TO THE EDITOR:
Among the numerous identified cytochrome b5 reductase 3 (CYB5R3) variants, T117S (rs1800457) stands out as a high-frequency variant (0.23 minor allele frequency), predominantly found in individuals of African ancestry and rarely (<1%) in other racial groups.1 A missense threonine-to-serine substitution at position 117 decreases its reductase activity ∼50% compared with wild-type (WT) CYB5R3.2 Diminished CYB5R3 reductase activity is associated with nitric oxide (NO) resistance observed in pulmonary arterial hypertension and other sickle cell disease (SCD) vasculopathies.3 Gordeuk et al reported that T117S lessens hemolytic anemia only in the presence of intact glucose-6-phosphate dehydrogenase (G6PD) activity (G6PD WT or A+/A– heterozygote) in persons of African ancestry.4 Hence, it has been theorized that the apparent selection advantage for T117S likely arises from the protection it offers to persons in malaria-endemic regions.4 Where G6PD activity is compromised, the protective effect for hypomorphic T117S against hemolytic anemia appears also compromised, as shown by Gordeuk et al.4 Deficient G6PD activity can allow oxidized heme levels of erythrocytes to exceed the methemoglobin reductase capacity of T117S. Under heightened levels of oxidative stress in conditions such as malaria and SCD, the systemic impact (ie, vascular and erythrocyte) of decreased CYB5R3 activity could promote hemolytic anemia.
Hydroxyurea (HU) is the first US Food and Drug Administration-approved drug for patients with SCD, extensively prescribed for the clinical advantages associated with fetal hemoglobin (HbF) induction.5-7 Substantial variability in HbF response to HU exists among patients, which complicates predicting who will experience a favorable HbF response. Moreover, there remains a great need to identify novel modifiers of HU-induced HbF in SCD.
CYB5R3 modulates redox signaling in erythrocytes and vascular cells by electron transfer reactions, using reduced nicotinamide adenine dinucleotide for electrons.8 CYB5R3 is known as a “resilience enzyme” under stress.8 Membrane-associated CYB5R3 exhibits diverse biological effects: coenzyme Q reduction in the plasma membrane, elongation and unsaturation of fatty acids, cholesterol biosynthesis, drug metabolism, and antioxidant activity through heme iron reduction as in hemoglobin, myoglobin, and soluble guanylate cyclase (sGC).1 Soluble CYB5R3 reduces methemoglobin in erythrocytes.1 It has been reported that NO–cyclic guanosine 3ʹ,5ʹ-monophosphate (cGMP) signaling plays a role via sGC in both steady- and stressed-state erythropoiesis9-11 and HbF induction.12 Because CYB5R3 maintains sGC in its active reduced heme state,8,13 it is possible that the reductase participates in HU-mediated HbF induction. Hence, we hypothesized that diminished CYB5R3 T117S reductase activity contributes to the observed interpatient variability in the HU-induced HbF response.
Aggregated, deidentified baseline data from the Treatment of Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy trial14 (Walk-PHaSST, NCT00492531) (Figure 1A) and the Pulmonary Hypertension and the Hypoxic Response in SCD study15 (PUSH, NCT00495638) were sourced to determine the effects of CYB5R3 T117S on HU-induced HbF in patients with SCD. Both multicenter studies had institutional review board approval of their participating institutions and written, informed consent for all patients.14,15
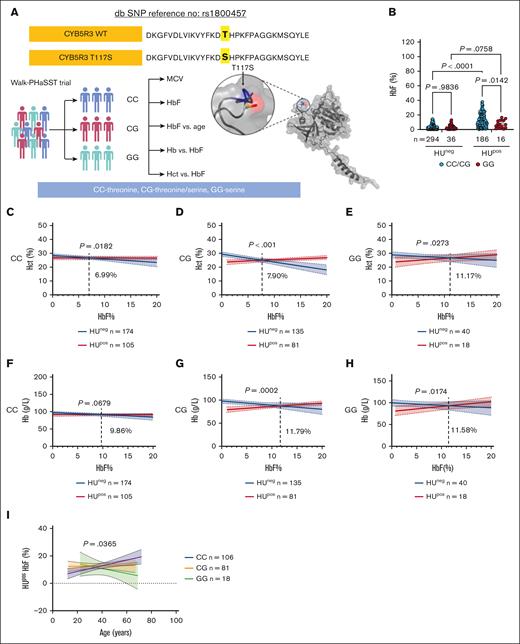
Clinical data showing CYB5R3 T117S lessens the HbF, Hct, and total Hb responses to HU treatment in Walk-PHaSST patients. (A) Experimental design for clinical data analysis. (B) HbF (%) in patient samples by different CYB5R3 genotypes and HU treatment status, analyzed using a 2-way analysis of variance (ANOVA). (C-E) Hct (%) vs HbF (%) for the different CYB5R3 genotypes (x-axis truncated at 20% HbF; full-scale x-axis data available in supplemental Figure 1C-E), analyzed by simple linear regression. (F-H) Hb (g/L) vs HbF (%) for the different CYB5R3 genotypes (x-axis truncated at 20% HbF; full-scale x-axis data available in supplemental Figure 1F-H), analyzed by simple linear regression. The dotted lines in these figures show where the 2 lines meet, and the point of intersection is mentioned adjacent to the line. (I) Percentage of HbF in HUpos patient samples vs age for the different CYB5R3 genotypes analyzed by simple linear regression. Data are expressed as mean ± standard error of the mean unless otherwise specified. A comparison of the linear regression lines for the differences between slopes used a method equivalent to analysis of covariance (ANCOVA). A value of P < .05 was considered significant. In all correlation graphs, the P value is for the difference between the slopes in each graph. db SNP, single nucleotide polymorphism database; HUneg, HU untreated; HUpos, HU treated; MCV, mean corpuscular volume.
Clinical data showing CYB5R3 T117S lessens the HbF, Hct, and total Hb responses to HU treatment in Walk-PHaSST patients. (A) Experimental design for clinical data analysis. (B) HbF (%) in patient samples by different CYB5R3 genotypes and HU treatment status, analyzed using a 2-way analysis of variance (ANOVA). (C-E) Hct (%) vs HbF (%) for the different CYB5R3 genotypes (x-axis truncated at 20% HbF; full-scale x-axis data available in supplemental Figure 1C-E), analyzed by simple linear regression. (F-H) Hb (g/L) vs HbF (%) for the different CYB5R3 genotypes (x-axis truncated at 20% HbF; full-scale x-axis data available in supplemental Figure 1F-H), analyzed by simple linear regression. The dotted lines in these figures show where the 2 lines meet, and the point of intersection is mentioned adjacent to the line. (I) Percentage of HbF in HUpos patient samples vs age for the different CYB5R3 genotypes analyzed by simple linear regression. Data are expressed as mean ± standard error of the mean unless otherwise specified. A comparison of the linear regression lines for the differences between slopes used a method equivalent to analysis of covariance (ANCOVA). A value of P < .05 was considered significant. In all correlation graphs, the P value is for the difference between the slopes in each graph. db SNP, single nucleotide polymorphism database; HUneg, HU untreated; HUpos, HU treated; MCV, mean corpuscular volume.
All baseline data for Walk-PHaSST patients (aged 12-70 years) and PUSH patients (aged 3-20 years) were acquired at steady state. We identified 584 Walk-PHaSST patients and 228 PUSH patients with sickle hemoglobin genotype (excluding sickle cell trait), with data for HU treatment status (currently on HU or not: HU treated, HUpos or HU untreated, HUneg) and HbF. Patients self-reported their HU therapy status, a possible limitation of this study because proper HU escalation and dosing adherence are difficult to assess. A comparison of mean corpuscular volume in HUpos and HUneg patients showed an enlarged mean corpuscular volume, as expected in HU-treated patients (supplemental Figure 1A). Blood measurements analyzed included HbF, total Hb, and hematocrit (Hct) (Figure 1A). Patients on transfusion protocols or HbA+ were statistically similar between CYB5R3 genotypes (supplemental Tables 1 and 2) and were included in all analyses. Genotyping for rs1800457 was performed as previously described.4
CD34+ hematopoietic stem cells (HSCs) from healthy non-SCD donors (Fred Hutchinson Cancer Research Center, Seattle, Washington) were cultured for 18 days in 3 phases for erythrocyte differentiation. CYB5R3 gene knockout (KO) using CRISPR occurred on day 3, and HU treatment (50 μM) every 2 days thereafter. KO was confirmed using western blot on day 15. F cell induction and erythrocyte differentiation were assessed using flow cytometry on day 18.
Data were analyzed using GraphPad Prism version 9, and statistics are expressed as mean ± standard error unless otherwise specified. F test was used to compare variances and Mann-Whitney for nonparametric data. Outliers (>interquartile range, 1.5; applied to all genotypes) were excluded (supplemental Table 3) for 2-way analysis of variance (ANOVA) of HbF percentage by CYB5R3 genotype and HU treatment status but not for linear regression analyses. Tukey test was used for 2-way ANOVA with multiple comparisons, which generated adjusted P values. A comparison of linear regression lines for differences between slopes used a method equivalent to the analysis of covariance. A value of P < .05 was considered significant.
Patients with SCD (SS, SC, S-beta+ thalassemia [S-beta+], and S-beta0 thalassemia) were stratified by T117S genotype: T117S/T117S homozygotes (GG), T117S/WT heterozygotes (CG), and WT/WT homozygotes (CC). Patients’ clinical measurements (supplemental Tables 1 and 2) were compared for the CYB5R3 genotype, irrespective of HU treatment status.
Among the Walk-PHaSST HUpos patients, HbF percentage of patients carrying WT CYB5R3 (CC and CG) was similar (P = .8334; supplemental Figure 1B), yet different from HbF percentage of patients homozygous for T117S (GG) (supplemental Figure 1B), suggesting a recessive T117S effect. Hence, the CC and CG groups were pooled to increase the power for analyzing HbF percentage by CYB5R3 genotype: CC/CG vs GG (Figure 1B). Walk-PHaSST HUpos patients showed a lower HbF percentage in the GG group than in the CC/CG group, whereas HUneg patients showed no effect of the CYB5R3 genotype on HbF percentage (Figure 1B). The HUpos data point to CYB5R3 modification of the HU-induced HbF response in patients with SCD, with a greater HU-induced HbF percentage in CC/CG patients (P = .0142) than in GG patients (P = .0758); a positive interaction (P = .0222) between the CYB5R3 genotype and HU-induced HbF was detected.
Similar analyses in PUSH HUpos patients (n = 81) showed no statistically significant effect of the CYB5R3 genotype on HbF percentage (supplemental Figure 2A). Furthermore, the overall fold difference (HUpos HbF/HUneg HbF) was higher in Walk-PHaSST (supplemental Figure 2B) than in PUSH patients (supplemental Figure 2C); 3.75-fold vs 1.68-fold, respectively. The contrast in HbF induction for Walk-PHaSST (mostly adult) and PUSH (children and adolescent) patients suggests age-dependent effects for CYB5R3 on HbF response to HU, which agrees with reported decreased CYB5R3 expression with aging.16
To determine the effects of HbF on Hct and total Hb, we used the larger Walk-PHaSST data set. A linear regression intrapatient analysis using Pearson correlation test (supplemental Table 4) of baseline Hct and total Hb vs HbF percentage for patients by CYB5R3 genotype showed different outcomes for HUneg and HUpos patients. For HUneg patients, Hct (Figure 1C-E) and total Hb (Figure 1F-H) vs HbF relationships were negative irrespective of the CYB5R3 genotype and counter to the reputed protective effect of HbF on Hct and Hb.17,18 Because patients with SC and S-beta+ typically exhibit mild baseline HbF levels, separate analyses were conducted with the exclusion of these patients. The resulting HUneg Hct/Hb vs HbF relationships became positive (supplemental Figure 3), suggesting a negative effect for patients with SC and S-beta+ on these relationships. For HUpos patients, the relationship between Hct (Figure 1C-E) and total Hb (Figure 1F-H) vs HbF was consistently positive. Importantly, stratification by the CYB5R3 genotype showed that the HbF percentage needed to elicit a positive Hct response in HUpos patients when compared with the HUneg cohort, progressively increased with the CYB5R3 T117S gene copy number by 1% to 4% (Figure 1C-E). A similar trend was noticed for total Hb when comparing the CG and GG groups vs CC (Figure 1F-H). This finding suggests that the more copies of the T117S allele carried by a patient with SCD, the less efficacious a given HbF percentage may be for preserving Hct and Hb levels.
A linear regression intrapatient analysis of HbF percentage vs age of Walk-PHaSST HUpos patients showed a negative effect for CYB5R3 T117S. For homozygous T117S, HbF percentage declined with increasing patient age (r = −0.2636; P = .2905). For WT CYB5R3, HbF percentage increased despite advancing age (r = 0.2767; P = .0041). For heterozygous T117S, the HbF percentage vs age relationship was intermediate (r = 0.0177; P = .8752). A comparison of the slopes for these disparate relationships revealed a statistically significant difference (P = .0365) (Figure 1I). Similar findings of a negative HbF percentage vs age relationship in Nigerians with SCD were reported by Olaniyi et al.19 Using a mouse model of accelerated aging, Ataei et al demonstrated the importance of a functional NO-sGC-cGMP pathway for resilience against nonatherosclerotic vascular aging. This is shown with Bay54-6544,20 which activates oxidized and apo-sGC, both of which can result from deficient CYB5R3 activity.13,21-23 CYB5R3 has also been shown to play a key role in aging and life span.16,24
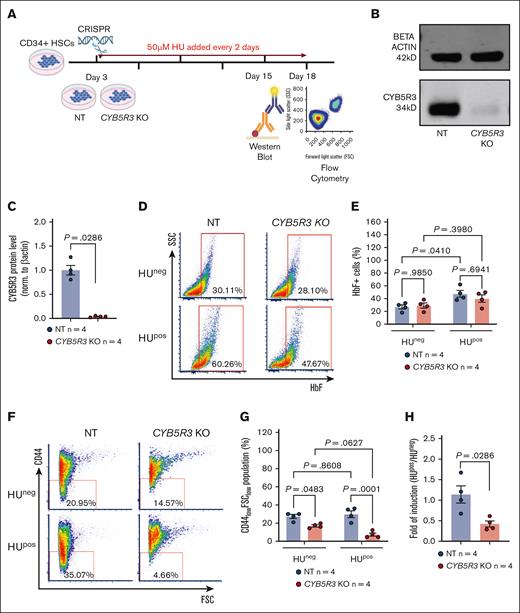
CD34+ HSCs were used to determine the effect of CYB5R3 on HU induction of F cells.25 CRISPR-created CYB5R3 nontargeting (NT) control and knockout (KO) CD34+ cells (Figure 2A) were compared, showing CYB5R3 protein depletion at day 15 of differentiation (P = .0286; Figure 2B-C) relative to NT cells. Flow cytometry was used to measure F cells and CD44lowFSClow populations corresponding to enucleated reticulocytes and mature erythrocytes26-28 on day 18 of differentiation. F-cell detection by flow cytometry requires at least 6 pg HbF per cell.29,30 CYB5R3 protein availability did not affect F cell levels in HUneg CD34+ cells (P = .9850; Figure 2D-E). However, in HUpos (50 μM) CD34+ cells, F cell induction was significant in NT cells (P = .0410), but not in KO cells (P = .3980; Figure 2D-E). This blunted F cell induction corresponded with fewer mature cells in HUposCYB5R3 KO than in NT cells (P = .0001; Figure 2F-G). Because HUneg KO and NT CD44lowFSClow cell populations were also different (P = .0483), normalization of HUpos measurements to their respective controls (HUpos/HUneg) was performed, revealing a significantly lower fold difference for KO (P = .0286) vs NT cells (Figure 2H). This suggests a potential role of CYB5R3 in HU-induced erythrocyte maturation.
In vitro data showing CYB5R3 KO in CD34+ HSCs lessens the F cell response to HU treatment. (A) Experimental design for CD34+ HSC study. (B-C) Western blot and quantification of CYB5R3 protein on day 15, analyzed by Mann-Whitney nonparametric test. (D-E) Representative flow plots and quantification showing the percentage of HbF+ cells on day 18 in the HUneg and HUpos groups, analyzed by 2-way ANOVA. (F-G) Representative flow plots and quantification showing the percentage of CD44lowFSClow populations on day 18 in the HUneg and HUpos groups, analyzed by 2-way ANOVA. (H) HUpos/HUneg ratio of the CD44lowFSClow population in NT control and KO cells, analyzed by Mann-Whitney nonparametric test. Data are expressed as mean ± standard error of the mean. A value of P < .05 was considered significant. SSC, side scatter; FSC, forward scatter; HUneg, HU untreated; HUpos, HU treated. Fluorochromes: HbF-APC, CD44-PE-Cy7.
In vitro data showing CYB5R3 KO in CD34+ HSCs lessens the F cell response to HU treatment. (A) Experimental design for CD34+ HSC study. (B-C) Western blot and quantification of CYB5R3 protein on day 15, analyzed by Mann-Whitney nonparametric test. (D-E) Representative flow plots and quantification showing the percentage of HbF+ cells on day 18 in the HUneg and HUpos groups, analyzed by 2-way ANOVA. (F-G) Representative flow plots and quantification showing the percentage of CD44lowFSClow populations on day 18 in the HUneg and HUpos groups, analyzed by 2-way ANOVA. (H) HUpos/HUneg ratio of the CD44lowFSClow population in NT control and KO cells, analyzed by Mann-Whitney nonparametric test. Data are expressed as mean ± standard error of the mean. A value of P < .05 was considered significant. SSC, side scatter; FSC, forward scatter; HUneg, HU untreated; HUpos, HU treated. Fluorochromes: HbF-APC, CD44-PE-Cy7.
Our novel findings indicate that the hypomorphic CYB5R3 T117S (1) tempers the HU-induced HbF response in patients with SCD and CD34+ HSCs, (2) attenuates the potency of HbF for protecting Hct and Hb, and (3) blunts HU-induced HbF with advancing age. The recognized role of NO-cGMP signaling in HU-induced HbF during stress erythropoiesis12 makes CYB5R3, through its regulation of the sGC redox state, a logical actor in HU-mediated HbF induction. It is noteworthy that deficient CYB5R3 activity, absent underlying oxidative pathology, does not intrinsically produce an obviously abnormal phenotype. Uncovering the molecular pathway by which CYB5R3 mediates these differential effects of HU on HbF induction and erythrocyte health, specifically under conditions of oxidative stress, such as SCD, requires additional studies. Indeed, the full extent of the effects of HU on erythrocyte preservation and underlying molecular activity remains incompletely understood.12,31 The effects of CYB5R3 on HU-induced HbF that we report here are based on a limited number of patients, are complex and vary with patient age and transfusion status. Quantitative trait loci (BCL11A, HMIP-2, and Xmn1-HBG2), which are well-recognized modifiers of gamma-globin induction, may underlie some of the CYB5R3 effects reported here.32 These reasons, albeit not exhaustive, are sufficient to warrant further studies into the mechanism of the effects of CYB5R3 on HbF induction by HU, which could lead to new insights and therapeutic approaches for SCD.
Acknowledgments: The authors thank the University of Pittsburgh and the Achievement Rewards for College Students Foundation for their continued support. The authors thank Sergei Nekhai for providing data from the Pulmonary Hypertension and the Hypoxic Response Study. M.T.G. is a coinventor of patents and patent applications directed at the use of recombinant neuroglobin and heme-based molecules as antidotes for carbon monoxide poisoning, which have been licensed by Globin Solutions, Inc. M.T.G. is a shareholder, advisor, and director in Globin Solutions, Inc. M.T.G. is also a coinventor of patents directed at the use of nitrite salts in cardiovascular diseases, which were previously licensed to United Therapeutics and now to Globin Solutions and Hope Pharmaceuticals. M.T.G. was the principal investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguat as a treatment for patients with sickle cell disease, which has now concluded. M.T.G. is a textbook author and receives royalties from MedMaster Inc, and a textbook editor with royalties from McGraw-Hill.
Financial support for this work was provided by National Institutes of Health grants R35 HL161177/R01 HL 153532 (A.C.S.) and R01 NS 129613 (A.C.S. and K.C.W.).
Contribution: F.A.C., A.C.S., and K.C.W. performed the experiments, analyzed and interpreted data, wrote the manuscript, and designed the research questions; M.S. and D.S. assisted with experiments; S.M.N. assisted with clinical data analysis and patient data collection; M.T.G. collected patient data; and all authors participated in manuscript review and edits.
Conflict-of-interest disclosure: A.C.S. received research funds from Bayer Pharmaceuticals and has an interest in Creegh Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Katherine C. Wood, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine; Heart, Lung, Blood and Vascular Medicine Institute, E1345 Biomedical Science Tower, 200 Lothrop St, Pittsburgh, PA 15216; email: katherine.wood@pitt.edu; and Adam C. Straub, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine; Heart, Lung, and Blood Vascular Medicine Institute, Center for Microvascular Research, E1345 Biomedical Science Tower, 200 Lothrop St, Pittsburgh, PA 15216; email: astraub@pitt.edu.
References
Author notes
Data are available on request from the corresponding authors, Katherine C. Wood (katherine.wood@pitt.edu) and Adam C. Straub (astraub@pitt.edu).
The full-text version of this article contains a data supplement.