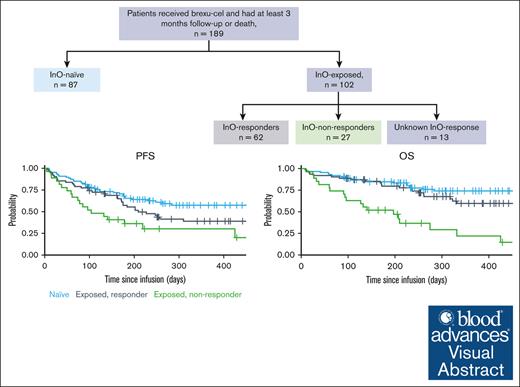
Key Points
InO-exposed patients with R/R B-ALL have inferior outcomes after brexu-cel, particularly those who were nonresponders to InO.
The negative association is unrelated directly to InO per se; rather, it reflects selection of patients with intrinsically adverse-risk ALL.
Visual Abstract
The effect of prior inotuzumab ozogamicin (InO) treatment on brexucabtagene autoleucel (brexu-cel) outcomes remains unclear in adults with acute lymphoblastic leukemia (ALL). We conducted a retrospective multicenter analysis of 189 patients with relapsed/refractory ALL treated with brexu-cel. Over half of the patients received InO before brexu-cel (InO exposed). InO-exposed patients were more heavily pretreated (P = .02) and frequently had active marrow disease before apheresis (P = .03). Response rate and toxicity profile after brexu-cel were comparable for InO-exposed and InO-naïve patients; however, consolidation therapy after brexu-cel response was used at a higher rate in InO-naïve patients (P = .005). With a median follow-up of 11.4 months, InO-exposed patients had inferior progression-free survival (PFS; P = .013) and overall survival (OS; P = .006) in univariate analyses; however, prior InO exposure did not influence PFS (hazard ratio, 1.20; 95% confidence interval, 0.71-2.03) in multivariate models. Within InO-exposed patients, InO responders had superior PFS (P = .002) and OS (P < .0001) relative to InO-refractory patients. The timing of administering InO did not affect brexu-cel outcomes, with comparable PFS (P = .51) and OS (P = .86) for patients receiving InO as bridging therapy or before apheresis. In conclusion, although InO exposure was associated with inferior survival outcomes after brexu-cel in unadjusted analyses, these associations were no longer significant in multivariate analyses, suggesting it is unlikely that InO negatively affects brexu-cel efficacy. Our data instead imply that InO-exposed recipients of brexu-cel tend to be higher-risk patients with intrinsic adverse leukemia biology.
Introduction
Over the last decade, the treatment landscape of relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) has considerably transformed with the approval of several effective targeted and immune-based salvage agents.1 Blinatumomab, a CD3-CD19 T-cell engager antibody, and inotuzumab ozogamicin (InO), an anti-CD22 antibody drug conjugate, have established superiority over multiagent chemotherapy in adults with R/R B-ALL in phase 3 studies.2,3 Nonetheless, remission duration after blinatumomab and InO is short, and relapse is common in the absence of a curative consolidative strategy afterward.2,4,5 Recently, the approval of CD19-targeted chimeric antigen receptor (CAR) T-cell therapy (CAR-T) in children and adults has generated substantial promise in the R/R B-ALL arena, considering outstanding remission rates in addition to remission durability in a subset of treated patients.6-9 Brexucabtagene autoleucel (brexu-cel) is the only US Food and Drug Administration–approved CD19-directed CAR-T for adults with R/R B-ALL.7,10 Among 55 patients with R/R B-ALL who underwent infusion treated in the phase 2 part of the ZUMA-3 study, 39 (71%) responded with a median duration of remission approaching 13 months.7 Real-world and registry data for brexu-cel use in adults with R/R B-ALL were reported by several groups, including the Center for International Blood and Marrow Transplant Research, the Real-World Outcomes Collaborative of CAR-T in Adult ALL (ROCCA), and the Group for Research on Adult Acute Lymphoblastic Leukemia, and they have validated brexu-cel high complete remission (CR) rate (range, 77%-90%), negative minimal residual disease (MRD) rates (92% and 82%) and survival outcomes (median overall survival [OS], 15.6 months).11-13
In contrast to InO and blinatumomab, CAR-T is an autologous cellular therapy product that requires manufacturing time (∼2-3 weeks) and is associated with unique immunologic toxicities similar to blinatumomab; nonetheless, they occur at higher rates and severity. With access to several targeted agents in R/R B-ALL, uncertainty persists regarding the optimal sequence of various salvage therapies. InO and brexu-cel share the same indication for R/R B-ALL, and brexu-cel–treated patients may have received prior InO treatment either before considering brexu-cel or after collecting T cells as a bridging therapy to maintain disease control while manufacturing CAR-T cells. Small scale studies have explored the influence of prior therapy with InO on post–CAR-T outcomes and have demonstrated mixed and inconclusive findings.8,14-16
Using a large population-based data set of adults with R/R B-ALL treated with commercial brexu-cel, we sought to understand the impact of prior InO therapy on brexu-cel outcomes. We additionally examined whether response to InO and/or timing of InO before brexu-cel influenced outcomes.
Methods
The ROCCA cohort was used for this analysis. ROCCA is a multicenter initiative that aggregates retrospective patient data that include 31 CAR-T centers across the United States, encompassing both academic and community-based institutions. Institutional review board approval was pursued at individual sites, and data-sharing agreements were formed between participating sites and Stanford University. Data were entered into a HIPAA-compliant database maintained by Stanford University.
The purpose of this study was to describe clinical characteristics, toxicity profiles, response rates, and survival outcomes among patients treated with brexu-cel for R/R B-ALL according to their prior treatment with InO.
Patients
Adult patients (aged ≥18 years) with R/R B-ALL who received brexu-cel as a standard-of-care therapy between October 2021 and August 2023 were included, with at least 3 months of follow-up after brexu-cel infusion or death. Data lock for analysis occurred on 30 October 2023. Individuals who were treated with brexu-cel as part of an expanded access protocol before US Food and Drug Administration approval were excluded.
Assessment of clinical outcomes
Response assessment, evaluated by bone marrow disease was performed around day +28 after brexu-cel, with MRD assessment performed per local institutional practices. MRD methodologies included flow cytometry, quantitative reverse transcription polymerase chain reaction for BCR::ABL1 fusion, or next-generation sequencing/ClonoSEQ (Adaptive Biotechnologies). When appropriate and per clinician discretion, involved areas of extramedullary disease (EMD) or central nervous system involvement were monitored longitudinally with imaging and/or cerebrospinal fluid evaluation. CR was defined as <5% lymphoblasts in the bone marrow, absence of circulating lymphoblasts or EMD, an absolute neutrophil count >1000 cells per μL, and a platelet count >100 000/μL. Complete response with incomplete hematologic recovery (CRi) was defined as CR with either platelet count <100 000 cells per μL and/or absolute neutrophil count <1000/μL. Response was defined as either CR or CRi. Central nervous system disease was graded by standard cytology metrics per the Children’s Oncology Group classification.17 Cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome were graded according to established criteria set by the American Society for Transplantation and Cellular Therapy.18
Statistical methodology
Progression-free survival (PFS) was measured from the time of CAR-T infusion to either disease relapse or death. OS was measured from the time of CAR-T infusion to death or last follow-up. Univariate cox regression models estimating hazard ratios (HRs) for PFS were constructed for patient, disease, and treatment variables of interest. Variables with a significance level of P value <.1 in univariate analyses, in addition to age and race/ethnicity (regardless of association in univariate analyses), were included in multivariate models of PFS. Kaplan-Meier unadjusted curves for PFS and OS were constructed with censoring at the time of last follow-up and stratified by InO exposure or response to InO during prior treatment. Kaplan-Meier unadjusted curves for PFS and OS were also stratified by the timing of InO exposure, defined as only during preapheresis or during bridging and/or before apheresis.
Results
One hundred eighty-nine patients received infusion with brexu-cel and were included in this analytic cohort. Over half (n = 102 [54%]) received InO at some time point before brexu-cel infusion (InO-exposed). Among these 102 patients, the median number of InO doses was 3; 74 patients (73%) received InO only before apheresis, whereas 28 patients (27%) received InO as a bridging therapy (ie, between apheresis and lymphodepletion) with (n = 16) or without InO administration (n = 12) before apheresis. The median numbers of administered InO doses were 3 (range, 1-22) in the preapheresis period and 2 (range, 1-3) during the bridging interval. Among InO-exposed patients, 62 patients (61%) were deemed responders (defined as achieving CR or CRi after treatment) to InO treatment, whereas 27 patients (26%) were considered refractory, and 13 patients had no reported response assessment after InO. The median time between preapheresis InO treatment and apheresis was 62 days (range, 9-1190), and that between the last dose of InO during bridging and CAR T-cell infusion was 20 days (range, 1-108) for patients who received InO before apheresis and patients who received InO as bridging therapy, respectively (Figure 1).
Patient demographic and disease characteristics for InO-exposed and InO-naïve patients are shown in Table 1. InO-exposed patients more frequently had Philadelphia chromosome (Ph)–negative ALL, including Ph-like disease (81% vs 59%; P = .003) and active marrow disease (>5% blasts) before apheresis (58% vs 40%; P = .03), and were more heavily pretreated (median prior lines of therapy, 4 vs 3; P = .02) than InO-naïve patients. Prior allogeneic hematopoietic cell transplantation (HCT; 45% vs 38%; P = .36) and blinatumomab therapy (61% vs 58%; P = .67) were not different between InO-naïve and InO-exposed patients. Other key patient and disease characteristics were not significantly different between the InO-exposed and InO-naïve patient cohorts. Among InO-exposed patients, those with responses to InO (as opposed to nonresponders) were more likely to have Ph+ disease (27% vs 4%; P = .03), lower rate of active marrow disease before apheresis (47% vs 89%; P < .0001), were more heavily pretreated (median prior lines of therapy, 4 vs 3; P = .002), and were more frequently recipients of prior allogeneic HCT (48% vs 7%; P = .0002; supplemental Table 1). Regarding the timing of InO administration, patients who received bridging InO had lower median number of prior lines of therapy (2 vs 4; P = .01) and trends toward more active disease (79% vs 50%; P = .07) and EMD (39% vs 20%; P = .05) before apheresis than patients who received InO before apheresis (supplemental Table 2).
Baseline demographic and clinical characteristics of 189 patients receiving brexucabtagene autoleucel infusion by prior InO treatment
| Characteristic . | Inotuzumab naïve, N (%) . | Inotuzumab-exposed, N (%) . | P-value . |
|---|---|---|---|
| Number of patients | 87 | 102 | |
| Median age, (range) years | 48 (18-79) | 45 (21-81) | 0.79 |
| 18-39 | 33 (38) | 44 (43) | 0.80 |
| 40-59 | 28 (32) | 30 (29) | |
| 60+ | 25 (29) | 28 (27) | |
| Sex, female | 37 (43) | 45 (44) | 0.83 |
| Race/ethnicity | 0.85 | ||
| Non-Hispanic White | 49 (56) | 55 (54) | |
| Hispanic | 26 (30) | 30 (29) | |
| Black | 6 (7) | 7 (7) | |
| Asian | 4 (5) | 8 (8) | |
| Other | 2 (2) | 1 (1) | |
| Disease Subtype | 0.003 | ||
| Ph+ | 36 (41) | 19 (19) | |
| Ph-like | 14 (16) | 20 (20) | |
| Ph-negative | 37 (43) | 63 (61) | |
| Pre-apheresis disease status | 0.03 | ||
| Active disease, >5% blasts | 35 (40) | 59 (58) | |
| CR, MRD+ or unknown | 32 (37) | 19 (19) | |
| CR, MRD-neg | 14 (16) | 14 (14) | |
| Missing | 6 (7) | 10 (10) | |
| Pre-apheresis bone marrow blast % | 0.002 | ||
| <25% | 59 (68) | 41 (40) | |
| 25-74% | 8 (9) | 25 (25) | |
| 75% | 5 (6) | 9 (9) | |
| Missing / not performed | 15 (17) | 27 (26) | |
| Extramedullary disease at apheresis | 0.33 | ||
| Yes | 17 (20) | 26 (25) | |
| No | 70 (80) | 76 (75) | |
| CNS involvement at apheresis | 0.37 | ||
| Yes | 18 (21) | 16 (16) | |
| No | 69 (79) | 86 (84) | |
| Number of prior lines of therapy, median (range) | 3 (2-9) | 4 (2-12) | 0.02 |
| Prior blinatumomab therapy | 53 (61) | 59 (58) | 0.67 |
| Prior HCT | 39 (45) | 39 (38) | 0.36 |
| Characteristic . | Inotuzumab naïve, N (%) . | Inotuzumab-exposed, N (%) . | P-value . |
|---|---|---|---|
| Number of patients | 87 | 102 | |
| Median age, (range) years | 48 (18-79) | 45 (21-81) | 0.79 |
| 18-39 | 33 (38) | 44 (43) | 0.80 |
| 40-59 | 28 (32) | 30 (29) | |
| 60+ | 25 (29) | 28 (27) | |
| Sex, female | 37 (43) | 45 (44) | 0.83 |
| Race/ethnicity | 0.85 | ||
| Non-Hispanic White | 49 (56) | 55 (54) | |
| Hispanic | 26 (30) | 30 (29) | |
| Black | 6 (7) | 7 (7) | |
| Asian | 4 (5) | 8 (8) | |
| Other | 2 (2) | 1 (1) | |
| Disease Subtype | 0.003 | ||
| Ph+ | 36 (41) | 19 (19) | |
| Ph-like | 14 (16) | 20 (20) | |
| Ph-negative | 37 (43) | 63 (61) | |
| Pre-apheresis disease status | 0.03 | ||
| Active disease, >5% blasts | 35 (40) | 59 (58) | |
| CR, MRD+ or unknown | 32 (37) | 19 (19) | |
| CR, MRD-neg | 14 (16) | 14 (14) | |
| Missing | 6 (7) | 10 (10) | |
| Pre-apheresis bone marrow blast % | 0.002 | ||
| <25% | 59 (68) | 41 (40) | |
| 25-74% | 8 (9) | 25 (25) | |
| 75% | 5 (6) | 9 (9) | |
| Missing / not performed | 15 (17) | 27 (26) | |
| Extramedullary disease at apheresis | 0.33 | ||
| Yes | 17 (20) | 26 (25) | |
| No | 70 (80) | 76 (75) | |
| CNS involvement at apheresis | 0.37 | ||
| Yes | 18 (21) | 16 (16) | |
| No | 69 (79) | 86 (84) | |
| Number of prior lines of therapy, median (range) | 3 (2-9) | 4 (2-12) | 0.02 |
| Prior blinatumomab therapy | 53 (61) | 59 (58) | 0.67 |
| Prior HCT | 39 (45) | 39 (38) | 0.36 |
Ph, Philadelphia chromosome; CR, complete response; MRD, measurable residual disease; CNS, central nervous system
Safety and toxicity
Any grade and grade 3 to 4 cytokine release syndromes occurred in 86 (85%) and 15 InO-exposed patients (15%) and 71 (83%) and 5 InO-naïve patients (6%), respectively. Any grade and grade 3 to 4 immune effector cell–associated neurotoxicity syndrome occurred in 55 (54%) and 37 InO-exposed (36%) and 50 (57%) and 22 InO-naïve patients (25%), respectively. Management of brexu-cel toxicity and length of hospitalization during brexu-cel therapy were not different for InO-exposed and InO-naive patients. Ten InO-exposed (9.8%) and 3 InO-naïve patients (3.4%) died during the first 30 days after brexu-cel infusion (P = .085; Table 2; supplemental Table 2). There were 4 deaths from sinusoidal obstruction syndrome after brexu-cel infusion, including 3 InO-exposed patients, and all 4 cases occurred after receiving hematopoietic stem cell transplantation.
Safety and toxicity summary for infused patients
| Event/grade . | Inotuzumab-naïve; N (%) . | Inotuzumab-exposed; N (%) . | P-value . |
|---|---|---|---|
| CRS, evaluable patients | |||
| Any | 71 (83) | 86 (85) | 0.63 |
| Grade 1-2 | 66 (93) | 71 (83) | 0.05 |
| Grade 3-4 | 5 (7) | 15 (17) | |
| Median days to onset | 5 | 5 | 0.47 |
| ICANS, evaluable patients | |||
| Any | 50 (57) | 55 (54) | 0.68 |
| Grade 1-2 | 28 (56) | 18 (33) | 0.02 |
| Grade 3-4 | 22 (44) | 37 (67) | |
| Median days to onset | 7 | 7 | 0.52 |
| Toxicity management, evaluable patients | |||
| Tocilizumab | 61 (70) | 68 (67) | 0.61 |
| Steroids | 58 (67) | 63 (62) | 0.48 |
| Anakinra | 17 (20) | 27 (26) | 0.26 |
| Hospital length of stay, median days (range) | 14 (4-91) | 15 (0-95) | 0.24 |
| Early death | |||
| Within 30 days of infusion | 3 (3) | 10 (10) |
| Event/grade . | Inotuzumab-naïve; N (%) . | Inotuzumab-exposed; N (%) . | P-value . |
|---|---|---|---|
| CRS, evaluable patients | |||
| Any | 71 (83) | 86 (85) | 0.63 |
| Grade 1-2 | 66 (93) | 71 (83) | 0.05 |
| Grade 3-4 | 5 (7) | 15 (17) | |
| Median days to onset | 5 | 5 | 0.47 |
| ICANS, evaluable patients | |||
| Any | 50 (57) | 55 (54) | 0.68 |
| Grade 1-2 | 28 (56) | 18 (33) | 0.02 |
| Grade 3-4 | 22 (44) | 37 (67) | |
| Median days to onset | 7 | 7 | 0.52 |
| Toxicity management, evaluable patients | |||
| Tocilizumab | 61 (70) | 68 (67) | 0.61 |
| Steroids | 58 (67) | 63 (62) | 0.48 |
| Anakinra | 17 (20) | 27 (26) | 0.26 |
| Hospital length of stay, median days (range) | 14 (4-91) | 15 (0-95) | 0.24 |
| Early death | |||
| Within 30 days of infusion | 3 (3) | 10 (10) |
CRS, cytokine release syndrome; ICANS, immune effector cell associated neurotoxicity syndrome; WBC, white blood cell.
Response and survival
The rates of CR/CRi after brexu-cel infusions were comparable for InO-exposed and InO-naïve patients (88.2% vs 91.6%, respectively; P = .47). Similarly, rates of MRD-negative remission did not differ based on InO exposure (75% vs 66%; P = .2). Among patients achieving CR/CRi after brexu-cel, a significantly greater proportion of InO-naïve patients (n = 37 [49%]) underwent either consolidation or maintenance therapy than InO-exposed patients (n = 20 [27%]; P = .005). Among InO-naïve patients, 20 received allogeneic HCT, 11 received tyrosine kinase inhibitors (TKIs), and 6 received other maintenance, whereas among InO-exposed patients, 10 received allogeneic HCT, 7 received TKIs, and 3 received other.
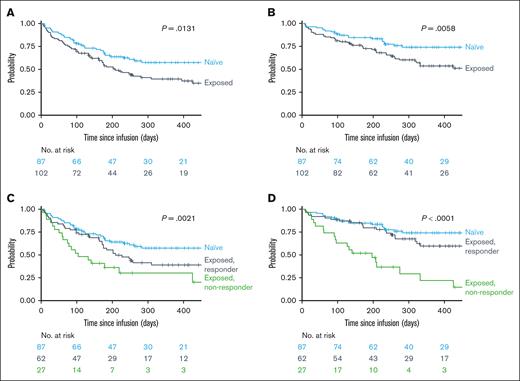
With a median follow-up from brexu-cel infusion of 11.4 months, the estimated 6- and 12-month PFS were 54% (95% confidence interval [CI], 43-63) and 39% (95% CI, 29-50) for InO-exposed and 65% (95% CI, 54-75) and 57% (95% CI, 45-68) for InO-naïve patients, respectively (Figure 2A). The 6- and 12-month OS were 73% (95% CI, 63-80) and 54% (95% CI, 42-64) for InO-exposed patients and 85% (95% CI, 75-91) and 74% (95% CI, 63-83) for InO-naïve patients, respectively (Figure 2B). In univariate survival analyses, pre–brexu-cel InO exposure was significantly associated with inferior PFS (P = .013) and OS (P = .006) compared with InO-naïve patients. When InO-exposed patients were stratified according to prior response to InO treatment (CR/CRi vs no response), InO responders had superior estimated 6- and 12-month PFS (57% and 39%) and OS (80% and 60%) relative to InO-refractory patients (PFS, 36% and 30%; OS, 52% and 22%), respectively (Figure 2C-D). The timing of pre-CAR InO therapy did not affect brexu-cel survival outcomes, with comparable OS (P = .86) and PFS (P = .51) for InO-exposed patients during bridging therapy and patients who received InO as a therapy before apheresis (supplemental Table 2; Figure 3A-B).
Post-brexu-cel survival outcomes based on InO-exposure and response. (A) PFS by prior InO exposure (naïve vs exposed). (B) OS by prior InO exposure (naïve vs exposed). (C) PFS by prior InO exposure and response to InO (naïve vs InO exposed and responded vs InO exposed and nonresponder). (D) OS by prior InO exposure and response to InO (naïve vs InO exposed and responded vs InO exposed and nonresponder).
Post-brexu-cel survival outcomes based on InO-exposure and response. (A) PFS by prior InO exposure (naïve vs exposed). (B) OS by prior InO exposure (naïve vs exposed). (C) PFS by prior InO exposure and response to InO (naïve vs InO exposed and responded vs InO exposed and nonresponder). (D) OS by prior InO exposure and response to InO (naïve vs InO exposed and responded vs InO exposed and nonresponder).
Multivariate analysis for association of demographic-, disease-, and treatment-specific characteristics, including prior InO response, with PFS in the study cohort.
Multivariate analysis for association of demographic-, disease-, and treatment-specific characteristics, including prior InO response, with PFS in the study cohort.
In adjusted multivariate models, prior InO exposure was no longer significantly associated with PFS (HR, 1.20; 95% CI, 0.71-2.03; supplemental Figure 1). Further, prior InO exposure demonstrated no significant association with PFS in a multivariate model, with InO exposure further stratified into InO responsive (HR, 1.30; 95% CI, 0.72-2.32) or InO refractoriness (HR, 1.67; 95% CI, 0.73-3.82; Figure 3). In this model, receipt of pre-CAR transplant (HR, 0.30; 95% CI, 0.16-0.57) and postresponse consolidation with either transplant (HR, 0.16; 95% CI, 0.07-0.36) or TKIs (HR, 0.15; 95% CI, 0.04-0.56) were associated with superior PFS, whereas administration of bridging therapy was associated with inferior PFS (HR, 1.80; 95% CI, 1.01-3.20; Figure 3).
Among brexu-cel responders, 24 InO-naïve (28%) and 37 InO-exposed patients (36%) relapsed afterward during the study follow-up (P = .20).
Discussion
In this analysis of a large multicenter cohort of adults treated with commercial brexu-cel for R/R B-ALL, we examined the relationship between prior InO exposure and outcomes after brexu-cel. This is of interest due to the conflicting data to date on whether InO exposure influences CAR T-cell outcomes. In this study, we demonstrate that over half of adults who received infusion with brexu-cel have previously received InO, either as a therapeutic line of treatment before apheresis, postapheresis bridging, or both. We found that InO-exposed and InO-naïve patients had comparable CR/CRi and MRD-negative responses after brexu-cel, but in unadjusted analyses, InO-exposed patients had inferior survival outcomes. However, multivariate models revealed that after adjusting for other key patient and disease characteristics, a significant association between pre–brexu-cel InO and survival outcomes was not present. Thus, the relationship between InO exposure and inferior brexu-cel outcomes is likely mediated by disease biology and intrinsic treatment resistance rather than a direct causative effect of InO on impaired brexu-cel activity.
Hypothetically, prior InO therapy could negatively hinder CAR T-cell expansion through depletion of malignant and normal B cells. With this speculation, we would anticipate that the administration of InO closer to the time of brexu-cel infusion would result in a more detrimental effect on long-term outcomes. We observed, however, no difference in outcomes among InO-exposed patients according to the timing of InO use (bridging vs preapheresis), suggesting that InO is unlikely to directly impair CAR T-cell function, even when administered as bridging therapy. It would be instructive to examine CAR T-cell expansion in populations of patients receiving InO at different times. The ROCCA registry currently does not include routine CAR T-cell expansion and persistence data, but this is certainly of interest.
Patients who were nonresponders to InO tended to have very poor survival outcomes after brexu-cel. This association further supports the hypothesis that inherently resistant disease biology confers poor outcomes with both targeted therapies. Similar findings have been demonstrated with blinatumomab and CAR T cells, in which nonresponders to blinatumomab were less likely to have favorable outcomes to tisagenleucleucel therapy.19 These findings raise the question of whether CAR T-cell therapies have utility in patients who are refractory to prior targeted immunotherapies or whether we are perhaps not applying novel therapies for B-ALL in the most effective sequence. Additional studies are necessary to answer these questions, but given the limited therapeutic options available for R/R B-ALL, we would not currently discourage consideration of brexu-cel in patients who previously did not respond to InO. Similarly, we would not recommend withholding InO if otherwise an appropriate option in order to somehow preserve or improve outcomes with subsequent brexu-cel.
We observed a comparable safety profile following brexu-cel for InO-exposed and InO-naïve patients, and prior InO therapy did not pose unusual or increased risks with brexu-cel treatment. Nonetheless, our study has illustrated a lower rate of patients achieving CR/CRi following brexu-cel transitioning to consolidation/maintenance therapy in InO-exposed compared to InO-naïve patients, despite comparable rates of pre-CAR transplant among the InO-exposed (38%) and InO-naïve (45%) cohorts. While the explanation for this observation is unclear, this could be related to treating physicians’ hesitancy to offer transplant for InO-exposed patients because of the concern of increased risk for sinusoidal obstruction syndrome,20,21 especially in the context of a second transplant in previously transplanted recipients. It is possible that the lower rate of post-CAR consolidation/maintenance therapy could have contributed to the inferior outcomes in InO-exposed patients, and hence, InO-exposed responders to brexu-cel may need innovative consolidative approaches that can be readily delivered to improve outcomes.
Although our analysis is one of the largest cohorts examining the impact of prior InO therapy on brexu-cel outcomes, as a retrospective study, there is inherent selection bias and heterogeneity in peri-CAR management. The number of patients who received InO as a bridging therapy in our analysis was small and included a portion of patients who received it in the preapheresis period, and this precludes providing a robust conclusion on the impact of bridging InO in this setting. Our study lacks data for CAR T-cell expansion and T-cell fitness, which are key correlates that could enhance our understanding of our clinical observations. Nevertheless, our study is a multicenter effort with detailed real-world pre– and post–CAR T-cell treatment information that is unlikely available in other registry databases.
In conclusion, InO-exposed patients with R/R B-ALL have inferior outcomes after brexu-cel, particularly those who were nonresponders to InO. This negative association is unrelated directly to InO per se but rather reflects the selection of a group of patients with intrinsically adverse-risk, resistant ALL, who are also less frequently able to receive post-CAR consolidative therapy. Optimizing pre– and post–brexu-cel management is warranted to advance post-CAR outcomes in InO-treated patients.
Authorship
Contribution: I.A., G.W.R., B.D.S., and L.S.M. contributed to the study design and data interpretation; A.Z. and K.M. performed statistical analyses; I.A. drafted the manuscript; and all authors provided study data and contributed to critical revision of the manuscript for intellectual content.
Conflict-of-interest disclosure: I.A. reports consultancy fees from Syndax, Wugen, Kite, Sobi, Jazz, Pfizer, and Takeda; consultancy fees and honoraria from Amgen; advisory board fees from Amgen, Pfizer, Jazz, Kite, Takeda, Syndax, Sobi, and Wugen; and research support from AbbVie and Macrogenics. R.F. reports research funding and advisory board membership fees from Kite/Gilead and research funding from Novartis. C.L. reports consultancy fees from Rigel Pharmaceuticals; being current equity holder in a publicly traded company, BioMarin; and advisory board fees from Autolus. A.S.A. reports research funding from Servier, ImmunoGen, OBI, Incyte, Seattle Genetics, and MacroGenics; honoraria and other including consulting fees and research funding from Kite; membership on an entity’s board of directors or advisory committees and research funding from GlycoMimetics; honoraria and research funding from Pfizer; honoraria from and membership on an entity’s board of directors or advisory committees in Jazz; honoraria from Beam, Nkarta, and Kura; honoraria from and membership on an entity’s board of directors or advisory committees in Taiho and Novartis; and honoraria and other including advisory board fees and research funding from Amgen. E.C. reports consultancy fees from AbbVie. M.S. reports consultancy fees from Jazz Pharmaceuticals, Kite, and Autolus. B.D. reports consultancy fees from BEAM Therapeutics, Pluri Biotech, Boxer Capital, Gamida Cell, Ellipsis Pharma, Lumanity, and Arivan; research funding from Atara, Molecular Templates, AstraZeneca, MEI, Gilead, Angiocrine, Adicet, Takeda, Poseida, Pfizer, Bristol Myers Squibb (BMS), Wugen, Orca Bio, Poseida, Allovir, and NCI; consultancy fees and honoraria from ADC Therapeutics; and consultancy fees, honoraria, and research funding from Janssen. N.M. reports membership on an entity’s board of directors or advisory committees in Anthem Inc. M.B. reports research funding from Novartis and Fate Therapeutics. P.S. reports honoraria from Autolus Therapeutics and BMS, and speakers' bureau fees from BMS and Sanofi. A.C.L. reports research funding from Amgen, Astellas, Autolus Therapeutics, Kadmon, Kite/Gilead, Pharmacyclics, and Talaris, and consultancy fees from AbbVie, Amgen, Actinium, BMS, Pfizer, Sanofi, and Takeda. A.L. reports consultancy fees from Pfizer and CTI Biopharma. R.T.H. reports research funding from Orca Bio. T.H. reports consultancy fees and research funding (to institution) from BeiGene. L.C.H. reports consulting fees from March Biosciences and speaker fees from Kite/Gilead. S.B.T. reports speakers' bureau fees from BMS and Jazz Pharmaceuticals, and advisory board fees from Autolus. M.M.S. reports speaker's bureau fees from BMS. C.J.L. reports consultancy fees from Fresenius Kabi; honoraria, advisory board member fees, and speaker's bureau fees from Kite Pharma; honoraria from BMS; consultancy fees, advisory board member fees, and honoraria from Sanofi; honoraria from Kadmon; and consultancy fees, advisory board member fees, and research funding from Incyte Corp. V.K.K. reports honoraria from Pfizer, Novartis, and Kite, and research funding from Incyte. D.K. reports consultancy fees and research funding from BMS. J.T.L. reports consultancy fees, membership on an entity’s board of directors or advisory committees in, and other fundings including travel, accommodations, and expenses from Adaptive Biotechnologies, and consultancy fees from Pfizer, Kite/Gilead, and Takeda. C.O. reports research funding from Novartis, Arog, Orca Bio, Jazz Pharmaceuticals, Pfizer, and Seagen. V.B. reports research funding from Citius, BMS, Incyte, and Gamida Cell; membership on an entity’s board of directors or advisory committees in AstraZeneca, ADC, and Allogene; other funding including data and safety monitoring board from Miltenyi; and advisory board member fees from AstraZeneca, Allogene, BeiGene, and CRISPR. W.S. reports consultancy fees from Kite and GlaxoSmithKline; consultancy fees and honoraria from Jazz Pharmaceuticals; honoraria from Amgen and Newave; research funding from Kura; and other funding including data safety monitoring board/advisory board fees from Servier. R.D.C. reports research funding from Servier, Incyte, Vanda Pharmaceuticals, and Merck; membership on an entity’s board of directors or advisory committees in PeproMene Bio and Autolus; consultancy fees, honoraria, and research funding from Kite/Gilead, Amgen, Jazz Pharmaceuticals, and Pfizer; and other interest including spouse being employed by and owned stock in Seagen within the last 24 months. V.P. reports consultancy fees and speakers' bureau fees from Servier, Amgen, Pfizer, Jazz Pharmaceuticals, Novartis, Genentech, and AbbVie. B.D.S. reports research funding from Incyte, Jazz Pharmaceuticals, Kite/Gilead, and Servier; honoraria from Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, and Gilead Sciences; current employment with Moffitt Cancer Center; other funding including travel, accommodations, and expenses from Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, and Kite/Gilead; membership on an entity’s board of directors or advisory committees and data and safety monitoring committee in PeproMene Bio; and consultancy fees from Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision BioSciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, and PeproMene Bio. L.S.M. reports consultancy from Amgen, Pfizer, and Autolus; research funding from BMS, Orca Bio, and Jasper; membership on an entity’s board of directors or advisory committees in and research funding from Adaptive; consultancy fees, honoraria, and research funding from Kite; and consultancy fees and research funding from Astellas. The remaining authors declare no competing financial interests.
Correspondence: Ibrahim Aldoss, Division of Leukemia, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA; email: ialdoss@coh.org.
References
Author notes
Data are available on reasonable request from the corresponding author, Ibrahim Aldoss (ialdoss@coh.org). Please note, individual participant data will not be shared.
The full-text version of this article contains a data supplement.