Key Points
SDoH affects disease severity in children with SCD (aged 0-6 years) despite evidence-based therapy and health care facility support.
Understanding SDoH is crucial for customizing interventions for individuals with SCD despite the limited applicability of results.
Visual Abstract
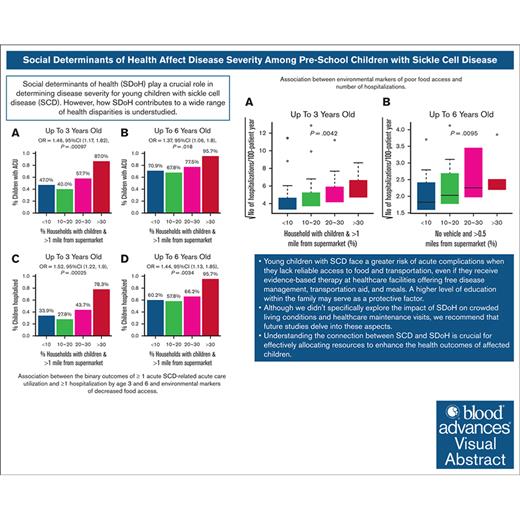
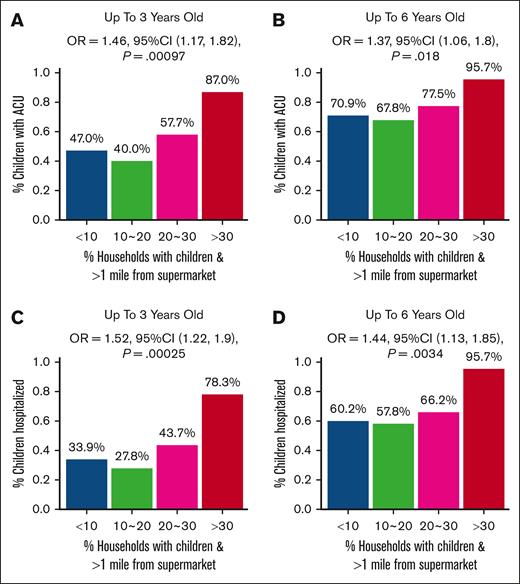
Individuals with sickle cell disease (SCD) face the burden of managing a lifelong chronic illness, increasing vulnerability to social determinants of health (SDoH). However, how SDoH contributes to health disparities is understudied. We hypothesized that preschool children with SCD living in poor neighborhoods with higher socio-economic distress would experience increased acute care utilization (ACU; described as emergency department visits plus hospitalizations) despite disease-modifying therapy. Participants' home addresses (aged 0-6 years) were mapped using census tract environmental data from the US Department of Agriculture Food Access Research Atlas. In multivariable analyses controlled for sickle genotype and disease-modifying therapies (hydroxyurea and chronic transfusion), SDoH indicators, that is, limited access to food, lack of vehicle, low income, and inadequate education, were associated with higher ACU. Living in households with children >1 mile from a supermarket was associated with more hospitalizations (odds ratio [OR], 1.44; 95% confidence interval [CI], 1.13-1.85) and ACU (OR, 1.37; 95% CI, 1.06-1.80) among children with SCD (aged <6 years). In households with at least 1 bachelor's degree, children with SCD experienced less ACU (OR, 0.67; 95% CI, 0.50-0.93) and hospitalizations (OR, 0.67; 95% CI, 0.49-0.92). Preschool children with SCD with limited access to food and transportation are at a higher risk of acute complications despite receiving free evidence-based therapy and social support. The family education level may have a protective effect. Although SDoH in crowded households and health care maintenance visits were not a focus of this study, future research should consider these factors. Understanding the SCD and SDoH association is crucial for directing resources to improve affected children's health.
Introduction
Social determinants of health (SDoH) are “the conditions in the environment where people are born, live, learn, work, play, worship, and age that affect a wide range of health functioning, quality-of-life outcomes, and risks.”1 The US Department of Health and Human Services identifies several socio-economic factors, such as household income, social cohesion, academic performance, access to health care, and discrimination, that disproportionately affect the health behaviors of individuals with chronic conditions such as sickle cell disease (SCD).1
SCD is a genetic blood disorder that causes multiple acute and chronic complications, such as vaso-occlusive events and progressive end-organ dysfunction, and requires lifelong clinical monitoring and treatment.2 In the United States, an estimated 100 000 individuals are affected by SCD, and about one-third are children.2 SCD mainly affects people of African descent and other ethnic minorities, who are more likely to experience socio-economic, environmental, racial, and nutritional hardships contributing to the complexity of managing this lifelong illness.3-7 Although there is an understanding that SDoH can have negative consequences on the health of young children with SCD,8-13 there has not been sufficient research to determine the extent to which environmental SDoH affects them, especially those children who receive comprehensive care with disease-modifying therapies. Further exploration is necessary to address whether preventive care can mitigate the impact of SDoH on young children.
Our study investigated how SDoH at the community level affected disease severity among children with SCD. We focused on SDoH essential to well-being in the patient's neighborhood,14 such as proximity to grocery stores, number of children in the household, vehicle ownership, neighborhood income, and education attainment. These factors were analyzed at the census tract level to determine how they might contribute to more frequent acute care utilization (ACU) in preschool children with SCD.
Methods
Study population and geographical setting
Participants included in the analysis were enrolled in the institutional review board–approved longitudinal clinical cohort study Sickle Cell Clinical Research and Intervention Program (SCCRIP)15 and were at least 3 years of age by the time of the data cutoff (31 December 2019). Hydroxyurea was provided as the standard of care at the maximum tolerated dose,16 and chronic transfusion therapy was provided to prevent primary and secondary strokes according to care guidelines.17
SCCRIP evaluates the health outcomes of patients with SCD throughout their life span to understand the disease progression and long-term effects of therapies and interpret the role of various genetic and environmental factors on health outcomes to improve their quality of life.15 All SCCRIP participants treated at St Jude Children's Research Hospital received free-of-charge care for SCD, including disease-modifying therapies (hydroxyurea and monthly erythrocyte transfusions whenever indicated) and support for transportation and meal services when they came for their appointments.18
The study participants were located in Memphis, 1 of the largest metropolitan areas in Tennessee, which has a high prevalence of food-insecure neighborhoods and poor public transportation systems in the United States.14,19 Memphis is located in Shelby County, which is predominantly urban, with some rural areas. However, several zip codes are considered food deserts. The US Census Bureau has identified 6 of 37 specific zip codes in Memphis that are particularly vulnerable to environmental hardships.20 A significant proportion of the SCCRIP study participants (43%) resided in these disadvantaged neighborhoods (supplemental Figure 1).
Data collection and study outcomes
Participants’ home addresses were geocoded and mapped at the census tract level to overlay environmental data provided by the United States Department of Agriculture Food Access Research Atlas 2017.21 This atlas serves as a data hub that provides various measures of SDoH, such as households below poverty level and poor food access, that can be used to study a wide range of health disparities and inequities among different populations.14 For this project, we obtained SDoH markers of low income and low access at the census tract level from the United States Department of Agriculture atlas, including distance to the nearest grocery store, household income, education, and transportation.
We used the SCCRIP database to gather information about participants’ demographics (age, sickle genotype, and sex), SCD treatment status (duration of hydroxyurea therapy and chronic transfusion), and health care use. We calculated the lifetime cumulative number of emergency department (ED) visits, hospitalizations, and ACU (ACU = ED visits + hospitalizations) resulting from SCD-related vaso-occlusive events such as acute chest syndrome, dactylitis, and vaso-occlusive crisis for each participant aged up to 3 and 6 years. Comprehensive acute care and SCD treatment data were available for patients aged 3 (100%) and 6 (88%) years. Because only 88% of the participants were at least 6 years old at the time of the analysis, we also analyzed their health use data and conducted a separate analysis of those aged ≤3 years (all with complete health use ascertainment) to strengthen the reliability of the findings.
Statistical analysis
Descriptive statistics, including mean and standard deviation for count and continuous variables and frequency for categorical variables, were reported by genotype and compared using the Wilcoxon rank-sum test for count and continuous variables with nonnormality of the data, and the Fisher exact test for categorical variables. Generalized linear regression models with quasi-Poisson and binomial link functions were used to investigate the association between environmental factors as continuous and categorical variables and count and binary outcomes, respectively. We checked the model overdispersion and zero-inflation for the count outcomes and calculated the variance inflation factor to check for multicollinearity.
To facilitate the interpretation of the environmental data, we divided the environmental variables into 4 groups based on the percentage of households affected by each determinant of interest within each census tract. We used a lower threshold of 10% because it was close to the mean values, and every 10% increase was used to create 3 additional categories: 20%, 30%, and >30%.22 To be precise, 10% was the lower threshold for the households per tract exposed to the SDoH factor. We then used 10% increments for the following 2 subsequent categories (20% and 30%). Because of the limited number of census tracts with households >40% with the SDoH factor of interest, we grouped the remaining census tracts to form a category of >30%. The acute care variables were examined independently, with a particular emphasis on ACU (ED visit + hospitalization) and cumulative hospitalizations. This approach enabled the identification of the factors that lead to overall acute outcomes, and those resulting in inpatient health use. As a result, it increased the granularity in the interpretations of findings. The analysis was adjusted to account for sickle genotype, hydroxyurea use, and chronic transfusions. The false discovery rate–adjusted P values, or q values, were calculated using the Benjamini and Hochberg procedure to account for multiple comparisons.
We further conducted a multivariate logistic regression analysis using the stepwise selection strategy to assess the environmental factors that met q value <0.1. All the analysis was performed on the entire cohort, comprising individuals who received or did not receive hydroxyurea therapy or chronic transfusions. This evaluated the odds of occurrence of any ED visit, hospitalization, and ACUs before ages 3 and 6 years because of clinical complications alone and for a combination of clinical and environmental variables. Model fits were assessed using pseudo-R2 values, likelihood ratio tests, and Akaike information criterion values. Finally, we generated the areas under the receiver operator characteristics curves to estimate whether environmental data can improve the accuracy of predicting ACU outcomes beyond demographics and medical factors alone.
Results
Participant characteristics
Of 501 children aged at least 3 years old, who lived in Tennessee and enrolled in SCCRIP, 435 had sufficient environmental data to be mapped to a census tract (Table 1). Whether included or excluded from the analyses, both groups were similar in every aspect, including demographics and clinical variables, except sickle genotype (supplemental Table 1).
Participant characteristics
| . | Sickle genotype . | P value . | ||
|---|---|---|---|---|
| All (N = 435) . | HbSS/SB0thalassemia (n = 259) . | HbSC/SB+thalassemia /other (n = 176) . | ||
| Demographics and health care use | ||||
| Sex (female) (%) | 225 (52) | 131 (51) | 94 (53) | .63 |
| Mean age, y (SD) | 5.7 (0.8) | 5.7 (0.8) | 5.8 (0.7) | .87 |
| Treatment with hydroxyurea (%) | 120 (28) | 109 (42) | 11 (6) | <.0001∗ |
| Mean duration of Hydroxyurea therapy, y (SD) | 0.7 (1.4) | 1.2 (1.6) | 0.1 (0.6) | <.0001∗ |
| Treatment with chronic transfusion (%) | 39 (9) | 39 (15) | 0 (0) | <.0001∗ |
| Mean no. of all acute care events (hospitalizations and ED visits) per patient-y (SD)† | 0.51 (0.66) | 0.61 (0.66) | 0.36 (0.62) | <.0001∗ |
| Mean no. of ED visits per patient-y (SD)† | 0.24 (0.37) | 0.26 (0.35) | 0.20 (0.41) | .001∗ |
| Mean number of hospitalizations per patient-y (SD)† | 0.30 (0.44) | 0.38 (0.49) | 0.18 (0.34) | <.0001 |
| No treatment (%) | 299 (69) | 134 (52) | 165 (94) | <.0001 |
| Treatment with chronic transfusion only (%) | 16 (4) | 16 (6) | 0 (0) | _ |
| Treatment with hydroxyurea only (%) | 97 (22) | 86 (33) | 11 (6) | _ |
| Treatment with both hydroxyurea and chronic transfusion | 23 (5) | 23 (9) | 0 (0) | _ |
| . | Sickle genotype . | P value . | ||
|---|---|---|---|---|
| All (N = 435) . | HbSS/SB0thalassemia (n = 259) . | HbSC/SB+thalassemia /other (n = 176) . | ||
| Demographics and health care use | ||||
| Sex (female) (%) | 225 (52) | 131 (51) | 94 (53) | .63 |
| Mean age, y (SD) | 5.7 (0.8) | 5.7 (0.8) | 5.8 (0.7) | .87 |
| Treatment with hydroxyurea (%) | 120 (28) | 109 (42) | 11 (6) | <.0001∗ |
| Mean duration of Hydroxyurea therapy, y (SD) | 0.7 (1.4) | 1.2 (1.6) | 0.1 (0.6) | <.0001∗ |
| Treatment with chronic transfusion (%) | 39 (9) | 39 (15) | 0 (0) | <.0001∗ |
| Mean no. of all acute care events (hospitalizations and ED visits) per patient-y (SD)† | 0.51 (0.66) | 0.61 (0.66) | 0.36 (0.62) | <.0001∗ |
| Mean no. of ED visits per patient-y (SD)† | 0.24 (0.37) | 0.26 (0.35) | 0.20 (0.41) | .001∗ |
| Mean number of hospitalizations per patient-y (SD)† | 0.30 (0.44) | 0.38 (0.49) | 0.18 (0.34) | <.0001 |
| No treatment (%) | 299 (69) | 134 (52) | 165 (94) | <.0001 |
| Treatment with chronic transfusion only (%) | 16 (4) | 16 (6) | 0 (0) | _ |
| Treatment with hydroxyurea only (%) | 97 (22) | 86 (33) | 11 (6) | _ |
| Treatment with both hydroxyurea and chronic transfusion | 23 (5) | 23 (9) | 0 (0) | _ |
Participants were aged 1 to 6 years. P value was calculated using Fisher exact test or χ2 test for the categorical variables, and Wilcoxon rank-sum or t test for the continuous variables, respectively.
SD, standard deviation.
False discovery rate–adjusted P values (<.05) or q value (<0.05).
Up to 6 years old.
All participants were African American, and their median age at the last follow-up was 5.7 years (range, 3-6). Approximately half (52%) of the participants were girls. Most participants had the severe SCD genotype (hemoglobin SS [HbSS]/HbSβ0thalassemia, 60%), 28% received treatment with hydroxyurea, and 39% received monthly erythrocyte transfusions. The mean number of ACU events was 0.51 per patient-year. Children with HbSS/HbSβ0thalassemia had higher ACUs, hydroxyurea use, and chronic transfusion use than those with other types of SCD.
Community level characteristics per census tract
The unemployment rate in the participants’ neighborhoods was ∼14.6%. Nearly 29% of households were below the federal poverty threshold, and ∼30.8% received food and nutrition services. Overall, 45.3% of households did not have a vehicle, and only 11.7% of adults in the area held a bachelor's degree.
Although genotype was found to be associated with ACU, it was not considered a confounding factor. This is because there were no variations in environmental factors based on genotypes among the participants in our cohort (Table 2). Because participants with HbSS/HbSβ0 thalassemia were not clustered in neighborhoods with higher poverty than those with HbSC, we combined participants with different genotypes in the primary analysis. Additionally, we conducted a subanalysis that only included participants with HbSS/HbSβ0thalassemia (supplemental Figures 2-3). The conclusions were similar to those obtained for all participants.
Neighborhood characteristics as per census tract
| . | Sickle genotype . | P value . | ||
|---|---|---|---|---|
| All (N = 435) . | HbSS/SB0thalassemia (n = 259) . | HbSC/SB+thalassemia/other (n = 176) . | ||
| General characteristics of a census tract | ||||
| Mean % of population below poverty rate (SD)∗ | 29.04 (14.15) | 29 (13.56) | 29.09 (14.99) | .97 |
| Mean % of unemployed population (SD)∗ | 14.61 (7.04) | 14.36 (6.77) | 14.96 (7.4) | .39 |
| Mean median household income ($) (SD)∗ | 37 418 (19 393) | 36 648 (17 697) | 38 494 (21 559) | .88 |
| Mean % received food and nutrition services (SD)∗ | 30.83 (14.29) | 31.25 (13.64) | 30.24 (15.17) | .59 |
| Distance to the nearest supermarket (miles)∗ | 2.84 (1.53) | 2.85 (1.54) | 2.81 (1.53) | .57 |
| Total housing units without a vehicle (SD) | 196.98 (160.22) | 206.1 (161.13) | 183.56 (158.36) | .12 |
| Educational attainment | ||||
| Mean % of individuals with a bachelor’s degree† | 11.72 (7.94) | 11.13 (7.37) | 12.56 (8.64) | .16 |
| Individuals with bachelor’s degree, n (%) | .63 | |||
| <10% in a census tract | 174 (49) | 106 (52) | 68 (46) | |
| ≥10%-20% | 136 (39) | 79 (38) | 57 (40) | |
| ≥20%-30% | 22 (6) | 11 (5) | 11 (7) | |
| ≥30% | 22 (6) | 11 (5) | 11 (7) | |
| Transportation and food access | ||||
| Mean % of households without a vehicle and living >0.5 miles from a supermarket | 0.09 (0.08) | 0.09 (0.08) | 0.08 (0.09) | .24 |
| % of households without a vehicle and living >0.5 miles from a supermarket, n (%) | .27 | |||
| <10% in a census tract | 280 (65) | 160 (62) | 120 (68) | |
| ≥10%-20% | 123 (28) | 82 (32) | 41 (23) | |
| ≥20%-30% | 19 (4) | 10 (4) | 9 (5) | |
| ≥30% | 13 (3) | 7 (2) | 6 (4) | |
| Mean % of households with children living >1.0 miles from a supermarket | 0.1 (0.11) | 0.1 (0.11) | 0.09 (0.1) | .51 |
| % of households with children living >1.0 miles from a supermarket, n (%) | .37 | |||
| <10% in a census tract | 251 (58) | 147 (57) | 104 (59) | |
| ≥10%-20% | 90 (21) | 52 (20) | 38 (22) | |
| ≥20%-30% | 71 (16) | 44 (17) | 27 (15) | |
| ≥30% | 23 (5) | 16 (6) | 7 (4) | |
| Health and safety | ||||
| Mean distance to the nearest hospital (miles) (SD)∗ | 2.86 (1.4) | 2.76 (1.39) | 3.01 (1.41) | .07 |
| . | Sickle genotype . | P value . | ||
|---|---|---|---|---|
| All (N = 435) . | HbSS/SB0thalassemia (n = 259) . | HbSC/SB+thalassemia/other (n = 176) . | ||
| General characteristics of a census tract | ||||
| Mean % of population below poverty rate (SD)∗ | 29.04 (14.15) | 29 (13.56) | 29.09 (14.99) | .97 |
| Mean % of unemployed population (SD)∗ | 14.61 (7.04) | 14.36 (6.77) | 14.96 (7.4) | .39 |
| Mean median household income ($) (SD)∗ | 37 418 (19 393) | 36 648 (17 697) | 38 494 (21 559) | .88 |
| Mean % received food and nutrition services (SD)∗ | 30.83 (14.29) | 31.25 (13.64) | 30.24 (15.17) | .59 |
| Distance to the nearest supermarket (miles)∗ | 2.84 (1.53) | 2.85 (1.54) | 2.81 (1.53) | .57 |
| Total housing units without a vehicle (SD) | 196.98 (160.22) | 206.1 (161.13) | 183.56 (158.36) | .12 |
| Educational attainment | ||||
| Mean % of individuals with a bachelor’s degree† | 11.72 (7.94) | 11.13 (7.37) | 12.56 (8.64) | .16 |
| Individuals with bachelor’s degree, n (%) | .63 | |||
| <10% in a census tract | 174 (49) | 106 (52) | 68 (46) | |
| ≥10%-20% | 136 (39) | 79 (38) | 57 (40) | |
| ≥20%-30% | 22 (6) | 11 (5) | 11 (7) | |
| ≥30% | 22 (6) | 11 (5) | 11 (7) | |
| Transportation and food access | ||||
| Mean % of households without a vehicle and living >0.5 miles from a supermarket | 0.09 (0.08) | 0.09 (0.08) | 0.08 (0.09) | .24 |
| % of households without a vehicle and living >0.5 miles from a supermarket, n (%) | .27 | |||
| <10% in a census tract | 280 (65) | 160 (62) | 120 (68) | |
| ≥10%-20% | 123 (28) | 82 (32) | 41 (23) | |
| ≥20%-30% | 19 (4) | 10 (4) | 9 (5) | |
| ≥30% | 13 (3) | 7 (2) | 6 (4) | |
| Mean % of households with children living >1.0 miles from a supermarket | 0.1 (0.11) | 0.1 (0.11) | 0.09 (0.1) | .51 |
| % of households with children living >1.0 miles from a supermarket, n (%) | .37 | |||
| <10% in a census tract | 251 (58) | 147 (57) | 104 (59) | |
| ≥10%-20% | 90 (21) | 52 (20) | 38 (22) | |
| ≥20%-30% | 71 (16) | 44 (17) | 27 (15) | |
| ≥30% | 23 (5) | 16 (6) | 7 (4) | |
| Health and safety | ||||
| Mean distance to the nearest hospital (miles) (SD)∗ | 2.86 (1.4) | 2.76 (1.39) | 3.01 (1.41) | .07 |
Data were available for 355 individuals.
Data were available for 354 individuals. P value was calculated using Fisher exact or χ2 test for the categorical variables, and Wilcoxon rank-sum or t test for the continuous variables, respectively.
Association between clinical and environmental variables with the occurrence of hospitalization and ACU in patients with SCD from multivariate analyses
| Aged up to 3 y . | Aged up to 6 y . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model description | Comparison model | LRT P | RN2 | AIC | AUC | Model description | Comparison model | LRT P | RN2 | AIC | AUC |
| ACU = ED visits + hospitalizations | ACU = ED visits + hospitalizations | ||||||||||
| Base model | NA | NA | 0.10 | 547 | 0.70 | Base model | NA | NA | 0.11 | 350 | 0.70 |
| Base model + E∗ | vs base model | 8 × 10–4 | 0.12 | 538 | 0.72 | Base model + E† | vs base model | .004 | 0.14 | 343 | 0.74 |
| Hospitalizations | Hospitalizations | ||||||||||
| Base model | NA | NA | 0.09 | 526 | 0.68 | Base model | NA | NA | 0.13 | 373 | 0.72 |
| Base model + E∗ | vs base model | 2 × 10–4 | 0.12 | 514 | 0.72 | Base model + E‡ | vs base model | 4 × 10–4 | 0.17 | 357 | 0.76 |
| Aged up to 3 y . | Aged up to 6 y . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model description | Comparison model | LRT P | RN2 | AIC | AUC | Model description | Comparison model | LRT P | RN2 | AIC | AUC |
| ACU = ED visits + hospitalizations | ACU = ED visits + hospitalizations | ||||||||||
| Base model | NA | NA | 0.10 | 547 | 0.70 | Base model | NA | NA | 0.11 | 350 | 0.70 |
| Base model + E∗ | vs base model | 8 × 10–4 | 0.12 | 538 | 0.72 | Base model + E† | vs base model | .004 | 0.14 | 343 | 0.74 |
| Hospitalizations | Hospitalizations | ||||||||||
| Base model | NA | NA | 0.09 | 526 | 0.68 | Base model | NA | NA | 0.13 | 373 | 0.72 |
| Base model + E∗ | vs base model | 2 × 10–4 | 0.12 | 514 | 0.72 | Base model + E‡ | vs base model | 4 × 10–4 | 0.17 | 357 | 0.76 |
AIC, Akaike information criterion value; AUC, areas under the receiver operator characteristics curves; base model, hydroxyurea treatment + chronic transfusion + genotype; E, environmental factors; LRT P, P value calculated using likelihood ratio test; NA, not applicable; RN2, pseudo-R2 (RN2) values calculated based on the logistic regression model.
E = % of households with children living >1 mile from a supermarket.
E = % of households with children living >1 mile from a supermarket + % of individuals with a bachelor’s degree.
E = % of households with children living >1 mile from a supermarket + % of individuals with a bachelor’s degree.
Neighborhood Level SDoH markers of low income and low access increase the prediction of ACUs in young children with SCD
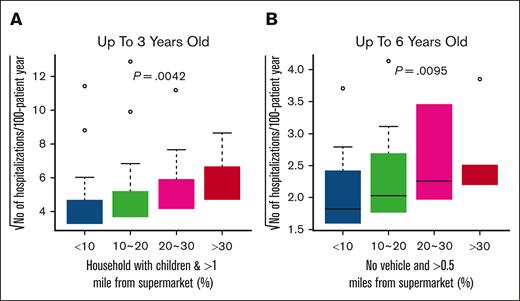
After adjusting for sickle cell genotype, hydroxyurea therapy, and chronic transfusion, we found that a significant number of households with children living >1 mile away from a supermarket were associated with increased hospitalizations by age 3 years at a significance level of q value <0.05 (Figure 1A; supplemental Table 2). Individuals living more than half a mile from a supermarket without vehicle access had increased hospitalizations by age 6 years at q value <0.1 (Figure 1B). With every 10% increase (<10%, ≥10%-20%, ≥20%-30%, and ≥30%) in the percentage of households with children living at least 1 mile away from a supermarket, the odds of occurrence of ACU (Figure 2A-B; supplemental Table 3) and hospitalizations (Figure 2C-D; supplemental Table 3) increased by 1.46 (95% confidence interval [CI], 1.17-1.82; P = .00097) and 1.52 (95% CI, 1.22-1.90; P = .00025), respectively, before 3 years; and 1.37 (95% CI, 1.06-1.80; P = .018) and 1.44 (95% CI, 1.13-1.85; P = .0034), respectively, before 6 years. The association results between continuous SDoH and categorized SDoH variables were consistent (supplemental Tables 2-3).
Association between environmental markers of poor food access and number of hospitalizations. The x-axis is the percentage of households with children who live >1 mile from a supermarket (A) and the number of housing units without vehicles located >0.5 miles from a supermarket (B). The analysis was subdivided based on 3 cutoff points: 10%, 20%, and 30%. The y-axis is the square root of the estimated number of hospitalizations up to 3 years (A) and 6 years (B) per 100-patient-year. P values were calculated using the generalized linear model (GLM), with adjustment for hydroxyurea exposure, chronic transfusions, and SCD genotype, by analyzing the categorized environmental variable as a dose effect of 0, 1, 2, and 3.
Association between environmental markers of poor food access and number of hospitalizations. The x-axis is the percentage of households with children who live >1 mile from a supermarket (A) and the number of housing units without vehicles located >0.5 miles from a supermarket (B). The analysis was subdivided based on 3 cutoff points: 10%, 20%, and 30%. The y-axis is the square root of the estimated number of hospitalizations up to 3 years (A) and 6 years (B) per 100-patient-year. P values were calculated using the generalized linear model (GLM), with adjustment for hydroxyurea exposure, chronic transfusions, and SCD genotype, by analyzing the categorized environmental variable as a dose effect of 0, 1, 2, and 3.
Association between the binary outcomes of ≥1 acute SCD–related ACU and greater hospitalization by age 3 and 6 years and environmental markers of decreased food access. The x-axis represents the percentage of households in the census tract that had children and were located beyond 1 mile from a supermarket, divided based on the cutoff point of 10%, 20%, and 30% of households in the census tract with these attributions. The y-axis is the proportion of patients with ACUs by age 3 years (A) and 6 years (B) and with hospitalizations by age 3 years (C) and by age 6 years (D). P values, ORs, and 95% CIs were calculated using a GLM, with adjustment for hydroxyurea exposure, chronic transfusion, and sickle genotype by analyzing the categorized environmental variable as a dose effect of 0, 1, 2, and 3.
Association between the binary outcomes of ≥1 acute SCD–related ACU and greater hospitalization by age 3 and 6 years and environmental markers of decreased food access. The x-axis represents the percentage of households in the census tract that had children and were located beyond 1 mile from a supermarket, divided based on the cutoff point of 10%, 20%, and 30% of households in the census tract with these attributions. The y-axis is the proportion of patients with ACUs by age 3 years (A) and 6 years (B) and with hospitalizations by age 3 years (C) and by age 6 years (D). P values, ORs, and 95% CIs were calculated using a GLM, with adjustment for hydroxyurea exposure, chronic transfusion, and sickle genotype by analyzing the categorized environmental variable as a dose effect of 0, 1, 2, and 3.
ACU and hospitalizations before age 6 years were less likely in neighborhoods with a higher percentage of adults holding a bachelor’s degree (q < 0.09) (supplemental Figure 4; supplemental Table 3). With every 10% increase in the proportion of individuals with bachelor’s degrees in the census tract (<10%, ≥10%-20%, ≥20%-30%, and ≥30%), the odds of ACU and hospitalizations before age 6 years decreased to 0.67 (95% CI, 0.50-0.93; P = .012) and 0.67 (95% CI, 0.49-0.92; P = .01), respectively. The association results between continuous SDoH and categorized SDoH variables were consistent (supplemental Tables 2-3).
In a multivariable analysis, after adjusting for sickle genotype, hydroxyurea therapy, and chronic transfusion, households with children living >1 mile away from a supermarket (odds ratio [OR], 1.38; 95% CI, 1.01-1.88; P = .04), and households with a bachelor’s degree (OR, 0.64; 95% CI, 0.46-0.87; P = .005), remained independent predictors of ACUs before age 6 years. Additionally, households without a vehicle and located >1 mile away from a supermarket (OR, 1.52; 95% CI, 1.14-2.02; P = .004) and households with a bachelor’s degree in the census tract (OR, 0.62; 95% CI, 0.46-0.85; P = .003) were also independent predictors of hospitalizations before the age of 6 years, along with sickle genotype, hydroxyurea therapy, and chronic transfusions.
Receiver operator characteristics curve for environmental data in predicting ACUs in those aged <6 years
We built 2 predictive models, using ACU and hospitalization data of children with SCD aged <6 years, that consisted of (1) clinical factors only (the base model = hydroxyurea treatment + chronic transfusion + sickle genotype) and (2) both clinical and environmental factors (the base model + environmental factors; Table 3). When the 2 models were compared, the pseudo-R2 score improved on average by 0.03, indicating a moderate enhancement in predicting the rates of ACU and hospitalizations after incorporating environmental factors into the base model. However, based on the likelihood ratio test conducted on both models (maximum P ≤ .004) and a decrease in the Akaike information criterion value for each comparison, it was evident that incorporating more environmental variables into clinical factors significantly enhanced the model fit, increasing the sensitivity of the model in predicting ACU.
Discussion
SDoH can have varying effects on the health outcomes of patients with SCD; however, the specific factors that influence these outcomes and modify disease severity are poorly understood. We examined how SDoH markers of low income and low access at the community level, such as the number of children in a household, proximity to the grocery stores, inadequate education, and vehicle availability, can contribute to significant variations in SCD-related ACU in a cohort of young children with SCD, after adjusting for the genotype and use of disease-modifying therapies.
The results suggested that children aged <6 years who lived in households with no transportation and poor food access were more likely to experience frequent hospitalizations than children in households with better access to these facilities, even with exposure to disease-modifying therapies. Despite free SCD treatment, such as hydroxyurea therapy and chronic transfusion, and social support services including free disease management, meals, and transportation,18 SDoH continued to have a significant impact on the patients’ health. In contrast, preschool children with SCD who had at least 1 family member with a bachelor’s degree experienced fewer hospitalizations and ACU, suggesting that the family’s educational level could play a protective role in determining the health outcomes for children with SCD.
Recent studies conducted in the United States, Brazil, and Nigeria have shown that SDoH at the patient and family level have a significant impact on disadvantaged families of children with SCD and increase their chances of experiencing more acute complications.8-13,23 However, other factors, such as the area of residence, transportation, food access, and education, have been understudied.24,25 Our research builds upon these earlier findings by demonstrating that specific SDoH indicators in the patients’ community, such as distance to the nearest supermarket, limited income, restricted access to a vehicle, and having children in the household, increase the likelihood of frequent hospitalizations among young children with SCD. Although the study participants lived in suburban areas of Memphis, these areas were identified as food deserts and lacked essential resources such as full-scale grocery stores, public transportation, and proper educational facilities. This meant that they had to travel longer distances to access these resources, which influenced their health outcomes. This highlights that a person’s area of residence could play a role in determining their health outcomes. There is an urgent need for a deeper understanding of all levels of SDoH that can influence health outcomes beyond the patient’s nucleus (patient and family) to maximize the health benefits for this vulnerable population.
The Centers for Disease Control and Prevention reports that 60% of births with SCD in the United States occur in regions in which the average household income falls below the national average.26 Financial constraints in these areas may compel families with SCD to settle for substandard housing in neighborhoods with limited opportunities.10 Moreover, they may rely on low-quality childcare settings, exposing their children to high social, clinical, and nutritional vulnerabilities.11,27-30 As a result, children may face stress, malnutrition, and infections that contribute to an inferior quality of life.11,27-29 These challenges become even more complex in adulthood,23 as managing chronic illnesses while sustaining a stable job and supporting family members becomes increasingly challenging.3 Therefore, it is crucial for researchers to prioritize studying the long-term effects of SDoH in patients with SCD. This will provide a comprehensive understanding of how these pathways interact with SCD outcomes, leading to the development and advocacy of multilevel interventions based on informed evidence.
The current health care system and innovation in research emphasize the development of novel therapies, such as genomic cures, but often overlook the importance of thoroughly comprehending and resolving fundamental issues in a patient’s environment that may compound or worsen health complications.31 Our research revealed that despite access to standardized medical care and assistance with transportation and meals,18 patients’ morbidity persisted when returning to an environment with multiple stressors, such as poverty and food insecurity, that may have initially led to hospitalization. Under these circumstances, the benefits of evidence-based treatment and supportive care were not fully realized. It is crucial to recognize that a patient is influenced by both their medical condition and the environment, which are interconnected,24,31 and that addressing environmental factors is essential to improving health outcomes.
Considering our findings and guidelines provided by professional organizations,32,33 health care providers must adopt a multifaceted approach to address the complex issue of SDoH. This requires ongoing awareness and assessment of SDoH through the development of interventions such as universal SDoH screening27 and accompanying social referral programs to understand illness from a global patient perspective. Clinical trials that reflect cultural diversity and collect SDoH data can also help measure the efficacy of different therapies across populations, improving health care outcomes. Collaboration between medical institutions globally can improve research infrastructure and knowledge sharing, leading to a better understanding of the disease and improved patient outcomes.
Improving the overall environment requires a comprehensive and structured approach through collaboration with community-based organizations encompassing various social interventions. These include improved public transportation, eliminating food deserts, providing better educational options, and creating higher-income-generating opportunities. Understanding how their conditions intersect with potential risk factors, such as poverty, infectious disease, and environmental triggers, is paramount to effectively delivering the best possible care to children with SCD.34
Limitations
Our research was specific to young children with SCD from birth to age 6 years who received care at a unique facility that provides free disease-modifying treatments, assistance with transportation (acute and nonacute visits) and meals, to demonstrate how SDoH can continue to affect health outcomes despite the level of care and social support provided during medical visits. The findings might not accurately reflect the experiences of older individuals with SCD, those who relocated to areas with better access to resources, such as full-service grocery stores, or those receiving care at facilities that do not offer similar social support. This could potentially limit the generalizability of the results. Because the environmental data we used were cross-sectional, we were unable to determine how the length of residence in the neighborhood might have affected acute care outcomes. In our analysis, we accounted for chronic transfusion, which could have resulted from inadequate health care access influenced by SDoH. This may have reduced some of our observations linking SDoH to health outcomes in the analysis. Furthermore, insufficient information on hydroxyurea adherence may have contributed to the results. However, it is important to note that medication adherence for children aged <6 years is generally above average in our population.16 Although in our study population, distance to the nearest hospital was not associated with ACU, in other populations, particularly those living in rural areas, it can be a surrogate for distance to a supermarket because of variations in demographics and geographical location and should be considered an SDoH influencing health outcomes. Investigation of the effect of living in crowded households and the impact of SDoH on health maintenance visits were not the focus of this analysis and should be considered in future studies. Lack of access to specialized health maintenance SCD care because of lack of providers or health facilities is an important SDoH that should also be studied.
Conclusion
Guideline-concordant standard-of-care therapies may not be enough to overcome the adverse health outcomes associated with environmental SDoH barriers in preschool children with SCD.31 To comprehensively enhance the well-being of patients with SCD, it is imperative to prioritize evidence-based care and investigate interventions that directly target SDoH. Health care providers can significantly affect the health outcomes of patients with SCD by using innovative, patient-centered approaches that account for the role of SDoH. SDoH can negatively affect a patient’s health; however, by incorporating SDoH data into clinical trial design and regular care assessments in the clinic,35 providers and researchers can better understand how these factors affect health outcomes. This knowledge enables them to tailor interventions based on each patient’s unique needs. By adopting these evidence-based approaches, health care providers and researchers can improve health equity and better address the needs of diverse patient populations, which is a crucial step toward achieving optimal health outcomes for all.
Acknowledgment
Agios Pharmaceuticals had no role in the study's design, analysis, or interpretation of the results or manuscript drafting.
Authorship
Contribution: H.K. drafted the initial concept, guided the methodology development process, drafted and finalized the manuscript, and responded to the queries during revision; G.K. conducted the statistical analyses, provided guidance on writing the methods and results sections, reviewed the manuscript, and provided feedback during the review process; J.S.P., J.D., W.C.W., J.H.E., J.G.G., and R.D. contributed to guiding the methodology, reviewed the manuscript, and provided feedback during the review process; and J.R.H. and J.S.H offered guidance on the concept and methodology, reviewed the manuscript, and provided feedback during the review process.
Conflict-of-interest disclosure: J.H.E. received research funding support from Global Blood Therapeutics, Forma Therapeutics, Pfizer, and Eli Lilly and Co; provided consultancy to Daiichi Sankyo, Esperion, and Global Blood Therapeutics; and received funding from the American Society of Hematology and the National Heart, Lung, and Blood Institute. After the completion of the activities in this manuscript, J.H.E. changed employers to Agios Pharmaceuticals and receives royalties from UpToDate. The remaining authors declare no competing financial interests.
Correspondence: Jason R. Hodges, Department of Hematology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; email: jason.hodges@stjude.org.
References
Author notes
J.S.H. and J.R.H are joint senior authors.
Data are available on request from the corresponding author, Jason R. Hodges (jason.hodges@stjude.org).
The full-text version of this article contains a data supplement.