Key Points
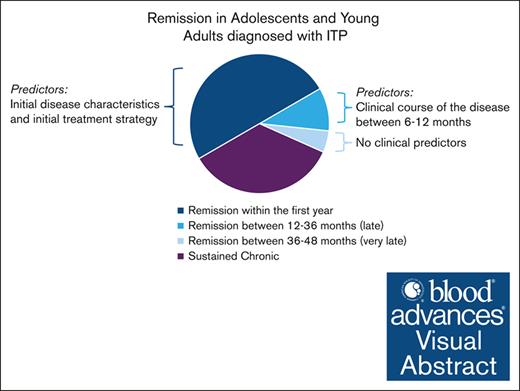
At 24-, 36-, and 48-month FU, 10% of AYAs with chronic ITP attain yearly SCROT.
Higher platelet counts and lower use of rescue therapy during the first year of the disease might be predictors of SCROT beyond 12 months.
Visual Abstract
Adolescents and young adults (AYAs) with immune thrombocytopenia (ITP) exhibit distinct clinical features and needs, defying categorization as either adults or children. Previous findings revealed a 50% risk of chronic disease at 12 months, yet the long-term course remains unclear. This study aimed to delineate the clinical and laboratory characteristics of AYAs with chronic primary ITP. Data from patients aged 12 to 25 years with chronic disease at 1 year were extracted from 3 registries (Pediatric and Adult Registry on Chronic ITP, CEREVANCE, and Cytopénies Auto-immunes Registre Midi-Pyrénéen), covering the period from 2004 to 2021. Sustained complete remission off treatment (SCROT) occurring beyond 12 months was defined as platelet count of >100 × 109/L without treatment for at least 12 months, independently of the previous treatment strategy. A total of 427 AYAs (64% female) with chronic primary ITP were included. Clinical information was available for ∼100% of patients at initial diagnosis and at 6- and 12-month follow-ups (FUs); and for 88%, 77%, and 59% at 24, 36, and 48 months, respectively. Over time, clinical features improved gradually, with fewer patients requiring treatment. Throughout the FU period, second-line drug use increased steadily among treated patients, without affecting the need for corticosteroids and IV immunoglobulins. The proportion of new patients achieving SCROT at 24-, 36-, and 48-month FU was 10% (38/375), 9.5% (31/327), and 12% (30/250), respectively, including 23 who underwent splenectomy. AYAs achieving SCROT between 12 and 36 months displayed higher platelet counts in the first year (excluding the initial period) and received fewer IV immunoglobulin treatments beyond 12 months compared with those with ongoing disease.
Introduction
Immune thrombocytopenia (ITP) exhibits age-dependent clinical characteristics, resulting in different management approaches in children and adults. However, defining ITP in 2 age categories only, children and adults, appears inappropriate because such a definition ignores the clinical characteristics and needs of adolescents and young adults (AYAs). AYAs are underrepresented in practice guidelines, and recommendations are biased by studies and publications focused either on adults with a mean age of >50 years or children with a mean age of <10 years.1,2 This gap can be partially explained by the 2 peaks of incidence: in children aged 1 to 5 years and adults aged >60 years,3 as well as by medical facilities (pediatric and adult hospitals) operating independently and rarely prioritizing the transitional age group. Undoubtedly, health-related quality of life is of particular interest in AYAs displaying an active and changeful life.4 Risk of chronic disease, physical activity limitation, fatigue, bleeding symptoms, and medical management have major effects on the mental health, social life, and professional goals of AYAs. Moreover, drug compliance and side effects may be challenging in this age group.5 Our previous study revealed that up to 2 of 3 AYAs requiring ITP therapy were exposed to corticosteroids, even at the 12-month follow-up (FU).6 Side effects of corticosteroids include mood disorders, muscle weakness, osteopenia, and body changes, such as weight gain, gynecomastia, acne, and hirsutism. These symptoms may have tremendous effects on AYAs.7 In 2019, the international consensus report on the investigation and management of primary ITP cautioned against a common mistake in ITP management: the excessive use of corticosteroids.5 Therefore, minimizing corticosteroid use and avoiding or delaying toxic treatments, such as splenectomy, must be part of the treatment strategy for AYAs with persistent or chronic ITP.8
In our previous publication on AYAs with newly diagnosed ITP,6 we emphasized the high risk of chronicity at 12 months (50%), consistent with the well-known heightened risk in children aged >10 years,2,9-12 thereby positioning AYAs between adults (70% prevalence of chronic disease) and children (20%). However, patients with initial severe thrombocytopenia receiving frontline treatment had a significant higher remission rate at 1 year than those initially managed with a watch-and-wait approach.6 Little is known about the course of chronic ITP in AYAs. In children with chronic ITP, age >10 years at ITP diagnosis and female sex negatively affect the long-term outcome in terms of bleeding severity, treatment escalation, and progression to secondary ITP.13 However, predictors of sustained complete remission off treatment (SCROT) after 12 months remain unclear. Studying the disease evolution of ITP in AYAs could help to better predict outcomes and to design age-adapted therapies.
Through an international collaboration of hematologists and by integrating 3 data sources, we aimed to describe clinical and laboratory data of AYAs with chronic primary ITP up to the 48-month FU.
Methods
Data sources
For this analysis, we extracted data of AYAs from the Pediatric and Adult Registry on Chronic ITP (PARC-ITP) by the Intercontinental Cooperative ITP Study Group from May 2004 to May 2021; the French national prospective OBS’CEREVANCE cohort, covering children (aged <18 years) with autoimmune cytopenia from 2004 to November 2021; and the Cytopénies Auto-immunes Registre Midi-Pyrénéen-France (CARMEN-France), which enrolls adults aged >18 years, from January 2013 to May 2021. Missing FU data were completed until April 2022. The local review boards and national or regional ethical committees approved data extraction from the 3 registries. Informed consent was obtained from all participants in the PARC-ITP and OBS’CEREVANCE cohorts, whereas all patients included in the CARMEN-France registry were informed and did not oppose data collection. Further information regarding the registries is provided in the supplemental Data.
Study population
We enrolled individuals diagnosed with ITP between the ages of 12 and 25 years, subsequently diagnosed with primary chronic ITP at 12 months. Chronic disease was defined as platelet count of <100 × 109/L or ongoing treatment at 12 months or documented relapse until 48 months (supplement Table 1). Primary ITP was defined according to the criteria set by the international working group,8 indicating isolated thrombocytopenia without the presence of secondary causes, as outlined in supplemental Table 2. Patients with a revised diagnosis of secondary ITP after 12 months were not excluded. Those patients presenting some immunopathological manifestations but without a concluding cause of secondary ITP were classified as primary. Pregnant women at ITP diagnosis and patients misdiagnosed with ITP were excluded, irrespective of timing.
Outcomes and definitions
This study aims to clinically describe AYAs with chronic primary ITP until 48 months FU and explore secondary ITP causes in patients initially diagnosed as primary. Alongside the age and sex of the cohort, the following parameters were analyzed at initial diagnosis and at the 6-, 12-, 24-, 36-, and 48-month FU periods: platelet count (unique value at FU), presence of bleeding (yes/no), bleeding site, and treatment between 2 FU periods.
Any FU visit at 3 to 9 months was designated as the 6-month FU visit. In analogy, any FU visits at 11 to 18, 20 to 30, 32 to 42, and 44 to 54 months were designated as 12-, 24-, 36- and 48-month FU visits, respectively. If multiple FU visits occurred within these ranges, the nearest 1 was considered. When the platelet count was documented as “normal,” the value 150 × 109/L was used. Bleeding was defined as any hemorrhagic event, regardless of bleeding intensity, extent, and frequency, and may have been self-reported or assessed at any clinical examination. Initial therapy was defined as ITP treatment initiated within 1 week of diagnosis. If treatment was initiated later, it was documented in the subsequent FU. Initial treatment lasting >4 weeks was documented as both initial treatment and subsequent 6 months FU treatment. IV immunoglobulin, including anti-D immunoglobulin, and corticosteroids were classified as first-line and/or rescue drugs. Rituximab, thrombopoietin-receptor agonists (TPO-RAs), splenectomy, hydroxychloroquine, and all other immunosuppressive drugs were defined as second-line drugs. Certain ITP drugs were not specifically identified in the questionnaire of PARC-ITP but were listed as “second-line drugs: other,” including dapsone, hydroxychloroquine, belimumab, fostamatinib, and some investigational drugs. The reported number of patients treated with hydroxychloroquine encompasses only patients of CARMEN and OBS'CEREVANCE and is therefore underestimated in our results section.
The final outcome was the percentage of AYAs with chronic primary ITP entering SCROT, along with the identification of potential factors associated with SCROT. SCROT was evaluated annually starting at 24 months, defined as a platelet count of ≥100 × 109/L without treatment in the preceding year and no documented relapse in subsequent FU. Relapses after 48 months were not evaluated. Patients entering SCROT between 12 and 36 months were classified as “late SCROT” and between 36 and 48 months as “very late SCROT.” Patients who received splenectomy after 12 months, with sustained platelet counts of ≥100 × 109/L and without needing further treatments for at least 1 year were included in the SCROT group. Patients with a platelet count of <100 × 109/L and/or receiving ITP treatment at FUs (≥24 months) were classified as having ongoing chronic ITP. The analysis of the “ongoing ITP” group was irrespective of the last available FU date.
Statistical analysis
Initial data and FU information until 48 months were analyzed using descriptive methods. Continuous variables were reported as medians with corresponding interquartile ranges, or as means with corresponding standard deviations, whereas categorical variables were reported as proportions.
For group comparisons (“ongoing chronic disease” vs “late and very late SCROT”), continuous variables were analyzed using the Wilcoxon rank-sum test, and categorical variables were analyzed with the χ2 test or Fisher exact test if N was <5. A P value <.05 was significant, a P value <.1 was defined as a trend. Age, sex, platelet count at 6 and 12 months, and treatment with IV immunoglobulin at 6 and 12 were analyzed as potential predictors of late SCROT, because this was already suggested in 2 retrospective studies.14,15 Odds ratios (ORs) were calculated using both univariate regression model (URM) and multivariable logistic regression model (MRM). MRM was performed with log-transformed platelet counts, including a 3-knot spline for continuous predictors,16 thereby accounting for the nonlinear components of the effects of continuous variables on the outcome. The OR of these potential predictors with their 95% confidence intervals were reported. All analyses were performed using excel and R version 4.1.2.
Results
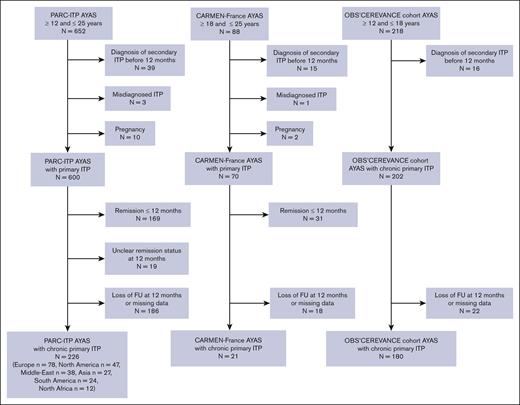
In November 2021, 958 AYAs with ITP were registered across all 3 databases: 652 from PARC-ITP, 88 from CARMEN-France, and 218 from OBS'CEREVANCE. Exclusions included 200 patients achieving stable remission by 12 months, as defined in our previous study,6 245 lost to FU or with missing data at 12 months, 70 with secondary ITP diagnosed within the first year, 4 with misdiagnosed ITP, and 12 who were pregnant at initial diagnosis (Figure 1). Finally, 427 AYAs (women, 64%) with chronic primary ITP (median age, 15 years [standard deviation, 2.7]) at diagnosis of chronic ITP (aged <18 years, n = 373; aged ≥18 years, n = 54) were analyzed. FU was available for 375 (88%), 327 (77%), and 250 (59%) patients at the 24-month, 36-month, and 48-month FU, respectively. The cohort included 7 patients with chronic ITP who underwent unsuccessful splenectomy before 12 months.
Flowchart of patient selection. Detailed patient selection process for each data source. Note that the OBS’CEREVANCE cohort enrolls only patients with chronic ITP. Loss at FU corresponds to patients with no information available at the 12-month FU. Missing data refer to patients with relevant information unavailable for the study or patients with no sufficient FU-time since diagnosis.
Flowchart of patient selection. Detailed patient selection process for each data source. Note that the OBS’CEREVANCE cohort enrolls only patients with chronic ITP. Loss at FU corresponds to patients with no information available at the 12-month FU. Missing data refer to patients with relevant information unavailable for the study or patients with no sufficient FU-time since diagnosis.
Clinical characteristics
At initial diagnosis, the median platelet count was 15 × 109/L (interquartile range, 7 × 109 to 35 × 109/L), and 237 of 427 (56%) patients had thrombocytopenia of <20 × 109/L. Overall, 325 (76%) patients had bleeding symptoms at presentation, whereas diagnosis was incidental (no bleeding) in 74 (17%) (missing data, n = 28). Among patients with bleeding (n = 325), 193 (59%) patients presented with wet bleeding, 3 (<1%) intracranial hemorrhage (ICH), and 126 (39%) with cutaneous bleeding only, (missing data, n = 3). For 170 of 427 (40%) patients, the initial management was “watch and wait” (no treatment in the first week). At 6- and 12-month, the median platelet count increased to 45 × 109/L and 51 × 109/L, respectively, and the percentage of individuals experiencing bleeding decreased from initial 76% to 59% and 51%, respectively. Use of ITP treatment was unchanged until 6 months (59%) but decreased to 49% between 6 and 12 months (Table 1).
Characteristics and treatment of AYAs with chronic primary ITP
| Patient characteristics . | Initial . | 6-mo FU . | 12-mo FU . | 24-mo FU . | 36-mo FU . | 48-mo FU . |
|---|---|---|---|---|---|---|
| No. of patients at FU∗ | 427 | 427 | 425 | 375 | 327 | 250 |
| Female (%) | 273 (64) | 273 (64) | 272 (64) | 240 (64) | 207 (63) | 155 (62) |
| Mean age (SD) | 14 (2.7) | 15 (2.7) | 16 (2.6) | 17 (2.3) | 18 (2.4) | |
| Platelet count (×109/L) at FU, median (IQR) | 15 (7-35) | 45 (21-79) | 51 (26-81) | 68 (30-115) | 76 (35-143) | 87 (43-169) |
| No. of patients with platelet count (%) | ||||||
| <20 × 109/L | 237 (56) | 88 (21) | 79 (19) | 57 (15) | 36 (11) | 18 (7) |
| 20 × 109 to 49 × 109/L | 107 (25) | 118 (28) | 110 (26) | 75 (20) | 58 (18) | 33 (13) |
| 50 × 109 to 99 × 109/L | 62 (15) | 105 (25) | 138 (32) | 103 (27) | 71 (22) | 56 (22) |
| >100 × 109/L | - | 72 (17) | 65 (15) | 104 (28) | 111 (34) | 82 (33) |
| No data on platelet count | 21 (5) | 44 (10) | 33 (8) | 36 (10) | 51 (16) | 61 (24) |
| Bleeding in the last FU period, yes (%)† | 325 (76) | 254 (59) | 215 (51) | 185 (49) | 132 (40) | 74 (30) |
| No (%)† | 74 (17) | 135 (32) | 157 (37) | 148 (39) | 140 (43) | 117 (47) |
| No data (%)† | 28 (7) | 38 (9) | 53 (12) | 42 (11) | 55 (17) | 59 (24) |
| Bleeding location‡(% of patients with bleeding) | ||||||
| Skin | 267 (82) | 207 (81) | 168 (78) | 142 (77) | 93 (70) | 52 (70) |
| Oral | 94 (29) | 76 (30) | 53 (25) | 45 (24) | 32 (24) | 22 (30) |
| Epistaxis | 99 (30) | 70 (28) | 57 (27) | 60 (32) | 36 (27) | 18 (24) |
| Gynecological (% of women with bleeding) | 73/209 (35) | 69/171 (40) | 50/144 (35) | 59/124 (48) | 43/90 (48) | 22/52 (42) |
| Gastrointestinal and/or hematuria | 10 (3) | 12 (5) | 10 (5) | 7 (4) | 5 (4) | 7 (10) |
| ICH | 3 (1) | 3 (1) | - | 2 (1) | - | - |
| Muscle and/or joint | 4 (1) | 4 (2) | 4 (2) | 2 (1) | - | - |
| Total wet bleeding§ | 193 (59) | 156 (61) | 124 (58) | 122 (66) | 87 (66) | 49 (66) |
| No data on bleeding site | 3 | 7 | 2 | 2 | 1 | - |
| Platelet count (×109/L) at FU, median (IQR) for patients with | ||||||
| Any bleeding | 11 (6-24) | 38 (16-70) | 38 (19-65) | 45 (22-95) | 42 (20-114) | 55 (23-140) |
| Only cutaneous bleeding | 16 (7-27) | 41 (21-75) | 40 (19-65) | 52 (28-98) | 55 (25-94) | 77 (41-193) |
| Wet or internal bleeding | 10 (5-21) | 32 (14-59) | 38 (19-66) | 41 (19-95) | 40 (18-149) | 43 (19-108) |
| No bleeding | 40 (20-65) | 58 (35-94) | 68 (45-97) | 77 (52-133) | 103 (60-172) | 114 (70-179) |
| Drug treatment in the last FU period, yes (%)† | 252 (59) | 254 (59) | 210 (49) | 177 (47) | 129 (39) | 85 (34) |
| No (watch and wait) (%)† | 170 (40) | 165 (39) | 206 (48) | 192 (51) | 191 (58) | 158 (63) |
| No data (%)† | 5 (1) | 8 (2) | 9 (2) | 6 (2) | 7 (2) | 7 (3) |
| Drug used (% of treated patients) | ||||||
| Corticosteroid | 129 (51%) | 188 (74%) | 127 (60%) | 84 (47%) | 40 (31%) | 31 (36%) |
| IV immunoglobulin and/or anti-D | 163 (65%) | 141 (56%) | 80 (38%) | 71 (40%) | 51 (40%) | 34 (40%) |
| Second line and/or third line | 10 (4%) | 59 (23%) | 76 (36%) | 95 (54%) | 79 (61%) | 55 (65%) |
| No data on drug used | 0 | 6 | 3 | 2 | 0 | 1 |
| Type of second-/third-line drugs | ||||||
| Hydroxychloroquine|| | 3 | 18 | 26 | 30 | 20 | 14 |
| TPO-RA | 2 | 17 | 17 | 20 | 22 | 9 |
| Azathioprine | - | 8 | 12 | 15 | 14 | 11 |
| Anti-CD20 (rituximab) | - | 5 | 9 | 17 | 12 | 8 |
| MMF | - | 4 | 6 | 4 | 7 | 6 |
| Vinka alkaloids | - | 5 | 4 | 3 | 1 | 1 |
| Tacrolimus/sirolimus | - | 3 | - | 1 | 1 | 1 |
| Other¶ | 6 | 14 | 14 | 20 | 12 | 10 |
| New splenectomy at each FU (not cumulative) | 0 | 3 | 4 | 16 | 11 | 13 |
| Platelet transfusion | 16 | 12 | 5 | 5 | 1 | 3 |
| Red blood cell transfusion total, (% represent proportion of female) | 16 (100%) | 5 (60%) | 4 (100%) | 5 (60%) | 2 (50%) | 2 (100%) |
| Patient characteristics . | Initial . | 6-mo FU . | 12-mo FU . | 24-mo FU . | 36-mo FU . | 48-mo FU . |
|---|---|---|---|---|---|---|
| No. of patients at FU∗ | 427 | 427 | 425 | 375 | 327 | 250 |
| Female (%) | 273 (64) | 273 (64) | 272 (64) | 240 (64) | 207 (63) | 155 (62) |
| Mean age (SD) | 14 (2.7) | 15 (2.7) | 16 (2.6) | 17 (2.3) | 18 (2.4) | |
| Platelet count (×109/L) at FU, median (IQR) | 15 (7-35) | 45 (21-79) | 51 (26-81) | 68 (30-115) | 76 (35-143) | 87 (43-169) |
| No. of patients with platelet count (%) | ||||||
| <20 × 109/L | 237 (56) | 88 (21) | 79 (19) | 57 (15) | 36 (11) | 18 (7) |
| 20 × 109 to 49 × 109/L | 107 (25) | 118 (28) | 110 (26) | 75 (20) | 58 (18) | 33 (13) |
| 50 × 109 to 99 × 109/L | 62 (15) | 105 (25) | 138 (32) | 103 (27) | 71 (22) | 56 (22) |
| >100 × 109/L | - | 72 (17) | 65 (15) | 104 (28) | 111 (34) | 82 (33) |
| No data on platelet count | 21 (5) | 44 (10) | 33 (8) | 36 (10) | 51 (16) | 61 (24) |
| Bleeding in the last FU period, yes (%)† | 325 (76) | 254 (59) | 215 (51) | 185 (49) | 132 (40) | 74 (30) |
| No (%)† | 74 (17) | 135 (32) | 157 (37) | 148 (39) | 140 (43) | 117 (47) |
| No data (%)† | 28 (7) | 38 (9) | 53 (12) | 42 (11) | 55 (17) | 59 (24) |
| Bleeding location‡(% of patients with bleeding) | ||||||
| Skin | 267 (82) | 207 (81) | 168 (78) | 142 (77) | 93 (70) | 52 (70) |
| Oral | 94 (29) | 76 (30) | 53 (25) | 45 (24) | 32 (24) | 22 (30) |
| Epistaxis | 99 (30) | 70 (28) | 57 (27) | 60 (32) | 36 (27) | 18 (24) |
| Gynecological (% of women with bleeding) | 73/209 (35) | 69/171 (40) | 50/144 (35) | 59/124 (48) | 43/90 (48) | 22/52 (42) |
| Gastrointestinal and/or hematuria | 10 (3) | 12 (5) | 10 (5) | 7 (4) | 5 (4) | 7 (10) |
| ICH | 3 (1) | 3 (1) | - | 2 (1) | - | - |
| Muscle and/or joint | 4 (1) | 4 (2) | 4 (2) | 2 (1) | - | - |
| Total wet bleeding§ | 193 (59) | 156 (61) | 124 (58) | 122 (66) | 87 (66) | 49 (66) |
| No data on bleeding site | 3 | 7 | 2 | 2 | 1 | - |
| Platelet count (×109/L) at FU, median (IQR) for patients with | ||||||
| Any bleeding | 11 (6-24) | 38 (16-70) | 38 (19-65) | 45 (22-95) | 42 (20-114) | 55 (23-140) |
| Only cutaneous bleeding | 16 (7-27) | 41 (21-75) | 40 (19-65) | 52 (28-98) | 55 (25-94) | 77 (41-193) |
| Wet or internal bleeding | 10 (5-21) | 32 (14-59) | 38 (19-66) | 41 (19-95) | 40 (18-149) | 43 (19-108) |
| No bleeding | 40 (20-65) | 58 (35-94) | 68 (45-97) | 77 (52-133) | 103 (60-172) | 114 (70-179) |
| Drug treatment in the last FU period, yes (%)† | 252 (59) | 254 (59) | 210 (49) | 177 (47) | 129 (39) | 85 (34) |
| No (watch and wait) (%)† | 170 (40) | 165 (39) | 206 (48) | 192 (51) | 191 (58) | 158 (63) |
| No data (%)† | 5 (1) | 8 (2) | 9 (2) | 6 (2) | 7 (2) | 7 (3) |
| Drug used (% of treated patients) | ||||||
| Corticosteroid | 129 (51%) | 188 (74%) | 127 (60%) | 84 (47%) | 40 (31%) | 31 (36%) |
| IV immunoglobulin and/or anti-D | 163 (65%) | 141 (56%) | 80 (38%) | 71 (40%) | 51 (40%) | 34 (40%) |
| Second line and/or third line | 10 (4%) | 59 (23%) | 76 (36%) | 95 (54%) | 79 (61%) | 55 (65%) |
| No data on drug used | 0 | 6 | 3 | 2 | 0 | 1 |
| Type of second-/third-line drugs | ||||||
| Hydroxychloroquine|| | 3 | 18 | 26 | 30 | 20 | 14 |
| TPO-RA | 2 | 17 | 17 | 20 | 22 | 9 |
| Azathioprine | - | 8 | 12 | 15 | 14 | 11 |
| Anti-CD20 (rituximab) | - | 5 | 9 | 17 | 12 | 8 |
| MMF | - | 4 | 6 | 4 | 7 | 6 |
| Vinka alkaloids | - | 5 | 4 | 3 | 1 | 1 |
| Tacrolimus/sirolimus | - | 3 | - | 1 | 1 | 1 |
| Other¶ | 6 | 14 | 14 | 20 | 12 | 10 |
| New splenectomy at each FU (not cumulative) | 0 | 3 | 4 | 16 | 11 | 13 |
| Platelet transfusion | 16 | 12 | 5 | 5 | 1 | 3 |
| Red blood cell transfusion total, (% represent proportion of female) | 16 (100%) | 5 (60%) | 4 (100%) | 5 (60%) | 2 (50%) | 2 (100%) |
anti-D, anti-D immunoglobulin; FU, follow-up; IQR, interquartile range; MMF, mycophenolate mophetil; SD, standard deviation.
Patients with available FU (also including patients with incomplete information). Two patients had no information at 12 months but had chronic ITP at a later FU.
% of patients with available FU (including patients with incomplete information). Bleeding and treatment information is given for the time between 2 FUs.
Oral bleeding includes spontaneous bleeding or after dental care. We included some rare cases of conjunctival bleeding or otorrhagia in this category. Epistaxis includes also rare cases of hemoptysis. Gynecological bleeding was defined as menorrhagia and/or metrorrhagia and included also patients with ovarian cyst hemorrhage. Gastrointestinal bleeding involved upper or lower gastrointestinal bleeding. ICH included also a single case of isolated retinal bleed.
Wet bleeding included all mucosal bleeding (oral, epistaxis, urological, gynecological, and gastrointestinal). Patients with different types of wet bleeding were counted once.
Hydroxychloroquine is only specified and documented in the OBS’CEREVANCE cohort and the CARMEN-France registry. Patients of the PARC-ITP receiving hydroxychloroquine are documented as having “other drugs.” Therefore, the number of patients receiving the drug might be underestimated.
Dapsone, cyclosporine, interferon, colchicine, plasmapheresis or protein A immunoadsorption, anti-CD52, infliximab, belimumab, golimumab, vedolizumab, ustekinumab, and others.
Bleeding likelihood consistently decreased in subsequent FUs, with only one-third experiencing symptoms between 36 and 48 months. Bleeding site distribution remained similar across all time points, with two-thirds of symptomatic patients experiencing wet bleeding. Skin bleeding was most frequent, followed by oral bleeding and/or epistaxis. Increased gynecological bleeding was the second most frequent site among females, reported in ∼20% of all women. Gastrointestinal hemorrhage and hematuria were rare, occurring in 3% to 9% of symptomatic patients. Six patients suffered from ICH within the first 6 months after diagnosis, 2 additional events occurred between 12 and 24 months, resulting in 1 fatality. Description of these cases are provided in the supplemental Data.
Use of drug treatment decreased from 49% at 12 months to 34% at 48 months FU. The proportion of second treatment lines among patients receiving treatment increased from 36% at 12-month FU to 65% at 48-month FU. However, >30% of treated patients still required rescue first-line drugs at each FU (Table 1). When comparing patients treated with TPO-RAs with others, irrespective of time point and duration, we observed no difference in the usage of rescue treatments across various FU periods (eg, between 12 and 24 months: 23% vs 19% received corticosteroids, respectively; and 15% vs 17% received IV immunoglobulin). Overall, 67 patients (16%) were managed with a watch-and-wait strategy until last available FU (37 patients with complete FU data until 48 months), whereas 167 (39%) received only first-line drugs and 188 (44%) second-line drugs until the last available FU, including 11 patients who received upfront second-line drugs without first-line drugs (missing data, n = 16 patients).
The most prescribed second-line drugs were hydroxychloroquine, TPO-RAs, azathioprine, and rituximab. In total, 47 patients received hydroxychloroquine, and 39 a TPO-RA during their illness. Most individuals receiving hydroxychloroquine were female (35/47 [74%]), registered by OBS'CEREVANCE (n = 42). Among them, 35 of 42 (83%) had associated immunopathological symptoms, including 13 with a positive antinuclear antibody (ANA) titer (supplement Table 2).
Forty patients underwent splenectomy beyond 12 months, uniformly distributed over all FUs (Table 1).
Late diagnosis of secondary ITP
Of 427 patients, 11 (2.6%) were diagnosed with secondary ITP between 12 and 48 months, 8 (73%) were girls, with a mean age of 14 years at initial ITP diagnosis. Two had common variable immunodeficiency, 4 developed autoimmune hemolytic anemia and were diagnosed with Evans syndrome, and 5 had systemic lupus erythematosus.
Analysis of patients achieving SCROT vs ongoing chronic disease
In total, 265 of 427 AYAs (62%) maintained a chronic disease until last available FU (24-48 months) and 99 (23%) entered SCROT between >12 and 48 months. The remission status in 63 AYAs (15%) was unclear because of missing information. The incidence of patients entering SCROT was constant over all FUs: 38 (10%), 31 (9.5%), and 30 (12%) patients between 12 and 24, 24 and 36, and 36 and 48 months, respectively. SCROT was independent of sex, age, initial clinical characteristics, platelet count at diagnosis, and initial treatment strategy. SCROT was attained in 23 of 40 patients with chronic ITP who underwent splenectomy.
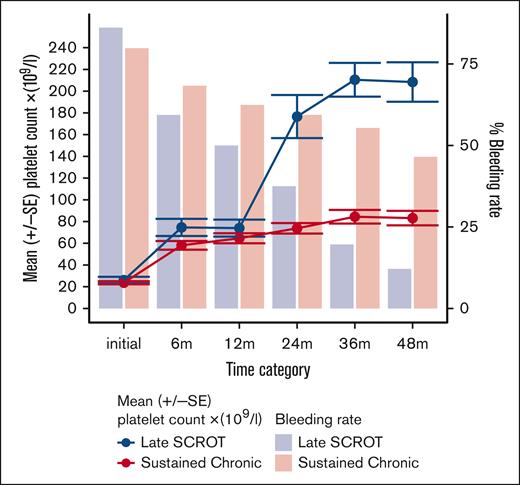
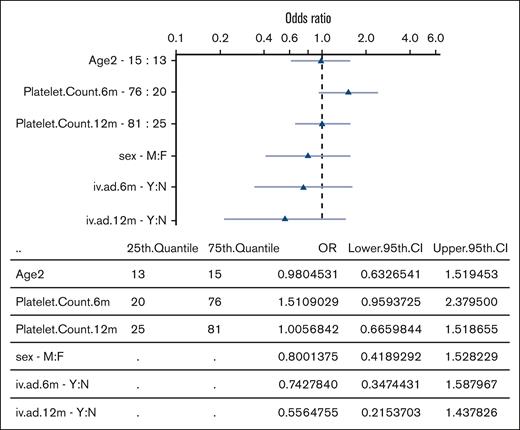
The clinical features of patients with ongoing chronic disease remained remarkably stable over time. Specifically, the percentage of patients reporting bleeding symptoms showed minimal variation between 6 and 36 months (ranging from 55% to 62%), slightly decreasing to 46% at the 48-month FU. Treatment requirement persisted throughout all FUs (∼50%), with a high proportion of patients managed with first-line drugs alone (41%, 35%, and 34% of patient treated at the 24-, 36-, and 48-month FU, respectively). Surprisingly, patients with “very late SCROT (>36 months)” exhibited similar clinical features to those of the “ongoing chronic disease” group until the 36-month FU, before entering remission. Platelet counts, bleeding tendency, and treatment needs showed remarkable parallels (Table 2). In contrast, patients with “late SCROT (between 12-36 months)” had significantly higher median platelet counts than the ongoing chronic disease group in the first year of the disease (58 × 109/L vs 40 × 109/L; P = .011 at 6 months, and 64 × 109/L vs 46 × 109/L; P = .05 at 12 months), respectively. Moreover, the late-SCROT group tended to have less bleeding at both 6 months (59% vs 68%; URM: OR, 1.48 [95% confidence interval (CI) 0.83-2.60]), and at 12 months (50% vs 62%; URM: OR, 1.66 [95% CI, 0.94-2.93]) and less need of rescue treatment with IV immunoglobulin at 6 months (27% vs 36%; URM: OR, 1.47 [95% CI, 0.82-2.74]) and 13% vs 22% at 12 months (URM: OR, 1.75 [95% CI, 0.85-4.01]) than the ongoing chronic group (Figure 2). Between 12 and 24 months the overall treatment requirement dropped significantly in the late SCROT group vs sustained chronic (37% vs 53%, P = .026; URM: OR, 1.93 [95% CI, 1.12-3.38]; P = .018). The MRM with a 3-knot spline showed that a high platelet count at 6 months and the absence of IV immunoglobulin use at 12 months shows a trend to predict late SCROT at 36 months (Figure 3).
Characteristics and treatment of AYAs with ongoing chronic disease at last available FU and entering SCROT
| Patient characteristics . | Ongoing chronic disease at last available FU . | Late SCROT (12-36 mo) . | Very late SCROT (36-48 mo) . |
|---|---|---|---|
| Total patients∗ | 265 | 69 | 30 |
| Female (%) | 171 (65) | 45 (65) | 18 (60) |
| Mean age at diagnosis, y (SD) | 14 (2.6) | 14 (2.9) | 14 (2.5) |
| Platelet count ×109/L, median (IQR) | |||
| Initial | 15 (8-31) | 18 (5-47) | 14 (10-37) |
| 6-mo FU | 40 (19-73) | 58 (25-96) | 45 (34-101) |
| 12-mo FU | 46 (23-79) | 64 (36-91) | 41 (28-80) |
| 24-mo FU | 56 (26-93) | 142 (84-215) | 44 (30-85) |
| 36-mo FU | 58 (31-99) | 171 (126-239) | 59 (26-90) |
| 48-mo FU | 62 (32-89) | 180 (144-247) | 162 (130-219) |
| Bleeding, yes (%)† | |||
| Initial | 198/248 (80) | 56/65 (86) | 22/26 (85) |
| 1-6 mo | 164/240 (68) | 38/64 (59) | 16/27 (59) |
| 6-12 mo | 143/229 (62) | 31/62 (50) | 15/25 (60) |
| 12-24 mo | 139/234 (59) | 24/64 (38) | 19/28 (68) |
| 24-36 mo | 103/186 (55) | 11/56 (20) | 18/28 (64) |
| 36-48 mo | 60/129 (47) | 4/33 (12) | 10/27 (37) |
| Drug treatment, n (% of all)† | |||
| Initial, yes | 152/262 (58) | 45/69 (65) | 14/29 (48) |
| Drug (%) | |||
| Corticosteroids | 68 (26) | 23 (33) | 9 (31) |
| IV immunoglobulin and/or anti-D | 108 (41) | 30 (43) | 8 (28) |
| Second or third line | 8 (3) | 1 (1) | - |
| 1-6 mo, yes | 152/258 (59) | 40 /66 (61) | 14/29 (48) |
| Drug (%) | |||
| Corticosteroids | 116 (45) | 30 (45) | 10 (34) |
| IV immunoglobulin and/or anti-D | 92 (36) | 18 (27) | 9 (31) |
| Second or third line | 37 (14) | 12 (18) | 2 (7) |
| 6-12 mo, yes | 130/259 (50) | 29/67 (43) | 15/29 (52) |
| Drug (%) | |||
| Corticosteroids | 71 (27) | 20 (30) | 12 (41) |
| IV immunoglobulin and/or anti-D | 56 (22) | 9 (13) | 4 (14) |
| Second or third line | 51 (20) | 12 (18) | 3 (10) |
| 12-24 mo, yes | 136/257 (53) | 25/68 (37) | 14/29 (48) |
| Drug (%) | |||
| Corticosteroids | 62 (24) | 12 (18) | 10 (34) |
| IV immunoglobulin and/or anti-D | 57 (22) | 13 (19) | 1 (3) |
| Second or third line | 80 (31) | 9 (13) | 6 (21) |
| 24-36 mo, yes | 109/224 (49) | 5/61 (8) | 15/28 (54) |
| Drug (%) | |||
| Corticosteroids | 31 (14) | 3 (5) | 6 (21) |
| IV immunoglobulin and/or anti-D | 44 (20) | 4 (7) | 3 (11) |
| Second or third line | 71 (32) | 0 | 8 (29) |
| 36-48 mo, yes | 82/167 (49) | 0/39 | 2/29 (7) |
| Drug (%) | |||
| Corticosteroids | 30 (18) | 0 | 1 (3) |
| IV immunoglobulin and/or anti-D | 32 (19) | 0 | 2 (7) |
| Second or third line | 54 (32) | 0 | 1 (3) |
| Patient characteristics . | Ongoing chronic disease at last available FU . | Late SCROT (12-36 mo) . | Very late SCROT (36-48 mo) . |
|---|---|---|---|
| Total patients∗ | 265 | 69 | 30 |
| Female (%) | 171 (65) | 45 (65) | 18 (60) |
| Mean age at diagnosis, y (SD) | 14 (2.6) | 14 (2.9) | 14 (2.5) |
| Platelet count ×109/L, median (IQR) | |||
| Initial | 15 (8-31) | 18 (5-47) | 14 (10-37) |
| 6-mo FU | 40 (19-73) | 58 (25-96) | 45 (34-101) |
| 12-mo FU | 46 (23-79) | 64 (36-91) | 41 (28-80) |
| 24-mo FU | 56 (26-93) | 142 (84-215) | 44 (30-85) |
| 36-mo FU | 58 (31-99) | 171 (126-239) | 59 (26-90) |
| 48-mo FU | 62 (32-89) | 180 (144-247) | 162 (130-219) |
| Bleeding, yes (%)† | |||
| Initial | 198/248 (80) | 56/65 (86) | 22/26 (85) |
| 1-6 mo | 164/240 (68) | 38/64 (59) | 16/27 (59) |
| 6-12 mo | 143/229 (62) | 31/62 (50) | 15/25 (60) |
| 12-24 mo | 139/234 (59) | 24/64 (38) | 19/28 (68) |
| 24-36 mo | 103/186 (55) | 11/56 (20) | 18/28 (64) |
| 36-48 mo | 60/129 (47) | 4/33 (12) | 10/27 (37) |
| Drug treatment, n (% of all)† | |||
| Initial, yes | 152/262 (58) | 45/69 (65) | 14/29 (48) |
| Drug (%) | |||
| Corticosteroids | 68 (26) | 23 (33) | 9 (31) |
| IV immunoglobulin and/or anti-D | 108 (41) | 30 (43) | 8 (28) |
| Second or third line | 8 (3) | 1 (1) | - |
| 1-6 mo, yes | 152/258 (59) | 40 /66 (61) | 14/29 (48) |
| Drug (%) | |||
| Corticosteroids | 116 (45) | 30 (45) | 10 (34) |
| IV immunoglobulin and/or anti-D | 92 (36) | 18 (27) | 9 (31) |
| Second or third line | 37 (14) | 12 (18) | 2 (7) |
| 6-12 mo, yes | 130/259 (50) | 29/67 (43) | 15/29 (52) |
| Drug (%) | |||
| Corticosteroids | 71 (27) | 20 (30) | 12 (41) |
| IV immunoglobulin and/or anti-D | 56 (22) | 9 (13) | 4 (14) |
| Second or third line | 51 (20) | 12 (18) | 3 (10) |
| 12-24 mo, yes | 136/257 (53) | 25/68 (37) | 14/29 (48) |
| Drug (%) | |||
| Corticosteroids | 62 (24) | 12 (18) | 10 (34) |
| IV immunoglobulin and/or anti-D | 57 (22) | 13 (19) | 1 (3) |
| Second or third line | 80 (31) | 9 (13) | 6 (21) |
| 24-36 mo, yes | 109/224 (49) | 5/61 (8) | 15/28 (54) |
| Drug (%) | |||
| Corticosteroids | 31 (14) | 3 (5) | 6 (21) |
| IV immunoglobulin and/or anti-D | 44 (20) | 4 (7) | 3 (11) |
| Second or third line | 71 (32) | 0 | 8 (29) |
| 36-48 mo, yes | 82/167 (49) | 0/39 | 2/29 (7) |
| Drug (%) | |||
| Corticosteroids | 30 (18) | 0 | 1 (3) |
| IV immunoglobulin and/or anti-D | 32 (19) | 0 | 2 (7) |
| Second or third line | 54 (32) | 0 | 1 (3) |
A total of 364 patients were analyzed here. For the remaining 63 patients no classification was possible (relevant information missing.)
Percentage of patients with complete information regarding the analyzed parameter at the defined time point. The total number of patients used as denominator may differ to the patient count reported at the top of the column.
Evolution of platelet counts and bleeding proportion according to remission status. Graphical evolution of the bleeding proportion and platelet count in AYAs with ongoing chronic disease vs AYAs with late SCROT, occurring between 12 and 36 months. The mean 6-month platelet count is higher in AYAs with late SCROT compared with those with ongoing chronic disease. m, months FU; SE, standard error.
Evolution of platelet counts and bleeding proportion according to remission status. Graphical evolution of the bleeding proportion and platelet count in AYAs with ongoing chronic disease vs AYAs with late SCROT, occurring between 12 and 36 months. The mean 6-month platelet count is higher in AYAs with late SCROT compared with those with ongoing chronic disease. m, months FU; SE, standard error.
OR for prediction of late remission in a logistic regression model. Graphical representation of the OR for predictors of late remission. Age, sex, 6- and 12-month platelet counts, and IV immunoglobulin or anti-D immunoglobulin use at 6 and 12 months were selected as potential predictors. Platelet count is log transformed. High 6-month platelet count and low use of IV immunoglobulin (or anti-D immunoglobulin) at 12 months shows a trend to predict late remission. Triangles represent the OR (log-scale), and light blue lines depict the 95% confidence interval of the OR. For continuous variables, the OR demonstrates the increase from the 25th to the 75th quantile. 6m, 6-month FU; 12m, 12-month FU; CI, confidence interval; iv.ad, IV immunoglobulin.
OR for prediction of late remission in a logistic regression model. Graphical representation of the OR for predictors of late remission. Age, sex, 6- and 12-month platelet counts, and IV immunoglobulin or anti-D immunoglobulin use at 6 and 12 months were selected as potential predictors. Platelet count is log transformed. High 6-month platelet count and low use of IV immunoglobulin (or anti-D immunoglobulin) at 12 months shows a trend to predict late remission. Triangles represent the OR (log-scale), and light blue lines depict the 95% confidence interval of the OR. For continuous variables, the OR demonstrates the increase from the 25th to the 75th quantile. 6m, 6-month FU; 12m, 12-month FU; CI, confidence interval; iv.ad, IV immunoglobulin.
Discussion
In this study, AYAs with chronic ITP demonstrate a gradual clinical improvement until 48 months of FU, characterized by fewer patients experiencing bleeding, increasing mean platelet counts, and reduced treatment use. A significant proportion of patients with ongoing chronic disease were managed solely with first-line drugs at various FU periods (>35% of treated patients), suggesting a potentially oligosymptomatic disease and an on-demand treatment approach.5 This is consistent with the findings of Ducassou et al, who reported that 45% of children with chronic ITP received first-line drugs only during FU.17 Bleeding sites among patients experiencing hemorrhage remained consistent across all FUs, with 60% wet bleeding, although the severity is unknown, except for gynecological bleeding, which increased over time, likely because of more girls entering puberty. ICH was rare in our cohort, with 6 of 8 events occurring within the first 6 months of the disease.6 This aligns with previous findings indicating that ICH risk is primarily observed in older populations (aged ≥60 years) and during the early stages of the disease.18-21
Despite the increasing use of second-line therapies in treated patients, IV immunoglobulin was still administered to 40% of them at all FUs. IV immunoglobulin serves as a standard rescue therapy, often used for significant bleeding or in the preoperative setting.5,22 This persistent use potentially suggests that second-line therapies fail to prevent platelet fluctuations, breakthrough bleeding events, or adequately address specific risk situations. It could also indicate unsuccessful attempts to discontinue ongoing treatment. Yet, the precise count of IV immunoglobulin administrations between FUs remains undisclosed, thus a potential decline in the effective use of IV immunoglobulin over the course of time cannot be excluded. Unfortunately, administration of corticosteroid remained relatively high at all FUs (31%-47% of all treated patients). Duration of treatment and cumulative dose was not evaluated, and guidelines regarding corticosteroid use differ greatly between children and adults,5,9 making it difficult to estimate the real toxic burden in AYAs. Although corticosteroids are effective, safe, convenient, and inexpensive first-line and/or rescue drugs,5,9,23 there is no evidence suggesting they improve health-related quality of life. However, because a significant number of patients were included 10 to 20 years ago, changes in practice may not yet be reflected in our registries. Notably, TPO-RAs were licensed for pediatric ITP between 2016 and 2018, potentially underrepresented in this analysis, thus, treatment strategies may have substantially evolved.
In adults, secondary ITP accounts for ∼20% of patients with ITP.3,24,25 Here, identification of a secondary cause of ITP was relatively rare after 12 months (2.6%). This result is reassuring for clinicians, who often worry about misdiagnosed cases of chronic ITP.
In our cohort, 99 AYAs (23%) of an initial 427 patients with chronic disease at 12 months achieved SCROT at later FUs, including 23 patients after splenectomy. However, it's worth noting that this number might be underestimated, because patients lost to FU are known to include a higher proportion of individuals attaining remission or with uncomplicated disease.2 Interpreting and comparing our results with published data pose challenges because of varying definitions and inclusion criteria. The definition of chronic ITP underwent a significant change in 2009, with chronicity previously defined as a platelet count of <150 × 109/L and a disease persisting for >6 months. The 2009 definition by Rodeghiero et al established chronicity as a platelet count of <100 × 109/L and a disease persisting for >12 months.8 Additionally, definition of sustained remission differs in terms of platelet count threshold, treatment status, and duration of remission. Furthermore, variations exist in inclusion criteria, subgroup analysis (eg, splenectomy cases), ethnicities, and data quality (prospective vs retrospective) across studies (Table 3).
Published remission rate for children and adults with chronic ITP
| Publication . | Study design . | Population (n) . | Inclusion criteria . |
| FU period . | CR rate at FU in patients with chronic ITP . |
|---|---|---|---|---|---|---|
| Sailer et al26 | Monocentric cohort, retrospective | Adults (n = 59) | Severe chronic ITP, nonsplenectomized (old definition) |
| 3 y | 0.5%/mo from 6 mo to 3 y |
| Bourgeois et al27 | Prospective | Children and adults (n = 47) | Chronic ITP (old definition) and refractory to splenectomy |
| Median FU 7.5 y after splenectomy | 57% |
| Schifferli et al2 | Prospective registry | Children and adults | Chronic ITP (new definition) |
| 2 y | 30% |
| Medeiros et al28 | Review of 9 studies | Children (n = 415) | Chronic ITP (old and new definition, and others) | Very heterogenous, (analysis includes 100 patients with splenectomy) | Heterogenous, up to 15 y | 40%-79% (for patients without splenectomy) |
| Blanchette et al29 | Review of 7 studies | Children (n = 337) | Chronic ITP (old and new, and others) |
| Heterogenous up to 20 y | 10%-64% |
| Jayabose et al30 | Retrospective | Children (n = 62) | Chronic ITP (old definition) |
| 4 y | 45%, CR without splenectomy (68% including patients with splenectomy) |
| Donato et al31 | Retrospective | Children (n = 230) | Nonsplenectomized chronic ITP (old definition) |
| 6 mo to 10 y | 27%, CR without splenectomy (using new definition of chronic ITP: 13.5% CR) (33% including patients with splenectomy) |
| Bansal et al, 2010 | Retrospective | Children (n = 270) | Chronic ITP (old definition) |
| Median FU, 30 mo | 25% (predicted CR rate at 5 y 30%, at 10 y 44%) |
| Rosthøj et al32 | Prospective cohort | Children (n = 96) | Chronic ITP (old definition) |
| 7 -93 mo (86 patients with 5 y FU) | 56% at 5 y (including patients with splenectomy) |
| Neunert et al 201346 | Prospective | Children (n = 343) | Persistent and chronic ITP (new definition) |
| 24 mo | 29% |
| Shim et al33 | Retrospective | Children (n = 47) | Chronic ITP (new definition) |
| Median, 4.4 y | 45% |
| Kim et al34 | Retrospective | Children (n = 41) | Chronic ITP (new definition) |
| 2-5 y | 76% |
| Ducassou et al17 | Prospective observational cohort | Children (n = 392) | Chronic ITP (new definition) |
| 5-20 y | 19.7%, cumulative at 5 y 39.2% cumulative at 10 y 61.2%, cumulative at 20 y |
| Pincez et al13 | Prospective observational cohort | Children (n = 846) | Chronic ITP (new definition) |
| Median FU for children 5.3 y | 23% |
| Publication . | Study design . | Population (n) . | Inclusion criteria . |
| FU period . | CR rate at FU in patients with chronic ITP . |
|---|---|---|---|---|---|---|
| Sailer et al26 | Monocentric cohort, retrospective | Adults (n = 59) | Severe chronic ITP, nonsplenectomized (old definition) |
| 3 y | 0.5%/mo from 6 mo to 3 y |
| Bourgeois et al27 | Prospective | Children and adults (n = 47) | Chronic ITP (old definition) and refractory to splenectomy |
| Median FU 7.5 y after splenectomy | 57% |
| Schifferli et al2 | Prospective registry | Children and adults | Chronic ITP (new definition) |
| 2 y | 30% |
| Medeiros et al28 | Review of 9 studies | Children (n = 415) | Chronic ITP (old and new definition, and others) | Very heterogenous, (analysis includes 100 patients with splenectomy) | Heterogenous, up to 15 y | 40%-79% (for patients without splenectomy) |
| Blanchette et al29 | Review of 7 studies | Children (n = 337) | Chronic ITP (old and new, and others) |
| Heterogenous up to 20 y | 10%-64% |
| Jayabose et al30 | Retrospective | Children (n = 62) | Chronic ITP (old definition) |
| 4 y | 45%, CR without splenectomy (68% including patients with splenectomy) |
| Donato et al31 | Retrospective | Children (n = 230) | Nonsplenectomized chronic ITP (old definition) |
| 6 mo to 10 y | 27%, CR without splenectomy (using new definition of chronic ITP: 13.5% CR) (33% including patients with splenectomy) |
| Bansal et al, 2010 | Retrospective | Children (n = 270) | Chronic ITP (old definition) |
| Median FU, 30 mo | 25% (predicted CR rate at 5 y 30%, at 10 y 44%) |
| Rosthøj et al32 | Prospective cohort | Children (n = 96) | Chronic ITP (old definition) |
| 7 -93 mo (86 patients with 5 y FU) | 56% at 5 y (including patients with splenectomy) |
| Neunert et al 201346 | Prospective | Children (n = 343) | Persistent and chronic ITP (new definition) |
| 24 mo | 29% |
| Shim et al33 | Retrospective | Children (n = 47) | Chronic ITP (new definition) |
| Median, 4.4 y | 45% |
| Kim et al34 | Retrospective | Children (n = 41) | Chronic ITP (new definition) |
| 2-5 y | 76% |
| Ducassou et al17 | Prospective observational cohort | Children (n = 392) | Chronic ITP (new definition) |
| 5-20 y | 19.7%, cumulative at 5 y 39.2% cumulative at 10 y 61.2%, cumulative at 20 y |
| Pincez et al13 | Prospective observational cohort | Children (n = 846) | Chronic ITP (new definition) |
| Median FU for children 5.3 y | 23% |
CR, complete remission; FU, follow-up.
Children exhibit a high likelihood of achieving remission during the disease course, especially within the first year (70%-80%).32,35 Remission data in chronic pediatric ITP using both old and new chronicity definitions show significant variability, ranging from 10% to 72% and 13.5% to 76%, respectively. Overall, remission rates typically fall within the range of 30% to 50% (Table 3).2,26-34,36 Conversely, evidence of treatment-free remission after 12 months is rare in adults, with some rare reports averaging ∼20% (Table 3). In our study, new SCROT percentages were evenly distributed across all years of FU (∼10% per year). A comparable rate of yearly remissions was reported in a retrospective pediatric study with remission defined as a platelet count of >100 × 109/L and no treatment for 3 months.30
Identifying predictors of remission is crucial for improving treatment decisions, patient counseling, and targeting quality of life. For patients with a high likelihood of remission, attempts to discontinue ongoing medications (eg, TPO-RAs) could be incorporated into treatment guidelines. By now, older age (>10 years), higher platelet counts, and/or absence of bleeding at diagnosis, and absence of preceding infection (in children only) are risk factors for chronicity.11,12,14,37-39 In our previous analysis of AYAs with newly diagnosed ITP,6 the 1-year remission rate varied depending on the initial treatment strategy. Predictive factors for late remission in chronic ITP are unclear. Our analysis revealed that initial disease characteristics, platelet count, and initial treatment did not influence the SCROT rate beyond 12 months, which is in line with the results of previous pediatric studies.30,36,40,41 Whether age and sex is a predictor of late remission in chronic pediatric ITP remains controversial. Some pediatric studies have reported that older age was associated with lower late remission rates 31,36,42; however, others did not find an association.15,30,40,41 Here, we found no difference in the SCROT rate in AYAs aged <18 years when compared with those aged ≥18 years, supporting the legitimacy of our AYAs group. In our study, AYAs with late SCROT, occurring between 12 and 36 months, tended to present a better disease course during the first year of the disease (excluding initial presentation) than AYAs with ongoing chronic disease, with higher platelet counts, less bleeding propensity, and reduced use of IV immunoglobulin between 6 and 12 months. This finding is in line with 3 pediatric studies that have reported better remission rates if the platelet count was >20 × 109/L at 6 months,32 >50 × 109/L14 or >60 × 109/L15 12 months after disease onset, and with the publication of Chotsampancharoen et al who found that the need for IV immunoglobulin at diagnosis of chronic ITP was an adverse prognostic marker for remission in 113 children with chronic ITP.15 This observation could be interpreted as indicative of an uncomplicated disease subtype: late SCROT may have inherently milder forms of the disease or may respond better to initial treatment strategies. Another possible explanation could be that these individuals display ongoing tolerance mechanisms in the first year. In contrast, patients with ongoing chronic disease exhibited a stable bleeding phenotype across all FU periods, with consistent rates of rescue treatments with corticosteroids or IV immunoglobulin, despite the escalating use of second-line therapies. This suggests the persistence of their disease and the limited effectiveness of current treatment strategies in mitigating bleeding episodes.
The AYA project comprises 3 distinct registries, each with unique characteristics, requiring specific adaptations. The primary challenges of this study revolved around harmonizing the description and analysis to accommodate the diverse methods of data collection across the 3 registries. For instance, in the PARC-ITP registry, bleeding data are aggregated as all events occurring between 2 FUs, whereas in the French registries, each bleeding event is recorded individually. In addition, some specific data couldn't be analyzed because of variations in recording methods and thresholds across registries; for example, ANA positivity that might be a predictor of chronic disease. It's also important to note that the interpretation of treatment types is influenced by geographic and institutional factors; for example, the use of hydroxychloroquine in France for adolescents or women with positive ANA at a stage of incomplete systemic lupus erythematosus, a practice not as common in other countries.43-45
However, this international study regroups a large sample size of patients afflicted by a rare disease, drawn from diverse geographic and ethnic backgrounds, and prospectively followed-up in real-life settings for 48 months. Additionally, the initial clinical characteristics of the cohort closely resemble those reported in our previous publication on AYAs with newly diagnosed ITP,6 demonstrating the consistency of our patient population even after inclusion of the OBS'CEREVANCE chronic ITP cohort.
To our knowledge, this study represents the first analysis of long-term FU data in the rare group of AYAs diagnosed with chronic primary ITP. Given that a considerable proportion of patients achieve SCROT after 12 months, clinicians should be encouraged to postpone splenectomy for as long as possible. Overall, even if AYAs have greater risk of chronic disease at 12 months, the clinical burden, late remission rate, and need of treatment seem to be closer to the pediatric population. Further investigations are needed to improve personalized disease management in ITP.
Acknowledgments
The authors thank all the investigators of the Pediatric and Adult Registry on Chronic ITP (PARC), Centre de Référence National des Cytopénies Auto-immunes de l'Enfant (CEREVANCE), and Cytopénies Auto-immunes Registre Midi-Pyrénéen-France (CARMEN) study groups. The authors thank Fiona Pugin and Andy Schötzau for the statistical analysis.
The adolescent and young adult project received financial support from the Platelet Disease Support Association (A.S.), Novartis Switzerland (grant [A.S.]), Stiftung hämatologische Forschung, Basel, Switzerland; Stiftung zur Förderung medizinischer und biologischer Forschung, Arlesheim, Switzerland; and from the Intercontinental Cooperative ITP Study Group. The CARMEN registry received support from the French National Society of Internal Medicine, Toulouse Referral Center for Autoimmune Cytopenias, Toulouse University Hospital, Amgen, CSL Behring, Grifols, and Novartis. The OBS’CEREVANCE cohort received support from French Ministry of Health (Programme Hospitalier de Recherche Clinique2005, Rare Disease Plan 2007, 2017, 2023), Association Bordelaise pour l'Avancement des Sciences Pediatriques research charity, Association pour la Recherche et les Maladies Hématologiques de l'Enfant research charity, Association Française du Syndrome d'Evans, and O'CYTO patients' associations.
Authorship
Contribution: A.S. was responsible for study conceptualization, methodology, investigation, data curation, formal analysis, funding acquisition, project administration, supervision, and writing of the manuscript; G.L.G. was responsible for investigation, data curation, formal analysis, writing of the manuscript, and funding acquisition; N.A. and G.M. were responsible for study conceptualization, methodology, investigation, data curation, review and editing, and supervision; B.G. was responsible for study conceptualization, review and editing, and supervision; T.L. and S.H. were responsible for investigation, data curation, and review and editing; H.F. was responsible for methodology, data curation, and review; and T.K. was responsible for study conceptualization, methodology, supervision, review and editing, and funding acquisition.
Conflict-of-interest disclosure: A.S. reports honoraria and research funding from Novartis; and reports honoraria from Sobi. G.M. received honoraria (consultancy) from Alpine, Amgen, Argenx, Grifols, Novartis, Sanofi, and Sobi; and is the coordinator of research studies granted by Amgen, Grifols, Novartis, and Sanofi. T.K. reports Novartis research funding; and reports honoraria (consultancy) from Argenx, Novartis, Sobi, Takeda, and UCB. The remaining authors declare no competing financial interest.
Correspondence: Alexandra Schifferli, Department of Hematology/Oncology, University Children's Hospital Basel, Spitalstrasse 33, 4056 Basel, Switzerland; email: alexandra.schifferli@ukbb.ch.
References
Author notes
A.S. and G.L.G. are joint first authors and contributed equally to this study.
For original data of the Pediatric and Adult Registry on Chronic ITP (PARC) registry, please contact the corresponding author, Alexandra Schifferli (alexandra.schifferli@ukbb.ch); for original data of the Cohorte observationelle du Centre de Référence National des Cytopénies Auto-immunes de l'Enfant (OBS'CEREVANCE), please contact Nathalie Aladjidi (nathalie.aladjidi@chu-bordeaux.fr); and for original data of the Cytopénies Auto-immunes Registre Midi-Pyrénéen-France (CARMEN), please contact Guillaume Moulis (moulis.g@chu-toulouse.fr).
The full-text version of this article contains a data supplement.