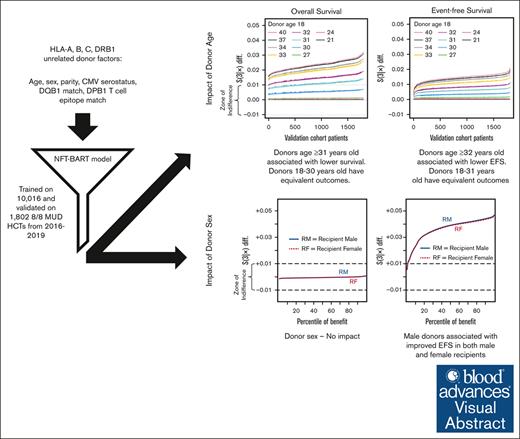
Key Points
Donors aged 18 to 30 years yield equivalent OS after matched unrelated donor transplantation based on a novel machine learning approach.
Male donors are associated with improved EFS, but donor sex does not associate with improved OS.
Visual Abstract
We investigated the impact of donor characteristics on outcomes in allogeneic hematopoietic cell transplantation (HCT) recipients using a novel machine learning approach, the Nonparametric Failure Time Bayesian Additive Regression Trees (NFT BART). NFT BART models were trained on data from 10 016 patients who underwent a first HLA-A, B, C, and DRB1 matched unrelated donor (MUD) HCT between 2016 and 2019, reported to the Center for International Blood and Marrow Transplant Research, then validated on an independent cohort of 1802 patients. The NFT BART models were adjusted based on recipient, disease, and transplant variables. We defined a clinically meaningful impact on overall survival (OS) or event-free survival (EFS; survival without relapse, graft failure, or moderate to severe chronic graft-versus-host disease) as >1% difference in predicted outcome at 3 years. Characteristics with <1% impact (within a zone of indifference) were not considered to be clinically relevant. Donor cytomegalovirus, parity, HLA-DQB1, and HLA-DPB1 T-cell epitope matching fell within the zone of indifference. The only significant donor factor that associated with OS was age, in which, compared with 18-year-old donors, donors aged ≥31 years old were associated with lower OS. Both donor age (≤32 years) and use of a male donor, regardless of recipient sex, improved EFS. We, therefore, recommend selecting the earliest available donor within the 18 to 30 years age range for HCT to optimize OS. If several donors in the 18 to 30 years age range are available, a male donor may be chosen to optimize EFS.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) represents a potentially curative treatment for numerous hematologic malignancies and blood disorders. Historically, the optimal donor is a fully HLA matched sibling donor, available for ≤30% of patients in need of HCT.1 In the absence of a matched sibling donor, alternative donor sources including mismatched related, matched and mismatched unrelated, and cord blood may be suitable. The importance of HLA matching in unrelated donor HCT was previously established, demonstrating that the optimal outcomes are achieved with a donor matched at HLA-A, B, C, and DRB1 (or 8/8 matched).2,3 The addition of HLA-DPB1 T-cell epitope permissive mismatching was shown to further improve outcomes in the unrelated donor setting.4,5 When multiple equivalently matched donors are available, prioritization based on non-HLA criteria have focused on younger donor age, cytomegalovirus (CMV) matching, sex matching, and donor parity, among other factors.6 A large historical (years 1999-2014) analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) demonstrated a linear relationship between donor age and OS, noting a 2% increase in mortality at 2 years after HCT for each 5-year increase in donor age.7 Recent research from our team confirmed the importance of donor age and lack of impact of other donor-related factors using a machine learning–based model on overall survival (OS); however, these findings were also based on older transplants (years 1999-2014), broad donor age categories, and limited follow-up (180 days).8
These findings on the impact of donor age have influenced donor selection and recruitment practices worldwide. The median age of donors supplied through the NMDP has decreased from 35 to 27 years from 2004 to 2023 (Martin Maiers, personal communication). Many registries have also reduced the upper age limit for recruitment to ≤40 years and the lower age limit as permitted under local age of majority restrictions (eg, 16 years in the United Kingdom vs 18 years in the United States). This clear demonstration of evidence into practice is both encouraging and has likely contributed to the improvements in outcomes over time. However, questions remain, including understanding the magnitude of effect with more granular differences in donor age and the impact of other donor factors on longer-term OS or other important outcomes such as event-free survival (EFS).
To address this gap in knowledge, we developed a novel statistical approach to further refine the understanding of the impact of various donor characteristics on outcomes after matched unrelated donor (MUD) HCT. The Nonparametric Failure Time Bayesian Additive Regression Trees (NFT BART), an advanced machine learning survival methodology, enables a more granular analysis of patient-specific outcomes and assessment of the impact of individual covariates across a large cohort.9 This study aims to enhance the precision of donor selection by using NFT BART to analyze a contemporary data set from the CIBMTR. By considering both OS and EFS and the impact of various donor attributes, we sought to establish a more robust framework for donor selection through consideration of clinically meaningful impacts on outcomes.
Methods
We selected patients receiving a first allogeneic HCT with an HLA-A, B, C, and DRB1 high-resolution matched (8/8) MUD from 2016 to 2019, reported to the CIBMTR. Donor characteristics included age, sex, parity, CMV status, HLA-DQB1 matching, and HLA-DPB1 T-cell epitope permissive mismatch.10 All patients provided informed consent for participation in the CIBMTR research database, and the study was approved by the NMDP Institutional Review Board.
NFT BART is a flexible machine learning method capable of handling time-to-event data without restrictive assumptions, such as proportionality, linearity, or homoskedasticity, and can automatically account for complex interactions.9 OS and EFS models were adjusted for patient, disease, and transplant factors to allow for the evaluation of the donor factors outlined above. Patient factors considered included age, race/ethnicity, sex, Karnofsky (adult)/Lansky (pediatric) performance score, HCT-comorbidity index, CMV serostatus, ventilation history, and history of fungal infection. Disease factors considered included disease, disease status, acute lymphoblastic leukemia (ALL)/acute myeloid leukemia (AML) cycles to achieve complete remission, Philadelphia chromosome status, ALL/AML/multiple myeloma cytogenetic risk score, ALL immunotype, AML progression from myelodysplastic syndrome (MDS), therapy-related AML or MDS and MDS predisposition, non-Hodgkin lymphoma subtype, and multiple myeloma International Staging System (ISS) type. Transplant factors considered included graft type, conditioning regimen intensity, prior autologous HCT, graft-versus-host disease (GVHD) prophylaxis, time from diagnosis to HCT, and year of HCT.11 Due to its Bayesian nonparametric underpinnings, NFT BART produces estimates for survival distribution, while naturally providing uncertainty of said estimates. The marginal effect of any given covariate can be summarized by Friedman partial dependence technique.12
A clinically meaningful impact on OS or EFS (survival without relapse, graft failure, or moderate to severe chronic GVHD) was defined as donor factors associated with >1% difference in predicted outcome at 3 years (corresponding to ∼10 days difference in restricted mean survival time within 3 years).13 Factors falling below this threshold were considered within a zone of indifference, given the negligible clinical impact, and set aside from further consideration.13
Results
The study population consisted of 11 818 patients. Population characteristics are provided in Table 1. The median patient age was 57 years (range, <1-82), whereas the median donor age was 27 years (range, 18-60). The majority of the patient population was non-Hispanic White (92%), male (58%), received transplant for an acute leukemia (ALL 13.0% and AML 39.8%) or MDS (19.9%), and mostly in early-stage disease (59.8%). Approximately half the population received myeloablative conditioning (49.6%), and the remainder received reduced-intensity (39.1%) or nonmyeloablative conditioning (11.3%). GVHD prophylaxis was predominately calcineurin inhibitor based (84.0%), with either methotrexate or mycophenolate mofetil or in combination with posttransplant cyclophosphamide (PTCy; 11.4%).
Population characteristics
| . | Training cohort (n = 10 016) . | Validation cohort (n = 1802) . | Total cohort . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Recipient age, y | |||
| 0-19 | 1159 (11.6%) | 179 (9.9%) | 1338 (11.3%) |
| 20-39 | 1470 (14.7%) | 255 (14.2%) | 1725 (14.6%) |
| 40-59 | 3167 (31.6%) | 537 (29.8%) | 3704 (31.3%) |
| 60-82 | 4220 (42.1%) | 831 (46.1%) | 5051 (42.7%) |
| Recipient sex, male | 5828 (58.2%) | 1059 (58.8%) | 6887 (58.3%) |
| Recipient race | |||
| White | 8993 (93.0%) | 1619 (92.8%) | 10612 (93.0%) |
| Black | 307 (3.2%) | 57 (3.3%) | 364 (3.2%) |
| Asian | 281 (2.9%) | 56 (3.2%) | 337 (3.0%) |
| Other | 86 (0.9%) | 13 (0.7%) | 99 (0.9%) |
| Missing | 349 | 57 | 406 |
| Recipient ethnicity | |||
| Hispanic | 764 (7.9%) | 143 (8.3%) | 907 (8.0%) |
| Missing | 359 | 81 | 440 |
| Donor age, y | |||
| 18-29 | 6499 (65.4%) | 1176 (65.7%) | 7675 (65.4%) |
| 30-39 | 2291 (23.0%) | 411 (22.9%) | 2702 (23.0%) |
| 40-49 | 855 (8.6%) | 148 (8.3%) | 1003 (8.5%) |
| 50-60 | 296 (3.0%) | 56 (3.1%) | 352 (3.0%) |
| Missing | 75 | 11 | 86 |
| Donor parity | |||
| Male | 6985 (70.2%) | 1245 (69.4%) | 8230 (70.0%) |
| Female-nulliparous | 1886 (18.9%) | 370 (20.6%) | 2256 (19.2%) |
| Female-parous | 1086 (10.9%) | 179 (10.0%) | 1265 (10.8%) |
| Missing | 59 | 8 | 67 |
| Sex matching, recipient/donor | |||
| Male/male | 4287 (43.1%) | 769 (42.9%) | 5056 (43.0%) |
| Male/female | 1508 (15.1%) | 286 (15.9%) | 1794 (15.3%) |
| Female/male | 2698 (27.1%) | 476 (26.5%) | 3174 (27.0%) |
| Female/female | 1464 (14.7%) | 263 (14.7%) | 1727 (14.7%) |
| Missing | 59 | 8 | 67 |
| CMV serostatus (recipient/donor) | |||
| −/− | 2783 (27.9%) | 503 (28.2%) | 3286 (28.0%) |
| −/+ | 1162 (11.7%) | 205 (11.5%) | 1367 (11.6%) |
| +/− | 3240 (32.5%) | 591 (33.1%) | 3831 (32.6%) |
| +/+ | 2777 (27.9%) | 487 (27.3%) | 3264 (27.8%) |
| Missing | 54 | 16 | 70 |
| DQB1 match | |||
| Yes | 9377 (95.6%) | 1699 (95.8%) | 11076 (95.6%) |
| Missing | 206 | 29 | 235 |
| DPB1 match or permissive mismatch | |||
| Yes | 6430 (75.4%) | 1171 (76.3%) | 7601 (75.5%) |
| Missing | 1490 | 267 | 1757 |
| Graft type | |||
| Bone marrow | 2239 (22.4%) | 384 (21.3%) | 2623 (22.2%) |
| Peripheral blood | 7777 (77.6%) | 1418 (78.7%) | 9195 (77.8%) |
| Karnofsky score | |||
| ≤60 | 144 (1.5%) | 25 (1.4%) | 169 (1.5%) |
| 70 | 1268 (12.9%) | 222 (12.6%) | 1490 (12.9%) |
| 80 | 2896 (29.5%) | 557 (31.6%) | 3453 (29.8%) |
| 90 | 3971 (40.5%) | 701 (39.7%) | 4672 (40.4%) |
| 100 | 1528 (15.6%) | 259 (14.7%) | 1787 (15.4%) |
| Missing | 209 | 38 | 247 |
| Disease∗ | |||
| ALL | 1290 (12.9%) | 251 (13.9%) | 1541 (13.0%) |
| AML | 3956 (39.5%) | 746 (41.4%) | 4702 (39.8%) |
| CLL | 139 (1.4%) | 26 (1.4%) | 165 (1.4%) |
| CML | 269 (2.7%) | 47 (2.6%) | 316 (2.7%) |
| MDS | 1988 (19.8%) | 362 (20.1%) | 2350 (19.9%) |
| MM | 128 (1.3%) | 26 (1.4%) | 154 (1.3%) |
| NHL | 617 (6.2%) | 94 (5.2%) | 711 (6.0%) |
| Other | 1629 (16.3%) | 250 (13.9%) | 1879 (15.9%) |
| Disease stage (ALL, AML, MDS only) | 6947 | 1306 | 8253 |
| Early | 4159 (59.9%) | 775 (59.3%) | 4934 (59.8%) |
| Intermediate | 1360 (19.6%) | 261 (20.0%) | 1621 (19.6%) |
| Advanced | 1428 (20.6%) | 270 (20.7%) | 1698 (20.6%) |
| Missing | 287 | 53 | 340 |
| Conditioning regimen | |||
| Myeloablative | 4974 (49.7%) | 885 (49.1%) | 5859 (49.6%) |
| Nonmyeloablative | 1122 (11.2%) | 213 (11.8%) | 1335 (11.3%) |
| Reduced intensity | 3920 (39.1%) | 704 (39.1%) | 4624 (39.1%) |
| GVHD prophylaxis | |||
| PTCy | 1130 (11.3%) | 216 (12.0%) | 1346 (11.4%) |
| Tac + MMF | 1097 (11.0%) | 209 (11.6%) | 1306 (11.1%) |
| Tac + MTX | 5425 (54.2%) | 965 (53.6%) | 6390 (54.1%) |
| Tac only | 1020 (10.2%) | 196 (10.9%) | 1216 (10.3%) |
| CSA + MMF | 430 (4.3%) | 84 (4.7%) | 514 (4.3%) |
| CSA + MTX | 356 (3.6%) | 61 (3.4%) | 417 (3.5%) |
| CSA only | 65 (0.6%) | 14 (0.8%) | 79 (0.7%) |
| Other | 493 (4.9%) | 57 (3.2%) | 550 (4.7%) |
| HCT-comorbidity index | |||
| 0 | 2093 (21.1%) | 333 (18.7%) | 2426 (20.7%) |
| 1-2 | 2938 (29.6%) | 547 (30.6%) | 3485 (29.7%) |
| 3-4 | 3050 (30.7%) | 539 (30.2%) | 3589 (30.6%) |
| 5+ | 1851 (18.6%) | 366 (20.5%) | 2217 (18.9%) |
| Missing | 84 | 17 | 101 |
| Time from diagnosis to HCT | |||
| 0-6 mo | 4311 (43.11%) | 754 (42.0%) | 5065 (43%) |
| >6-12 mo | 2511 (25.1%) | 475 (26.4%) | 2986 (25.3%) |
| >12-18 mo | 785 (7.9%) | 151 (8.4%) | 936 (7.9%) |
| >18-24 mo | 457 (4.6%) | 89 (5.0%) | 546 (4.6%) |
| >24 mo | 1931 (19.3%) | 327 (18.2%) | 2258 (19.2%) |
| Missing | 21 | 6 | 27 |
| Transplant year | |||
| 2016 | 2347 (23.4%) | 419 (23.3%) | 2766 (23.4%) |
| 2017 | 2527 (25.2%) | 438 (24.3%) | 2965 (25.1%) |
| 2018 | 2593 (25.9%) | 450 (25.0%) | 3043 (25.7%) |
| 2019 | 2549 (25.4%) | 495 (27.5%) | 3044 (25.8%) |
| . | Training cohort (n = 10 016) . | Validation cohort (n = 1802) . | Total cohort . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Recipient age, y | |||
| 0-19 | 1159 (11.6%) | 179 (9.9%) | 1338 (11.3%) |
| 20-39 | 1470 (14.7%) | 255 (14.2%) | 1725 (14.6%) |
| 40-59 | 3167 (31.6%) | 537 (29.8%) | 3704 (31.3%) |
| 60-82 | 4220 (42.1%) | 831 (46.1%) | 5051 (42.7%) |
| Recipient sex, male | 5828 (58.2%) | 1059 (58.8%) | 6887 (58.3%) |
| Recipient race | |||
| White | 8993 (93.0%) | 1619 (92.8%) | 10612 (93.0%) |
| Black | 307 (3.2%) | 57 (3.3%) | 364 (3.2%) |
| Asian | 281 (2.9%) | 56 (3.2%) | 337 (3.0%) |
| Other | 86 (0.9%) | 13 (0.7%) | 99 (0.9%) |
| Missing | 349 | 57 | 406 |
| Recipient ethnicity | |||
| Hispanic | 764 (7.9%) | 143 (8.3%) | 907 (8.0%) |
| Missing | 359 | 81 | 440 |
| Donor age, y | |||
| 18-29 | 6499 (65.4%) | 1176 (65.7%) | 7675 (65.4%) |
| 30-39 | 2291 (23.0%) | 411 (22.9%) | 2702 (23.0%) |
| 40-49 | 855 (8.6%) | 148 (8.3%) | 1003 (8.5%) |
| 50-60 | 296 (3.0%) | 56 (3.1%) | 352 (3.0%) |
| Missing | 75 | 11 | 86 |
| Donor parity | |||
| Male | 6985 (70.2%) | 1245 (69.4%) | 8230 (70.0%) |
| Female-nulliparous | 1886 (18.9%) | 370 (20.6%) | 2256 (19.2%) |
| Female-parous | 1086 (10.9%) | 179 (10.0%) | 1265 (10.8%) |
| Missing | 59 | 8 | 67 |
| Sex matching, recipient/donor | |||
| Male/male | 4287 (43.1%) | 769 (42.9%) | 5056 (43.0%) |
| Male/female | 1508 (15.1%) | 286 (15.9%) | 1794 (15.3%) |
| Female/male | 2698 (27.1%) | 476 (26.5%) | 3174 (27.0%) |
| Female/female | 1464 (14.7%) | 263 (14.7%) | 1727 (14.7%) |
| Missing | 59 | 8 | 67 |
| CMV serostatus (recipient/donor) | |||
| −/− | 2783 (27.9%) | 503 (28.2%) | 3286 (28.0%) |
| −/+ | 1162 (11.7%) | 205 (11.5%) | 1367 (11.6%) |
| +/− | 3240 (32.5%) | 591 (33.1%) | 3831 (32.6%) |
| +/+ | 2777 (27.9%) | 487 (27.3%) | 3264 (27.8%) |
| Missing | 54 | 16 | 70 |
| DQB1 match | |||
| Yes | 9377 (95.6%) | 1699 (95.8%) | 11076 (95.6%) |
| Missing | 206 | 29 | 235 |
| DPB1 match or permissive mismatch | |||
| Yes | 6430 (75.4%) | 1171 (76.3%) | 7601 (75.5%) |
| Missing | 1490 | 267 | 1757 |
| Graft type | |||
| Bone marrow | 2239 (22.4%) | 384 (21.3%) | 2623 (22.2%) |
| Peripheral blood | 7777 (77.6%) | 1418 (78.7%) | 9195 (77.8%) |
| Karnofsky score | |||
| ≤60 | 144 (1.5%) | 25 (1.4%) | 169 (1.5%) |
| 70 | 1268 (12.9%) | 222 (12.6%) | 1490 (12.9%) |
| 80 | 2896 (29.5%) | 557 (31.6%) | 3453 (29.8%) |
| 90 | 3971 (40.5%) | 701 (39.7%) | 4672 (40.4%) |
| 100 | 1528 (15.6%) | 259 (14.7%) | 1787 (15.4%) |
| Missing | 209 | 38 | 247 |
| Disease∗ | |||
| ALL | 1290 (12.9%) | 251 (13.9%) | 1541 (13.0%) |
| AML | 3956 (39.5%) | 746 (41.4%) | 4702 (39.8%) |
| CLL | 139 (1.4%) | 26 (1.4%) | 165 (1.4%) |
| CML | 269 (2.7%) | 47 (2.6%) | 316 (2.7%) |
| MDS | 1988 (19.8%) | 362 (20.1%) | 2350 (19.9%) |
| MM | 128 (1.3%) | 26 (1.4%) | 154 (1.3%) |
| NHL | 617 (6.2%) | 94 (5.2%) | 711 (6.0%) |
| Other | 1629 (16.3%) | 250 (13.9%) | 1879 (15.9%) |
| Disease stage (ALL, AML, MDS only) | 6947 | 1306 | 8253 |
| Early | 4159 (59.9%) | 775 (59.3%) | 4934 (59.8%) |
| Intermediate | 1360 (19.6%) | 261 (20.0%) | 1621 (19.6%) |
| Advanced | 1428 (20.6%) | 270 (20.7%) | 1698 (20.6%) |
| Missing | 287 | 53 | 340 |
| Conditioning regimen | |||
| Myeloablative | 4974 (49.7%) | 885 (49.1%) | 5859 (49.6%) |
| Nonmyeloablative | 1122 (11.2%) | 213 (11.8%) | 1335 (11.3%) |
| Reduced intensity | 3920 (39.1%) | 704 (39.1%) | 4624 (39.1%) |
| GVHD prophylaxis | |||
| PTCy | 1130 (11.3%) | 216 (12.0%) | 1346 (11.4%) |
| Tac + MMF | 1097 (11.0%) | 209 (11.6%) | 1306 (11.1%) |
| Tac + MTX | 5425 (54.2%) | 965 (53.6%) | 6390 (54.1%) |
| Tac only | 1020 (10.2%) | 196 (10.9%) | 1216 (10.3%) |
| CSA + MMF | 430 (4.3%) | 84 (4.7%) | 514 (4.3%) |
| CSA + MTX | 356 (3.6%) | 61 (3.4%) | 417 (3.5%) |
| CSA only | 65 (0.6%) | 14 (0.8%) | 79 (0.7%) |
| Other | 493 (4.9%) | 57 (3.2%) | 550 (4.7%) |
| HCT-comorbidity index | |||
| 0 | 2093 (21.1%) | 333 (18.7%) | 2426 (20.7%) |
| 1-2 | 2938 (29.6%) | 547 (30.6%) | 3485 (29.7%) |
| 3-4 | 3050 (30.7%) | 539 (30.2%) | 3589 (30.6%) |
| 5+ | 1851 (18.6%) | 366 (20.5%) | 2217 (18.9%) |
| Missing | 84 | 17 | 101 |
| Time from diagnosis to HCT | |||
| 0-6 mo | 4311 (43.11%) | 754 (42.0%) | 5065 (43%) |
| >6-12 mo | 2511 (25.1%) | 475 (26.4%) | 2986 (25.3%) |
| >12-18 mo | 785 (7.9%) | 151 (8.4%) | 936 (7.9%) |
| >18-24 mo | 457 (4.6%) | 89 (5.0%) | 546 (4.6%) |
| >24 mo | 1931 (19.3%) | 327 (18.2%) | 2258 (19.2%) |
| Missing | 21 | 6 | 27 |
| Transplant year | |||
| 2016 | 2347 (23.4%) | 419 (23.3%) | 2766 (23.4%) |
| 2017 | 2527 (25.2%) | 438 (24.3%) | 2965 (25.1%) |
| 2018 | 2593 (25.9%) | 450 (25.0%) | 3043 (25.7%) |
| 2019 | 2549 (25.4%) | 495 (27.5%) | 3044 (25.8%) |
CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma.
Other diseases included inherited erythrocyte abnormalities, inherited immune syndrome, severe aplastic anemia, and other nonmalignant conditions.
The NFT BART model was trained on ∼85% of the population (n = 10 016) and validated on the remainder (n = 1802). The training and validation cohorts were well balanced for clinical characteristics (Table 1). NFT BART was used to generate predictions for OS and EFS as previously described.11 To describe the impact of various donor factors on OS/EFS, outcomes for each level of a donor factor are predicted, while holding all other patient, disease, and transplant characteristics constant. The vast majority (95.6%) of the population was HLA-DQB1 matched, and the impact of mismatching fell within the zone of indifference for both OS and EFS. Donor CMV status/match, HLA-DPB1 T-cell epitope permissive match/mismatch, and donor parity had minimal impact (<1%) on OS and EFS (data not shown).11
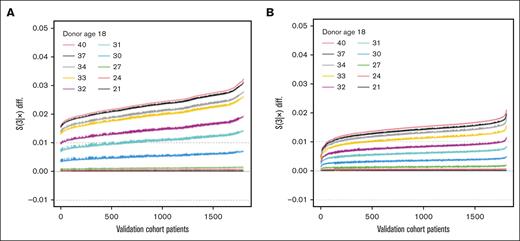
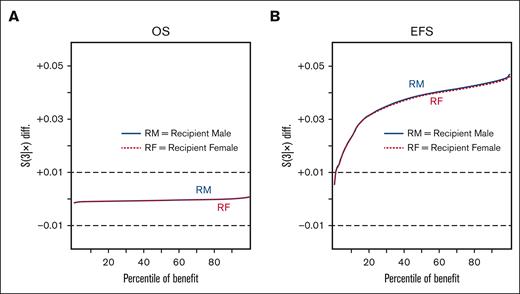
The impact of donor age was tested based on comparisons with an 18-year-old reference for the following cut points; ages 21, 24, 27, 30, 31, 32, 33, 34, 37, and 40 years. Compared with 18-year-old donors, donors aged 21 to 30 years fell within the zone of indifference, whereas donors aged ≥31 years were associated with an increased risk of mortality (Figure 1A) or those aged ≥32 years with worse EFS (Figure 1B). Donor sex fell within the zone of indifference for OS (Figure 2A), but use of a male donor was associated with improved EFS (Figure 2B). The impact of donor male sex ranged from ∼1% to 4.5% improvement (Figure 2B), in which donor age ≥32 years was associated with an increased risk ranging from ∼1% to 2% at 3 years after HCT (Figure 1B). Therefore, the youngest readily available male (aged 18-32 years) is generally preferable due to an EFS benefit; in the absence of a younger male donor, a younger female (aged ≤30 years) is preferred to optimize OS.
Impact of donor age. Waterfall plots of OS (A) and EFS (B) differentials based on predictions for the validation set. The OS and EFS differentials are noted on the vertical axis, with a zone of indifference denoted by the dashed gray horizontal lines at ±0.01, and predicted difference for each patient included in the validation set plotted along the x-axis. Differentials for an older donor vs an 18-year-old donor; donor ages (lines) are shown in ascending sequence: 21, 24, 27, 30, 31, 32, 33, 34, 37, and 40 years.
Impact of donor age. Waterfall plots of OS (A) and EFS (B) differentials based on predictions for the validation set. The OS and EFS differentials are noted on the vertical axis, with a zone of indifference denoted by the dashed gray horizontal lines at ±0.01, and predicted difference for each patient included in the validation set plotted along the x-axis. Differentials for an older donor vs an 18-year-old donor; donor ages (lines) are shown in ascending sequence: 21, 24, 27, 30, 31, 32, 33, 34, 37, and 40 years.
Impact of donor sex. Waterfall plots of OS (A) and EFS (B) differentials based on predictions for the validation set. The OS and EFS differentials are noted on the vertical axis, with a zone of indifference denoted by the dashed gray horizontal lines at ±0.01, and the percentile of benefit on the horizontal axis. Differentials for a male donor vs a female; recipient male (female) with a solid blue line (dotted red line): in the left panel, the percentage of those who benefit from a male vs female donor for recipient males (females) in blue (red).
Impact of donor sex. Waterfall plots of OS (A) and EFS (B) differentials based on predictions for the validation set. The OS and EFS differentials are noted on the vertical axis, with a zone of indifference denoted by the dashed gray horizontal lines at ±0.01, and the percentile of benefit on the horizontal axis. Differentials for a male donor vs a female; recipient male (female) with a solid blue line (dotted red line): in the left panel, the percentage of those who benefit from a male vs female donor for recipient males (females) in blue (red).
Discussion
This advanced machine learning approach provides new insights into the impact donor characteristics on MUD HCT outcomes. The key finding was that donors from age 18 to 30 years are associated with comparable OS, whereas male donors are associated with improved EFS using our definition. This should provide clinical teams greater flexibility in selecting donors within this age range based on the availability for cell collection within a desired time frame. Although the lack of an impact of donor CMV status on OS has been described previously,7,14 many centers continue to prioritize CMV in donor selection. This may be more relevant in the PTCy setting in which there is an increased risk of CMV infection.15 However, those findings are from the era before the ready availability of effective CMV prophylaxis with the approval of letermovir in 2017.16 Further studies on the impact of donor and recipient CMV serostatus are warranted in setting of letermovir prophylaxis. The lack of a benefit of HLA-DPB1 T-cell epitope–defined permissive mismatching on OS was also previously shown in a large analysis of donor characteristics7 and is now replicated here in a more contemporary cohort.
This study has limitations. It is an observational registry study. The analysis was limited to MUD transplants, and the large majority of patients were non-Hispanic White and received transplant from donors aged <35 years. Outcomes considered were limited to OS and EFS (defined as survival in the absence of graft failure, relapse-malignant disease only, and moderate/severe chronic GVHD). Other factors, such as ABO match, cell dose, donor/recipient weight difference, and treatment for GVHD were incomplete in the available data set and, therefore, not considered in the models. PTCy was used in only 11.4% of the patients, so additional data will be needed to determine whether predictions hold in a data set containing more PTCy-treated patients. Although a recent analysis from the EBMT Acute Leukemia Working Party noted a similar 30-year-old optimal donor age cut point in patients receiving 10/10 or 9/10 unrelated donor HCT with PTCy for AML.17
A novel aspect of this study was defining a zone of indifference for clinically relevant findings that is particularly important for guiding clinical practice. By limiting clinically relevant donor characteristics or factors to those associated with >1% improvement in OS at 3 years (or >10 days in restricted mean survival time), statistically significant associations of negligible clinical benefit can be deprioritized. This is critically important for eliminating delays in donor selection, as well as providing support for consideration of other factors such as large weight discrepancies or logistic issues. Recent results from the Blood and Marrow Transplant Clinical Trials Network 1702 study demonstrate that patient condition and disease status are the primary factors associated with delays to HCT.18 Further delaying transplant by prolonging the donor search over a minor age (in the 18-30 years range), or even a sex difference, compounds this risk.
Based on these results, when the goal is to optimize OS, patients with multiple 8/8 unrelated donors should go to HCT as soon as ready using the earliest available donor within the 18 to 30 years age range. If several donors in that age range are available, a male donor is preferred to optimize EFS.
Acknowledgments
CIBMTR is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research. Support is also provided by the Medical College of Wisconsin, NMDP, Gateway for Cancer Research, and Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc; Amgen; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio; Blueprint Medicines; Bristol Myers Squibb; CareDx; CSL Behring; CytoSen Therapeutics; DKMS; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; LAB; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; medac GmbH; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miller Pharmacal Group, Inc; Miltenyi Biotec; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Optum Health; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; REGiMMUNE; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi; Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
Authorship
Contribution: S.R.S., R.S., M.M., B.R.L., B.E.S., P.L., and S.M.D. designed research; C.B. and M.H. prepared data files for analysis; R.S. and B.R.L. analyzed data; S.R.S. wrote the manuscript; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen R. Spellman, Center for International Blood and Marrow Transplant Research, NMDP, 500 N 5th St, Minneapolis, MN 55401; email: sspellma@nmdp.org.
References
Author notes
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accord with the National Institutes of Health data sharing policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR releases only deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.