MIR155HGΔexon3 T cells provide protection against lethal acute GVHD in a xenogeneic mouse model of disease.
MIR155HGΔexon3 T cells maintain beneficial graft-versus-leukemia response in vivo.
Visual Abstract
Acute graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic cell transplantation (allo-HCT). Using preclinical mouse models of disease, previous work in our laboratory has linked microRNA-155 (miR-155) to the development of acute GVHD. Transplantation of donor T cells from miR-155 host gene (MIR155HG) knockout mice prevented acute GVHD in multiple murine models of disease while maintaining critical graft-versus-leukemia (GVL) response, necessary for relapse prevention. In this study, we used clustered, regularly interspaced, short palindromic repeats (CRISPR)/Cas9 genome editing to delete miR-155 in primary T cells (MIR155HGΔexon3) from human donors, resulting in stable and sustained reduction in expression of miR-155. Using the xenogeneic model of acute GVHD, we show that NOD/SCID/IL2rγnull (NSG) mice receiving MIR155HGΔexon3 human T cells provide protection from lethal acute GVHD compared with mice that received human T cells with intact miR-155. MIR155HGΔexon3 human T cells persist in the recipients displaying decreased proliferation potential, reduced pathogenic T helper–1 cell population, and infiltration into GVHD target organs, such as the liver and skin. Importantly, MIR155HGΔexon3 human T cells retain GVL response significantly improving survival in an in vivo model of xeno-GVL. Altogether, we show that CRISPR/Cas9–mediated deletion of MIR155HG in primary human donor T cells is an innovative approach to generate allogeneic donor T cells that provide protection from lethal GVHD while maintaining robust antileukemic response.
Introduction
Acute graft-versus-host disease (GVHD) is a T-cell–mediated immunological complication arising in patients receiving an allogeneic hematopoietic cell transplantation (allo-HCT) and one of the primary causes of nonrelapse mortality.1,2 The pathogenesis of acute GVHD involves the recognition of HLA-mismatched host tissues by immunocompetent T cells present in the donor graft, leading to activation, proliferation, and migration of donor T cells to GVHD target organs—primarily the liver, skin, and gastrointestinal tract. This ultimately leads to severe organ damage because of combination of inflammatory cytokine secretion and direct cytotoxic effects.1-6 Conversely, these same donor T cells are critical to eradicate residual hematologic malignancy via the graft-versus-leukemia (GVL) effect in order to prevent relapse.4,7 Standard GVHD prophylactic regimens include combinations of calcineurin inhibitors along with antimetabolites.8 Recent clinical trials using posttransplantation cyclophosphamide as prophylaxis decreased acute and chronic GVHD incidence but not relapse.9-11 Comprehensive preclinical12-14 and clinical studies15 on the role of CD28 costimulation in T-cell alloreactivity led to the Food and Drug Administration approval of abatacept as the first drug to prevent acute GVHD. Abatacept decreased incidence of acute but not chronic GVHD without improving relapse rate or viral infections.16-19 Therefore, to improve patient outcomes after allo-HCT, novel strategies that prevent donor T-cell–mediated host tissue damage while permitting GVL are highly needed.
MicroRNAs (miR) are small, noncoding RNAs that impart posttranscriptional gene regulation through sequence-specific messenger RNA silencing or degradation, resulting in reduced target protein translation.20,21 miRs regulate various cellular processes including cell growth, differentiation, development, and apoptosis and have gained recognition for playing critical roles in the development and function of the immune system.22 One of the most well-defined miRs, microRNA-155 (miR-155), is encoded by its host gene, MIR155HG or B-cell integration cluster, which is composed of 3 exons that span a 13-kb region within human chromosome 21. The mature miR-155 transcript is encoded by the pre-miR-155 region located within exon 3.23,24
Early work has established the role of miR-155 in both innate and adaptive immunity.25,26 Mice harboring a genomic deletion of miR-155 (BICKO/MIR155HGKO) are viable with impaired T-cell responses; MIR155HGKO T cells show attenuated inflammatory tumor necrosis factor α and interferon gamma (IFN-γ) cytokine release when stimulated with an antigen.25,27 Additionally, MIR155HGKO CD4+ T cells show T helper–2 cell (Th2) bias, secreting higher amounts of interleukin-10 (IL-10) and IL-4 and low tumor necrosis factor α and IFN-γ25-27 cytokines. Corresponding to its role in inflammation, miR-155 positively regulates pathogenic T-cell responses in experimental autoimmune encephalomyelitis.28-30 During acute GVHD, miR-155 dysregulation occurs in both donor and recipient immune cells.27,31,32 Expression of miR-155 increases in activated dendritic cells (DCs) and transplanting MIR155HGKO DCs decreases GVHD severity through impaired DC migration and reduced inflammasome activation.31 Previously, we showed that miR-155 is upregulated in donor T cells in both mice and humans with acute GVHD. Moreover, transplantation of T cells from MIR155HGKO donor mice prevents acute GVHD in multiple murine models of disease27,32 while maintaining beneficial GVL response.32 Treatment of mice after transplantation with antisense oligonucleotides targeting miR-155 (anti-miR-155) decreases acute GVHD; however, efficiency of targeting miR-155 was low, and responses were not robust.32 These marginal increments in survival were maintained in additional experiments, despite using anti-miR-155 then undergoing clinical trials33 and a dose or schedule optimized in mice (unpublished data). Oligonucleotide inhibitors of miR-155 showed promise in phase 1 safety trials33 but failed phase 2 efficacy trials limiting the examination of miR-155 contribution to human T-cell function and translation of preclinical studies.
To overcome this problem, we deployed a CRISPR (clustered, regularly interspaced, short palindromic repeats)/Cas9 strategy to delete miR-155 at the genome level in donor T cells. Using the xenogeneic model of acute GVHD and GVL, we show that genetically engineered human donor T cells harboring a genomic deletion of MIR155HG (MIR155HGΔexon3) provide protection from lethal acute GVHD while maintaining GVL response. MIR155HGΔexon3 human T cells persist in the recipients displaying decreased proliferation potential, proinflammatory Th1 population and infiltration into GVHD target organs, including the liver and skin. This study establishes that genomic deletion of MIR155HG in donor T cells via genetic engineering is a novel and feasible strategy to prevent acute GVHD, effectively decoupling GVHD and GVL responses.
Methods
Isolation and activation of human peripheral blood T lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation from buffy coats of healthy human donors obtained from Versiti. T lymphocytes were isolated from PBMCs using human Pan T-cell isolation kit (Miltenyi Biotec). Isolated T cells confirmed to be >95% pure (CD3+) and viable (live/dead) were seeded at a density of 1 × 106 cells per mL and activated using Dynabeads Human T-Activator CD3/CD28 (Thermo Fisher Scientific) at 0.5 × 106 cells per mL of media (RPMI + 20% fetal bovine serum + 1% penicillin-streptomycin + 1% glutamine) + 30 U/mL recombinant human IL-2 (R&D Systems). Cells were incubated at 37°C in 5% CO2 in a humidified incubator for 48 hours. T-cell activation was confirmed by staining for early activation marker CD69 as well as CD25, and only those donors that showed robust activation (>95% CD69 and/or CD25+) were used for transfection (supplemental Figure 1A).
CRISPR/Cas9 gene editing of MIR155HG
Guide RNAs were designed using Integrated DNA Technologies (IDT) and CHOPCHOP34 web tools, and those predicted to have lowest off-target binding and highest on-target efficiency while binding to the specific region of interest were selected. Guide RNAs were purchased from IDT as single guide RNAs (sgRNAs) which contain both the crRNA and tracrRNA sequences and chemical modifications for stability. crRNA targeting sequences are listed in supplemental Table 1. CRISPR mutagenesis was performed on activated T cells using ribonucleoprotein (RNP) complex following previously published protocols.35,36 Briefly, 2 sgRNAs (Alt-R CRISPR-Cas9 sgRNA, IDT) and electroporation enhancer (Alt-R Cas9 Electroporation Enhancer, IDT) were incubated with Alt-R S.p. Cas9 Nuclease V3 (IDT 1081059) in buffer T (Thermo Fisher Scientific) at room temperature for 10 minutes in ∼1.4 to 1 ratio to form the RNP complex. RNP complex was mixed with activated T cells (1 × 106) and electroporation was performed using the Neon Transfection System (Thermo Fisher Scientific). Electroporation conditions were 1600 V, 10 milliseconds, and 3 pulses using buffer T. Electroporated cells were seeded at a density of 0.5 × 106 cells/mL in a 6-well plate in RPMI-1640 medium supplemented with 20% fetal bovine serum and 1% L-glutamine (antibiotic free). The flask was incubated at 37°C in 5% CO2 in a humidified incubator. Fresh antibiotic-containing media (as for activation of T cells) with IL-2 were added, and cells expanded for 7 days for in vivo experiments, pretransplant immunophenotyping and for cytotoxic T lymphocyte (CTL)–associated experiments.
Xenogeneic acute GVHD mouse models
Mice underwent transplantation under standard protocols approved by the Institutional Animal Care and Use Committee at The Ohio State University. Only age- and sex-matched NSG mice were used. Briefly, 10-week recipient NSG mice were irradiated with 100 cGy the day before transplantation. Equal numbers of MIR155HGΔexon3 or nontargeting control (NT) CRISPR/Cas9–edited T cells from healthy donors (5 × 106 or 10 × 106) were administered on the day of the transplantation through tail-vein injection.
Xenogeneic GVL mouse models
Irradiated (100 cGy) age- and sex-matched NSG mice were injected with 5000 green fluorescent protein–containing (GFP+) luciferase–transduced MOLM-13 human acute myeloid leukemia (AML) cells 1 day before transplantation. On the day of transplantation, mice were IV injected with 20 × 106 thawed T-cell–depleted PBMCs (TCD-PBMCs) alone or with 5 × 106 autologous MIR155HGΔexon3 or NT CRISPR/Cas9–edited T cells from healthy donors in the ratio of TCD-PBMCs to T cells at 4:1. Tumor persistence was tracked by whole-body IVIS imaging. TCD-PBMCs and MOLM-13 cells (leukemia alone) served as the control group. MOLM-13–induced leukemic death was defined by the occurrence of either macroscopic tumor nodules in liver and/or spleen or hind limb paralysis.
Flow cytometry analysis
Cells were stained at various time points to assess purity, viability, activation, and phenotype. Approximately 0.5 × 106 to 1 × 106 T cells were stained with surface antibodies and viability dyes following manufacturer’s protocols. Intracellular cytokine staining was performed by T-cell stimulation with 1× cell stimulation cocktail (ThermoFisher Scientific) for 5 hours. After 1 hour, 1× protein transport inhibitor cocktail (ThermoFisher Scientific) was added. Cells were fixed and permeabilized (eBioscience Permeabilization buffer [1×]) followed by staining of intracellular cytokines or transcription factors. Single-cell suspensions of liver used for flow cytometry analysis were derived using commercially available liver dissociation kit (Miltenyi Biotec). Absolute cell numbers were enumerated using CountBright Plus Absolute Counting Beads (Invitrogen). Absolute counts were calculated according to manufacturer protocol. Analysis was performed on the fluorescence-activated cell sorting LSR Fortessa flow cytometer (Becton Dickinson) or the Aurora (Cytek) depending on the size of the panel. Data analysis was performed using FlowJo (Tree Star). Cells from peripheral bleeds were analyzed using a modified version37 of OMIP-042. Representative gating strategies (supplemental Figure 1B) and antibodies used (supplemental Table 2) can be found in the supplemental Data.
Detailed methods can be found in the supplemental Data.
Results
CRISPR/Cas9–targeted deletion of MIR155HG results in sustained downregulation of miR-155 with minimal off-target effects.
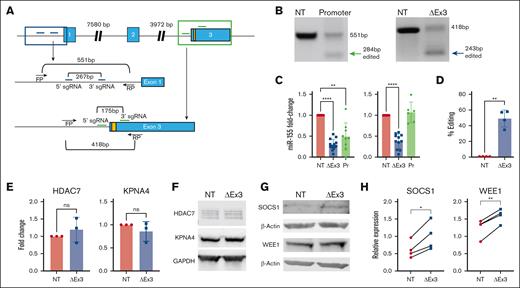
We designed pairs of sgRNA34 to target MIR155HG. Schema of MIR155HG loci identifying the pairs of gRNAs flanking regions of interest are shown in Figure 1A and genomic polymerase chain reaction (PCR) primers used to confirm deletion are listed in supplemental Table 1. Activated, viable human T cells were electroporated with sgRNAs NT, exon 3 targeting (MIR155HGΔexon3), or promoter targeting (MIR155HGΔpromoter) and HiFi Cas9 enzyme38,39 as an RNP complex.35,39-41 Predicted genomic deletions were confirmed using genomic PCR 72 hours after transfection from NT vs MIR155HGΔexon3 (418 vs 243 bp) and NT vs MIR155HGΔpromoter (551 vs 284 bp) genomic DNA (Figure 1B). Quantitative reverse transcriptase–PCR showed a significant reduction in miR-155 expression at 72 hours (MIR155HGΔexon3 vs NT = 0.33 vs 1, P < .0001; MIR155HGΔpromoter vs NT = 0.48 vs 1, P < .01; Figure 1C). Reduction in expression of miR-155 (MIR155HGΔexon3 vs NT = 0.45 vs 1, P < .0001) remained significantly low through 7 days of expansion only in MIR155HGΔexon3 cells (Figure 1C) and, therefore, only exon 3 targeting guides were chosen for further studies. Quantification of editing efficiency was performed using droplet digital PCR that showed a mean 49.05% editing using exon 3 targeting guides (P < .01; Figure 1D). Whole-genome sequencing was performed on DNA from unedited and MIR155HGΔexon3 samples to identify off-target effects and data analyzed using Churchill.42 Putative off-target mutations of moderate or high impact are listed in supplemental Table 2. Quantitative reverse transcriptase–PCR was performed in unstimulated vs CD3/CD28–stimulated T cells, and among the 6 genes identified (HDAC7, KPNA4, MCC, OLFML2A, HRNR, and IGFN1), only HDAC7 and KPNA4 were found to be expressed in T cells (data not shown) corroborating publicly available data sets.43,44 No changes in gene expression of HDAC7 (MIR155HGΔexon3 vs NT = 1.19 vs 1, not significant, ns) and KPNA4 (0.86 vs 1, ns, Figure 1E) or protein levels (Figure 1F) were detected between the NT and MIR155HGΔexon3 T cells. To test the functional consequence of MIR155HG editing and subsequent miR-155 downregulation, we assessed the expression of 2 evolutionary conserved and bona fide miR-155 targets,29,45 Suppressor of cytokine signaling (SOCS1) and WEE1, both at protein level. We observed an increase in both SOCS1 and WEE1 protein expression in MIR155HGΔexon3 compared with NT T cells confirming functional miR-155 deletion (P = .02, Figure 1G-H).
CRISPR/Cas9–mediated deletion of MIR155HG results in downregulation of miR-155 expression with minimal off-target effects. (A) MIR155HG gene locus with 3 exons in blue shaded boxes and promoter region is shown. Yellow shaded box within exon 3 denotes pre-miR-155. Guide RNAs (gRNAs) targeting promoter region are shown in dark blue, and gRNAs targeting miR-155 sequence in exon 3 junction are shown in green. (B) Genomic PCR performed 72 hours after transfection. One representative donor is shown. (C) Validation of sgRNA pairs to target MIR155HG. Fold change in miR-155 expression measured using quantitative reverse transcriptase–PCR (qRT-PCR) at 72 hours (left) and 7 days after transfection (right). (D) On-target efficiency was quantified by droplet digital PCR performed 72 hours after transfection. (E) qRT-PCR, n = 3 donors and (F) Western blot analysis of identified off-target genes from a single representative donor. (G) Western blot analysis SOCS1 and WEE1 from a single representative donor. (H) Densitometric quantification of panel G, n = 4 donors. Data are combined from 3 to 16 independent donors, each symbol represents an individual donor (∗∗P < .01, ∗∗∗∗P < .0001).
CRISPR/Cas9–mediated deletion of MIR155HG results in downregulation of miR-155 expression with minimal off-target effects. (A) MIR155HG gene locus with 3 exons in blue shaded boxes and promoter region is shown. Yellow shaded box within exon 3 denotes pre-miR-155. Guide RNAs (gRNAs) targeting promoter region are shown in dark blue, and gRNAs targeting miR-155 sequence in exon 3 junction are shown in green. (B) Genomic PCR performed 72 hours after transfection. One representative donor is shown. (C) Validation of sgRNA pairs to target MIR155HG. Fold change in miR-155 expression measured using quantitative reverse transcriptase–PCR (qRT-PCR) at 72 hours (left) and 7 days after transfection (right). (D) On-target efficiency was quantified by droplet digital PCR performed 72 hours after transfection. (E) qRT-PCR, n = 3 donors and (F) Western blot analysis of identified off-target genes from a single representative donor. (G) Western blot analysis SOCS1 and WEE1 from a single representative donor. (H) Densitometric quantification of panel G, n = 4 donors. Data are combined from 3 to 16 independent donors, each symbol represents an individual donor (∗∗P < .01, ∗∗∗∗P < .0001).
Transplantation of MIR155HGΔexon3 T cells prevents lethal acute GVHD in a xenogeneic model
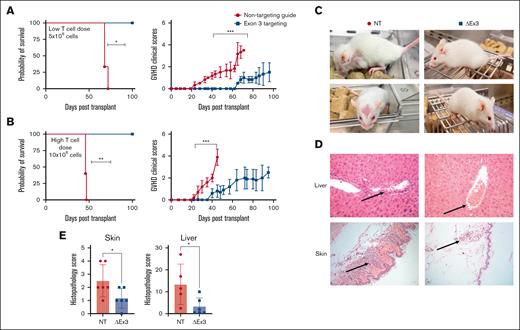
To evaluate in vivo function of edited T cells, we used a xenogeneic model of acute GVHD, in which NSG mice underwent transplantation with equal numbers of MIR155HGΔexon3 or NT T cells. Recipients of MIR155HGΔexon3 T cells survived significantly longer than mice receiving NT T cells (median survival MIR155HGΔexon3 vs NT = not reached vs 68 days, P < .05; Figure 2A-B left panels) and showed significantly decreased acute GVHD clinical scores (Figure 2A-B right panels). Crucially, improvement in survival was maintained even when we increased T-cell dose from low (5 × 106 cells; Figure 2A) to high (10 × 106 cells; Figure 2B). NT T-cell–recipient mice showed decreased overall activity with severe hunching, poor fur texture, and decreased skin integrity, whereas MIR155HGΔexon3-recipient mice resembled healthy mice (Figure 2C). Histopathological analysis revealed dramatically lower levels of T cells infiltrating classical target organs for acute GVHD, liver and skin,46 with correspondingly lower acute GVHD scores in recipients of MIR155HGΔexon3 compared with NT T cells (P = .04 and P = .03; Figure 2D-E).
MIR155HGΔexon3 human T cells protects from acute GVHD in a xenogeneic model of disease. Xenogeneic GVHD transplants were performed as described in methods. NSG mice were injected with (A) 5 × 106 (n = 3 per cohort) and (B) 10 × 106 MIR155HGΔexon3 or NT human T cells (n = 5 per cohort). Survival curves (left) and acute GVHD clinical scores (right). Data combined from 2 independent donors, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. (C) Representative images of NT (left) and MIR155HGΔexon3 (right) recipient mice when the GVHD scores reached ≥4 and mice met euthanization criteria in the NT cohort. (D) Hematoxylin and eosin staining of liver (top) and skin (bottom) sections from mice receiving MIR155HGΔexon3 (right) or NT T cells (left). (E) Pathology scores for liver and skin from MIR155HGΔexon3 and NT-recipient mice at time of euthanasia (∗P < .05).
MIR155HGΔexon3 human T cells protects from acute GVHD in a xenogeneic model of disease. Xenogeneic GVHD transplants were performed as described in methods. NSG mice were injected with (A) 5 × 106 (n = 3 per cohort) and (B) 10 × 106 MIR155HGΔexon3 or NT human T cells (n = 5 per cohort). Survival curves (left) and acute GVHD clinical scores (right). Data combined from 2 independent donors, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. (C) Representative images of NT (left) and MIR155HGΔexon3 (right) recipient mice when the GVHD scores reached ≥4 and mice met euthanization criteria in the NT cohort. (D) Hematoxylin and eosin staining of liver (top) and skin (bottom) sections from mice receiving MIR155HGΔexon3 (right) or NT T cells (left). (E) Pathology scores for liver and skin from MIR155HGΔexon3 and NT-recipient mice at time of euthanasia (∗P < .05).
MIR155HGΔexon3 T cells persist, with lower Th1-cell population and decreased infiltration into GVHD target organs
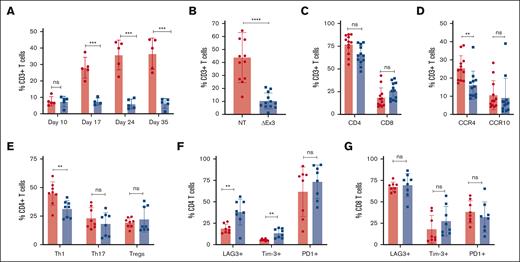
Analysis of weekly peripheral bleeds of recipient mice showed a consistent increase in percentage of CD3+ NT T cells over time whereas levels of MIR155HGΔexon3 T cells persisted, resulting in a significant increase of CD3+ NT T cells compared with MIR155HGΔexon3 T cells (Figure 3A-B). There was no significant difference in the CD4 and CD8 subsets of T cells (Figure 3C). Analysis of homing receptors CCR4 and CCR10 revealed a significant reduction in CCR4 (P = .005) but not CCR10 expressing CD3+ T cells in mice that received MIR155HGΔexon3 compared with those receiving NT T cells (Figure 3D). In addition, there was a significant reduction in the percentage of circulating Th1 (CXCR5−CCR6−CXCR3+CCR10−, P < .05) but not Th17 T cells (CXCR5−CCR6+CCR4+CXCR3+/−CCR10+) in mice that received MIR155HGΔexon3 compared with those who received NT T cells (gating strategy in supplemental Figure 1 B). Similarly, the CD25hiCD127lo regulatory T (Treg)–cell percentages were similar between MIR155HGΔexon3 and NT T-cell–recipient mice groups (Figure 3E). Furthermore, our data show a significant increase in expression of exhaustion markers LAG3 and Tim-3 (P < .01) whereas increase in programmed cell death protein 1 (PD1) expression did not reach statistical significance in MIR155HGΔexon3 CD4 T cells compared with NT T cells (Figure 3F). Interestingly, there was no difference in expression of exhaustion markers on CD8 T cells (Figure 3G). Pretransplantation immunophenotyping did not reveal any significant difference between MIR155HGΔexon3 and NT T cells before injection into NSG mice (supplemental Figure 2).
MIR155HGΔexon3 T cells persist with lower Th1 cell population in circulation. Flow cytometry was performed on peripheral blood cells obtained from facial bleeds of xeno-GVHD mice that received 10 × 106 MIR155HGΔexon3 or NT human T cells. Each point represents an independent mouse receiving either MIR155HGΔexon3 or NT control human T cells. (A) Percentage of CD3+ T cells over time from a single representative donor is shown, n = 5 per cohort. (B-G) Mice were bled through cardiac puncture on day 35 after transplantation, data combined from 2 to 3 independent donors, n = 8 to 12 per cohort. (B) Percentage CD3+ T cells. (C) Percentage CD4+ and CD8+ T cells subsets. (D) CCR4 and CCR10 expressing CD3+ T cells. (E) Proportion of Th1, Th17, and Treg CD4+ T-cell subtypes. LAG3, Tim3, and PD1 expression on (F) CD4+ T cells and (G) CD8+ T cells (∗P < .05, ∗∗P < .01, ∗∗∗P < .001).
MIR155HGΔexon3 T cells persist with lower Th1 cell population in circulation. Flow cytometry was performed on peripheral blood cells obtained from facial bleeds of xeno-GVHD mice that received 10 × 106 MIR155HGΔexon3 or NT human T cells. Each point represents an independent mouse receiving either MIR155HGΔexon3 or NT control human T cells. (A) Percentage of CD3+ T cells over time from a single representative donor is shown, n = 5 per cohort. (B-G) Mice were bled through cardiac puncture on day 35 after transplantation, data combined from 2 to 3 independent donors, n = 8 to 12 per cohort. (B) Percentage CD3+ T cells. (C) Percentage CD4+ and CD8+ T cells subsets. (D) CCR4 and CCR10 expressing CD3+ T cells. (E) Proportion of Th1, Th17, and Treg CD4+ T-cell subtypes. LAG3, Tim3, and PD1 expression on (F) CD4+ T cells and (G) CD8+ T cells (∗P < .05, ∗∗P < .01, ∗∗∗P < .001).
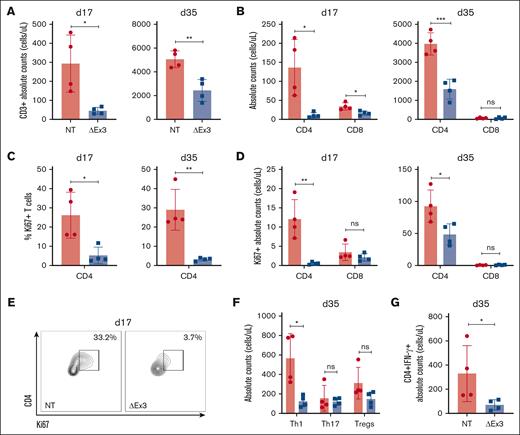
Furthermore, to evaluate T-cell infiltration into GVHD target organs, NSG mice receiving NT or MIR155HGΔexon3 were harvested on days 17 and 35 after transplantation and absolute counts of T cells infiltrating the liver were enumerated. At both time points, CD3+ MIR155HGΔexon3 T cells present in the liver were significantly lower than NT T cells (day 17, P = .04 and day 35, P = .004; Figure 4A). Moreover, the numbers of MIR155HGΔexon3 CD4+ T cells were significantly reduced as compared with that of NT CD4+ T cells at both time points (day 17, P = .04 and day 35, P = .0009) whereas MIR155HGΔexon3 CD8+ T cells were significantly lower at day 17 (P = .02), with approximately undetectable levels of CD8 T cells in both groups at day 35 (Figure 4B). Proliferative capacity was evaluated by Ki67 expression that showed significantly reduced percentages and absolute counts of Ki67+ MIR155HGΔexon3 CD4+ T cells at both day 17 (P = .03 and P = .01, respectively) and day 35 (P = .01 and P = .03, respectively) after transplantation compared with control (Figure 4C-E). Interestingly, we did not observe this trend in CD8+ T cells at either time points. Aligning with peripheral bleed data, we show a significant reduction in MIR155HGΔexon3 CD4+ Th1 subset as compared with NT (P = .03), but similar absolute counts of Th17 and Treg-cell populations (Figure 4F). Concordantly, total IFN-γ secreting MIR155HGΔexon3 CD4+ T cells were significantly lower than NT IFN-γ+ CD4+ T cells (P = .02; Figure 4G). Taken together, our data substantiate that MIR155HGΔexon3 deletion reduces T-cell infiltration into a primary site of disease pathogenesis, the liver, displaying reduced proliferation potential and effector Th1 cytokine secretion.
MIR155HGΔexon3 T cells display markedly reduced proliferation and Th1 population in GVHD target organ. Xenogeneic GVHD transplants were performed as before, and recipient NSG mice were euthanized at either day 17 or day 35 after transplantation to obtain single-cell suspension from GVHD target organ liver for flow cytometric analyses. N = 4 mice for each time point, data combined from 2 independent donors. All data are shown as absolute counts (cells per μL). (A) CD3+ T cells in liver on day 17 (left) and day 35 (right) after transplantation. (B) CD4+ and CD8+ T-cell subsets. (C) Percentage Ki67 expression on CD4+ T cells. (D) Absolute numbers of Ki67+ CD4+ and Ki67+ CD8+ T cells at day 17 (left) and day 35 (right) after transplantation. (E) Representative day 17 contour plots from panel C. (F) Proportion of Th1, Th17, and Treg CD4+ T-cell subtypes on day 35 after transplantation. (G) IFN-γ expressing CD4+ T cells on day 35 after transplantation.
MIR155HGΔexon3 T cells display markedly reduced proliferation and Th1 population in GVHD target organ. Xenogeneic GVHD transplants were performed as before, and recipient NSG mice were euthanized at either day 17 or day 35 after transplantation to obtain single-cell suspension from GVHD target organ liver for flow cytometric analyses. N = 4 mice for each time point, data combined from 2 independent donors. All data are shown as absolute counts (cells per μL). (A) CD3+ T cells in liver on day 17 (left) and day 35 (right) after transplantation. (B) CD4+ and CD8+ T-cell subsets. (C) Percentage Ki67 expression on CD4+ T cells. (D) Absolute numbers of Ki67+ CD4+ and Ki67+ CD8+ T cells at day 17 (left) and day 35 (right) after transplantation. (E) Representative day 17 contour plots from panel C. (F) Proportion of Th1, Th17, and Treg CD4+ T-cell subtypes on day 35 after transplantation. (G) IFN-γ expressing CD4+ T cells on day 35 after transplantation.
MIR155HGΔexon3 T cells retain GVL response in vivo
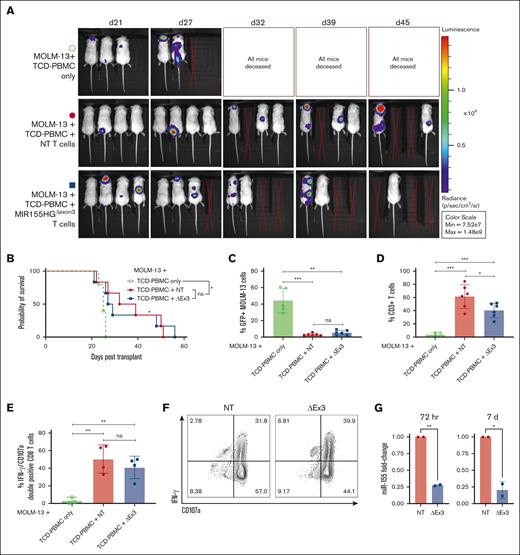
The primary goal of an allo-HCT is the elimination of residual malignant cells in the recipient via a donor antileukemic response. Thus, to investigate GVL capacity of MIR155HG-edited T cells, we used a xeno-GVL model. Briefly, NSG mice were irradiated and injected with GFP+ luciferase–transduced FLT3-ITD+ MOLM-13 AML cell line along with TCD-PBMCs alone or TCD-PBMCs with either MIR155HGΔexon3 or NT T cells from 2 independent donors. Mice receiving control or MIR155HGΔexon3 T cells showed reduced luminescence via whole-body bioluminescent imaging when compared with mice receiving TCD-PBMCs alone (Figure 5A), with significantly improved survival (P < .05; Figure 5B). Flow cytometric analysis of GFP+ MOLM-13 tumor burden in splenocytes of recipient mice confirmed that T-cell–receiving mice were able to clear MOLM-13 leukemic cells (NT/MIR155HGΔexon3 T cells vs TCD-PBMCs P = .003; Figure 5C). In line with our previous results, levels of donor T cells were lower in mice that received MIR155HGΔexon3 than those who received NT T cells (percent CD3+ splenic T cells, 61.23 vs 40.40, P = .04; Figure 5D). Importantly, lower percentages of MIR155HGΔexon3 T cells did not hinder tumor clearance (% splenic GFP+ MOLM-13 cells, MIR155HGΔexon3 vs NT = 5.28 vs 3.19, ns; Figure 5C). To evaluate CTL function of donor CD8+ T cells, splenocytes from GVL mice were isolated and IFN- γ production and degranulation was analyzed. Donor CD8+ T cells from NT and MIR155HGΔexon3 T cells showed comparable expression of IFN- γ and CD107a, suggesting MIR155HGΔexon3 deletion does not disrupt the CTL function in vivo (Figure 5E-G) and in vitro (supplemental Figure 3).
MIR155HGΔexon3 T cells retain beneficial GVL effect. NSG mice were irradiated and injected with GFP+ luciferase–transduced FLT3-ITD+ MOLM-13 AML cells along with TCD-PBMCs alone (open green circles) or TCD-PBMCs with either NT (filled red circles) or MIR155HGΔexon3 T cells (filled blue boxes). (A) Whole-body bioluminescent signal intensity of recipient NSG mice (n = 3 or 4) from 1 representative donor. Mice were imaged on indicated days. (B) Survival curve from 2 independent donors (n = 5 or 6). (C-D) Splenocytes were isolated at time of death and (C) MOLM-13 leukemic burden evaluated by GFP positivity and (D) percentage live CD3+ T cells were evaluated using flow cytometry (n = 5 or 6 combined) from 2 independent transplants or donors. (E) Percentage IFN-γ and CD107a double-positive CD8+ T cells in splenocytes. (F) Representative contour plots of panel E. (G) Fold change in miR-155 expression measured by qRT-PCR at 72 hours (left) and 7 days (right) after transfection of human donors used for GVL transplants (∗P < .05, ∗∗P < .01, ∗∗∗P < .001).
MIR155HGΔexon3 T cells retain beneficial GVL effect. NSG mice were irradiated and injected with GFP+ luciferase–transduced FLT3-ITD+ MOLM-13 AML cells along with TCD-PBMCs alone (open green circles) or TCD-PBMCs with either NT (filled red circles) or MIR155HGΔexon3 T cells (filled blue boxes). (A) Whole-body bioluminescent signal intensity of recipient NSG mice (n = 3 or 4) from 1 representative donor. Mice were imaged on indicated days. (B) Survival curve from 2 independent donors (n = 5 or 6). (C-D) Splenocytes were isolated at time of death and (C) MOLM-13 leukemic burden evaluated by GFP positivity and (D) percentage live CD3+ T cells were evaluated using flow cytometry (n = 5 or 6 combined) from 2 independent transplants or donors. (E) Percentage IFN-γ and CD107a double-positive CD8+ T cells in splenocytes. (F) Representative contour plots of panel E. (G) Fold change in miR-155 expression measured by qRT-PCR at 72 hours (left) and 7 days (right) after transfection of human donors used for GVL transplants (∗P < .05, ∗∗P < .01, ∗∗∗P < .001).
Discussion
An allo-HCT is the most effective treatment for many patients with leukemias, especially adults with AML. However, the overall survival of patients with AML who underwent allo-transplantation remains dismal at only 50% at 3 years owing to unacceptably high rates of relapse and treatment-related mortality caused by GVHD and infections, highlighting the need for more effective strategies to improve posttransplant outcomes.1,47,48 Several preclinical studies have demonstrated the significance of miRs in the development of acute GVHD,49-53 leading to the identification of miRs as a novel target for GVHD prevention. We have previously shown that miR-155 is required for acute GVHD development, and transplantation of MIR155HGKO T cells prevented murine acute GVHD while maintaining beneficial GVL response.27,32 However, the therapeutic efficacy of miR-155 antisense technology was low, suggesting that better miR-155 targeting strategies were required for clinical translation of these studies.
The CRISPR/Cas9 system uses a nuclease, Cas9, guided by sgRNA to precisely modify the human genome and is an elegant therapeutic approach for correcting monogenic disorders.54 Electroporation of a complex of recombinant Cas9 with either in vitro transcribed or synthetic sgRNA has overcome challenges related to earlier approaches using viral vector or plasmid delivery.35,54,55 This approach has resulted in high efficiency (50%-90%) in multiple targets, such as CXCR4 and CCR555-59 in human T cells. Use of the high-fidelity Cas9 (HiFi Cas9)– guided CRISPR system efficiently engineers primary murine and human T cells,38,60 significantly reducing off-target effects without affecting on-target efficiency.60 A recent phase 1 clinical trial to assess the safety and feasibility of CRISPR/Cas9 gene editing of T cells showed that modified T cells were well-tolerated with durable engraftment,61 encouraging further exploration of CRISPR-engineered immunotherapies.
In this study, we used CRISPR/Cas9 genome editing to delete MIR155HG in primary human T cells and show that MIR155HG regulates the incidence and severity of acute GVHD in a xenogeneic model. To our knowledge, our approach is the first to translate genetic MIR155HGKO murine studies into human T cells using an alternative approach of genome editing, thus filling the gap in knowledge on the role of miR-155 in human T-cell function.
miRs exert their function by binding to messenger RNAs in a sequence-specific manner and repressing translation of target proteins. Studies have shown that miR-155 promotes inflammation in part by downregulating SOCS129,62 protein. Moreover, miR-155 induces mutator activity, linking inflammation and cancer in part by inhibiting translation of WEE1, a cell-cycle regulator.45 In agreement with these studies, we observed an increase in SOCS1 and WEE1 protein expression in MIR155HGΔexon3 T cells, which we used as a functional readout for successful MIR155HG editing.
Chemokine receptors and adhesion molecules regulate migration of T lymphocytes to target organs, playing a critical role in GVHD pathogenesis. Blocking chemokine receptor CCR5 with maraviroc decreases acute GVHD incidence in allo-HCT recipients63 whereas use of natalizumab that blocks α4 chain of α4β7 integrin impairs T-cell homing to the intestine, showing limited protection against intestinal GVHD.64,65 Our results can be explained mechanistically by impact of miR-155 on T-cell homing to acute-GVHD target organs. Notably, we observed a significant downregulation of CCR4 but not CCR10 protein expression in donor MIR155HGΔexon3 T cells after engraftment. In both mice and humans, CCR4 has been identified as a crucial mediator of skin-specific Th lymphocyte homing,66 resulting in enhanced skin inflammation.67 Thus, modulating T-cell trafficking to GVHD target organs by targeting CCR4 in human donor T cells has clinical implications for acute GVHD without interfering with GVL responses.
Several studies have shown that miR-155 expression is increased in different activated immune cell populations.30 Aberrant expression of miR-155 promotes inflammation in multiple autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus,30 skewing T-cell differentiation toward a proinflammatory Th1 phenotype.29,68 We observed a significant decrease in circulating and target organ infiltrating Th1 but not Th17 T cells in MIR155HGΔexon3 recipients compared with mice receiving NT T cells. Interestingly, we do not observe these differences in vitro, before injecting the cells into NSG mice. These findings are in line with the first published reports that showed no difference in Th1/Th2 cells in vitro between miR-155–expressing and miR-155–deleted T cells.25,26 Additionally, we show that MIR155HG-deleted T cells expand normally under homeostatic conditions in vitro but exhibit lower proliferation potential in vivo under inflammatory conditions. Surprisingly, we observed an increase in expression of coinhibitory receptors Tim-3 and Lag3 but not PD1 on CD4 MIR155HGΔexon3 vs NT T cells with no differences in CD8 T cells. This pattern may denote lower activation status or induction of tolerance in MIR155HGΔexon3 CD4+ T cells;69-72 however, further investigation is required as to the functional consequence of our findings because coinhibitory receptor expression alone is not enough to denote exhaustion. Taken together, our results suggest that MIR155HGΔexon3 deletion in donor T cells permits T-cell survival and persistence but limits Th1 proliferation and proinflammatory IFN-γ secretion at the target tissue without affecting Th17 and Treg-cell numbers, thereby reducing incidence and severity of GVHD.
Providing protection against acute GVHD without abrogating beneficial GVL responses has been a major challenge limiting the efficacy of allo-HCT therapy. Here, we show for the first time that MIR155HGΔexon3 human T cells blunt acute GVHD responses with significantly improved survival while retaining positive GVL responses in vivo in a xenogeneic model. The caveat to using the MOLM-13 model is that all mice eventually succumb to leukemia as opposed to murine GVL models32,73,74 in which allogeneic T-cell transfer induces strong GVL responses, completely eradicating tumor. Despite using the highly aggressive MOLM-13 leukemia cell line, our results demonstrate that MIR155HGΔexon3 T cells show potent cytotoxic function in vitro and in vivo with effective killing of AML cells and efficient degranulation and IFN-γ production by MIR155HGΔexon3 T cells as compared with NT T cells, significantly improving survival compared with mice that did not receive T cells.
Our proof-of-concept studies show the feasibility of generating MIR155HG-deleted allogeneic donor T cells as an approach to prevent GVHD while preserving antileukemic response. However, questions remain regarding the different mechanism(s) by which MIR155HGΔexon3 provides protection from GVHD. The addition of TCD-PBMCs was necessary in the xeno-GVL model, indicating the need for human antigen presenting cells to elicit a robust GVL response against MOLM-13 AML cells. This was markedly different from the xeno-GVHD model in which human T cells were necessary and sufficient for causing GVHD. The discrepancies between the GVL and GVHD models raise the possibility that MIR155HGΔexon3 T cells may require TCD-PBMCs to induce GVHD. This is a potential limitation of our study and a subject of ongoing investigations in our laboratory. Encouragingly, previously published data with murine MIR155HGKO CD4 and CD8 T cells have shown that recipients are protected from GVHD even in the presence of wild-type T-cell–depleted bone marrow.27 Establishing tolerance is a crucial step to prevent acute GVHD, with several studies showing that early adoptive transfer of Treg cells and/or expansion of Tregs can prevent acute GVHD development.75-78 Studies using global MIR155HGKO murine models have shown that loss of miR-155 leads to reduced Treg-cell population in the thymus and periphery without affecting suppressive function.62,79,80 Our study distinguishes itself from prior research by specifically targeting the deletion of MIR155HG in peripheral T cells. In contrast, previous studies investigating the role of miR-155 in immune response modulation have predominantly used genetic knockout mice, which inherently cannot exclude its influence on T-cell development.25,26,29,62,81,82 Nonetheless, Treg functional studies will be important to further understand the impact of MIR155HG genetic deletion on human T-cell function.
In conclusion, our preclinical data strongly support the innovative approach of genomic deletion of MIR155HG to generate allogeneic donor T cells that provide protection from lethal GVHD and preserve GVL. This strategy could also be used to blunt acute GVHD responses arising after donor lymphocyte infusion and for generating chimeric antigen receptor T-cell therapies that possess targeted antileukemic properties.
Acknowledgments
The authors thank the Pelotonia Institute for Immuno-Oncology (PIIO) and the Immune Monitoring and Discovery Platform for spectral flow cytometry. The authors thank the Target Validation Shared Resource at The Ohio State University (OSU) Comprehensive Cancer Center (CCC) for providing the NSG mice used in the preclinical studies described herein. They also thank the Gene Editing Shared Resource at the OSU CCC, CRISPR/Gene editing core, and Institute for Genomic Medicine at Nationwide Children’s Hospital, Columbus, OH for whole-genome sequencing.
This work was supported by the OSU Leukemia Research Program, OSU Drug Development Institute, OSU CCC start-up funds, National Institutes of Health (NIH) R01CA252469, R01HL163849, American Cancer Society RSG RSG-22-053-01-IBCD (P.R.); NIH T32CA090223 fellowship to K.B. and R01CA240612 (R.G.). Research reported in this publication was supported by the OSU CCC and the NIH under grant number P30 CA016058. This research was made possible through resources, expertise, and support provided by the PIIO, which is funded by the Pelotonia community and the OSU CCC.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: L.N.-C. and S.K. performed the T-cell isolations, CRISPR transfections, in vivo murine acute GVHD experiments, ex vivo fluorescence-activated cell sorting (FACS) analyses, genomic PCR, real-time PCR analyses, in vitro cytotoxic T-cell lymphocyte assays, in vivo xeno–graft-versus-leukemia experiments and wrote the manuscript; K.M.B. designed the single guide RNA probes, performed T-cell isolations, CRISPR transfection experiments, in vivo murine acute GVHD experiments and wrote the manuscript; K.J.S. and S.K. (New York University) performed histopathological analysis; S.K., M.E.D., M.C., and P.M. performed droplet digital PCR experiments; R.K., M.A.W., S.S., and O.T. performed T-cell isolations and CRISPR transfections; Y.G. performed western blot experiments and ex vivo FACS analyses; M.N.K. performed the off-target analyses; M.K., H.K.C., and W.K.C. provided discussion and edited the manuscript; R.G. designed the study, supervised research, and edited the manuscript; P.R. designed the study, supervised research, analyzed and interpreted the data, wrote, and edited the manuscript.
Conflict-of-interest disclosure: P.R. and R.G. have filed a provisional patent application for the use of genetically modified cells comprising a deletion in MIR155HG to prevent GVHD; H.K.C. has served on advisory boards or consulting for Incyte, Sanofi, and Actinium and research support from Opna. The remaining authors declare no competing financial interests.
Correspondence: Parvathi Ranganathan, Division of Hematology, Department of Internal Medicine, The Ohio State University, 460 W. 12th Ave BRT420, Columbus, OH 43210; email: parvathi.ranganathan@osumc.edu.
References
Author notes
L.N.-C. and S.K. are both first authors and contributed equally to this study.
Sequencing data were deposited in BioProject (accession number PRJNA1060736). Original WGS data and other forms of data are available upon request from the corresponding author, Parvathi Ranganathan (parvathi.ranganathan@osumc.edu).
The full-text version of this article contains a data supplement.