TO THE EDITOR:
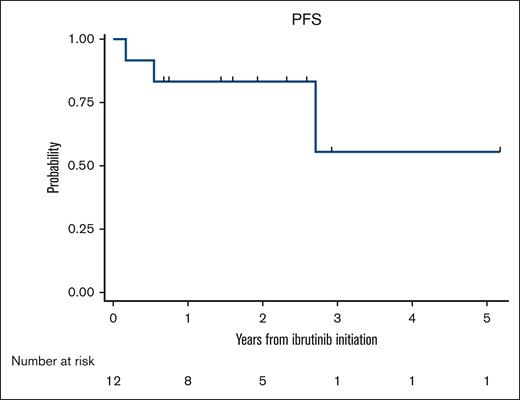
Marginal zone lymphoma (MZL) constitutes ∼8%-12% of all B-cell non-Hodgkin lymphomas1-3 and is classified into 3 specific subtypes: extranodal MZL of mucosa-associated lymphoid tissue, splenic MZL, and nodal MZL.2 The optimal frontline management of MZL is not well defined, and the current treatment recommendations are largely adapted from those for follicular lymphoma. For patients requiring treatment, options can range from local excision, radiation therapy, or rituximab (R) monotherapy to R-based chemoimmunotherapy (CIT). With the advent of novel agents, the outcomes of MZL have improved significantly over the past decade.4 In the only randomized study to date comparing R-chemotherapy and R monotherapy, R-chemotherapy (R-chlorambucil) showed significantly better event-free survival and progression-free survival (PFS).5 However, chlorambucil is rarely used as a chemotherapy backbone in the United States, with R-bendamustine (BR) being the most commonly used regimen.6 Given the long-term survival of these patients, administering myelosuppressive chemotherapy such as BR in the first-line setting could limit future treatment options at relapse. Ibrutinib, a first-in-class covalent Bruton tyrosine kinase inhibitor (BTKi) was approved for relapsed or refractory (R/R) MZL based on the results of a phase 2 clinical trial, in which the overall response rate (ORR) was 48%.7 The final analysis of the study showed an ORR of 58% with durable responses.8 Comparable outcomes were seen with ibrutinib in R/R MZL in a real-world study as well.9 However, the outcomes associated with ibrutinib in the first-line setting are unknown. Hence, we sought to evaluate the real-world outcomes of patients with MZL treated with ibrutinib in the first-line setting. This was a multicenter retrospective cohort study and included adult patients (≥18 years) with MZL diagnosed on or after 1 January 2010 at 10 US medical centers. To be eligible for the analysis, patients should have received ibrutinib in the first-line setting. All staging procedures (eg, bone marrow evaluations) and treatment assessments were conducted in accordance with local practice. The study was approved by the institutional review boards at all participating sites and was performed in compliance with the Declaration of Helsinki. The primary objective of the study was to evaluate the ORR, whereas the secondary objectives included PFS and identifying predictors for complete response (CR). PFS was defined as the time from the start of ibrutinib therapy until lymphoma relapse/progression or death from any cause, censoring at the last clinical assessment if no progression or death occurred. Demographic and disease characteristics were summarized using descriptive statistics. PFS was estimated using the Kaplan-Meier method. To evaluate the associations between patients’ clinicopathological factors and CR, Poisson regression models with robust error variance were used to estimate the relative risk and 95% confidence intervals. This analytic approach provides an unbiased estimate of relative risk when the outcome is common (>10%).10 All statistical tests were 2-sided with a type-1 error of 0.05. Twelve patients met the inclusion criteria. The median age at the start of ibrutinib therapy was 68 years (52-86 years), with 4 patients (33%) aged 75 years or older. There was a male predominance (n = 7) with Eastern Cooperative Oncology Group performance statuses of 0 and 1 in 6 patients each. Most patients had stage III to IV disease (83%). Nodal MZL was the most common subtype (n = 5), followed by splenic MZL (n = 4), and extranodal MZL (n = 3). The median follow-up was 28 months (range, 9-62 months) from the initiation of ibrutinib therapy. Table 1 shows the baseline characteristics. All 12 patients were evaluable for response assessment. Among these, 10 patients achieved a response (ORR 83% with a CR rate of 42%; n = 5) and 2 had stable disease (17%). All patients aged 75 years or older achieved a response (n = 4 of 4) with 3 CR and 1 partial response. Among the 10 patients who achieved a response, only 1 patient subsequently experienced disease progression at 2.5 years after response evaluation. There were no significant baseline clinicopathological factors that predicted CR to ibrutinib in the first-line setting (supplemental Table 1). Among the patients responding to ibrutinib, only 1 patient discontinued therapy after 15 cycles of ibrutinib because of dementia. The median PFS was not reached (Figure 1). The 1- and 3-year PFS rates were 83.3% (95% confidence interval, 48.2%-95.6%) and 55.6% (95% confidence interval, 8.6%-86.9%), respectively. In this case series evaluating the initial experience of patients with MZL treated with ibrutinib in a first-line setting, we found high (83% ORR) and strong (42% ORR) response rates. Furthermore, the responses were durable. We did not find any baseline clinicopathological characteristics that predicted CR to ibrutinib in this study.
Baseline characteristics
| Variable . | All patients N = 12 (%) . |
|---|---|
| Median age, range (y) | 68 (52-86) |
| Sex, n (%) | |
| Males | 7 (58) |
| Females | 5 (42) |
| BMI < 30 kg/m2, n (%) | 8 (67) |
| ECOG PS, n (%) | |
| 0 | 6 (50) |
| 1 | 6 (50) |
| B symptoms, n (%) | 3 (25) |
| MZL subtype, n (%) | |
| EMZL | 3 (25) |
| NMZL | 5 (42) |
| SMZL | 4 (33) |
| Stage, n (%) | |
| I-II | 2 (17) |
| III-IV | 10 (83) |
| WBC, ×103/μL, median, range | 7.9 (14.8-32.4) |
| Hgb, g/dL, median, range | 12.1 (10.1-15) |
| LDH > ULN | 3 (25) |
| Albumin < ULN | 2 (17) |
| BM involvement | |
| No | 4 (33%) |
| Yes | 6 (50%) |
| Not done | 2 (17) |
| Monoclonal protein | |
| No | 6 (50) |
| Yes | 2 (17) |
| Not checked | 4 (33) |
| 17p del/TP53 mutation | |
| No | 5 (42) |
| Yes | 0 (0) |
| Not checked | 7 (58) |
| Complex cytogenetics∗ | |
| No | 5 (83) |
| Yes | 1 (17) |
| Variable . | All patients N = 12 (%) . |
|---|---|
| Median age, range (y) | 68 (52-86) |
| Sex, n (%) | |
| Males | 7 (58) |
| Females | 5 (42) |
| BMI < 30 kg/m2, n (%) | 8 (67) |
| ECOG PS, n (%) | |
| 0 | 6 (50) |
| 1 | 6 (50) |
| B symptoms, n (%) | 3 (25) |
| MZL subtype, n (%) | |
| EMZL | 3 (25) |
| NMZL | 5 (42) |
| SMZL | 4 (33) |
| Stage, n (%) | |
| I-II | 2 (17) |
| III-IV | 10 (83) |
| WBC, ×103/μL, median, range | 7.9 (14.8-32.4) |
| Hgb, g/dL, median, range | 12.1 (10.1-15) |
| LDH > ULN | 3 (25) |
| Albumin < ULN | 2 (17) |
| BM involvement | |
| No | 4 (33%) |
| Yes | 6 (50%) |
| Not done | 2 (17) |
| Monoclonal protein | |
| No | 6 (50) |
| Yes | 2 (17) |
| Not checked | 4 (33) |
| 17p del/TP53 mutation | |
| No | 5 (42) |
| Yes | 0 (0) |
| Not checked | 7 (58) |
| Complex cytogenetics∗ | |
| No | 5 (83) |
| Yes | 1 (17) |
BM, bone marrow; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; EMZL, extranodal MZL; Hgb, hemoglobin; LDH, lactate dehydrogenase; NMZL, nodal MZL; PS, performance status; SMZL, splenic MZL; ULN, upper limit of normal per institutional standard; WBC, white blood cell.
Only among those who had BM involvement.
R monotherapy has shown significant activity in patients with MZL11,12; however, the responses are neither deep nor durable.11 Although the addition of chemotherapy to R increases its efficacy,5,6 it comes at the price of higher and considerable toxicity, limiting the administration of CIT for older and frail patients. In our study, we found that ibrutinib produced excellent response rates in older patients; however, the results need to be interpreted with caution, given the small sample size. In a phase 2 study that evaluated the outcomes of patients treated with ibrutinib in R/R MZL (n = 60), the CR rate was only 10%, with a median PFS of 15.7 months.8 The CR rate was 26% with zanubrutinib in the MAGNOLIA trial (n = 68), with a 1-year PFS rate of 82.5% at a median follow-up of 15.7 months.13 In a multicenter retrospective study that evaluated the outcomes of patients with R/R MZL treated with ibrutinib (n = 119), the CR rate was comparable (17%) with that observed in the phase 2 study but with a longer PFS (median PFS of 29 months).9 In contrast, the CR rate was 42% in this study, in which ibrutinib was administered in the first-line setting, with median PFS not reached. This might be related to the altered tumor microenvironment when ibrutinib is administered at later lines of therapy. This hypothesis is further supported by the fact that patients with R/R MZL treated with ibrutinib had a CR rate of 19% among those who had received prior R monotherapy, whereas the CR rate was 8% among those who had received prior R-chemotherapy.8 A phase 3 clinical trial was started in 2019, studying ibrutinib + R vs ibrutinib + placebo in treatment-naïve MZL (NCT04212013); however, the study is currently not recruiting. This is because of the voluntary withdrawal of ibrutinib from the market by the company in the United States for mantle cell lymphoma and MZL based on the results of the SHINE and SELENE trials. In the SHINE trial,14 patients with previously untreated mantle cell lymphoma were randomized to receive ibrutinib + BR vs placebo + BR. The study met its primary end point of PFS benefit; however, patients who received the addition of ibrutinib to CIT experienced increased adverse reactions compared with those who received placebo. In the SELENE trial,15 patients with R/R FL and MZL who received prior anti-CD20–containing CIT were randomized to receive ibrutinib + CIT (BR or R-cyclophosphamide, doxorubicin, vincristine, prednisone) vs placebo + CIT. The study did not meet the primary end point of PFS benefit. Next-generation BTKis, such as zanubrutinib (RITZ trial, NCT05735834) and pirtobrutinib (N. Epperla, oral communication, 14 September 2023), are currently being studied in the first-line setting. Our study is limited by its retrospective design, nonuniform selection of first-line therapy, and small sample size. We did not collect data on the safety or toxicities associated with ibrutinib because the primary goal of the study was to understand the activity of ibrutinib in the first-line setting. Other limitations include missing data on prognostic factors such as monoclonal protein16 and 17p del/TP53 mutation precluding our ability to study the impact of these variables on response and survival. In conclusion, to our knowledge this is the first study to report the outcomes of patients with MZL treated with BTKi (ibrutinib) in a first-line setting. The outcomes are superior when patients with MZL are treated with ibrutinib as first-line compared with later lines at relapse and the study supports the hypothesis of studying BTKis in first-line MZL. We await the results of first-line BTKi-based clinical trials to validate our findings.
Contribution: N.E. contributed to the conception and design of the study and prepared the first draft of the manuscript; N.E. and Q.Z. contributed to the data analysis; and all other authors contributed to data collection, assembly, and interpretation, provided critical and insightful comments, and approved the final manuscript.
Conflict-of-interest disclosure: N.E. received research funding from BeiGene, AstraZeneca, and Eli Lilly; served on the speakers’ bureau for Incyte, BeiGene, and Novartis; and reported honoraria/consulting/advisory boards for Merck, ADC Therapeutics, Ipsen, and Eli Lilly. P.T. reported honoraria/consulting/advisory boards for TG Therapeutics, ADC Therapeutics, Genentech, Genmab, and Eli Lilly. B.C. received research funding from Genentech, Acerta, Triphase, MorphoSys, Seagen, Millenium, Bristol Myers Squibb, F. Hoffman-La Roche, and served on the advisory board for Genentech and ADC Therapeutics. S.K.B. reported honoraria from Acrotech, Affimed, Daiichi Sankyo, Kyowa Kirin, Janssen, and Seagen. A.J.O. received research funding and honoraria from Genmab, Precision Bio, Adaptive Biotechnologies, Celldex, Acrotech Biopharma, Schrodinger, TG Therapeutics, and Genentech. N.L.B. received research funding from ADC Therapeutics, Autolus, Bristol Myers Squibb, Celgene, Forty Seven, Genentech, Immune Design, Janssen, Merck, Millennium, Pharmacyclics, Affirmed Therapeutics, Dynavax, Gilead, MedImmune, Novartis, and served on the consulting/advisory boards for Kite Pharma, Pfizer, ADC Therapeutics, Roche/Genentech, Seattle Genetics, BTG, and Acerta. The remaining authors declare no competing financial interests.
Correspondence: Narendranath Epperla, Division of Hematology, Department of Medicine, The Ohio State University, 2121 Kenny Rd, Columbus, OH 43221; email: narendranath.epperla@osumc.edu.
References
Author notes
Data are available upon reasonable request from the corresponding author, Narendranath Epperla (narendranath.epperla@osumc.edu).
The full-text version of this article contains a data supplement.