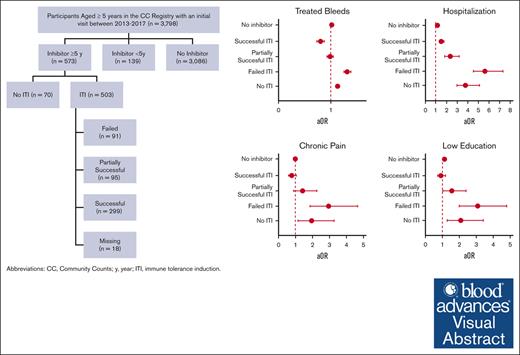
Immune tolerance induction was associated with less bleeding, less health care use, less chronic pain, and improved function.
Immune tolerance induction was not associated with unemployment or mortality.
Visual Abstract
Although the near-term benefit of immune tolerance induction (ITI) for the treatment of people with severe hemophilia A with inhibitor is apparent, the magnitude of the longer-term impact of ITI on clinical outcomes remains undefined. We examined the association between receiving ITI and the success of ITI on clinical outcomes including (1) clinical events, (2) health care use, (3) quality of life/function, (4) socioeconomic status, and (5) death, using the Community Counts (CC) registry of US Hemophilia Treatment Centers between 2013 and 2017. Multivariate logistic regression, negative binomial, and Poisson models were used. Included in this study were 3659 people with severe hemophilia A with median age of 21 years when entering the CC registry. Among 576 participants with inhibitors, 485 had received ITI (84%). ITI was successful in 299 (61.7%) and partially successful or failed in 95 (19.5%) or 91 (18.7%), respectively. Those that received ITI had fewer treated bleeds, less chronic pain, better function, and higher educational attainment than those not receiving ITI. Successful vs partially successful and failed ITI was associated with fewer treated bleeds, less health care use, less chronic pain, better function, and fewer missed days of school or work. Mortality was not associated with ITI, regardless of its success. Those with successful ITI had similar rates of treated bleeds, chronic pain, and health care use as those with no inhibitors. Undergoing ITI, particularly if successful, improved clinical outcomes but not mortality. These findings support decision making regarding initiation of ITI and inform future clinical trials.
Introduction
Formation of an immunogenic response to factor VIII (FVIII) remains a major complication of hemophilia A treatment.1,2 Neutralizing anti-FVIII antibody (inhibitor) leads to a lack of response to FVIII replacement therapy. Immune tolerance induction (ITI) is the main mode of inhibitor eradication.3 Although standard of care, not all people with hemophilia/families are offered ITI, choose to pursue ITI, or can adhere to the regimen because it requires frequent infusions of FVIII and can be difficult to complete. The benefit of ITI in restoring the capacity to prevent and treat bleeding with FVIII has been understood by patients and providers alike. However, now that emicizumab, an effective therapy for maintenance of hemostasis for people with hemophilia A including those with inhibitor, is available, the utility of ITI has been reevaluated and is debated.4,5
Markers of less-than-optimal care, in addition to a high annualized bleed rate, typically result from musculoskeletal destruction and include chronic pain, functional limitations, and subsequent joint procedures and/or disability. Additional consequences include use of the emergency department (ED) or hospitalization for bleeding-related events and inability to engage with school or work, leading to low educational attainment, unemployment, and missed days of school or work. The greatest risk of inadequate treatment is death. Although inhibitors were previously associated with increased mortality,6 more recent analyses of mortality have yielded conflicting results.7,8
Although the benefits of ITI in restoring responsiveness to FVIII are well known, there is limited understanding of the magnitude of its benefit and impact on long-term and patient-reported outcomes. Understanding the impact of ITI on markers of less-than-optimal care before emicizumab can inform how ITI is used in the future with or without emicizumab. We hypothesized that these clinical outcomes are better (lower health care use, less functional limitations, fewer joint procedures, less disability, greater educational attainment, less unemployment, and fewer missed days of school or work) among those that received ITI, most notably if ITI was successful.
Methods
Study population
This study used data from the Community Counts (CC) Registry for Bleeding Disorders Surveillance, a collaborative project of the US Hemophilia Treatment Center Network, American Thrombosis & Hemostasis Network, and Centers for Disease Control and Prevention.9 The CC registry collects data from people with hemophilia who received care from 139 hemophilia treatment centers (HTCs) across the United States and authorized to have their information included in the registry. The CC registry began in 2012 as an extension of the previous Universal Data Collection program.9 Because the data obtained for this study were deidentified, the study was considered nonhuman subject research by the Emory University institutional review board.
In the CC registry, the clinical histories of people with hemophilia were collected along with contemporary sociodemographic, clinical, and laboratory data on initial visit forms.9 During the initial visit, participants were enrolled in the registry, and historical and contemporary data were collected on these forms. It may, or may not, have been the first visit at the HTC. Surveillance forms containing updated clinical information (eg, inhibitor status and treatment) were obtained on an approximately annual basis. Information on deaths was captured from 30 September 2011 via a standardized mortality form.
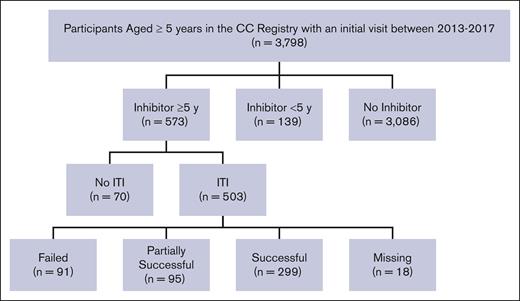
In the registry there were 3798 participants aged ≥5 years with an HTC visit between 2013 and 2017 (Figure 1). Participants aged ≥5 years with and without inhibitors were selected to allow ample time to receive and respond to ITI and to ensure a comparable age distribution between groups. The 5-year cutoff point was also chosen because it allowed enough time for outcomes to be measured. Time since inhibitor onset was based on the difference between the participant’s age at entering the registry and the age at inhibitor detection. If the age of inhibitor onset was unknown on the initial form, participants were included if they had an inhibitor history and were aged ≥18 years, or if they were identified as having an inhibitor for ≥5 years on subsequent forms. Inhibitors were defined based on documentation of at least 2 positive inhibitor titers (>1 Nijmegen-Bethesda units or ≥0.5 chromogenic Bethesda units) or documentation of at least 1 positive inhibitor titer and inhibitor-related treatment (ITI, bypassing therapy use, or immune modulation therapy; supplemental Table 1). We excluded 139 participants because they had an inhibitor history for <5 years (Figure 1). Of the remaining participants, 3086 (81.2%) people had not developed inhibitor, hereafter referred to as “people without inhibitors,” and 573 (15.0%) participants had an inhibitor history recorded ≥5 years before their initial visit, referred to as “people with inhibitors.” We examined ITI treatment and outcomes reported between 2013 and 2017 because we were interested in the period before the US Food and Drug Administration’s approval of emicizumab.10
Study population selection among people with severe hemophilia A in the CC registry (2013-2017). CC, Community Counts; y, year; ITI, immune tolerance induction.
Study population selection among people with severe hemophilia A in the CC registry (2013-2017). CC, Community Counts; y, year; ITI, immune tolerance induction.
Measures
Among people with inhibitors, ITI treatment was considered along with response, categorized as: successful tolerance, partially successful tolerance, and failed. People reported to be receiving FVIII replacement therapy at standard doses were considered to have achieved successful tolerance, and those using FVIII replacement therapy but requiring increased dosing, were considered to have partial tolerance (supplemental Table 1). What constituted a standard or increased dose was at the discretion of the HTC. If bypassing agents were still administered, ITI was considered failed. ITI tolerance was coded based on treatment information when a participant entered the registry.
Outcomes included self-reported dimensions of (1) clinical events; (2) health care use; (3) quality of life/function; (4) socioeconomic status, based on the initial registry form; and (5) death, based on the mortality form filled out by HTC staff. Details on outcomes are outlined in supplemental Table 1. Briefly, clinical events included the number of bleeds treated in the past 12 months and history of any invasive joint procedure (yes/no). Health care use included self-reported ED visits or hospitalization in the past 12 months. Quality-of-life measures included chronic pain in the past 12 months and limited functional status. For functional status, participants were asked how pain, loss of motion or weakness due to joint disease currently influenced their overall activity level; people who said that they currently “require assistance” or were “limited” in activities were coded as having limited function, whereas those responding “unrestricted” were coded as not being limited. Missed days were assessed among those who stated in the initial form that they were a student or employed by asking the number of school or workdays missed because of a bleeding disorder in the past 12 months. Measures of socioeconomic status included educational attainment, employment, and disability. Education was analyzed among adults aged ≥22 years because those <22 may still be in school, and low education was defined as having a high school degree or less. Employment was assessed among working-aged adults, defined as those aged 22 to 64 years. Disability was defined as people who reported that they were “unemployed because of disability” or were <65 years of age and insured with Medicare. Mortality was defined as dying from any cause between 30 September 2001 (when mortality data collection began) and 31 December 2018 (the end of study period). Person years (PYs) were computed from the year at which a person entered the CC registry until the year of death or end of the study period (2018). If a person died during the same year of entering the CC registry, a half (0.5) PY was assigned because exact dates of death (months, days) were not available.
Covariates included age at initial registry visit (5-10, 11-20, 21-30, 31-40, 41-60, and ≥60 years), race and ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, and other), and prior infection with HIV (yes/no) or hepatitis C (HCV; yes/no). Insurance was coded as commercial, Medicaid, Medicare, uninsured, or other.
Statistical analyses
Sociodemographic characteristics and outcomes according to ITI treatment and tolerance were compared using 2-sided Fisher's exact, χ2, or F tests (α = .05). Several treatment/inhibitor categories were analyzed with multivariable models. Combinations of ITI treatment/tolerance (no ITI; or successful, partially successful, and failed ITI) were compared with a group of people without an inhibitor history. Among people with an inhibitor history, receiving ITI vs no ITI treatment was compared to determine the net association of ITI with clinical outcomes. Among people receiving ITI, successful vs partial/failed ITI were also compared to determine whether clinical outcomes varied according to ITI response. In sensitivity analyses, tolerance was categorized according to the most recent follow-up form during 2013 to 2017 to better understand the influence of initial vs eventual ITI tolerance on outcomes. As noted earlier, follow-up forms are collected on an annual basis, to capture changes in treatments and outcomes.
Multivariate logistic regression models, accounting for age, HIV/HCV infection, and race and ethnicity were used to estimate adjusted odds ratios (aORs) and corresponding 95% confidence intervals (CIs) for all outcomes except the number of treated bleeds, missed school/workdays, and mortality. For the number of treated bleeds and missed days, zero-inflated negative binomial models were used to estimate adjusted rate ratios (aRRs) to account for overdispersion as >50% of participants reported having no treated bleeds or missed days of school or work, and the variance of these outcomes exceeded the mean. For mortality, crude and age-adjusted death rates were computed as the number of deaths per PY, and aRRs were computed with Poisson models.
Results
Among the 3659 participants included in the study, the median age when entering the CC registry was 21 years (interquartile range [IQR], 13-33; Table 1), and was slightly lower in those with (19 years) vs without (22 years) inhibitor (P < .001). In most people with inhibitor (77%), inhibitor was detected at aged ≤5 years, with a median age of inhibitor detection of 1.8 years (IQR, 1-4.5). Among all participants included in the study, 12.0% and 29.2% had been infected with HIV and HCV, respectively; these proportions were higher in people without (HIV, 13.5%; HCV, 30.5%), than with (HIV 5.5%; HCV 24%), inhibitor (Table 1).
Study population characteristics according to inhibitor status and ITI treatment among people with severe hemophilia A
| Categories . | Participants with, and without, an inhibitor . | ITI treatment among participants with an inhibitor . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | No inhibitor . | Inhibitor . | Inhibitor vs no inhibitor . | No ITI . | ITI . | ITI vs no ITI . | Failed . | Partially successful . | Successful . | Successful vs partial vs failed . | |
| N = 3659 . | n = 3086 . | n = 576 . | P value . | n = 70 . | n = 485 . | P value . | n = 91 . | n = 95 . | n = 299 . | P value . | |
| n (%) | |||||||||||
| Race/ethnicity∗ | <.001 | .71 | .009 | ||||||||
| Asian | 173 (4.8) | 145 (4.8) | 30 (5.3) | † | 24 (5) | 6 (6.6) | † | 14 (4.7) | |||
| Black | 529 (14.6) | 415 (13.6) | 115 (20.2) | 15 (21.4) | 95 (19.8) | 17 (18.7) | 25 (26.9) | 53 (18.0) | |||
| Hispanic | 540 (14.9) | 437 (14.3) | 102 (17.9) | 15 (21.4) | 83 (17.3) | 20 (22) | 14 (15.1) | 49 (16.6) | |||
| White | 2350 (65) | 2031 (66.6) | 321 (56.3) | 36 (51.4) | 275 (57.4) | 48 (52.7) | 50 (53.8) | 177 (60.0) | |||
| Insurance | .002 | <.001 | < .001 | ||||||||
| Commercial | 1862 (52) | 1601 (53) | 260 (45.9) | 22 (32.4) | 231 (48.2) | 39 (43.3) | 48 (51.1) | 144 (48.8) | |||
| Medicaid | 1209 (33.7) | 979 (32.4) | 227 (40) | 22 (32.4) | 200 (41.8) | 40 (44.4) | 44 (46.8) | 116 (39.3) | |||
| Medicare | 313 (8.7) | 265 (8.8) | 53 (9.3) | 19 (27.9) | 28 (5.8) | † | † | 20 (6.8) | |||
| Other | 199 (5.6) | 173 (5.7) | 27 (4.8) | † | 20 (4.2) | † | † | 15 (5.1) | |||
| HIV | 424 (12) | 395 (13.2) | 31 (5.5) | <.001 | 13 (19.7) | 16 (3.4) | <.001 | † | † | 10 (3.4) | < .001 |
| HCV | 1039 (29.2) | 915 (30.5) | 135 (24) | .001 | 40 (59.7) | 82 (17.2) | <.001 | 18 (20) | 10 (10.9) | 54 (18.3) | < .001 |
| Prophylaxis‡ | 3085 (84.3) | 2451 (79.5) | 412 (71.5) | <.001 | 32 (35.2) | 380 (78.4) | <.001 | 40 (44.0) | 74 (77.9) | 266 (89.0) | < .001 |
| Median (IQR) | |||||||||||
| Age at enrollment, y | 21 (13-33) | 22 (13-34) | 19 (12-28) | <.001 | 34 (19-52) | 18 (12-26) | <.001 | 19 (11-28) | 17 (10-23) | 18 (12-26) | .068 |
| Age of inhibitor detection, y | -- | -- | 1.8 (1-4.5) | -- | 6 (2.2- 22) | 1.7 (1- 3.8) | <.001 | 1.6 (0.9-4.2) | 1.2 (0.8-2.3) | 1.8 (1-3.8) | .351 |
| Categories . | Participants with, and without, an inhibitor . | ITI treatment among participants with an inhibitor . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | No inhibitor . | Inhibitor . | Inhibitor vs no inhibitor . | No ITI . | ITI . | ITI vs no ITI . | Failed . | Partially successful . | Successful . | Successful vs partial vs failed . | |
| N = 3659 . | n = 3086 . | n = 576 . | P value . | n = 70 . | n = 485 . | P value . | n = 91 . | n = 95 . | n = 299 . | P value . | |
| n (%) | |||||||||||
| Race/ethnicity∗ | <.001 | .71 | .009 | ||||||||
| Asian | 173 (4.8) | 145 (4.8) | 30 (5.3) | † | 24 (5) | 6 (6.6) | † | 14 (4.7) | |||
| Black | 529 (14.6) | 415 (13.6) | 115 (20.2) | 15 (21.4) | 95 (19.8) | 17 (18.7) | 25 (26.9) | 53 (18.0) | |||
| Hispanic | 540 (14.9) | 437 (14.3) | 102 (17.9) | 15 (21.4) | 83 (17.3) | 20 (22) | 14 (15.1) | 49 (16.6) | |||
| White | 2350 (65) | 2031 (66.6) | 321 (56.3) | 36 (51.4) | 275 (57.4) | 48 (52.7) | 50 (53.8) | 177 (60.0) | |||
| Insurance | .002 | <.001 | < .001 | ||||||||
| Commercial | 1862 (52) | 1601 (53) | 260 (45.9) | 22 (32.4) | 231 (48.2) | 39 (43.3) | 48 (51.1) | 144 (48.8) | |||
| Medicaid | 1209 (33.7) | 979 (32.4) | 227 (40) | 22 (32.4) | 200 (41.8) | 40 (44.4) | 44 (46.8) | 116 (39.3) | |||
| Medicare | 313 (8.7) | 265 (8.8) | 53 (9.3) | 19 (27.9) | 28 (5.8) | † | † | 20 (6.8) | |||
| Other | 199 (5.6) | 173 (5.7) | 27 (4.8) | † | 20 (4.2) | † | † | 15 (5.1) | |||
| HIV | 424 (12) | 395 (13.2) | 31 (5.5) | <.001 | 13 (19.7) | 16 (3.4) | <.001 | † | † | 10 (3.4) | < .001 |
| HCV | 1039 (29.2) | 915 (30.5) | 135 (24) | .001 | 40 (59.7) | 82 (17.2) | <.001 | 18 (20) | 10 (10.9) | 54 (18.3) | < .001 |
| Prophylaxis‡ | 3085 (84.3) | 2451 (79.5) | 412 (71.5) | <.001 | 32 (35.2) | 380 (78.4) | <.001 | 40 (44.0) | 74 (77.9) | 266 (89.0) | < .001 |
| Median (IQR) | |||||||||||
| Age at enrollment, y | 21 (13-33) | 22 (13-34) | 19 (12-28) | <.001 | 34 (19-52) | 18 (12-26) | <.001 | 19 (11-28) | 17 (10-23) | 18 (12-26) | .068 |
| Age of inhibitor detection, y | -- | -- | 1.8 (1-4.5) | -- | 6 (2.2- 22) | 1.7 (1- 3.8) | <.001 | 1.6 (0.9-4.2) | 1.2 (0.8-2.3) | 1.8 (1-3.8) | .351 |
Data were collected from the CC registry between 2013 to 2017. The following number of patients were missing data on race/ethnicity (n = 44); insurance (n = 77); HIV infection (n = 114); HCV infection (n = 99).
“Other” race/ethnicities are “non-Hispanic,” and data are not included because of small sample sizes.
Data suppressed because of small sample sizes of <5.
Continuous prophylaxis as reported by the HTC.
Among persons with inhibitors, most (87.8%) received ITI, and those receiving ITI were younger (median age, 18 years) at enrollment than people who had not received ITI (median age, 34 years; P < .001; Table 1). Among people receiving ITI, 61.6% had successful tolerance, 19.6% partially successful tolerance, and 18.8% had failed ITI. The median age was similar across ITI tolerance categories (successful, partially successful, and failed; Table 1). Most of those with successful (89.0%) or partially successful (77.9%) ITI were receiving continuous prophylaxis whereas less than half of those with failed (44.0%) or no (35.2%) ITI received prophylaxis treatment (Table 1).
Clinical events
The median number of treated bleeds in the past 12 months was 3 bleeds (IQR, 1-10), ranging from 2 to 3 bleeds in people with no inhibitors and those with successful or partially successful ITI, to 6 bleeds in people with inhibitors and no or failed ITI (P < .001; Table 2). Those with successful ITI had similar treated bleeding rates as people with no inhibitors, and 18% less treated bleeds relative to people with inhibitors and failed or partially successful ITI (aOR, 0.82; 95% CI, 0.76-0.89); Figure 2; Table 3). Overall, receipt of ITI was associated with a nearly 30% lower bleeding rate than with no ITI (aOR, 0.71; 95% CI, 0.67-0.74; Table 3).
Outcomes according to inhibitor history and ITI treatment among people with severe hemophilia A
| . | Participants with, and without, an inhibitor . | ITI treatment among participants with inhibitor . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | No inhibitor . | Inhibitor . | Inhibitor vs no inhibitor . | No ITI . | ITI . | No ITI vs ITI . | Successful ITI . | Partially successful ITI . | Failed ITI . | Successful ITI vs partially successful/failed ITI . | |
| N = 3659 . | n = 3086 . | n = 576 . | P value . | n = 70 . | n = 485 . | P value . | n = 91 . | n = 95 . | n = 299 . | P value . | |
| Clinical outcomes | |||||||||||
| Median number of treated bleeds (IQR) | 3 (1-10) | 3 (1-10) | 3, (1-9) | .688 | 6 (2-19) | 3 (1-8) | .081 | 3 (1-8) | 2 (0-7) | 6 (4-15) | <.001 |
| Joint procedures, % (any) | 28.5 | 28.6 | 28.0 | .781 | 39.1 | 25.7 | .020 | 23.9 | 22.1 | 38.5 | .132 |
| Health care use | |||||||||||
| ED visits, % (past 12 mo) | 21.8 | 20.4 | 29.2 | <.001 | 31.9 | 29.1 | .639 | 21.7 | 30.5 | 48.9 | <.001 |
| Hospitalization, % (past 12 mo) | 12.0 | 10.1 | 21.3 | <.001 | 33.8 | 19.6 | .007 | 13.4 | 20.4 | 38.9 | <.001 |
| Quality of life | |||||||||||
| Chronic pain, % (past 12 mo) | 39.8 | 39.6 | 41.0 | .494 | 67.7 | 36.6 | <.001 | 37.8 | 56.8 | 65.9 | .002 |
| Limited function, % (current) | 24.7 | 24.1 | 27.7 | .064 | 55.2 | 23.0 | <.001 | 17.9 | 19.3 | 43.8 | <.001 |
| Median number of missed days, past 12 mo (IQR)∗ | 0 (0-2) | 0 (0-2) | 0 (0-4) | <.001 | 5 (0-20) | 0 (0-2) | .182 | 0 (0-2) | 1 (0-2) | 2 (0- 10) | <.001 |
| Socioeconomic status | |||||||||||
| Unemployed, %† | 37.6 | 35.9 | 48.3 | .001 | 51.3 | 41.7 | .108 | 39.8 | 32.1 | 58.1 | .099 |
| Disabled, % | 13.5 | 13.3 | 15.1 | .233 | 18.0 | 10.1 | .002 | 12.3 | 11.4 | 8.9 | .410 |
| Low education, %‡ | 37.0 | 36.6 | 39.1 | .469 | 37.2 | 34.8 | .531 | 30.3 | 39.3 | 38.2 | .529 |
| Mortality | |||||||||||
| Deaths, n | 61 | 48 | 13 | 6 | 7 | 6 | -- | -- | -- | ||
| PYs | 9748 | 8152 | 1596 | 242.5 | 1304 | ||||||
| Crude rate (per 1000 PYs) | 6.3 | 5.8 | 8.2 | .550 | 24.7 | 5.4 | .012 | ||||
| Age-adjusted rate (per 1000 PYs) | 25.1 | 21.9 | 46.2 | .231 | 46.8 | 26.9 | .477 | ||||
| . | Participants with, and without, an inhibitor . | ITI treatment among participants with inhibitor . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | No inhibitor . | Inhibitor . | Inhibitor vs no inhibitor . | No ITI . | ITI . | No ITI vs ITI . | Successful ITI . | Partially successful ITI . | Failed ITI . | Successful ITI vs partially successful/failed ITI . | |
| N = 3659 . | n = 3086 . | n = 576 . | P value . | n = 70 . | n = 485 . | P value . | n = 91 . | n = 95 . | n = 299 . | P value . | |
| Clinical outcomes | |||||||||||
| Median number of treated bleeds (IQR) | 3 (1-10) | 3 (1-10) | 3, (1-9) | .688 | 6 (2-19) | 3 (1-8) | .081 | 3 (1-8) | 2 (0-7) | 6 (4-15) | <.001 |
| Joint procedures, % (any) | 28.5 | 28.6 | 28.0 | .781 | 39.1 | 25.7 | .020 | 23.9 | 22.1 | 38.5 | .132 |
| Health care use | |||||||||||
| ED visits, % (past 12 mo) | 21.8 | 20.4 | 29.2 | <.001 | 31.9 | 29.1 | .639 | 21.7 | 30.5 | 48.9 | <.001 |
| Hospitalization, % (past 12 mo) | 12.0 | 10.1 | 21.3 | <.001 | 33.8 | 19.6 | .007 | 13.4 | 20.4 | 38.9 | <.001 |
| Quality of life | |||||||||||
| Chronic pain, % (past 12 mo) | 39.8 | 39.6 | 41.0 | .494 | 67.7 | 36.6 | <.001 | 37.8 | 56.8 | 65.9 | .002 |
| Limited function, % (current) | 24.7 | 24.1 | 27.7 | .064 | 55.2 | 23.0 | <.001 | 17.9 | 19.3 | 43.8 | <.001 |
| Median number of missed days, past 12 mo (IQR)∗ | 0 (0-2) | 0 (0-2) | 0 (0-4) | <.001 | 5 (0-20) | 0 (0-2) | .182 | 0 (0-2) | 1 (0-2) | 2 (0- 10) | <.001 |
| Socioeconomic status | |||||||||||
| Unemployed, %† | 37.6 | 35.9 | 48.3 | .001 | 51.3 | 41.7 | .108 | 39.8 | 32.1 | 58.1 | .099 |
| Disabled, % | 13.5 | 13.3 | 15.1 | .233 | 18.0 | 10.1 | .002 | 12.3 | 11.4 | 8.9 | .410 |
| Low education, %‡ | 37.0 | 36.6 | 39.1 | .469 | 37.2 | 34.8 | .531 | 30.3 | 39.3 | 38.2 | .529 |
| Mortality | |||||||||||
| Deaths, n | 61 | 48 | 13 | 6 | 7 | 6 | -- | -- | -- | ||
| PYs | 9748 | 8152 | 1596 | 242.5 | 1304 | ||||||
| Crude rate (per 1000 PYs) | 6.3 | 5.8 | 8.2 | .550 | 24.7 | 5.4 | .012 | ||||
| Age-adjusted rate (per 1000 PYs) | 25.1 | 21.9 | 46.2 | .231 | 46.8 | 26.9 | .477 | ||||
Data were collected from the CC registry between 2013 to 2017; the following number of participants had missing data on ED visits (n = 55); hospitalization (n = 47), bleeding events (n = 754), joint procedures (n = 66); CVAD (n = 149); ICH (n = 126); chronic pain (n = 96); limited function (n = 107), employment (n = 267); and education (n = 114). Bold values are statistically significant.
CVAD, central venous access device inserted; HS, high school; ICH, intracerebral hemorrhage.
Missed days of school and work among students and employed adults, respectively.
Among adults aged 22 to 64 years, unemployed includes those who are unemployed and able, disabled/not able to work, retired, or are still in school.
Defined as high school degree or less, among adults aged ≥22 years.
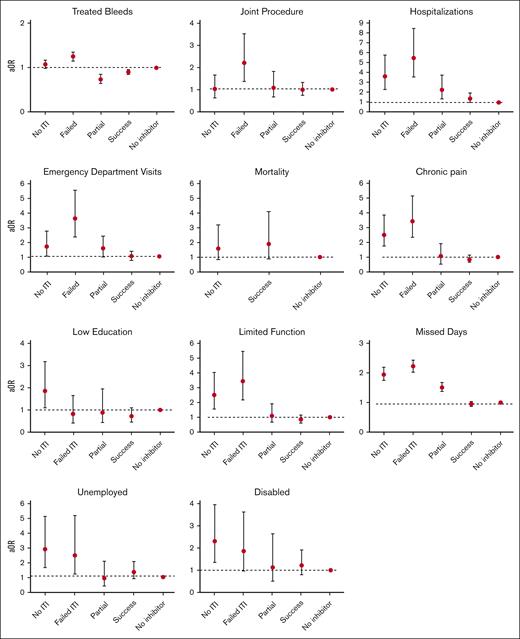
Outcomes according to inhibitor history and ITI treatment in the CC Registry (2013-2017).
Outcomes according to inhibitor history and ITI treatment in the CC Registry (2013-2017).
Health care use, quality of life, clinical events, socioeconomic status, and mortality according to ITI treatment among people with severe hemophilia A with inhibitors
| Number included . | ITI vs no ITI . | Successful ITI vs partially successful or failed ITI . | ||||
|---|---|---|---|---|---|---|
| 555 . | 485 . | |||||
| aOR . | 95% CI . | aOR . | 95% CI . | |||
| Clinical events | ||||||
| Number of treated bleeds∗ | 0.71 | 0.67 | 0.74 | 0.82 | 0.76 | 0.89 |
| Joint procedure | 1.29 | 0.61 | 2.76 | 0.60 | 0.38 | 0.95 |
| Health care use | ||||||
| Hospitalization | 0.30 | 0.15 | 0.58 | 0.36 | 0.23 | 0.58 |
| ED visit | 0.58 | 0.30 | 1.11 | 0.42 | 0.28 | 0.64 |
| Quality of life | ||||||
| Chronic pain | 0.35 | 0.17 | 0.73 | 0.40 | 0.26 | 0.60 |
| Limited function | 0.32 | 0.16 | 0.65 | 0.38 | 0.24 | 0.63 |
| Missed days∗,† | 0.89 | 0.60 | 1.31 | 0.56 | 0.50 | 0.62 |
| Socioeconomic status | ||||||
| Unemployed‡ | 0.42 | 0.15 | 1.22 | 0.81 | 0.42 | 1.57 |
| Disabled | 1.11 | 0.41 | 3.04 | 0.81 | 0.40 | 1.66 |
| Low education§ | 0.36 | 0.14 | 0.93 | 0.86 | 0.43 | 1.74 |
| aRR | 95% CI | aRR | 95% CI | |||
| Death‖ | 0.97 | 0.33 | 2.81 | -- | -- | |
| Death among people aged ≥20 y¶ | 0.68 | 0.20 | 2.31 | -- | -- | |
| Number included . | ITI vs no ITI . | Successful ITI vs partially successful or failed ITI . | ||||
|---|---|---|---|---|---|---|
| 555 . | 485 . | |||||
| aOR . | 95% CI . | aOR . | 95% CI . | |||
| Clinical events | ||||||
| Number of treated bleeds∗ | 0.71 | 0.67 | 0.74 | 0.82 | 0.76 | 0.89 |
| Joint procedure | 1.29 | 0.61 | 2.76 | 0.60 | 0.38 | 0.95 |
| Health care use | ||||||
| Hospitalization | 0.30 | 0.15 | 0.58 | 0.36 | 0.23 | 0.58 |
| ED visit | 0.58 | 0.30 | 1.11 | 0.42 | 0.28 | 0.64 |
| Quality of life | ||||||
| Chronic pain | 0.35 | 0.17 | 0.73 | 0.40 | 0.26 | 0.60 |
| Limited function | 0.32 | 0.16 | 0.65 | 0.38 | 0.24 | 0.63 |
| Missed days∗,† | 0.89 | 0.60 | 1.31 | 0.56 | 0.50 | 0.62 |
| Socioeconomic status | ||||||
| Unemployed‡ | 0.42 | 0.15 | 1.22 | 0.81 | 0.42 | 1.57 |
| Disabled | 1.11 | 0.41 | 3.04 | 0.81 | 0.40 | 1.66 |
| Low education§ | 0.36 | 0.14 | 0.93 | 0.86 | 0.43 | 1.74 |
| aRR | 95% CI | aRR | 95% CI | |||
| Death‖ | 0.97 | 0.33 | 2.81 | -- | -- | |
| Death among people aged ≥20 y¶ | 0.68 | 0.20 | 2.31 | -- | -- | |
Data were collected from the CC registry between 2013 to 2017; models are adjusted for: age, HIV and HCV infection, and race/ethnicity. Bold values are statistically significant.
Abbreviations are explained in Table 2.
Log binomial models to account for overdispersion.
Among students and employed adults.
Among adults aged 22 to 64 years.
Defined as those with a high school degree or less, among adults aged ≥22 years.
Poisson models are adjusted for: age, HIV, HCV infection, and race/ethnicity.
Poisson models are that restricted on age and are adjusted for HIV, HCV infection, and race/ethnicity.
Approximately 30% of participants (28.5%) reported ever having a joint procedure; this proportion ranged from ≤30% in people with no inhibitor and people with successful and partially successful ITI, to nearly 40% in those with failed (38.5%) or no (39.1%) ITI (Table 2). Overall, successful ITI was associated with 40% (aOR, 0.60; 95% CI, 0.38-0.95) lower odds of having a joint procedure relative to failed or partially successful ITI (Table 3). However, when all people with inhibitors who had received ITI were grouped together, joint procedures were similar to those in the no-ITI group, owing to the relatively high frequency of joint procedures among people with failed ITI (38.5%).
Health care use
ED visits and hospitalizations were relatively frequent, with 21.8% and 12.0% of participants reporting at least 1 of these outcomes in the 12 prior months, respectively (Table 2). ED visits were more common in participants with no (31.9%), partially successful (30.5%), or failed (48.9%) ITI than in those with successful ITI (21.7%) or no inhibitors (20.4%). In adjusted analyses, the odds of having an ED visit were significantly greater among people with inhibitors with no ITI (aOR, 1.70; 95% CI, 1.06-2.73), failed ITI (aOR, 3.59; 95% CI, 2.35-5.50), or partially successful ITI (aOR, 1.56; 95% CI, 1.00-2.45) relative to people with no inhibitors (Figure 2). Similarly, the odds of a hospitalization were more than twice as high among people with inhibitors and no ITI (aOR, 3.64; 95% CI, 2.26-5.86), failed ITI (aOR, 5.48; 95% CI, 3.52-8.54), or partially successful ITI (aOR, 2.21; 95% CI, 1.31-3.73) relative to people with no inhibitors. Overall, those with successful ITI had a similar number of ED visits and hospitalizations compared with people with no inhibitors (Figure 2) and a lower number of hospitalization and ED visits than people with inhibitors and partially successful or failed ITI treatment (Table 3).
Quality of life
Chronic pain and limited function were reported in 39.8% and 24.7% of participants, respectively (Table 2). The odds of these 2 quality-of-life measures were 2 to 3 times higher among people with inhibitor and failed ITI relative to people with no inhibitor (Figure 2). People with successful ITI had consistently lower chronic pain (aOR, 0.40; 95% CI, 0.26-0.60) and less limited function (aOR, 0.38; 95% CI, 0.24-0.63) relative to people with inhibitor and partially successful or failed ITI (Table 3). Among those who were students or who were employed, the median number of missed days of school or work, respectively, because of a bleeding disorder was 0 (IQR, 0-2) but was slightly higher among people with inhibitor and no ITI (median, 5; IQR, 0-20) or failed ITI (median, 2; IQR, 0-10). Overall, people with inhibitor and successful ITI had fewer missed days (aRR, 0.56; 95% CI, 0.50-0.62) relative to those with partially successful or failed ITI.
Socioeconomic status
Among adults in our study, 37.6% were unemployed and 37.0% had lower educational attainment (defined as a high school degree or less; Table 2). People with inhibitor and no ITI were more likely to be unemployed and to have lower educational attainment, relative to people with no inhibitor (Figure 2). Among people with inhibitors, those receiving ITI were less likely to have lower educational attainment relative to people not receiving ITI (aOR, 0.36; 95% CI, 0.14-0.93). Among those that received ITI, educational attainment did not vary by ITI response. Approximately 13.5% of participants were coded as being disabled, and there were no significant differences according to ITI treatment in adjusted analyses.
Mortality
There were 61 total deaths reported in our study, with a crude death rate of 6.3 per 1000 PYs (Table 2). The death rate was 5.4 per 1000 PYs among people with inhibitor who received ITI, 6.3 per 1000 PYs in people with no inhibitor, and 24.7 per 1000 PYs in people with inhibitor that did not receive ITI (P = .012). There were too few deaths to separately report on partially successful ITI and failed ITI. Age-adjusted death rates were 21.9, 26.9, and 46.8 per 1000 PYs in those with no inhibitor, with inhibitor that received ITI, and with inhibitor that did not receive ITI, respectively. In unadjusted models, death rates among people with inhibitor who received ITI were significantly lower (relative risk [RR], 0.22; 95% CI, 0.07-0.68) than among people with inhibitor who did not receive ITI. However, after accounting for age, HCV and HIV infection, race, and ethnicity, death rates were similar (Table 3).
Sensitivity analyses
In the main analysis, receipt of ITI and outcomes were based on the initial visit forms. When ITI receipt and outcomes were categorized according to participants’ most recent visit form during the study period (2013-2017), there was a slightly lower sample size because more registry participants were missing ITI response information on their most recent vs initial forms. The average time between the initial and most recent form was 3.8 years (standard deviation, 2.2). Based on the most recent forms, the number of treated bleeds (aOR, 0.70; 95% CI, 0.60-0.82), hospitalization (aOR, 0.28; 95% CI, 0.14-0.56), chronic pain (aOR, 0.43; 95% CI, 0.19-0.95), and limited function (aOR, 0.41; 95% CI, 0.19-0.89) were significantly lower among people with ITI relative to those who did not receive ITI, similar to that observed in the main analyses (supplemental Table 2). When successful ITI was compared with failed/partial ITI using the most recent forms, the number of treated bleeds was lower, and chronic pain, limited function, and missed days were higher in those with successful ITI, similar to the main analysis. In the sensitivity analysis, joint procedures and hospitalizations were not significantly different when successful vs partial/failed ITI were compared.
Discussion
This study was undertaken to understand and quantify the effect of ITI on clinically relevant outcomes in the absence of emicizumab and showed that not receiving ITI and failed ITI were associated with more bleeding events, higher health care use and chronic pain, and lower function relative to people without inhibitor. There was also evidence that successful ITI was associated with fewer joint procedures and missed days of school or work and improved educational attainment relative to partially successful or failed ITI.
Bleeding, most often musculoskeletal bleeding, leads to pain, disability, loss of function, and reduced quality of life. Accordingly, preventing joint bleeding is fundamental to optimizing care for persons with hemophilia, and bleeding events are the primary clinical outcome of interest when evaluating treatment quality. In our study cohort, there was a median of 3 treated bleeding events during the past 12 months in all participants, with 3 for those without inhibitors and 6 for people with inhibitors (no ITI or failed ITI). Our findings align with those of the Cost of Haemophilia in Europe: a Socioeconomic Survey (CHESS) study,11 in which people with inhibitors experienced more than twice the mean annual number of bleeds compared with those without inhibitors. In our study, bleeding rates in those with successful and partially successful ITI were akin to people without inhibitors, suggesting that successful or partially successful ITI can normalize bleeding rates. In the International ITI study,12 participants that achieved successful tolerance and who were observed for 12 months after completion of ITI (n = 46) had an annualized median bleeding rate of 1.8 (IQR, 0.000-3.48) and 0.000 (IQR, 0.000-2.77) in the low- and high-dose arms, respectively, which also aligns with our study. Before emicizumab, the main difference for those with and without inhibitor was the capacity to undertake adequate bleeding prevention with continuous prophylaxis. We saw this impact in this cohort with >80% of participants without active inhibitor receiving continuous prophylaxis whereas <50% of those with active inhibitor (failed ITI or no ITI) received continuous prophylaxis. Emicizumab equalizes the ability to undertake effective prophylaxis regardless of inhibitor status.
In addition to understanding the number of bleeding events, the severity of the bleeding event can be inferred from the number of ED visits and hospitalizations, which were common in our study population. ITI was associated with a 70% reduction in hospitalization relative to non-ITI, and successful ITI nearly restored the odds of hospitalization during a 12-month period to that seen among people without inhibitor. Although previous studies have not examined the association between ITI and ED visits or hospitalization, the CHESS study reported that people with inhibitor had more than double the rate of hospitalizations compared with those without inhibitor (1.83 vs 0.64, respectively).11 This difference mirrors that seen in this study between people without inhibitor and those with inhibitor that did not receive ITI. Given the high cost of ED visits and hospitalization, preventing these types of events would, in part, offset the added cost of ITI. Emicizumab may be also capable of limiting the severity of bleeding events; however, bleeding events in people with persistent inhibitor require treatment with recombinant activated FVII, which is likely to be less effective and more burdensome to deliver than treatment with FVIII.
In the primary analysis, there was a 40% reduction in the odds of undergoing a joint procedure among those with successful ITI compared with those with partially successful or failed ITI. Receiving ITI was not associated with a reduction in joint procedures and the proportion of those with inhibitors that have joint procedures was similar in the failed-ITI and no-ITI groups. The impact of ITI on joint procedures did not closely mirror that of the bleeding rate, likely because of the variable impact of bleeding on joint damage in individuals. For some, a single bleed is sufficient to lead to hemophilic arthropathy and subsequent joint replacement. For others, minimal damage is seen even after repeated joint bleeding.12 For these reasons, the impact of ITI on joint procedures may be slightly less robust than its impact on bleeding events. Additionally, joint procedures typically occur at a more advanced age and the average age in our cohort was 21 years, potentially limiting the ability to detect a difference. Additionally, surgery in a person with persistent inhibitors is more difficult and may create a negative selection bias, thereby limiting the ability to use joint procedures as a marker of joint health and treatment quality.
Pain is a lagging indicator for optimal hemophilia care, with reduced function ensuing. When bleeding is not well controlled, pain increases and becomes chronic and function declines. In this study, chronic pain was experienced in 67.7% of those that did not receive ITI, and a dose-dependent effect was seen in those that received ITI. Accordingly, compared with failed ITI, successful or partially successful ITI resulted in 60%, 62%, and 44% lower odds of chronic pain, limited function, and missing school/work, respectively. In the CHESS study, it was reported that 32.8% of people without inhibitors experienced moderate or severe chronic pain compared with 53.5% of people with inhibitors. Overall, this study supports that ITI, both successful and partially successful, reduces the likelihood of developing chronic pain.
Educational attainment is a less commonly reported hemophilia outcome. We found that for approximately one-third of participants, the education attainment was a high school degree or less. As reported in US 2021 census data, 37% of the US population aged >25 years did not acquire education attainment higher than a high school diploma or its equivalent.13 Thus, our data are consistent with that seen in the general population, suggesting that hemophilia does not reduce educational attainment. In a study of persons with hemophilia in The Netherlands, similar educational attainment was seen between those with hemophilia and the general population.14 In contrast, other studies of children with chronic health conditions have demonstrated reduced educational attainment.15 There is limited prior knowledge of how inhibitor presence affects educational attainment in those with severe hemophilia A and inhibitor. In this study, ITI was associated with 64% lower odds of lower educational attainment relative to those who did not receive ITI; however, educational attainment was not statistically significantly different in those with successful ITI vs those with partially successful or failed ITI.
In this study, we did not observe a difference in employment or disability according to ITI treatment. However, based on the data on chronic pain, limited function, and missed days of school/work, it is possible that people with inhibitor or failed ITI may be underemployed or have fewer positive experiences in the workplace because of their hemophilia that could be mitigated by ITI. Other studies of employment have demonstrated lower employment among persons with hemophilia with severe disease than among person of the general population.14 Unemployment has also been associated with increased pain and functional disability after adjusting for joint status as measured by the hemophilia joint health score,16 suggesting employment may mediate quality-of-life and, thus, is a useful long-term outcome.
The presence of inhibitors was previously associated with higher mortality,6 although recent cohort studies suggested no difference.7 Given the lack of underlying difference in mortality between those with and without inhibitors, there is less opportunity for ITI to affect mortality, as seen in this study. However, it is worth noting that the cohort may have been too young to exclude an impact on mortality later in life; thus, there is value in continuing to assess the impact of major therapies, such as ITI, on mortality.
There are several limitations of the study. The dates that ITI began and ended were not recorded, and thus the precise timing of outcomes in relation to ITI were not known nor was completion of ITI confirmed. However, ITI is typically administered within the first few years of inhibitor development and among our study participants, there was an average of 17 years between inhibitor development and the collection of outcomes. Furthermore, we selected participants who had inhibitor for at least 5 years to ensure sufficient time for ITI to be undertaken and for clinical events to occur. The number of people with incident inhibitors was insufficient to support analysis of a subgroup with more precise timing of inhibitor development, ITI, and outcomes, which would provide a more precise temporal assessment of clinical outcomes. Several characteristics, such as HCV status and health insurance were collected at the time of enrollment in the registry and may not reflect what was observed at the time of ITI.
In conclusion, in the absence of emicizumab, ITI appears to improve important outcomes, with a greater impact when successful. Although emicizumab has supported improved bleeding prevention in people with hemophilia A and persistent inhibitors, breakthrough bleeds still occur in many.12 Given the challenges of effectively treating breakthrough bleeds in persons with hemophilia A with inhibitors, we hypothesize that outcomes including chronic pain, functional limitations, and increased health care use will continue to be negatively affected by persistent inhibitor, thus arguing for the continued use of ITI in participants with inhibitor. Future studies of ITI with emicizumab should be extended to include longer-term outcomes and cohorts that do not receive ITI.
Acknowledgments
Data reported in this publication were collected through Community Counts, a Centers for Disease Control and Prevention Public Health Surveillance Project for Bleeding Disorders.
Community Counts is a project supported by cooperative agreement NU27DD000020 awarded to the American Thrombosis and Hemostasis Network in partnership with the US Hemophilia Treatment Center Network. The cooperative agreement is an annual financial assistance award totaling $4 300 000, which is 100% funded by the Centers for Disease Control and Prevention and the US Department of Health and Human Services. The writing of this manuscript had no direct funding.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention, the Department of Health and Human Services, the American Thrombosis and Hemostasis Network, or the US Hemophilia Treatment Center Network.
Authorship
Contribution: C.L.K. conceived the project and created the analysis plan; and S.A.F. and C.L.K. performed the analysis and drafted the manuscript.
Conflict-of-interest disclosure: C.L.K. has received honoraria for participation in advisory boards from Biomarin, Pfizer, Genentech, and Spark. S.A.F. declare no competing financial interests.
Correspondence: Christine L. Kempton, Emory University School of Medicine, Hemophilia of Georgia Center for Bleeding & Clotting Disorders, Suite 1075, 550 Peachtree St, Atlanta, GA 30308; email: ckempto@emory.edu.
References
Author notes
Community Counts registry data are available from the Centers for Disease Control and Prevention with approval.
The full-text version of this article contains a data supplement.