloodadvances.2023012242/2/m_blooda_adv-2023-012242-gr1.jpeg?Expires=1769249372&Signature=CsPf4ZTHo-9pdKZNrGgFacSeBMFg92SCIfDQX4TsfygMsE25hKG3~XEa5SwAb4k2HwbF7I1ptB4~Uc2gUQQ4yquNRgO90hKP9eBvVDeM1DkLnylkR2Z7kgXGWRlbeMibp28NxjPjPee73K~PiB2iSoNIlplp56MGVvF4VsgxMKAXYRpooXs~gxfI5eOqogFmHeNXqlaAYt1QoGiLZp9WgSwZw~FSbi5mIDa6WyeEW9rkEldO~sDZRPMXTwcvLgS6jF-yZSa28M7BE1mxTdUYiEPTNaPjiHI6sT2uVFbm2AqKQOanMMb3P9wqC4gqVMEF6Emi7MjhLwQxji549fRSVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)