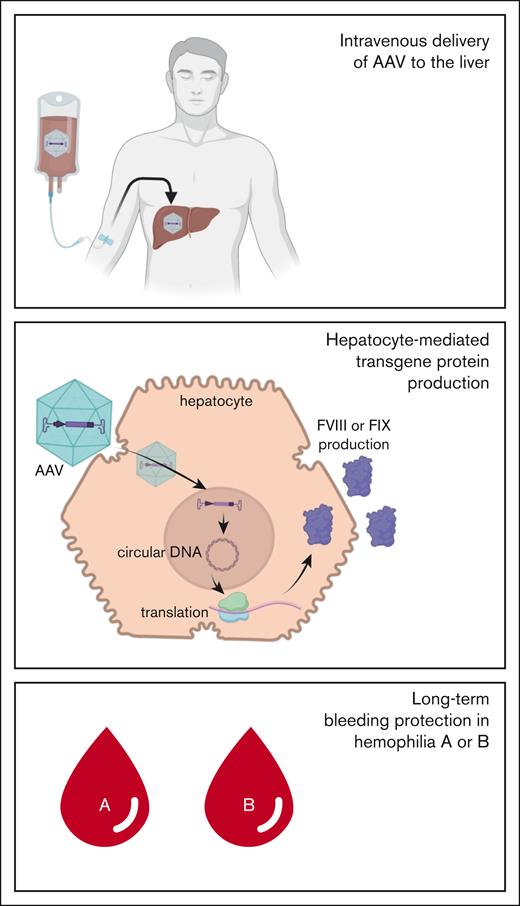
Concept
Gene therapy with adeno-associated virus (AAV) vectors treats hemophilia A or B by delivering a functional F8 or F9 gene to hepatocytes. This single infusion enables endogenous production of factor VIII (FVIII) or FIX protein, reducing bleeding in patients (Figure)
Evolution
Long-term clinical studies have provided insight into the efficacy, safety, and durability of AAV-mediated gene therapies for hemophilia A1,2 and B.3-5 Gene therapies have now been approved for hemophilia A (valoctocogene roxaparvovec) and hemophilia B (etranacogene dezaparvovec and fidanacogene elaparvovec) in select regions.
Application
Future steps
Improvements in AAV technology, protein expression, and immunogenicity may make AAV-mediated gene therapies more efficient, longer lasting, and safer for people with hemophilia A or B.
Authorship
A single infusion of AAV-particles containing cDNA encoding factor VIII or factor IX allows for production of these proteins in hepatocytes, resulting in long-term bleeding protection in hemophilia A or B.
A single infusion of AAV-particles containing cDNA encoding factor VIII or factor IX allows for production of these proteins in hepatocytes, resulting in long-term bleeding protection in hemophilia A or B.
Conflict-of-interest disclosure: P.J.L. receives research funding to institute from BioMarin, Sanofi, Sobi, and Roche. S.F. is employee of BioMarin Pharmaceutical Inc.