Subjects with ADAN may show neutrophil counts similar to those with SCNP, complicating differential diagnoses.
Differentially expressed cytokines and higher nonclassical monocyte and antineutrophil antibody numbers suggest immune dysregulation in ADAN.
Visual Abstract
ACKR1/DARC-associated neutropenia (NP; ADAN; Online Mendelian Inheritance in Man 611862), caused by a variation in the ACKR1/DARC gene (rs2814778), is common in persons of African or Middle Eastern descent. In a cohort of 66 genetically confirmed subjects with ADAN, we show that absolute neutrophil counts (ANCs) may occasionally be lower than previously recognized (0.1 × 109-0.49 × 109/L for 9% of the subjects), which is similar to ANCs in severe congenital NP (SCNP). ANCs often normalized during inflammation, even mild. Individuals with ADAN (of 327 observed person-years) showed no cases of myelodysplastic syndrome (MDS), which is frequently encountered in SCNP. Unexpectedly, 22% presented with autoantibodies to neutrophils, compared with <1% in controls. Compared with healthy donors, subjects with ADAN demonstrated significantly lower human cationic antimicrobial protein-18/pro-leucin leucin-37 plasma levels; higher levels of nonclassical, proinflammatory, 6-sulfo LacNac-expressing monocytes; and differentially expressed plasma levels of 28 of the 239 analyzed cytokines related to immunity/inflammation, cell signaling, neutrophil activation, and angiogenesis. Collectively, more severe neutropenia in ADAN than previously assumed may complicate differential diagnoses compared with other SCNPs, and various (auto)immune/inflammatory reactions with a distinct profile may be a cause or consequence of this hereditary neutropenia.
Introduction
ACKR1/DARC-associated neutropenia (NP), ADAN,1 previously called ethnic benign NP (Online Mendelian Inheritance in Man 611862), is an inherited NP considered to be mild to moderate and not associated with an increased disposition for bacterial infections.2-5 This is in contrast to severe congenital NPs (SCNPs) caused by ELANE or HAX1 mutations, in which such infections cause considerable morbidity and mortality.6 SCNPs also show a propensity to transform into myelodysplastic syndromes (MDS) or acute leukemia,6,7 but data on MDS/leukemia in ADAN are lacking.
ADAN, prevalent in Africa and the Near/Middle East and in the descendants of these populations worldwide, is due to a single nucleotide polymorphism (SNP) in the promoter region of the ACKR1/DARC gene (rs2814778).8 This SNP is also linked to the Duffy null trait and is associated with protection against Plasmodium vivax and P knowlesi infections.2-5
In 2017, Duchene et al9 suggested that the ACKR1/DARC SNP is associated with enhanced neutrophil transendothelial migration and subsequent accumulation in tissues, for example, the spleen. Moreover, ADAN neutrophils showed increased surface expression of CD16 and some serum molecules involved in neutrophil migration, for example, matrix metallopeptidase 9.9,10 Hence, it was hypothesized that ADAN may not be a true NP but rather an altered neutrophil compartmentalization and that neutrophils were activated, suggesting an autoimmune/inflammatory propensity.
Here, we sought answers to the questions raised by these findings. The issues concerned the clinical presentations and associated immune reactions in ADAN.
First, we assessed whether blood absolute neutrophil counts (ANC) were in mild or moderate NP ranges (1.0-1.6 and 0.5 × 109-0.99 × 109/L, respectively), as previously suggested,2-5,11,12 or whether lower ANCs (<0.5 × 109/L) could occur, as proposed in serologically diagnosed subjects with ADAN.13 This is important to know to avoid unnecessary diagnostic procedures, for example, bone marrow examination (BMEs).14 Conversely, if ADAN ANCs were within the reference values used in Sweden, for example, due to transient inflammatory reactions, it is also of significance because it may obscure the ADAN diagnosis. Consequently, the range and variability of ANCs need re-evaluation in genetically defined individuals with ADAN.
Secondly, the supposed benign nature of ADAN is based on the observed absence of enhanced susceptibility to bacterial infections.5,15,16 Nonetheless, the HIV acquisition rate,1-4 morbidity/mortality in lung injuries,17 and incidence of triple-negative breast cancer18 were all reported to be higher in individuals with ADAN than in the controls. This poses the question of whether the incidence or severity of various morbidities in ADAN differs from that in other NPs and healthy populations. A related question is whether the proposed neutrophil accumulation in ADAN tissues is associated with a proinflammatory profile, including a more aggressive course of autoimmune disorders.2,9 In addition, incidences of MDS and acute or chronic leukemia, which are frequent in other inherited NPs, are unknown in ADAN.6,7
The third issue is related to the immune/inflammatory reactions in ADAN. Previous reports have shown altered blood cytokine levels and responses to endotoxin in ADAN,19 as well as increased CD16 expression in neutrophils.9,10 This suggested that studies of immune/inflammatory phenomena, for example blood cytokine levels, might disclose shifts in subpopulations of other myeloid cells, for example, monocytes20 and other autoimmune reactions, including autoantibodies to neutrophils.
Here, we present investigations of these questions in a cohort of individuals with genetically defined ADAN, followed up for a median of >5 years.
Materials and methods
Ethics
This study was approved by the Swedish Ethics Review Authority (Dnr 2019-03532, Dnr 2014/988-31, and Dnr 2016/1105-32), and subjects were included in the study in accordance with routines specified in the decision of the ethical committee. The study was conducted in accordance with the Declaration of Helsinki.
The databases
Two databases were used. The first, the Stockholm Neutropenia Study, is described in supplemental Material. Briefly, all subjects referred to us because of NP were included in the database. Supplemental Figure 1 shows the evaluation algorithm.21 Those with a family based in Africa, the Near/Middle East, and the Mediterranean area as well as those with familial NP were considered for genetic ADAN screening (supplemental Material provides the details). Migrants from the Near/Middle East and East Africa (supplemental Table 1) constituted the majority of referrals from general practitioners/other hospitals from 2010 to 2018. Individuals with ADAN were investigated and followed up by the authors. The clinical follow-up was until 31 December 2019. A total of 327 person-years formed the evaluation basis (mean value, 5.2 years per subject).
The second database included records from the Platelet and Granulocyte Laboratory, Clinical Immunology, and Transfusion Medicine (TRoLL), Karolinska University Hospital, Stockholm, Sweden. TRoLL performed all polymerase chain reaction tests for the ADAN SNPs. Because these samples originated from various regions in Sweden, clinical data were unavailable for 24 individuals with ADAN.
Healthy control cohorts
Three cohorts served as controls to the ADAN cohort: (1) 94 healthy blood donors for comparisons of pro-leucin leucin (LL)-37 levels, recruited for method development as previously described;22 (2) 102 healthy blood donors as controls for the frequency of anti-neutrophil antibodies (see supplemental Material for details); and (3) 20 healthy donors included in a study on the role of natural killer (NK) cells in NP23 for comparisons of immune cell subsets and protein plasma analyses. All controls were recruited from Swedish individuals (mostly having Swedish/European ancestry) and checked for health according to criteria to be blood donors (cohorts 1 and 2) or by direct questions at the time of inclusion (cohort 3) and were expected to have a normal ANC.
Inclusions and exclusions
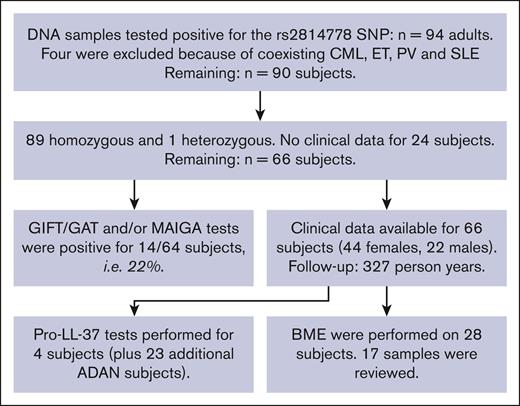
The patients included and the flow of the study are described in Figure 1. Inclusion ended on 31 July 2018. Only adults (ie, aged 18 years and older) were included. Most subjects were also tested for the presence of anti-neutrophil antibodies.
ADAN study flowchart for inclusion and exclusions from the study. CML, chronic myelogenous leukemia; ET, essential thrombocythemia; PV, polycythemia vera; SLE, systemic lupus erythematosus.
ADAN study flowchart for inclusion and exclusions from the study. CML, chronic myelogenous leukemia; ET, essential thrombocythemia; PV, polycythemia vera; SLE, systemic lupus erythematosus.
ANCs and leukocyte subtype
A total of 417 ANC analyses obtained from routine analyses formed the basis of the reported ANC. Median values for all ANC analyses for each individual determined the severity grading as follows: <0.1 × 109 to 0.49 × 109, severe NP; 0.5 × 109 to 0.99 × 109, moderate NP; 1.0 × 109 to 1.49 × 109, mild NP; and 1.5 × 109 to 1.6 × 109/L, borderline NP).21 Monocyte and lymphocyte counts were annotated only if they were outside the reference values of the Stockholm County Health System.
BME
Infection propensity measurements
Infections were determined or inferred from the patient’s history and available documentation in the TakeCare (Stockholm, Sweden) computerized patient record system, in which all health data made by >90% of physicians in Stockholm County, Sweden, have been entered since the beginning of the year 2000. Bacterial infection susceptibility was graded as follows: no apparent susceptibility, <2 peroral antibiotic treatment courses per 5 years; mild susceptibility, >2 peroral antibiotic treatment courses per 5 years; moderate susceptibility, need for hospitalization and IV antibiotic treatment; severe infection propensity, death due to infection. A total of 327 person-years formed the basis (mean, 5.2 years per person) for the evaluation.
ACKR1/DARC-variation analysis
For the ACKR1/DARC-polymorphism analysis, DNA was extracted from the peripheral blood samples. The readout was the presence or absence of the rs2814778 SNP and/or the wild-type allele. See supplemental Material for details.
Blood monocyte phenotyping
Cryopreserved peripheral blood mononuclear cells (PBMC) samples from 7 patients with ADAN and 11 age-matched healthy controls were thawed, stained, and analyzed for monocyte subsets, dendritic cells, and NK cells, as described in the supplemental Material.
Plasma protein analyses
Plasma C-reactive protein (CRP) levels were analyzed using routine techniques (the reference value is <3 mg/L). CRP levels and ANCs were obtained simultaneously when a person with ADAN sought medical care for minor infectious/inflammatory conditions (eg, upper respiratory tract infections).
GIFT/GAT/MAIGA and LSM to test for anti-neutrophil antibodies
EDTA plasma was tested for the presence of anti-neutrophil antibodies using Granulocyte Agglutination Test (GAT) and Granulocyte Immunofluorescence Test (GIFT), as described.26 Some control samples were also analyzed using a bead-based screening method on the Luminex platform LabScreen Multi (Thermo Fisher Scientific). Neutrophil-reactive samples were in all cases tested for the presence of anti-HLA-antibodies (supplemental Methods) because HLA antibodies can give false-positive reactions in both GAT and GIFT. If positive GAT and/or GIFT results were noted and/or the person had HLA antibodies, a monoclonal antibody–specific immobilization of granulocyte antigens (MAIGA) test was used for detection of specific antibodies to CD11a/b, CD16, CD18, and CD177.26 Thus, if GIFT/GAT and HLA results were all positive, the neutrophil autoantibody result was considered negative, unless specific antibodies to the aforementioned epitopes were detected.
hCAP-18 or pro-LL-37 ELISA
Luminex
Granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), and tumor necrosis factor (TNF) levels were analyzed with the Luminex methodology using Milliplex kits and Belysa software (Merck Life Science, Solna, Sweden).
Proximity ligation assay
In a subset of 7 individuals with ADAN and 20 healthy controls, plasma samples were analyzed externally using a proximity extension assay (Olink Proteomics). A total of 263 plasma proteins were analyzed for each sample (supplemental Table 3). Of these, 14 proteins were excluded because of >50% missing values. Missing values for 12 of the remaining 249 proteins were replaced with limit of detection values. The log2-transformed arbitrary unit Normalized Protein eXpression (Olink arbitrary unit, which is in log2 scale) was used for all calculations and visualization. Gene Ontology analysis was performed using GeneOntology/Panther.
Statistical analyses
Statistical analyses were performed using the Statistica software, as indicated. Data are presented as means/medians, depending on data skewness. Student t test or nonparametric tests were used for comparisons, and Spearman test was used for correlation. Prism 9 (GraphPad Software, Inc, San Diego, CA) was used to analyze the flow cytometry data using the Mann-Whitney test. For plasma protein analysis (Olink), statistical analyses and visualization were performed using R with the ggplot2 and dunn.test packages (Wilcoxon rank-sum tests) and Prism 9.
Results
Patient cohort
A total of 94 subjects included in the Stockholm Neutropenia Study cohort tested positive for the rs2814778 SNP in the DARC/ACKR1 gene up to 31 July 2018 (Figure 1). Of these, 93 were homozygous and 1 was heterozygous for the rs2814778 SNP. Four patients were excluded from further clinical evaluations because they were followed up or treated for systemic lupus erythematosus, chronic myeloid leukemia, polycythemia vera, essential thrombocythemia, or disorders that can affect both ANC and outcomes. Clinical details were available for 66 subjects (67% females; Table 1). The median age at diagnosis was 41 years (range, 18-93 years). The original nationalities (n = 20 countries) are given in supplementary Table 1, representing the influx of migrants to Sweden over the past decades, often from areas with armed conflicts.
Characteristics of all individuals with genetically defined ADAN
| . | All n = 90 . | Clinically characterized n = 66 . | Positive results for neutrophil autoantibodies n = 14 . |
|---|---|---|---|
| Age, y, median (and range) | 42 (18-93) | 41 (18-93) | 34 (21-63) |
| Sex, females/males (%) | 69/31 | 67/33 | 64/36 |
| ANC follow-up, y, median (range) | — | 4 (1-20) | N/A |
| Antibiotic follow-up, y, median (range) | — | 7 (2-11) | N/A |
| Number of ANC analyses per subject, median, (range) | — | 4 (1-33) | N/A |
| ANC mean (SD) | — | 1.0 (0.29)∗ | 1.0 (0.29) |
| . | All n = 90 . | Clinically characterized n = 66 . | Positive results for neutrophil autoantibodies n = 14 . |
|---|---|---|---|
| Age, y, median (and range) | 42 (18-93) | 41 (18-93) | 34 (21-63) |
| Sex, females/males (%) | 69/31 | 67/33 | 64/36 |
| ANC follow-up, y, median (range) | — | 4 (1-20) | N/A |
| Antibiotic follow-up, y, median (range) | — | 7 (2-11) | N/A |
| Number of ANC analyses per subject, median, (range) | — | 4 (1-33) | N/A |
| ANC mean (SD) | — | 1.0 (0.29)∗ | 1.0 (0.29) |
All were included (n = 90), 66 were also clinically characterized, and 14 tested positive for neutrophil autoantibodies (GAT, GIFT, and MAIGA).
N/A, not applicable; SD, standard deviation.
In the subgroup with negative results for anti-neutrophil autoantibodies, the mean ANC was 1.0 × 109/L (SD, 0.37 × 109/L); when compared with the subgroup with detected autoantibodies, no statistically significant difference was noted (P > .05).
None of the 50 patients with records of abdominal palpation or imaging showed any evidence of splenomegaly.
ANC in ADAN may occasionally be lower than previously recognized
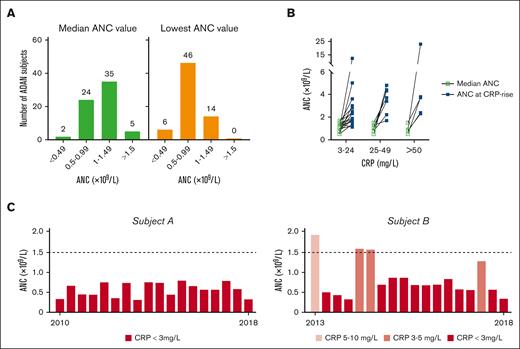
To assess the range of ANCs in our cohort of genetically defined individuals with ADAN, we used ANCs retrieved from medical records for a median time of 4 years (range, 1-20 years). We found a total of 417 measurements, with a median of 6.5 analyses per subject (range, 1-33 ANC examinations per person). All 66 clinically evaluable individuals with ADAN were neutropenic, that is, ANC < 1.6 × 109/L on at least 1 occasion. The median and mean ANCs for all 66 individuals with ADAN were 1.0 × 109 and 0.95 × 109/L, respectively. The 66 median individual ANCs were then distributed into NP severity strata (Figure 2A). Two subjects displayed median values below 0.5 × 109/L but had separate ANCs in the moderate/mild NP strata. However, when the lowest-recorded and available individual ANC at any time was classified, 6 of the 66 individuals (9.1%) fulfilled the criteria for severe NP, and 46 individuals (70%) had moderate NP, emphasizing that persons with ADAN occasionally displayed very low ANCs (Figure 2A). Thus, even those with an occasional ANC < 0.5 often had near-normal ANCs at other times.
Blood ANCs of individuals with ADAN. (A) ANCs of 66 individuals with ADAN, grouped according to the NP strata as severe (0-0.49 × 109/L), moderate (0.5-0.99 × 109/L), mild (1.0-1.49 × 109/L), and borderline (1.50-1.60 × 109/L) NP. The median ANCs are shown in the left panel, and the lowest-recorded ANC values during the observation time are shown in the right panel. The numbers above the bars denote the number of subjects in that stratum. (B) Increase in the ANC at the time of simultaneous recorded low, moderate, or marked CRP increases. The graph is based on available data from 13 individuals (1-5 independent samples per subject) for which CRP levels and ANCs were analyzed simultaneously when persons with ADAN sought medical care for minor infectious/inflammatory conditions (eg, upper respiratory tract infections). ANC and CRP levels did not correlate statistically when removing an outlier. (C) All recorded ANCs for 2 individuals with ADAN followed up for 5 and 8 years, respectively. Light red bars depict ANC when the CRP was raised (according to the inserted explanation). Red bars represent ANC obtained at normal CRP values. ANC levels for subject A remained stable over the individual follow-up period (however, short periods with concomitantly raised ANC and CRP values were noted). This patient remained in excellent health, displaying a normal pro-LL-37 plasma value, a normal BM sample, and whole-genome sequencing showing no other NP-related mutations/variants.
Blood ANCs of individuals with ADAN. (A) ANCs of 66 individuals with ADAN, grouped according to the NP strata as severe (0-0.49 × 109/L), moderate (0.5-0.99 × 109/L), mild (1.0-1.49 × 109/L), and borderline (1.50-1.60 × 109/L) NP. The median ANCs are shown in the left panel, and the lowest-recorded ANC values during the observation time are shown in the right panel. The numbers above the bars denote the number of subjects in that stratum. (B) Increase in the ANC at the time of simultaneous recorded low, moderate, or marked CRP increases. The graph is based on available data from 13 individuals (1-5 independent samples per subject) for which CRP levels and ANCs were analyzed simultaneously when persons with ADAN sought medical care for minor infectious/inflammatory conditions (eg, upper respiratory tract infections). ANC and CRP levels did not correlate statistically when removing an outlier. (C) All recorded ANCs for 2 individuals with ADAN followed up for 5 and 8 years, respectively. Light red bars depict ANC when the CRP was raised (according to the inserted explanation). Red bars represent ANC obtained at normal CRP values. ANC levels for subject A remained stable over the individual follow-up period (however, short periods with concomitantly raised ANC and CRP values were noted). This patient remained in excellent health, displaying a normal pro-LL-37 plasma value, a normal BM sample, and whole-genome sequencing showing no other NP-related mutations/variants.
The ANC varied considerably because the lowest-recorded ANC was 0.1 and the highest was 23 × 109/L. ANC elevations (often >1.6 × 109/L) coincided (but not always) with simultaneously increased plasma CRP levels (often small; Figure 2B). Figure 2C shows examples of 2 series of ANCs for subjects A and B, with normal or occasionally elevated CRP levels, respectively. Thus, based on 13 of 66 individuals with documented ANC rises during CRP elevations, inflammatory reactions caused by, for example, mild respiratory infections, may confer ANC normalizations, disguising the ADAN diagnosis.
No apparent bacterial infection propensity in ADAN
Infection susceptibility was graded according to criteria in “Materials and methods.” Eleven individuals with ADAN had evidence of past hepatitis B infection but none with hepatitis C. One had been treated for schistosomiasis and 1 for Salmonellosis; NP persisted before and after treatment for these disorders. However, 4 of the 66 subjects had mild/moderate susceptibility to suspected bacterial agents, whereas the rest showed no evidence of increased infection propensity (supplemental Material). Thus, 1 subject presented with upper respiratory tract infections, 1 with bronchiectasis, requiring repeated peroral antibiotics and filgrastim treatment (before the ADAN diagnosis), and 1 was hospitalized for postpartum infection, requiring IV antibiotics. Those with infections did not display lower mean ANCs than the others (data not shown). The antibiotic prescription numbers for subjects with ADAN did not exceed those for the general Swedish population (supplemental Material). The COVID-19 pandemic started after closing the files for this study.
Lack of MDS pathology in individuals with ADAN and other morbidities
Other diseases recorded during the 327 person-years included a wide spectrum of disorders without apparent segregation into any specific pathology (supplemental Table 2). Of interest, 1 developed polycythemia vera, 1 developed essential thrombocythemia, and 1 developed chronic myelogenous leukemia. Four had autoimmune disorders (thyroiditis [2], bullous pemphigoid [1], and systemic lupus erythematosus [1]), but none had rheumatoid arthritis or Sjögren syndrome. One was diagnosed with lung cancer. None had evidence of cardiovascular disease (except hypertension).
Importantly, BMEs were performed for 28 persons as part of the routine work up, often because of a clinical suspicion of MDS. Upon systematical re-evaluation of the 17 available BM samples and clinical follow-up, no evidence of diagnostic dysplastic features, maturation arrest in the myeloid series, abnormal cellularity, or evolution toward MDS was detected (supplemental Material). Thus, we found no evidence of pathologies related to MDS in the examined individuals with ADAN.
Detection of autoantibodies against neutrophils in a subset of individuals with ADAN
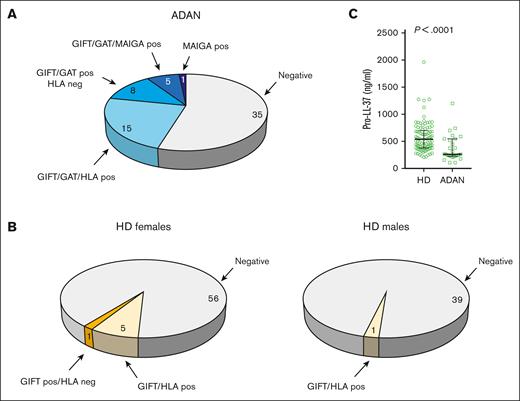
Tests for autoantibodies against neutrophils are part of the early workup for a NP case (supplemental Figure 1; supplemental Material). Of the 90 adult individuals with ADAN in this study, 64 underwent tests for autoantibodies using GIFT, GAT, and/or MAIGA analysis. All individuals were simultaneously tested for HLA antibodies, which can cause false-positive GIFT and GAT results.
Twenty-eight individuals with ADAN showed a positive GIFT/GAT result (Figure 3A). Fifteen of them also had HLA antibodies, and their GIFT/GAT results, therefore, could not be evaluated (see “Materials and methods”). The remaining 13 tested positive for either GAT, GIFT, or both. Of these 13, 5 subjects also tested positive for MAIGA (including CD11a/b, CD16a/b, and CD177).
Detection of autoantibodies to neutrophils in a subset of individuals with ADAN and lower pro-LL-37 levels than in controls. (A) The outcome of tests for anti-neutrophil antibodies in 64 individuals with ADAN tested. (B) Fractions of healthy female and male blood donors with antibodies against either HLA class I or human neutrophil antigen (HNA). One of the 40 males and 6 of the 62 females were positive for GIFT. GIFT-positive samples were subjected to LabScreen Multi (LSM), a bead-based screening method on the Luminex platform (Thermo Fisher Scientific) capable of differentiating HNA and HLA class I antibodies in setting of alloimmunization. GIFT reactivity in all donors except 1 could be explained by HLA class I antibodies and only 1 of the female donors showed weak reactivity against HNA in LSM. (C) Plasma levels of hCAP-18/pro-LL-37 in the ADAN group and in healthy donors. Pos, positive result; neg, negative result.
Detection of autoantibodies to neutrophils in a subset of individuals with ADAN and lower pro-LL-37 levels than in controls. (A) The outcome of tests for anti-neutrophil antibodies in 64 individuals with ADAN tested. (B) Fractions of healthy female and male blood donors with antibodies against either HLA class I or human neutrophil antigen (HNA). One of the 40 males and 6 of the 62 females were positive for GIFT. GIFT-positive samples were subjected to LabScreen Multi (LSM), a bead-based screening method on the Luminex platform (Thermo Fisher Scientific) capable of differentiating HNA and HLA class I antibodies in setting of alloimmunization. GIFT reactivity in all donors except 1 could be explained by HLA class I antibodies and only 1 of the female donors showed weak reactivity against HNA in LSM. (C) Plasma levels of hCAP-18/pro-LL-37 in the ADAN group and in healthy donors. Pos, positive result; neg, negative result.
One additional person tested negative for GIFT and GAT but positive for MAIGA. Thus, 14 (22%) of the tested individuals with ADAN showed evidence of the presence of autoantibodies against neutrophils (Figure 3A). To compare these results with those of individuals with non-ADAN, we performed GIFT on 102 healthy blood donors and found a corresponding figure of <1% (Figure 3B).
One of the 4 persons with increased infection propensity (see above) tested positive for CD16 autoantibodies, but the other 3 tested negative in the MAIGA test. None with anti-CD11a/b antibodies had evidence of increased infection propensity. The mean ANC of the subgroup with autoantibodies to neutrophils did not differ from that of the subgroup without detectable autoantibodies (Table 1). Thus, those with positive results for autoimmune NP exhibited no particular infection propensity or more severe NP than those without autoantibodies.
Reduced plasma levels of hCAP-18 /Pro-LL-37 indicates slightly perturbed myelopoiesis in subjects with ADAN
To investigate whether neutrophil maturation was perturbed in patients with ADAN, we measured plasma levels of hCAP18/pro-LL-37. This peptide has previously been shown to have high specificity for the detection of NPs caused by reduced neutrophil maturation, for example, SCNP.22,27 Because the pro-LL-37 method has only been used in our clinical laboratory since February 2018, only 3 of the 90 patients in our retrospective cohort were analyzed for plasma pro-LL-37 levels. To complement this analysis, we, therefore, included samples from 24 additional patients with ADAN homozygous for the rs2814778 gene variation. When this group of 27 patients was compared using the reference material of 94 healthy blood donors, the levels were reduced in the patients with ADAN (Figure 3C). None of the individuals with ADAN showed values approaching the very low values characterizing SCNP, yet 17 of the 27 displayed values below the lower reference value (269 ng/mL). These data support recent findings from our laboratory showing reduced pro-LL-37 levels in a large fraction of 226 patients with NP analyzed in clinical routine.22
Increased fractions of CD14+CD16+ monocytes in individuals with ADAN
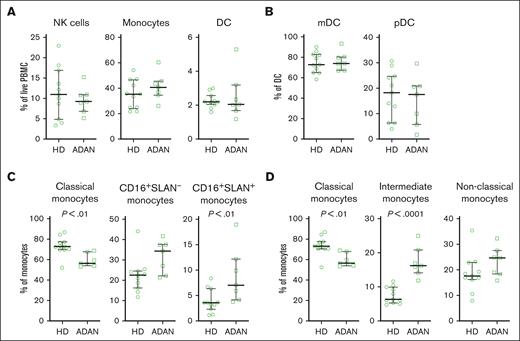
Because compensatory blood monocytosis is frequent in SCNP,6 we analyzed the peripheral blood monocyte counts in 66 individuals with ADAN. No one had counts outside the reference value range for blood monocytes or lymphocytes (data not shown). Moreover, upon phenotyping PBMCs from a subset of individuals with ADAN (n = 7), we found normal frequencies of monocytes, dendritic cells, and NK cells (Figure 4A-B; supplemental Figure 2). However, we observed a reduced fraction of CD14+CD16– classical monocytes and an increased fraction of CD14+CD16+ nonclassical monocytes, particularly, those that were 6-sulfo LacNac (SLAN) positive (Figure 4C-D), as compared with healthy donors (n = 11), indicating that the monocyte population is skewed toward proinflammatory responses.
Increased fractions of CD14+CD16+ monocytes in individuals with ADAN. Cells from individuals with ADAN (ADAN) (n = 7) and age-matched healthy donors (HDs) (n = 11) were analyzed by flow cytometry. (A) Frequency of monocytes, DC, and NK cells among the PBMC. (B) The frequencies of myeloid DC (mDC) and plasmacytoid DC (pDC) among DCs. (C-D) Monocyte subsets defined according to their expression of CD16 and SLAN or in (D) according to the traditional gating based on CD14 and CD16 expression. Statistical significance of the differences between mean values was calculated using the Mann-Whitney test, and P-values < 0.05 are indicated.
Increased fractions of CD14+CD16+ monocytes in individuals with ADAN. Cells from individuals with ADAN (ADAN) (n = 7) and age-matched healthy donors (HDs) (n = 11) were analyzed by flow cytometry. (A) Frequency of monocytes, DC, and NK cells among the PBMC. (B) The frequencies of myeloid DC (mDC) and plasmacytoid DC (pDC) among DCs. (C-D) Monocyte subsets defined according to their expression of CD16 and SLAN or in (D) according to the traditional gating based on CD14 and CD16 expression. Statistical significance of the differences between mean values was calculated using the Mann-Whitney test, and P-values < 0.05 are indicated.
Altered levels of plasma proteins relevant for cell signaling, neutrophil activation, and angiogenesis in individuals with ADAN
Against the background of more severe NP than previously recognized, lower hCAP18/pro-LL-37 levels, shift in monocyte subpopulations, and presence of autoantibodies to neutrophil antigens, we investigated whether signaling systems for neutrophil generation and immune regulation were perturbated in ADAN.
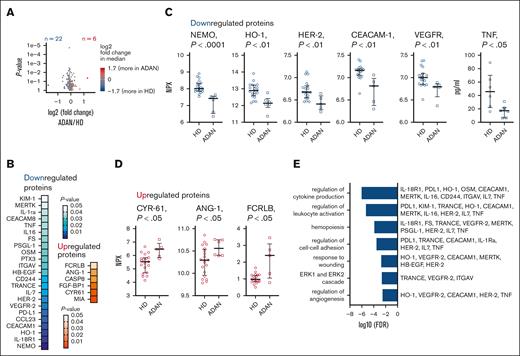
In a subset of individuals with ADAN, a large-scale screening of 249 plasma proteins was performed (supplemental Table 3). Of these, 28 plasma proteins were significantly altered in primary statistical analysis (Figure 5A). Among the downregulated proteins were NEMO/IKKB, HO-1, HER-2, CEACAM-1, and vascular endothelial growth factor receptor 2, and upregulated proteins included ANG-1, CYR61, and FCRLB (Figure 5B-D). In additional neutropoiesis-focused plasma protein analyses, no difference was found in plasma levels of G-CSF or M-CSF in individuals with ADAN compared with those in healthy controls (supplemental Table 4). In contrast, TNF levels were significantly lower in the subjects with ADAN (Figure 5C). Enrichment analysis highlighted regulation of extracellular signal-regulated kinase cascade, angiogenesis, neutrophil-activation, and cytokine-signaling processes as being altered in ADAN (Figure 5F). The levels of several plasma proteins correlated (supplemental Figure 3), for example, levels of KIM-1/HAVCR1 (a protein involved in cell injury and viral recognition) correlated significantly with levels PSGL1, ITGAV (neutrophil adhesion molecules), and HO-1 (involved in the prevention of vascular inflammation). Collectively, this indicates dysregulation of plasma protein networks in ADAN with relevance to cell signaling, neutrophil activation, and angiogenesis.
Altered levels of plasma proteins relevant for cell signaling, neutrophil activation, and angiogenesis in individuals with ADAN. Analysis of 249 plasma proteins using proximity ligation assay (Olink) and ELISA. (A-E) Twenty-eight proteins significantly differed in individuals with ADAN from those in HDs, of which 22 were downregulated and 6 were upregulated in the group with ADAN. (A) Volcano plot showcasing the fold change in altered proteins between those with ADAN and HDs. (B) Significance heat map of the 28 proteins. (C-D) Raw NPX values or concentrations (TNF) for selected proteins. (E) Selected identified terms in Gene Ontology analysis (biological process) and the contributing proteins. Nonparametric testing (Wilcoxon rank-sum test). HDs (n = 6-20) and individuals with ADAN (n = 5-7). Olink Proteomics arbitrary unit, in Log2 scale. Samples for plasma Olink and other inflammation/autoimmune analyses were obtained at apparently infection-free intervals, as assessed by CRP and the treating physician. FDR, false discovery rate; NPX, Normalized Protein eXpression.
Altered levels of plasma proteins relevant for cell signaling, neutrophil activation, and angiogenesis in individuals with ADAN. Analysis of 249 plasma proteins using proximity ligation assay (Olink) and ELISA. (A-E) Twenty-eight proteins significantly differed in individuals with ADAN from those in HDs, of which 22 were downregulated and 6 were upregulated in the group with ADAN. (A) Volcano plot showcasing the fold change in altered proteins between those with ADAN and HDs. (B) Significance heat map of the 28 proteins. (C-D) Raw NPX values or concentrations (TNF) for selected proteins. (E) Selected identified terms in Gene Ontology analysis (biological process) and the contributing proteins. Nonparametric testing (Wilcoxon rank-sum test). HDs (n = 6-20) and individuals with ADAN (n = 5-7). Olink Proteomics arbitrary unit, in Log2 scale. Samples for plasma Olink and other inflammation/autoimmune analyses were obtained at apparently infection-free intervals, as assessed by CRP and the treating physician. FDR, false discovery rate; NPX, Normalized Protein eXpression.
Discussion
Here, we demonstrate that NP may be more severe in genetically defined individuals with ADAN than previously assumed,11,12 extending a previous observation.13 Thus, episodes of severe NP were found in ∼10% of individuals with ADAN and were not associated with compensatory monocytosis (as in SCNP).6 Furthermore, ADAN did not associate with a propensity for severe bacterial infections.
The remarkable variability of the ANC, as previously noted in ADAN,11,12 might be partly explained by minor, transient inflammatory conditions, for example, common colds, increasing the ANC even to normal levels. In order to critically assess NP diagnosis, it is advisable to perform concomitant CRP analysis.
MDS/acute leukemias were not observed in this cohort; however, longer follow-ups might be needed to estimate the risk of possible malignant transformation.6,7,18 The size of our cohort with ADAN did not allow for deep analyses of outcomes for other diseases, for example, severe viral infections, pulmonary injuries, cancer, and autoimmune disorders.2-5,17,18,28-32 Separate studies are needed to determine whether the 4 of 66 individuals with ADAN with chronic myeloid neoplasms as well as 5 of 66 with autoimmune disorders represent a difference from expected rates in an ethnic matched control population. For instance, triple-negative breast cancer is reported to be more prevalent in individuals with ADAN than in Black individuals without ADAN.17 Likewise, disparities for African-Americans concerning the rates and severity of prostate cancer, multiple myeloma, and systemic sclerosis lack data on ADAN.2-5,17,18,28-32 Nonetheless, such data may be of interest in relation to the proposed increased neutrophil transmigration and accumulation in tissues in ADAN9, which might raise concerns about an exaggerated inflammatory reaction in a variety of disorders.
Our study also focused on the (auto)immune/inflammatory reactions that appear to partly explain the DARC/ACKR1 SNP rs2814778 phenotype. Previously, enhanced neutrophil CD16 expression,9 matrix metallopeptidase 9 plasma increases,10 and aberrant plasma cytokine reactions to lipopolysaccharide19 have been observed. Here, we describe a shift in the subset composition of monocytes that we analyzed in 7 subjects with ADAN. Specifically, those with ADAN had reduced fractions of classical monocytes (CD14+CD16–), whereas nonclassical CD14+CD16+ monocytes were increased, in particular those that were SLAN positive. These monocytes can produce high amounts of TNF, interleukin-12 (IL-12), and IL-23 when activated, and their likely function is that of “patrolling” in the blood.33 Their frequency in the peripheral blood has also been found to be increased in other diseases, for example, in HIV34 and diffuse large B-cell lymphoma.35 The mechanisms that regulate the relative expansion of this population, however, are still unclear.
Unexpectedly, we found that 22% of our subjects with ADAN presented with autoantibodies to neutrophils, whereas the corresponding figure for healthy controls was <1%. Subjects with ADAN who tested positive for autoantibodies did not display more severe NP or increased propensity for infection. Positivity for antibodies against CD16, the most common antigen in autoimmune NPs, is intriguing, considering the reported increased expression of neutrophil CD16,9 a marker for an activated ADAN neutrophil phenotype.9 Notably, autoimmune NPs are now described in various genetically defined immunodeficiencies, for example, in TACI mutations, supporting notions that germ line gene variations, including ADAN, may associate with autoimmune NP.36
Recently, we reported reduced levels of hCAP-18/pro-LL-37 in an unselected NP cohort.22 Here, we extended these data showing that individuals with ADAN follow the same trend. Pro-LL-37 is known to reflect the rate of myelopoiesis in the BM.22,27,37 The reason why plasma pro-LL-37 levels primarily reflect myelopoiesis and not the ANC of peripheral blood despite being stored in specific neutrophil granules remains to be explained.
The cytokine sink hypothesis, previously suggested to be part of the spectrum of ADAN immune aberrations, refers to the immune/inflammatory effects of reduced clearance of CXCL and CCL from the blood because of the absence of cytokine-transporting ACKR1/DARC molecules in early erythroid cells.38 Here, however, ADAN plasma concentrations of ACKR1-ligands, for example, CCL-2, -3, -7, -11, −17 or CXCL-1, -3, -5, -6, -8, -9, and -11 (supplementary Table 3), were similar to those in healthy controls, suggesting that the cytokine sink hypothesis is a minor consequence of various ADAN immune reactions. Instead, the ADAN plasma profile was characterized by altered levels of 28 other proteins related to cell signaling, neutrophil activation, and angiogenesis. Of the 28, 12 of the proteins have been implicated in inflammatory/immune reactions (NEMO, HO-1, CYR61, HB-EGF, ITGAV, PTX3, PSGL-1, IL-16, CEACAM8, IL-1RA, FCRLB, and MERTK), and 3 in angiogenesis/apoptosis (vascular endothelial growth factor receptor 2, ERBB2/HER2, and FGF-BP1), whereas 3 have been associated in both processes (CEACAM1, KIM-1/HAVRC1, and ANG-1/ANGPT). A further discussion of the potential role of the 12 proteins in NP mechanisms and monocyte subsets is given in supplemental Table 3.
In conclusion, our study has highlighted that (1) individuals with ADAN might present with lower ANCs, (2) with possible wider immune dysregulation than previously assumed, and (3) that further tests for autoimmune reactions might disclose whether ADAN is prone to various autoimmune/inflammatory phenomena and, finally, whether the prevalence/incidence or severity of various diseases is influenced by ADAN gene variation.18,28-32 More data are needed on susceptibility to viral infections, such as COVID-19, in which an increase in immature activated neutrophils with immunosuppressive features has been associated with critical outcomes.28
Acknowledgments
The authors are grateful for the discussion on ADAN in Eu-Net INNOCHRON.
This study was supported by grants to P.H. from the Swedish Cancer Society (2015/727; 4-331/2019; and 4-640/2022), Radiumhemmets Forskningsfonder (2019-2021 and 2022-2024), the Stockholm City Council (FoUI-952144 and FoUI-955130), CIMED (FoUI-955282) and Karolinska Institutet Strategic Research Area Stem Cells and Regenerative Medicine, and the work of E.S. was supported by grants to Karl-Johan Malmberg laboratory from the Swedish Research Council, the Swedish Cancer Society, and the Karolinska Institutet.
Authorship
Contribution: J.P. and P.H. designed research; J.P., H.L., C.C.N., and S.D. collected clinical data; M.K., S.M., A.M., and P.R. analyzed samples; E.S. analyzed protein data; J.P. and E.S. performed statistical analysis; J.P., E.S., and P.H. wrote the manuscript; and all authors analyzed and interpreted data.
Conflict-of-interest disclosure: J.P. is a consultant to Chiesi Canada, Ltd. E.S. is a consultant to Fate Therapeutics. S.M. is a consultant to XNK Therapeutics AB. All relationships have been reviewed and managed by Karolinska Institute, in accordance with its conflict-of-interest policies. The remaining authors declare no competing financial interests.
Correspondence: Jan Palmblad, The Hematology Center R51, Karolinska University Hospital Huddinge, S-141 86 Stockholm, Sweden; email: jan.palmblad@ki.se.
References
Author notes
Data are available on request from the corresponding author, Jan Palmblad (jan.palmblad@ki.se).
The full-text version of this article contains a data supplement.