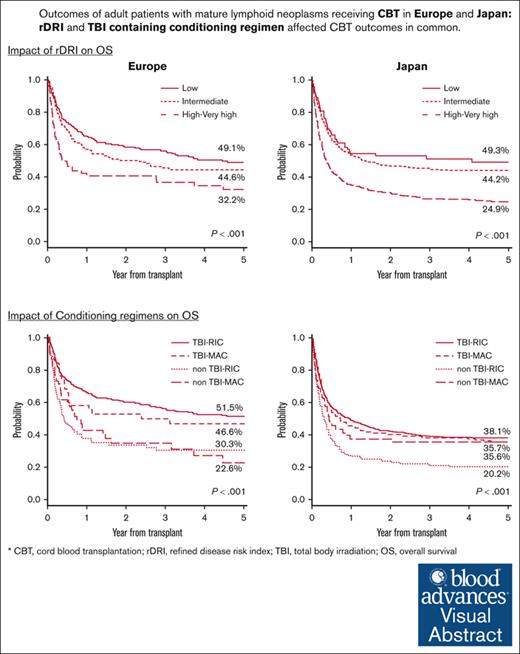
Despite different characteristics and transplant protocols, patients with lymphoma from Europe and Japan have similar outcomes.
TBI–containing regimens positively affected survival in both (European, Japanese) cohorts, whereas the effect of HLA mismatches differed.
Visual Abstract
To clarify the different characteristics and prognostic factors of cord blood transplantation (CBT) in adult patients with lymphoid neoplasms in Europe and Japan, we conducted a collaborative study. Patients aged 18-75 years receiving their first CBT (Europe: single CBT, n = 192; double CBT, n = 304; Japan: single CBT, n = 1150) in 2000-2017 were analyzed. Fewer patients with Hodgkin lymphoma (Europe vs Japan, 26% vs 5%), and older patients (≥50 years) (39% vs 59%) with a higher refined disease risk index (rDRI) (high-very high: 49% vs 14%) were included in the Japanese registry. High-very high rDRI was associated with inferior overall survival (OS) (vs low rDRI, Europe: hazard ratio [HR], 1.87; P = .001; Japan: HR, 2.34; P < .001) with higher progression/relapse risks. Total body irradiation (TBI)–containing conditioning contributed to superior OS both in Europe (vs TBI–reduced-intensity conditioning [RIC], non-TBI-RIC: HR, 1.93; P < .001; non-TBI–Myeloablative conditioning [MAC]: HR, 1.90; P = .003) and Japan (non–TBI-RIC: HR, 1.71; P < .001; non–TBI-MAC: HR 1.50, P = .007). The impact of HLA mismatches (≥2) on OS differed (Europe: HR, 1.52; P = .007; Japan: HR, 1.18; P = .107). CBT for lymphoid neoplasms, especially in those with high rDRI showed poor outcomes despite all the different characteristics in both registries. TBI should be considered in conditioning regimens to improve these outcomes. The different impacts of HLA mismatches call attention to the fundamental differences among these populations.
Introduction
The rise of novel strategies, such as the use of posttransplant cyclophosphamide–based haploidentical transplant, have gradually changed the role of umbilical cord blood (CB) as an alternative donor source.1-6 Nevertheless, improving the outcomes of cord blood transplantation (CBT) is very important because CB remains a good alternative graft source, in particular when other suitable donors are not available in a timely fashion. In a previous collaborative study between the Eurocord/European Society for Blood and Marrow Transplantation (Eurocord/EBMT) and the Japanese Society for Transplantation and Cellular Therapy (JSTCT)/Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT) focusing on leukemia, we observed that the factors affecting CBT outcomes were similar between the 2 registries despite different clinical practices and ethnicities.7 Meanwhile, we have limited knowledge about the differences between allogeneic hematopoietic cell transplantation (allo-HCT) performed for patients with mature lymphoid neoplasm in Western countries and Japan. Because the effects of allo-HCT, such as the risk of nonrelapse mortality (NRM) and graft-versus-host disease (GVHD), differ between patients suffering from leukemia and those suffering from lymphoid neoplasms, we performed a new study focusing, this time, on CBT outcomes in adult patients with lymphoid neoplasms. The aim of this study was to comprehensively examine the characteristics and outcomes of patients who received CBT for lymphoid neoplasms in Europe or Japan and to determine the CBT prognostic factors in each registry, which could potentially help improve transplant strategies and outcomes.
Patients and methods
Data collection
Transplant data were obtained from the Eurocord/EBMT Registry and Transplant Registry Unified Management Program of JSTCT/JDCHCT. All patients provided written informed consent for research. The study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of Eurocord, Kyoto University Graduate School of Medicine, and the Data Management Committees of JSTCT and JDCHCT.
Inclusion and exclusion criteria
Patients aged 18 to 75 years with mature lymphoid neoplasms who received their first unrelated CBT using unmanipulated single or double CB unit(s) between 2000 and 2017 were eligible for this study. CBTs combined with other cell sources were excluded. Patients with adult T-cell leukemia/lymphoma or immature lymphoid malignancies were also excluded.
Definitions and end points
Overall survival (OS) was defined as the time from transplantation to the last date of follow-up or death. Relapse/progression was defined as any recurrence or progression of lymphoid neoplasm after transplantation. Relapse/progression-free survival (PFS) was defined as lymphoid neoplasm progression/relapse and death as events. NRM was defined as death without evidence of lymphoid neoplasm progression or relapse. Neutrophil engraftment was defined as the first day of neutrophil count ≥0.5 × 109 /L for 3 consecutive days, without evidence of autologous reconstitution or graft rejection within the first 100 days of CBT. Acute and chronic GVHD were diagnosed and graded using standard criteria.8,9 Myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC) regimens were classified as reported by the transplant centers following the EBMT and Japanese Transplant Registry Unification Management Program guidelines and according to standard criteria.10 To standardize the data, HLA typing was classified considering low-resolution typing for HLA-A and HLA-B and high-resolution typing for HLA-DRB1 in both cohorts.
The primary objectives of this study were to, independently, describe the characteristics of patients who received their first CBT for lymphoid neoplasms in either Eurocord/EBMT or JSTCT/JDCHC transplant centers and to identify the common and different prognostic factors for outcomes in respective to the 2 registries. The secondary end points were to describe the probability of OS and PFS, the cumulative incidence of progression/relapse, NRM, neutrophil engraftment, and acute and chronic GVHD.
Statistical analysis
OS and PFS were evaluated using the Kaplan-Meier method, and lymphoid neoplasm progression/relapse and NRM were calculated based on the cumulative incidence function (CIF) to account for competing risks.11 The effects of patient and transplant characteristics on the outcomes of interest were assessed using the Cox proportional hazard model for OS and PFS, Fine and Gray proportional subhazards model for progression/relapse, NRM, neutrophil engraftment, and acute and chronic GVHD.12 The competing events were death without progression/relapse for progression/relapse, death without engraftment for engraftment, progression/relapse for NRM, and death without acute or chronic GVHD for acute and chronic GVHD. Chronic GVHD was assessed for patients who survived for at least 100 days after transplantation. Covariates considered were transplant year (continuous variable), patient sex, patient age (<50 or ≥50 years old), Karnofsky Performance Status (40-80, 90-100), hematopoietic cell transplantation-specific comorbidity index (Japanese cohort only), history of autologous hematopoietic stem cell transplantation (HCT) before CBT, refined disease risk index (rDRI), patients’ cytomegalovirus infection status, total nucleated cell (TNC) counts (quartile in each registry), CD34+ cell counts (quartile in each registry), number of HLA mismatches (<2 or ≥2 locus mismatches out of 6 loci), the combination of intensity of conditioning regimen (RIC or MAC) and the use of total body irradiation (TBI) (TBI-RIC, TBI-MAC, non–TBI-RIC, non–TBI-MAC), use of anti-thymocyte globulin (ATG) as a GVHD prophylaxis (European cohort only), GVHD prophylactic regimen other than ATG. For variables with >5% missing values, the missing data were included as a separate category. Covariates were selected in the preceding multivariate analysis for each registry in a stepwise manner, with a variable retention criterion of P value <.05. All covariates selected from 1 or both registries were included in the corresponding subsequent multivariate analysis. Variables with P values <.05 were considered significant.
All statistical analyses were performed using commercial software Stata (version 13, Stata Corp, College Station, TX) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan),13 which is a graphic user interface for R (R Foundation for Statistical Computing, version 3.1.1, Vienna, Austria).
Results
Patients’ characteristics
A total of 496 patients (single CBT, n = 192; double CBT, n = 304) from the European registry and 1150 patients (single CBT only) from the Japanese registry were included. Table 1 shows patient and CBT characteristics. The distributions of lymphoid neoplasm subtypes were different between Europe (mature B-cell neoplasms, n = 247 [49.8%]; mature T/NK-cell neoplasms, n = 98 [19.8%]; Hodgkin lymphoma [HL], n = 131 [26.4%], considering both single and double CBT) and Japan (mature B-cell neoplasms, n = 658 [57.2%]; mature T/NK-cell neoplasms, n = 401 [34.9%]; HL, n = 58 [5.0%]) with different frequency of autologous stem cell transplantation (Europe: 55.0%; Japan: 26.8%) before CBT (supplemental Table 1). The Japanese cohort comprised older patients >50 years of age (59.0% vs 39.1%) with a higher rDRI (high-very high: 49.3% vs 13.7%) (Table 1; supplemental Table 2). Median TNC counts were 4.38 (interquartile range [IQR]: 3.69-5.35) × 107/kg in Europe, and it was 2.66 (IQR: 2.27-3.17) × 107/kg in Japan (only single CBT). Median CD34+ cell counts were 1.76 (IQR: 1.19-2.51) × 105/kg in Europe and 0.81 (IQR: 0.60-1.15) × 105/kg in Japan. Mycophenolate mofetil (MMF) in combination with a calcineurin inhibitor (CI) was used for GVHD prophylaxis more frequently in Europe (Europe: 72.7%; Japan: 27.8%) and methotrexate (MTX) with CI almost only in Japan (Japan: 46.0%). ATG was rarely used in the Japanese cohort (0.3%) whereas it was used in 30.4% of patients in the European cohort. The median follow-up time for survivors was 4.6 years in the European and 3.7 years in the Japanese cohorts.
Patients’ characteristics
| . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||
|---|---|---|---|---|
| n∗ . | %† . | n∗ . | %† . | |
| Disease subtype | ||||
| B-cell lymphoma | 247 | 49.8 | 658 | 57.2 |
| Mature T/NK-cell lymphoma | 98 | 19.8 | 401 | 34.9 |
| Hodgkin lymphoma | 131 | 26.4 | 58 | 5.0 |
| NHL (not specified) | 20 | 4.0 | 33 | 2.9 |
| Year of transplantation | ||||
| 2000-2004 | 9 | 1.8 | 140 | 12.2 |
| 2005-2009 | 196 | 39.5 | 258 | 22.4 |
| 2010-2014 | 256 | 51.6 | 445 | 38.7 |
| 2015-2017 | 35 | 7.1 | 307 | 26.7 |
| Patient sex | ||||
| Male | 312 | 62.9 | 695 | 60.4 |
| Female | 183 | 36.9 | 455 | 39.6 |
| Missing | 1 | 0.2 | 0 | 0.0 |
| Age at CBT | 46 (IQR, 32-56) | 53 (IQR, 42-60) | ||
| Age‡(<50 or ≥50) | ||||
| 18-49 | 302 | 60.9 | 471 | 41.0 |
| 50-70 | 194 | 39.1 | 679 | 59.0 |
| KPS | ||||
| 40-80 | 90 | 18.1 | 368 | 32.0 |
| 90-100 | 278 | 56.0 | 694 | 60.3 |
| Missing | 128 | 25.8 | 88 | 7.7 |
| rDRI | ||||
| Low | 208 | 41.9 | 84 | 7.3 |
| Intermediate | 191 | 38.5 | 458 | 39.8 |
| High-very high | 68 | 13.7 | 567 | 49.3 |
| Missing | 29 | 5.8 | 41 | 3.6 |
| History of auto-HCT | ||||
| No | 223 | 45.0 | 842 | 73.2 |
| Yes | 273 | 55.0 | 308 | 26.8 |
| Double or single | ||||
| Single | 192 | 38.7 | 1150 | 100.0 |
| Double | 304 | 61.3 | 0 | 0.0 |
| TNCs at collection per recipient weight (×107/kg) | 4.38 (IQR, 3.69-5.35)§ | 2.66 (IQR, 2.27-3.17) | ||
| Number of CD34+ cells at collection per recipient weight (×105/kg) | 1.76 (IQR, 1.19-2.51)| | 0.81 (IQR, 0.60-1.15) | ||
| Number of HLA-MM | ||||
| 0-1 | 122 | 24.6 | 214 | 18.6 |
| 2- | 307 | 61.9 | 817 | 71.0 |
| Missing | 67 | 13.5 | 119 | 10.3 |
| Conditioning | ||||
| MAC | ||||
| Non-TBI | 53 | 10.7 | 92 | 8.0 |
| TBI | 38 | 7.7 | 280 | 24.3 |
| Missing | 13 | 2.6 | 0 | 0.0 |
| RIC | ||||
| Non-TBI | 83 | 16.7 | 175 | 15.2 |
| TBI | 301 | 60.7 | 602 | 52.3 |
| Missing | 8 | 1.6 | 1 | 0.1 |
| GVHD prophylaxis | ||||
| CI + MMF | 359 | 72.4 | 320 | 27.8 |
| CI + MTX | NA | NA | 529 | 46.0 |
| CI + steroid | 62 | 12.5 | NA | NA |
| Others | 75 | 15.1 | 301 | 26.2 |
| Use of ATG | ||||
| No | 279 | 56.3 | 1067 | 92.8 |
| Yes | 151 | 30.4 | 3 | 0.3 |
| Missing | 66 | 13.3 | 80 | 7.0 |
| Patient CMV status | ||||
| Positive | 206 | 41.5 | 848 | 73.7 |
| Negative | 269 | 54.2 | 200 | 17.4 |
| Missing/not performed | 21 | 4.2 | 102 | 8.9 |
| . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||
|---|---|---|---|---|
| n∗ . | %† . | n∗ . | %† . | |
| Disease subtype | ||||
| B-cell lymphoma | 247 | 49.8 | 658 | 57.2 |
| Mature T/NK-cell lymphoma | 98 | 19.8 | 401 | 34.9 |
| Hodgkin lymphoma | 131 | 26.4 | 58 | 5.0 |
| NHL (not specified) | 20 | 4.0 | 33 | 2.9 |
| Year of transplantation | ||||
| 2000-2004 | 9 | 1.8 | 140 | 12.2 |
| 2005-2009 | 196 | 39.5 | 258 | 22.4 |
| 2010-2014 | 256 | 51.6 | 445 | 38.7 |
| 2015-2017 | 35 | 7.1 | 307 | 26.7 |
| Patient sex | ||||
| Male | 312 | 62.9 | 695 | 60.4 |
| Female | 183 | 36.9 | 455 | 39.6 |
| Missing | 1 | 0.2 | 0 | 0.0 |
| Age at CBT | 46 (IQR, 32-56) | 53 (IQR, 42-60) | ||
| Age‡(<50 or ≥50) | ||||
| 18-49 | 302 | 60.9 | 471 | 41.0 |
| 50-70 | 194 | 39.1 | 679 | 59.0 |
| KPS | ||||
| 40-80 | 90 | 18.1 | 368 | 32.0 |
| 90-100 | 278 | 56.0 | 694 | 60.3 |
| Missing | 128 | 25.8 | 88 | 7.7 |
| rDRI | ||||
| Low | 208 | 41.9 | 84 | 7.3 |
| Intermediate | 191 | 38.5 | 458 | 39.8 |
| High-very high | 68 | 13.7 | 567 | 49.3 |
| Missing | 29 | 5.8 | 41 | 3.6 |
| History of auto-HCT | ||||
| No | 223 | 45.0 | 842 | 73.2 |
| Yes | 273 | 55.0 | 308 | 26.8 |
| Double or single | ||||
| Single | 192 | 38.7 | 1150 | 100.0 |
| Double | 304 | 61.3 | 0 | 0.0 |
| TNCs at collection per recipient weight (×107/kg) | 4.38 (IQR, 3.69-5.35)§ | 2.66 (IQR, 2.27-3.17) | ||
| Number of CD34+ cells at collection per recipient weight (×105/kg) | 1.76 (IQR, 1.19-2.51)| | 0.81 (IQR, 0.60-1.15) | ||
| Number of HLA-MM | ||||
| 0-1 | 122 | 24.6 | 214 | 18.6 |
| 2- | 307 | 61.9 | 817 | 71.0 |
| Missing | 67 | 13.5 | 119 | 10.3 |
| Conditioning | ||||
| MAC | ||||
| Non-TBI | 53 | 10.7 | 92 | 8.0 |
| TBI | 38 | 7.7 | 280 | 24.3 |
| Missing | 13 | 2.6 | 0 | 0.0 |
| RIC | ||||
| Non-TBI | 83 | 16.7 | 175 | 15.2 |
| TBI | 301 | 60.7 | 602 | 52.3 |
| Missing | 8 | 1.6 | 1 | 0.1 |
| GVHD prophylaxis | ||||
| CI + MMF | 359 | 72.4 | 320 | 27.8 |
| CI + MTX | NA | NA | 529 | 46.0 |
| CI + steroid | 62 | 12.5 | NA | NA |
| Others | 75 | 15.1 | 301 | 26.2 |
| Use of ATG | ||||
| No | 279 | 56.3 | 1067 | 92.8 |
| Yes | 151 | 30.4 | 3 | 0.3 |
| Missing | 66 | 13.3 | 80 | 7.0 |
| Patient CMV status | ||||
| Positive | 206 | 41.5 | 848 | 73.7 |
| Negative | 269 | 54.2 | 200 | 17.4 |
| Missing/not performed | 21 | 4.2 | 102 | 8.9 |
auto-HCT, autologous HCT; CMV, human cytomegalovirus; dCBT, transplantation using double CB units; HLA-MM, HLA mismatches; KPS, Karnofsky Performance Status; NA, not available; NHL, non-Hodgkin lymphoma; sCBT, transplantation using single CB unit.
n indicates the number of patients with each characteristics.
% indicates the percentage of patients in each group.
Indicates patients' age at CBT.
sCBT: 3.76 (IQR: 3.70-5.24), dCBT: 4.94 (IQR: 3.70-5.34).
sCBT: 1.51 (IQR: 1.19-2.50), dCBT: 1.88 (IQR: 1.20-2.51).
OS and progression/relapse-free survival
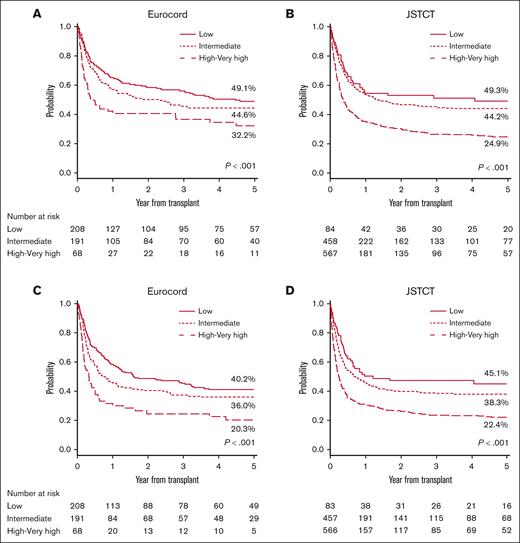
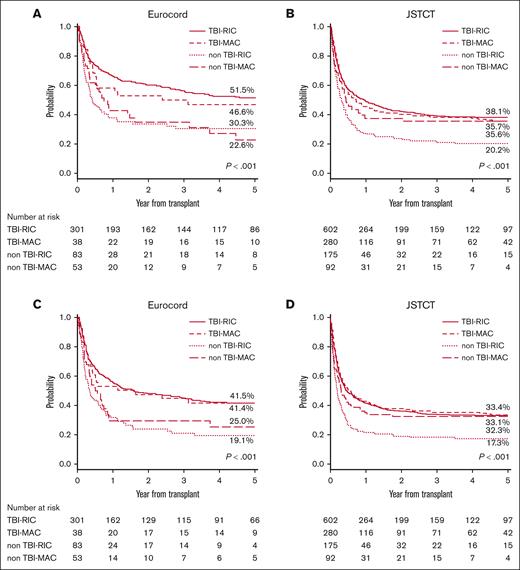
The 5-year OS and PFS in the European registry were 44.1% (low rDRI, 49.1%; intermediate rDRI, 44.6%; high-very high rDRI, 32.2%) and 35.3% (low rDRI, 40.2%; intermediate rDRI, 36.0%; high-very high rDRI, 20.3%), respectively. Whereas in the Japanese registry, the 5-year OS was 34.4% (low rDRI, 49.3%; intermediate rDRI, 44.2%; high-very high rDRI, 24.9%), and the 5-year PFS was 30.5% (low rDRI, 45.1%; intermediate rDRI, 38.3%; high-very high rDRI, 22.4%). A significant effect of rDRI and the use of TBI in the conditioning regimen on OS and PFS was observed in both registries. Compared with low rDRI, high-very high rDRI had a significant negative impact on OS (Europe: hazard ratio [HR], 1.87; P = .001; Japan: HR, 2.34; P < .001) and PFS (Europe: HR, 1.89; P < .001; Japan: HR, 2.35; P < .001) (Tables 2 and 3, respectively), resulting in poor survival probability for patients in this group (Figure 1). Patients with intermediate rDRI showed comparable outcomes with those with low rDRI in Europe (OS: HR, 1.19; P = .239; PFS: HR, 1.18; P = .218), but not in Japan, where a statistically significant negative effect of intermediate compared with low rDRI in PFS was also observed (OS: HR, 1.36; P = .071; PFS: HR, 1.46; P = .022) (Tables 2 and 3). The 5-year OS according to the conditioning regimen for the European and Japanese cohorts were TBI-RIC 51.5% and 38.8%, TBI-MAC 46.6% and 35.6%, non–TBI-RIC 30.3% and 20.2%, and non–TBI-MAC 22.6% and 35.7%, respectively (Figure 2). The 5-year PFS according to the respective conditioning regimen groups TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC were 33.1%, 33.4%, 17.3%, and 32.3% in the European cohort and 41.4%,31.1%, 19.1%, and 25.1%, respectively, in the Japanese cohort (Figure 2). The positive effect of TBI–containing regimens was also confirmed in multivariate analyses for OS (vs TBI-RIC, Europe: non–TBI-RIC: HR, 1.93; P < .001; non–TBI-MAC: HR, 1.90; P = .003; Japan: non–TBI-RIC: HR, 1.71; P < .001; non–TBI-MAC: HR, 1.50; P = .007) and PFS (Europe: non–TBI-RIC: HR, 1.84; P < .001; non–TBI-MAC: HR, 1.73; P = .010; Japan: non–TBI-RIC: HR, 1.74; P < .001; non–TBI-MAC: HR, 1.36; P = .037). HLA mismatches ≥ 2 (vs <2) had a significant negative impact on OS (Europe: HR, 1.52; P = .007; Japan: HR, 1.18; P = .107) and PFS only in the European cohort, whereas age at CBT (≥50 vs <50 years) and GVHD prophylaxis (CI plus MTX vs CI plus MMF) had a significant negative impact only in the Japanese cohort (Tables 2 and 3). Low-dose TBI (<4 Gy) showed positive effect on OS (vs non-TBI regimens, Europe: HR, 0.48; P < .001; Japan: HR, 0.60; P < .001) and PFS (vs non-TBI regimens, Europe: HR, 0.53; P < .001; Japan: HR, 0.57; P < .001) in patients receiving the RIC regimen in both registries. The positive impact of high-dose TBI (≥12 Gy) was significant only in Japanese cohorts receiving the MAC regimen (vs non-TBI regimens, OS: HR, 0.58; P = .005; PFS: HR, 0.62; P = .007) (supplemental Table 5). The details of the analysis of the effect of conditioning regimens on outcomes according to the rDRI groups are provided in supplemental Tables 3 and 4. Non-TBI regimens showed a more evident adverse effect on OS (vs TBI-RIC, Europe: non–TBI-RIC: HR, 2.96; P = .004; non–TBI-MAC: HR, 2.84; P = .027) and PFS (vs TBI-RIC, Europe: non–TBI-RIC: HR, 2.58; P = .014; non–TBI-MAC: HR, 2.78; P = .046; Japan: non–TBI-RIC: HR, 1.81; P < .001; non–TBI-MAC: HR, 1.56; P = .026) for patients with high-very high rDRI.
Overall survival
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Year of transplant, continuous variable | 0.99 | (0.95-1.03) | .648 | 0.96 | (0.94-0.98) | <.001 |
| Age∗(<50 or ≥50) | ||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||
| 50-70 | 1.28 | (0.98-1.67) | .074 | 1.48 | (1.26-1.74) | <.001 |
| rDRI | ||||||
| Low | 1.00 | Reference | 1.00 | Reference | ||
| Intermediate | 1.19 | (0.89-1.58) | .239 | 1.36 | (0.97-1.91) | .071 |
| High or very high | 1.87 | (1.31-2.69) | .001 | 2.34 | (1.69-3.25) | <.001 |
| Conditioning | ||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||
| TBI-MAC | 1.15 | (0.71-1.86) | .580 | 1.05 | (0.85-1.28) | .663 |
| Non–TBI-RIC | 1.93 | (1.38-2.70) | <.001 | 1.71 | (1.40-2.10) | <.001 |
| Non–TBI-MAC | 1.90 | (1.25-2.90) | .003 | 1.50 | (1.12-2.03) | .007 |
| GVHD prophylaxis | ||||||
| CI + MMF | 1.00 | Reference | 1.00 | Reference | ||
| CI + MTX | NA | NA | NA | 1.46 | (1.20-1.78) | <.001 |
| CI + steroid | 1.18 | (0.78-1.79) | .444 | NA | NA | NA |
| Others | 1.32 | (0.92-1.89) | .128 | 1.68 | (1.35-2.08) | <.001 |
| Number of HLA-MM | ||||||
| 0-1 | 1.00 | Reference | 1.00 | Reference | ||
| 2- | 1.52 | (1.12-2.07) | .007 | 1.18 | (0.97-1.44) | .107 |
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Year of transplant, continuous variable | 0.99 | (0.95-1.03) | .648 | 0.96 | (0.94-0.98) | <.001 |
| Age∗(<50 or ≥50) | ||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||
| 50-70 | 1.28 | (0.98-1.67) | .074 | 1.48 | (1.26-1.74) | <.001 |
| rDRI | ||||||
| Low | 1.00 | Reference | 1.00 | Reference | ||
| Intermediate | 1.19 | (0.89-1.58) | .239 | 1.36 | (0.97-1.91) | .071 |
| High or very high | 1.87 | (1.31-2.69) | .001 | 2.34 | (1.69-3.25) | <.001 |
| Conditioning | ||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||
| TBI-MAC | 1.15 | (0.71-1.86) | .580 | 1.05 | (0.85-1.28) | .663 |
| Non–TBI-RIC | 1.93 | (1.38-2.70) | <.001 | 1.71 | (1.40-2.10) | <.001 |
| Non–TBI-MAC | 1.90 | (1.25-2.90) | .003 | 1.50 | (1.12-2.03) | .007 |
| GVHD prophylaxis | ||||||
| CI + MMF | 1.00 | Reference | 1.00 | Reference | ||
| CI + MTX | NA | NA | NA | 1.46 | (1.20-1.78) | <.001 |
| CI + steroid | 1.18 | (0.78-1.79) | .444 | NA | NA | NA |
| Others | 1.32 | (0.92-1.89) | .128 | 1.68 | (1.35-2.08) | <.001 |
| Number of HLA-MM | ||||||
| 0-1 | 1.00 | Reference | 1.00 | Reference | ||
| 2- | 1.52 | (1.12-2.07) | .007 | 1.18 | (0.97-1.44) | .107 |
95% CI, 95% confidence interval; HLA-MM, HLA mismatches; NA, not available.
Results of the analysis statistically significant (with P < .05) are highlighted in bold.
Indicates patients' age at CBT.
Relapse/progression-free survival
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Year of transplant, continuous variable | 0.99 | (0.95-1.03) | .542 | 0.96 | (0.95-0.98) | <.001 |
| Age∗(<50 or ≥50) | ||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||
| 50-70 | 1.06 | (0.82-1.37) | .648 | 1.41 | (1.21-1.65) | <.001 |
| rDRI | ||||||
| Low | 1.00 | Reference | 1.00 | Reference | ||
| Intermediate | 1.18 | (0.91-1.55) | .218 | 1.46 | (1.06-2.02) | .022 |
| High or very high | 1.89 | (1.34-2.66) | <.001 | 2.35 | (1.71-3.23) | <.001 |
| Conditioning | ||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||
| TBI-MAC | 0.97 | (0.61-1.55) | .909 | 0.94 | (0.77-1.14) | .514 |
| Non–TBI-RIC | 1.84 | (1.33-2.53) | <.001 | 1.74 | (1.43-2.12) | <.001 |
| Non–TBI-MAC | 1.73 | (1.14-2.61) | .010 | 1.36 | (1.02-1.82) | .037 |
| GVHD prophylaxis | ||||||
| CI + MMF | 1.00 | Reference | 1.00 | Reference | ||
| CI + MTX | NA | NA | NA | 1.47 | (1.22-1.79) | <.001 |
| CI + steroid | 0.86 | (0.56-1.31) | .496 | NA | NA | NA |
| Others | 1.19 | (0.84-1.69) | .318 | 1.70 | (1.38-2.10) | <.001 |
| TNCs at collection per recipient weight (×107/kg)† | ||||||
| -First quartile | 1.00 | Reference | 1.00 | Reference | ||
| First-second quartile | 0.67 | (0.47-0.96) | .028 | 0.93 | (0.75-1.14) | .465 |
| Second-third quartile | 0.84 | (0.59-1.18) | .314 | 0.91 | (0.74-1.12) | .358 |
| Third quartile - | 0.88 | (0.61-1.26) | .482 | 0.98 | (0.80-1.21) | .874 |
| Number of HLA-MM | ||||||
| 0-1 | 1.00 | Reference | 1.00 | Reference | ||
| 2- | 1.36 | (1.03-1.81) | .030 | 1.17 | (0.96-1.42) | .119 |
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Year of transplant, continuous variable | 0.99 | (0.95-1.03) | .542 | 0.96 | (0.95-0.98) | <.001 |
| Age∗(<50 or ≥50) | ||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||
| 50-70 | 1.06 | (0.82-1.37) | .648 | 1.41 | (1.21-1.65) | <.001 |
| rDRI | ||||||
| Low | 1.00 | Reference | 1.00 | Reference | ||
| Intermediate | 1.18 | (0.91-1.55) | .218 | 1.46 | (1.06-2.02) | .022 |
| High or very high | 1.89 | (1.34-2.66) | <.001 | 2.35 | (1.71-3.23) | <.001 |
| Conditioning | ||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||
| TBI-MAC | 0.97 | (0.61-1.55) | .909 | 0.94 | (0.77-1.14) | .514 |
| Non–TBI-RIC | 1.84 | (1.33-2.53) | <.001 | 1.74 | (1.43-2.12) | <.001 |
| Non–TBI-MAC | 1.73 | (1.14-2.61) | .010 | 1.36 | (1.02-1.82) | .037 |
| GVHD prophylaxis | ||||||
| CI + MMF | 1.00 | Reference | 1.00 | Reference | ||
| CI + MTX | NA | NA | NA | 1.47 | (1.22-1.79) | <.001 |
| CI + steroid | 0.86 | (0.56-1.31) | .496 | NA | NA | NA |
| Others | 1.19 | (0.84-1.69) | .318 | 1.70 | (1.38-2.10) | <.001 |
| TNCs at collection per recipient weight (×107/kg)† | ||||||
| -First quartile | 1.00 | Reference | 1.00 | Reference | ||
| First-second quartile | 0.67 | (0.47-0.96) | .028 | 0.93 | (0.75-1.14) | .465 |
| Second-third quartile | 0.84 | (0.59-1.18) | .314 | 0.91 | (0.74-1.12) | .358 |
| Third quartile - | 0.88 | (0.61-1.26) | .482 | 0.98 | (0.80-1.21) | .874 |
| Number of HLA-MM | ||||||
| 0-1 | 1.00 | Reference | 1.00 | Reference | ||
| 2- | 1.36 | (1.03-1.81) | .030 | 1.17 | (0.96-1.42) | .119 |
95% CI, 95% confidence interval; HLA-MM, HLA mismatches; NA, not available.
Results of the analysis statistically significant (with P < .05) are highlighted in bold.
Indicates patients' age at CBT.
TNCs at collection per recipient weight (×107/kg) was categorized into 4 groups using the quartiles as referred in the Table 1.
OS and progression/relapse-free survival stratified by rDRI. Probability of OS stratified by rDRI was grouped into 3 (low, intermediate, and high-very high) in the European cohort (A) and in the Japanese cohort (B). Probability of PFS stratified by rDRI was grouped into 3 (low, intermediate, high-very high) in the European cohort (C) and in the Japanese cohort (D).
OS and progression/relapse-free survival stratified by rDRI. Probability of OS stratified by rDRI was grouped into 3 (low, intermediate, and high-very high) in the European cohort (A) and in the Japanese cohort (B). Probability of PFS stratified by rDRI was grouped into 3 (low, intermediate, high-very high) in the European cohort (C) and in the Japanese cohort (D).
OS and progression/relapse-free survival stratified by conditioning regimen. Probability of OS stratified by conditioning regime was grouped into 4 (TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC) in the European cohort (A) and in the Japanese cohort (B). Probability of PFS stratified by conditioning regimen in the European (C) and Japanese cohorts (D).
OS and progression/relapse-free survival stratified by conditioning regimen. Probability of OS stratified by conditioning regime was grouped into 4 (TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC) in the European cohort (A) and in the Japanese cohort (B). Probability of PFS stratified by conditioning regimen in the European (C) and Japanese cohorts (D).
GVHD as the cause of death was more frequently observed in the Eurocord cohort, whereas the progression of lymphoid neoplasms was more frequently observed in the Japanese cohort. The primary cause of death is provided in supplemental Table 6.
Relapse/progression
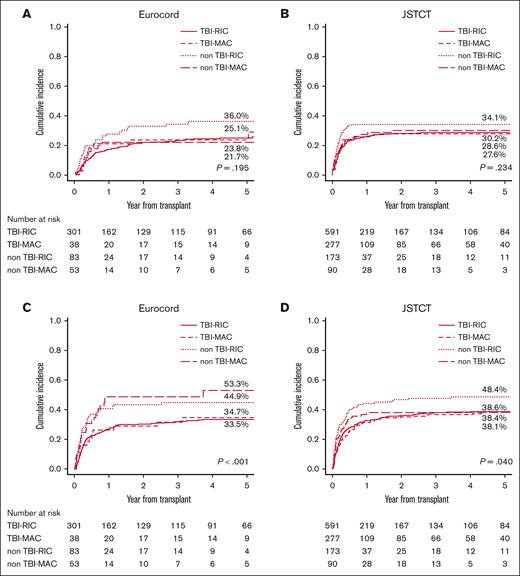
The 5-year CIF for relapse/progression was 26.7% in the European cohort and 29.4% in the Japanese cohort. High-very high rDRI (vs low rDRI) had a negative effect in both cohorts (Europe: HR, 2.04; P = .007; Japan: HR, 2.96; P < .001) with very high incidences of progression (Europe: 40.6%; Japan: 34.1%) (supplemental Figure 1), whereas the impact of intermediate rDRI was statistically significant only in Japan (Europe: HR, 1.48; P = .064; Japan: HR, 2.01; P = .022) (Table 4). In both cohorts, most of the patients who experienced relapse or progression (Europe: 73.5%, Japan; 92.1%) had the event within 1 year after the transplant. Age ≥ 50 years (vs <50 years) was identified as a protective factor for relapse only in the European cohort, whereas a history of autologous HCT had a statistically positive effect only in the Japanese cohort (Table 4). A negative effect tendency of non–TBI-RIC was observed in both cohorts in the univariate analysis (Figure 3A-B), but in the multivariate analysis, it was borderline statistically significant only in the Japanese cohort (HR, 1.36; P = .052) (Table 4). A higher dose of TBI ≥4 Gy was associated with a lower progression risk in the Japanese patients receiving RIC (HR, 0.72; P = .048), whereas no difference was observed between different TBI doses in the European registry (HR, 0.73; P = .425) (supplemental Table 5).
Disease relapse/progression
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age∗(<50 or ≥50) | ||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||
| 50-70 | 0.58 | (0.38-0.88) | .010 | 0.82 | (0.65-1.04) | .103 |
| rDRI | ||||||
| Low | 1.00 | Reference | 1.00 | Reference | ||
| Intermediate | 1.48 | (0.98-2.24) | .064 | 2.01 | (1.11-3.67) | .022 |
| High or very high | 2.04 | (1.21-3.42) | .007 | 2.96 | (1.64-5.34) | <.001 |
| Conditioning | ||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||
| TBI-MAC | 0.77 | (0.39-1.53) | .461 | 0.83 | (0.62-1.11) | .204 |
| Non–TBI-RIC | 1.19 | (0.74-1.90) | .469 | 1.36 | (1.00-1.84) | .052 |
| Non–TBI-MAC | 0.67 | (0.35-1.30) | .234 | 1.05 | (0.68-1.62) | .817 |
| History of auto-HCT | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.94 | (0.64-1.37) | .743 | 0.65 | (0.49-0.86) | .002 |
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age∗(<50 or ≥50) | ||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||
| 50-70 | 0.58 | (0.38-0.88) | .010 | 0.82 | (0.65-1.04) | .103 |
| rDRI | ||||||
| Low | 1.00 | Reference | 1.00 | Reference | ||
| Intermediate | 1.48 | (0.98-2.24) | .064 | 2.01 | (1.11-3.67) | .022 |
| High or very high | 2.04 | (1.21-3.42) | .007 | 2.96 | (1.64-5.34) | <.001 |
| Conditioning | ||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||
| TBI-MAC | 0.77 | (0.39-1.53) | .461 | 0.83 | (0.62-1.11) | .204 |
| Non–TBI-RIC | 1.19 | (0.74-1.90) | .469 | 1.36 | (1.00-1.84) | .052 |
| Non–TBI-MAC | 0.67 | (0.35-1.30) | .234 | 1.05 | (0.68-1.62) | .817 |
| History of auto-HCT | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.94 | (0.64-1.37) | .743 | 0.65 | (0.49-0.86) | .002 |
95% CI, 95% confidence interval; auto-HCT, autologous HCT.
Results of the analysis statistically significant (with P < .05) are highlighted in bold.
Indicates patients’ age at CBT.
Relapse/progression and nonrelapse mortality stratified by the conditioning regimen. Cumulative incidence of relapse or disease progression stratified by conditioning regimen was grouped into 4 (TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC) in the European cohort (A) and in the Japanese cohort (B). Cumulative incidence of non-relapse mortality stratified by conditioning regimen was grouped into 4 (TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC) in the European cohort (C) and in the Japanese cohort (D).
Relapse/progression and nonrelapse mortality stratified by the conditioning regimen. Cumulative incidence of relapse or disease progression stratified by conditioning regimen was grouped into 4 (TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC) in the European cohort (A) and in the Japanese cohort (B). Cumulative incidence of non-relapse mortality stratified by conditioning regimen was grouped into 4 (TBI-RIC, TBI-MAC, non–TBI-RIC, and non–TBI-MAC) in the European cohort (C) and in the Japanese cohort (D).
Non relapse/progression mortality
The 5-year CIF for transplant-related mortality was 37.9% in the European cohort and 40.1% in the Japanese cohort, which was relatively high, regardless of the rDRI (supplemental Figure 2). Age ≥50 years (vs <50) was the only factor having statistically significant negative effect in both cohorts (Table 5). HLA mismatches ≥2 and the use of ATG showed a negative impact only in the European cohort, whereas GVHD prophylaxis with CI plus MTX was only observed in the Japanese cohort (Table 5). Regarding the conditioning regimen, the negative effect of non–TBI-RIC (vs TBI-RIC, HR, 1.45; P = .005) was significant in the Japanese cohort. The impact of TBI was positive in Japanese patients who had received the RIC regimen, regardless of the TBI dose (vs non-TBI regimens, low-dose <4 Gy: HR, 0.62; P = .012; high dose ≥4 Gy: HR, 0.74; P = .042). A negative impact of non–TBI-MAC in the European cohort was also observed (HR, 1.63; P = .075), although it did not reach statistical significance (Figure 3C-D; Table 5; supplemental Table 5).
Nonrelapse mortality
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |||
| Age∗(<50 or ≥50) | ||||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||||
| 50-70 | 1.65 | (1.19-2.30) | 0.003 | 1.67 | (1.35-2.07) | <.001 | ||
| Conditioning | ||||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||||
| TBI-MAC | 1.09 | (0.57-2.06) | 0.799 | 1.08 | (0.84-1.39) | .550 | ||
| Non–TBI-RIC | 1.11 | (0.67-1.85) | 0.678 | 1.45 | (1.12-1.89) | .005 | ||
| Non–TBI-MAC | 1.63 | (0.95-2.78) | 0.075 | 1.17 | (0.80-1.72) | .420 | ||
| Number of HLA-MM | ||||||||
| 0-1 | 1.00 | Reference | 1.00 | Reference | ||||
| 2- | 1.71 | (1.13-2.60) | 0.011 | 1.03 | (0.80-1.31) | .844 | ||
| GVHD prophylaxis | ||||||||
| CI + MMF | 1.00 | Reference | 1.00 | Reference | ||||
| CI + MTX | NA | NA | NA | NA | 1.48 | (1.15-1.90) | .002 | |
| CI + steroid | 0.98 | (0.56-1.69) | 0.935 | NA | NA | NA | NA | |
| Others | 1.14 | (0.71-1.82) | 0.594 | 1.84 | (1.41-2.40) | <.001 | ||
| Use of ATG | ||||||||
| No | 1.00 | Reference | 1.00 | Reference | ||||
| Yes | 1.54 | (1.02-2.31) | 0.040 | NA | NA | NA | NA | |
| Patient CMV status | ||||||||
| Negative | 1.00 | Reference | 1.00 | Reference | ||||
| Positive | 1.41 | (1.01-1.96) | 0.045 | 0.91 | (0.71-1.17) | .459 | ||
| Variables . | EUROCORD (N = 496) . | JSTCT (N = 1150) . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |||
| Age∗(<50 or ≥50) | ||||||||
| 18-49 | 1.00 | Reference | 1.00 | Reference | ||||
| 50-70 | 1.65 | (1.19-2.30) | 0.003 | 1.67 | (1.35-2.07) | <.001 | ||
| Conditioning | ||||||||
| TBI-RIC | 1.00 | Reference | 1.00 | Reference | ||||
| TBI-MAC | 1.09 | (0.57-2.06) | 0.799 | 1.08 | (0.84-1.39) | .550 | ||
| Non–TBI-RIC | 1.11 | (0.67-1.85) | 0.678 | 1.45 | (1.12-1.89) | .005 | ||
| Non–TBI-MAC | 1.63 | (0.95-2.78) | 0.075 | 1.17 | (0.80-1.72) | .420 | ||
| Number of HLA-MM | ||||||||
| 0-1 | 1.00 | Reference | 1.00 | Reference | ||||
| 2- | 1.71 | (1.13-2.60) | 0.011 | 1.03 | (0.80-1.31) | .844 | ||
| GVHD prophylaxis | ||||||||
| CI + MMF | 1.00 | Reference | 1.00 | Reference | ||||
| CI + MTX | NA | NA | NA | NA | 1.48 | (1.15-1.90) | .002 | |
| CI + steroid | 0.98 | (0.56-1.69) | 0.935 | NA | NA | NA | NA | |
| Others | 1.14 | (0.71-1.82) | 0.594 | 1.84 | (1.41-2.40) | <.001 | ||
| Use of ATG | ||||||||
| No | 1.00 | Reference | 1.00 | Reference | ||||
| Yes | 1.54 | (1.02-2.31) | 0.040 | NA | NA | NA | NA | |
| Patient CMV status | ||||||||
| Negative | 1.00 | Reference | 1.00 | Reference | ||||
| Positive | 1.41 | (1.01-1.96) | 0.045 | 0.91 | (0.71-1.17) | .459 | ||
95% CI, 95% confidence interval; CMV, Human cytomegalovirus; HLA-MM, HLA mismatches; NA, not available.
Results of the analysis statistically significant (with P < .05) are highlighted in bold.
Indicates patients' age at CBT.
Neutrophil engraftment
The 60-days CIF for neutrophil engraftment was 86.8% in the European registry and 82.0% in the Japanese cohort. Age ≥50 years (vs age <50, Europe: HR, 0.74; P = .006; Japan: HR, 0.80; P = .002), intermediate (only in the European cohort: HR, 0.78; P = .028) and high-very high rDRI (vs low rDRI, Europe: HR, 0.63; P = .011; Japan: HR, 0.75; P = .025) showed adverse effect on neutrophil engraftment (supplemental Table 7). TBI-MAC in the European cohort (HR, 0.66; P = .026) and non–TBI-RIC (HR, 0.74; P = .002) in the Japanese cohort had a negative impact on engraftment. The positive effect of the recent year of transplantation (HR, 1.04; P < .001), higher CD34+ cell counts, and negative impact of CI+MTX prophylaxis (vs CI plus MMF, HR, 0.66; P < .001) were significant only in the Japanese cohort (supplemental Table 7).
Acute and chronic GVHD
The 100-days CIF of grade 2 to 4 and grade 3 to 4 acute GVHD was 33.0% and 16.5% in the European cohort and 37.4% and 13.9% in the Japanese cohort, respectively. HLA mismatches ≥2 were significantly associated with the risk of grades 3 to 4 acute GVHD in both cohorts (vs HLA<2, Europe: HR, 2.08; P = .028; Japan: HR, 2.77; P = .001), whereas the effect on grade 2 to 4 acute GVHD was only significant in the Japanese cohort (HR, 1.75; P < .001, supplemental Table 8). The use of ATG was associated with a lower risk of grade 3 to 4 acute GVHD (HR, 0.22; P < .001) in the European cohort, and a higher number of CD34+ cell and male gender (HR, 1.61; P = .008) was associated with a higher risk of grade 3 to 4 acute GVHD only in the Japanese cohort (supplemental Table 8).
The 5-year CIF of chronic and extensive chronic GVHD was 35.0% and 15.3% in the European cohort, and 30.3% and 14.0% in the Japanese cohort, respectively. The non–TBI-RIC conditioning regimen was associated with a decreased risk of chronic GVHD in the European cohort (vs TBI-RIC, HR, 0.40; P = .014), whereas no significant impact of the conditioning regimen on chronic GVHD was observed in the Japanese cohort (supplemental Table 9). None of the studied factors were found to be statistically significant associated with the occurrence of extensive chronic GVHD.
Discussion
To our knowledge, this was the first study to simultaneously analyze CBT outcomes in adult patients with lymphoid neoplasms from 2 registries comprising ethnically different populations. Our study reinforced the knowledge that the characteristics of patients receiving CBT for lymphoid neoplasms largely differ between Europe and Japan, which was reflected in the different distributions of lymphoma subtypes between registries.14,15 Autologous transplantation before allo-HCT was observed more frequently in European patients, which was possibly due to the higher frequency of patients with HL in that cohort than in the Japanese cohort. The Japanese cohort comprised older patients with a higher rDRI (high-very high: 49% vs 13%) than the European cohort, a similar tendency of what has been observed in the setting of leukemia in a previous collaborative study between the Eurocord and Japanese groups.7 Regarding the characteristics of CB units, the tendencies to use units with higher TNC and CD34+ cell counts and fewer HLA mismatches were more clearly observed in the European cohort, even when considering only a single CBT. This tendency could be partly explained by the different recommendations for the minimum required doses for TNC and CD34+,16-21 as well as the acceptable degree of HLA mismatches22-25 for transplant between Europe and Japan.
Despite these differences, transplant outcomes were similar between the 2 cohorts, with high frequencies of NRM and progression/relapse.
Refined DRI showed strong effect on transplant outcomes. Patients with intermediate or low rDRI had acceptable outcomes, whereas those with high-very high rDRI had a poor prognosis, supporting the known impact of the lymphoma stage.26,27 NRM did not seem to have significantly decreased over the years (data not shown), contrary to what has been observed in a previous study,28-32 which might be attributed, in part, to the type of patients included in our study and to the decreasing number of patients with lymphoma in Europe receiving CBT over time. Another possibility is that the impact of improved transplant strategies might be lessened in patients with lymphoma in comparison to patients with other hematologic malignancies, given their frequent history of heavy treatments, consequent organ toxicity, and susceptibility to infections.
Consistent with previous reports on analysis including other hematologic diseases,29-31 the use of ATG showed negative impact on NRM despite a lower incidence of GVHD, suggesting that use of ATG might be detrimental and should be cautiously used in this setting. Regarding HLA mismatches, we found that their impact differed between the 2 registries. In the European cohort, HLA mismatches >2 showed a significantly negative impact on OS and PFS. This could be partly attributed to the incidence of acute GVHD in patients receiving CB units with higher degree of HLA mismatches. The incidence of acute GVHD grade 2 has been shown in a previous study in the setting of leukemia to have a protective effect on the survival of Japanese patients, whereas the opposite effect was observed in the European cohort.33-35 Due to the nature of the available data for our analyses, we could not investigate the potential impact of HLA-mismatch at each locus on GVHD and graft-versus-lymphoma, as demonstrated in previous reports.36-40
An important factor unveiled in our study was that TBI-RIC and TBI-MAC were significantly associated with superior survival in both cohorts. The superiority of TBI–containing regimens in CBT has been previously described in some studies including both lymphoid and other hematological malignancies.41-44 Nonetheless, controversial results in the use of TBI have been reported in some other studies, including more patients with myeloid malignancies and/or receiving allo-HCT from donor sources other than CB;17,45,46 therefore, our findings suggest that the positive impact of TBI might be a distinctive feature of CBT for lymphoid neoplasms. In our study, patients who received TBI showed lower NRM and relapse/progression risks than those who did not. Improved neutrophil engraftment in the TBI cohorts47 could have been a contributing factor for the reduced NRM,41,42 although this effect was not statistically significant in our analysis. The mechanisms underlying the superiority of TBI–containing regimens for patients with lymphoma remain to be clarified, but the benefit of using TBI, especially low doses in RIC, was evident in the 2 registries, suggesting that it should be more broadly considered in the conditioning of patients with lymphoid neoplasms.
It is important to note that, in addition to the recommendations regarding caution with the use of ATG, careful assessment of HLA disparities, and to preconize the use of TBI for patients with lymphoma, it is of utmost importance to follow current CBT guidelines for CB unit selection, cell dose recommendations, conditioning regimens, and GVHD prophylaxis to further improve outcomes.17,43-46,48
Conclusion
In conclusion, CBT is an acceptable choice for patients with lymphoid neoplasms, especially those with low or intermediate rDRI, despite different transplant strategies and patient characteristics. Meanwhile, strategical improvements to reduce NRM and lymphoid neoplasm progression risks remain urgent issues, especially in those with high-very high rDRI. The effect of HLA mismatches on CBT outcomes in patients with lymphoid neoplasm differed between Europe and Japan, which calls for careful attention when examining the impact of this factor on transplant outcomes of patients from different ethnicities. The most evident factor contributing to superior outcomes was the use of a TBI–containing conditioning regimen, which was a modifiable factor. Therefore, we suggest considering its use, whenever possible, in CBT protocols for patients with lymphoid neoplasms.
Acknowledgments
The authors are grateful to all physicians, nurses, and other medical staff for their dedicated care of patients and donors throughout transplantation. The authors also thank all members who were involved in data management in each transplant center, The European Society for Blood & Marrow Transplantation, and the Japanese Data Center for Hematopoietic Cell Transplantation for their contribution to the transplantation registries.
This work was supported in part by Japanese Society for the Promotion of Science KAKENHI grant 21K08391 (J.K.) and Japanese Agency for Medical Research and Developement grant 21ek0510034h0001 (J.K.).
Authorship
Contribution: M.W. and F.V. designed research and performed analysis; M.W., F.V., J.K., B.C., G.M.S., A.R., and V.R. wrote the manuscript; E.G., R.S., H.R., F.K., E.K., S.T., C.K., M.M.R-F., S.O., P.C., J.S., S.F., J.C., N.M., N.U., Y.S., T.K., T.I., T.F., M.M., and R.P.d.L. were responsible for the data collection at each center and helped with the analysis; F.V. and Y.A. were responsible for data management and helped write the manuscript; and J.K. and E.G. organized and supervised this research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junya Kanda, Department of Hematology and Oncology, Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaramachi, Kyoto, Japan 606-8507; email: jkanda16@kuhp.kyoto-u.ac.jp.
References
Author notes
Data are available on request from the corresponding author, Junya Kanda (jkanda16@kyoto-u.ac.jp).
The full-text version of this article contains a data supplement.