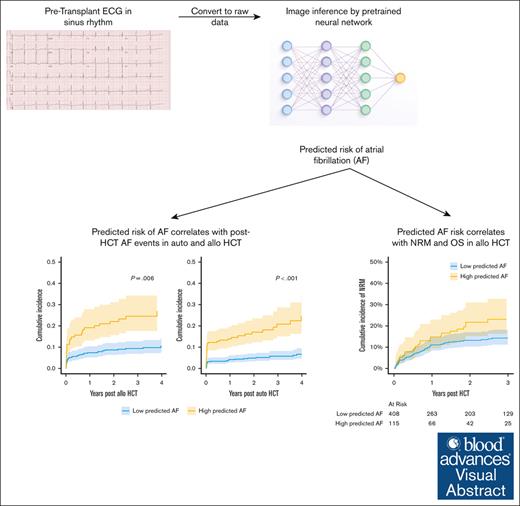
Key Points
AI interpretation of pretransplant ECG images is a novel diagnostic tool to predict transplant arrhythmia risk.
AI-ECG prediction of AF also informed the risk of mortality after allo transplantation.
Visual Abstract
Artificial intelligence (AI)–enabled interpretation of electrocardiogram (ECG) images (AI-ECGs) can identify patterns predictive of future adverse cardiac events. We hypothesized that such an approach would provide prognostic information for the risk of cardiac complications and mortality in patients undergoing hematopoietic cell transplantation (HCT). We retrospectively subjected ECGs obtained before HCT to an externally trained, deep-learning model designed to predict the risk of atrial fibrillation (AF). Included were 1377 patients (849 autologous [auto] HCT and 528 allogeneic [allo] HCT recipients). The median follow-up was 2.9 years. The 3-year cumulative incidence of AF was 9% (95% confidence interval [CI], 7-12) in patients who underwent auto HCT and 13% (10%-16%) in patients who underwent allo HCT. In the entire cohort, pre-HCT AI-ECG estimate of AF risk correlated highly with the development of clinical AF (hazard ratio [HR], 7.37; 95% CI, 3.53-15.4; P < .001), inferior survival (HR, 2.4; 95% CI, 1.3-4.5; P = .004), and greater risk of nonrelapse mortality (NRM; HR, 95% CI, 3.36; 1.39-8.13; P = .007), without increased risk of relapse. Association with mortality was only noted in allo HCT recipients, where the risk of NRM was greater. The use of cyclophosphamide after transplantation resulted in greater 90-day incidence of AF (13% vs 5%; P = .01) compared to calcineurin inhibitor–based graft-versus-host disease prophylaxis, corresponding to temporal changes in AI-ECG AF prediction after HCT. In summary, AI-ECG can inform risk of posttransplant cardiac outcomes and survival in HCT patients and represents a novel strategy for personalized risk assessment.
Introduction
Hematopoietic cell transplantation (HCT) is an effective therapy for patients with hematologic malignancy; however, cardiac toxicity can occur either from chemotherapy or secondary to other post-HCT complications.1 Cardiac arrhythmias, most prominently atrial fibrillation (AF), are prevalent after HCT and are associated with prolonged hospitalization, increased risk of admission to an intensive care unit, and increased risk of nonrelapse mortality (NRM).2-6 Despite the clinical and prognostic significance of AF after HCT, current available tools for pretransplant prediction of AF and other cardiac toxicities after HCT are lacking.7 In particular, a point-of-care tool that predicts arrhythmia risk without extensive outpatient arrhythmia monitoring would aid treatment planning before intensive chemotherapy.
Artificial intelligence (AI) is an emerging tool that may be used to enhance clinician appraisal of biometric data. Deep-learning approaches, when applied to electrocardiogram (ECG) waveform data (AI-ECG), demonstrate reliable discriminatory capability in classification of existing cardiac disorders such as arrhythmia, left ventricular systolic dysfunction, cardiac amyloidosis, valvular disease, and others.8-11 More recently, a convolutional neural network–based model, trained using a large repository of ECG records from general medical and surgical patients, was capable of accurate prediction of future risk of AF.8 Whether such a tool can subsequently generalize to a heterogenous population of patients undergoing cancer therapy is unknown. To test these hypotheses, we conducted a cohort study of patients who underwent autologous (auto) or allogeneic (allo) HCT at a single center. This model system was selected given the relatively high burden of AF in HCT recipients and the standard use of ECG as a pre-HCT appraisal tool. Because there is a strong association between AF and mortality in recipients of HCT, we further hypothesized that AI-ECG would predict treatment-related toxicity and survival in this population.2,4 Finally, pre-HCT echocardiograms and routine ECG parameters were correlated with AI-ECG risk to infer the relationship between model estimates and cardiac electromechanical findings that associate with AF.12
Methods
Study design
We performed a retrospective study of adult patients undergoing auto or allo HCT at Memorial Sloan Kettering Cancer Center between 2014 and 2022 (allo) or 2016 and 2022 (auto) with a diagnosis of plasma cell dyscrasia (auto) or acute leukemia, myelodysplastic syndrome, or myelodysplastic syndrome/myeloproliferative overlap disorders (allo). Patients with lymphoma were excluded from this analysis due to the high degree of overlap between auto HCT and allo HCT procedures. Patients in permanent or chronic AF or with no available ECG at Memorial Sloan Kettering Cancer Center within 180 days before HCT were excluded from the analyses. Digital ECG records stored in the MUSE data management system (GE HealthCare, Chicago, IL) were extracted and converted to comma separated variable formatted files before model inference. Pre-HCT ECGs and echocardiography data obtained before the start of conditioning chemotherapy, but not greater than 180 days before HCT, were included in the analyses. The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center. Consent for retrospective research was provided by patients.
Auto and allo HCT treatment procedures
Conditioning for patients with plasma cell dyscrasias was with single-agent melphalan 120 to 200 mg/m2. Patients with identified cardiac or renal dysfunction or patients with reduced performance status received reduced-dose melphalan (120-160 mg/m2). Conditioning therapy for allo HCT was as previously described.13 Patients who underwent Allo HCT received graft-versus-host disease (GVHD) prophylaxis with a calcineurin inhibitor (CNI; tacrolimus or cyclosporine) combined with either methotrexate (CNI-based) or posttransplant cyclophosphamide (PTCY)–based regimen14 or with allograft T-cell depletion (TCD) using CD34+ selection without routine administration of pharmacologic immunosuppression after HCT.15 Acute and chronic GVHD, disease risk, and disease staging criteria were defined using standard guidelines.16,17 Supportive care for allo and auto HCT recipients was consistent with international guidelines as previously described.18,19
AI-ECG model output, end point determination, and biostatistical methods
The AI-ECG AF prediction model was trained on a cohort of patients distinct from the current analysis and included patients derived from a general population cohort.8 The Memorial Sloan Kettering Cancer Center cohort ECG labels were assigned by a single cardiology specialist who evaluated the ECG at the time of acquisition. The AI-ECG tool outputs a continuous variable from 0 to 1 representative of the probability of future AF. During initial model development, the optimal cut point for dichotomization into “low” and “high” risk groups was found to be 0.11. We assessed the AI-ECG AF prediction both as a continuous variable as well as a dichotomized variable using the initial cut point that was identified during the model development, which was performed on an independent cohort outside of this study.8 Clinical end points were determined from the time of HCT. Multivariable regression models were made using a forward selection procedure, retaining clinical covariables with P value < .10 on univariable analysis. Covariates considered included cardiovascular and noncardiovascular comorbidities as well as key transplant characteristics provided in Table 1. Cardiovascular comorbidities were obtained from patient clinical records. Cox proportional hazards models and competing risk regression models were fitted using a forward selection procedure, retaining clinically pertinent variables, with significant variables on univariate analyses (P < .05). All analyses were performed using R.
Patient characteristics in the allo and auto cohorts
| . | Allo HCT (n = 528) . | Auto HCT (n = 849) . |
|---|---|---|
| Age (IQR), y | 63 (54-68) | 62 (56-68) |
| Sex, female | 230 (44%) | 348 (41%) |
| Disease | ||
| Acute lymphocytic leukemia | 71 (13%) | — |
| Acute myeloid leukemia | 291 (55%) | — |
| MDS or MDS/MPN overlap | 166 (31%) | — |
| Amyloidosis | — | 37 (4.4%) |
| Monoclonal immunoglobulin deposition | — | 1 (0.1%) |
| Multiple myeloma | — | 811 (95%) |
| HCT-CI | ||
| 0-2 | 273 (52%) | 397 (47%) |
| ≥3 | 255 (48%) | 448 (53%) |
| Vascular disease | 58 (11%) | 95 (11%) |
| AF history | 34 (6.4%) | 54 (6.4%) |
| Diabetes mellitus | 55 (10%) | 98 (12%) |
| Regimen | ||
| Busulfan and melphalan based | 131 (25%) | 0 (0%) |
| Busulfan based | 76 (14%) | 0 (0%) |
| High-dose TBI based | 88 (17%) | 0 (0%) |
| Low-dose TBI based | 20 (3.8%) | 0 (0%) |
| Melphalan based | 213 (40%) | 849 (100%) |
| HLA matching | ||
| HLA matched | 390 (74%) | — |
| HLA mismatched | 138 (26%) | — |
| GVHD prophylaxis | ||
| CNI | 190 (36%) | — |
| PTCY | 158 (30%) | — |
| TCD | 180 (34%) | — |
| . | Allo HCT (n = 528) . | Auto HCT (n = 849) . |
|---|---|---|
| Age (IQR), y | 63 (54-68) | 62 (56-68) |
| Sex, female | 230 (44%) | 348 (41%) |
| Disease | ||
| Acute lymphocytic leukemia | 71 (13%) | — |
| Acute myeloid leukemia | 291 (55%) | — |
| MDS or MDS/MPN overlap | 166 (31%) | — |
| Amyloidosis | — | 37 (4.4%) |
| Monoclonal immunoglobulin deposition | — | 1 (0.1%) |
| Multiple myeloma | — | 811 (95%) |
| HCT-CI | ||
| 0-2 | 273 (52%) | 397 (47%) |
| ≥3 | 255 (48%) | 448 (53%) |
| Vascular disease | 58 (11%) | 95 (11%) |
| AF history | 34 (6.4%) | 54 (6.4%) |
| Diabetes mellitus | 55 (10%) | 98 (12%) |
| Regimen | ||
| Busulfan and melphalan based | 131 (25%) | 0 (0%) |
| Busulfan based | 76 (14%) | 0 (0%) |
| High-dose TBI based | 88 (17%) | 0 (0%) |
| Low-dose TBI based | 20 (3.8%) | 0 (0%) |
| Melphalan based | 213 (40%) | 849 (100%) |
| HLA matching | ||
| HLA matched | 390 (74%) | — |
| HLA mismatched | 138 (26%) | — |
| GVHD prophylaxis | ||
| CNI | 190 (36%) | — |
| PTCY | 158 (30%) | — |
| TCD | 180 (34%) | — |
Values are reported as median (IQR) or n (%).
IQR, interquartile range; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; TBI, total body irradiation.
Results
Baseline characteristics
The patient cohort included 1377 patients who underwent either auto HCT (n = 849) or allo HCT (n = 528). A total of 13 auto and 6 allo HCT recipients were found in AF at HCT evaluation and were not considered in the subsequent analysis. Demographic and oncologic baseline characteristics are provided in Table 1. The median follow-up was 2.9 years (interquartile range, 1.6-4.5). The 3-year overall survival (OS) and event-free survival were 61% (95% confidence interval [CI], 56-65) and 54% (95% CI, 50-59) in the allo HCT cohort, respectively, and 82% (95% CI, 79-85) and 74% (95% CI, 71-78) in the auto HCT cohort, respectively. The 3-year NRM was 16% (95% CI, 13-20) and 4% (95% CI, 2-5) in allo HCT recipients and auto HCT recipients, respectively. In the allo cohort, there were significant differences in baseline characteristics of the patient cohort based on GVHD prophylaxis selection (supplemental Table 1). Patients who underwent TCD were younger, had lower HCT comorbidity index (HCT-CI) scores, and lower-risk disease. Full-intensity (myeloablative) conditioning was used in all TCD patients, 33% of CNI-treated patients, and 44% of PTCY-treated patients. HLA mismatched grafts (<8/8 matched at HLA-A, -B, -C, or -DRB1) were more likely to be used with PTCY (71.5%) than with CNI-based (6.3%) or TCD transplants (7.2%).
Using the ECG obtained during the HCT workup, patients were dichotomized to high- or low-risk groups based on a predefined cut point identified during the model development. High risk was defined as an AI-ECG AF prediction >0.11. Patient characteristics according to AI-ECG AF risk group are shown in Table 2. Patients at high AI-ECG–predicted AF risk were older and had more frequent prior history of diabetes mellitus, AF, and coronary or peripheral vascular disease than lower-risk patients. The distribution of ECG sampling after HCT is provided in supplemental Figure 1. The median number of post-HCT ECGs obtained per patient was 9 (interquartile range, 4-19).
Baseline characteristics based on pre-HCT AI-ECG–predicted risk of AF
| . | Overall cohort . | Low AI-ECG–predicted AF . | High AI-ECG–predicted AF . | P value . |
|---|---|---|---|---|
| (N = 1377) . | (n = 1031) . | (n = 346) . | ||
| Transplant type | .13 | |||
| Allo | 529 (38%) | 408 (40%) | 121 (35%) | |
| Auto | 848 (62%) | 623 (60%) | 225 (65%) | |
| Patient age | 62 (55-68) | 61 (54-67) | 65 (60-70) | <.001 |
| Female | 578 (42%) | 483 (47%) | 95 (27%) | <.001 |
| Diabetes mellitus | 153 (11%) | 99 (9.6%) | 54 (16%) | .002 |
| Vascular disease | 153 (11%) | 82 (8.0%) | 71 (21%) | <.001 |
| AF | 88 (6.4%) | 32 (3.1%) | 56 (16%) | <.001 |
| Disease histology | <.001 | |||
| Acute leukemia | 363 (26%) | 281 (27%) | 82 (24%) | |
| Plasma cell disease | 848 (62%) | 623 (60%) | 225 (65%) | |
| MDS | 166 (12%) | 127 (12%) | 39 (11%) | |
| High-risk (3+) HCT-CI | 703 (51%) | 493 (48%) | 210 (61%) | <.001 |
| Remission status at HCT | 1200 (87%) | 903 (88%) | 297 (86%) | .4 |
| . | Overall cohort . | Low AI-ECG–predicted AF . | High AI-ECG–predicted AF . | P value . |
|---|---|---|---|---|
| (N = 1377) . | (n = 1031) . | (n = 346) . | ||
| Transplant type | .13 | |||
| Allo | 529 (38%) | 408 (40%) | 121 (35%) | |
| Auto | 848 (62%) | 623 (60%) | 225 (65%) | |
| Patient age | 62 (55-68) | 61 (54-67) | 65 (60-70) | <.001 |
| Female | 578 (42%) | 483 (47%) | 95 (27%) | <.001 |
| Diabetes mellitus | 153 (11%) | 99 (9.6%) | 54 (16%) | .002 |
| Vascular disease | 153 (11%) | 82 (8.0%) | 71 (21%) | <.001 |
| AF | 88 (6.4%) | 32 (3.1%) | 56 (16%) | <.001 |
| Disease histology | <.001 | |||
| Acute leukemia | 363 (26%) | 281 (27%) | 82 (24%) | |
| Plasma cell disease | 848 (62%) | 623 (60%) | 225 (65%) | |
| MDS | 166 (12%) | 127 (12%) | 39 (11%) | |
| High-risk (3+) HCT-CI | 703 (51%) | 493 (48%) | 210 (61%) | <.001 |
| Remission status at HCT | 1200 (87%) | 903 (88%) | 297 (86%) | .4 |
MDS, myelodysplastic syndrome.
Clinical AF incidence and AI-ECG model characteristics
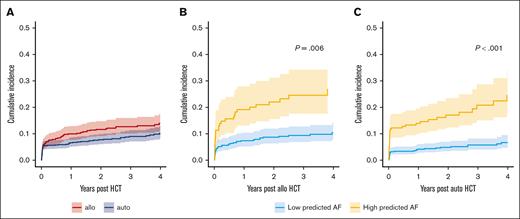
The cumulative incidence of clinically manifest AF after HCT was 11% (Figure 1A) and did not vary significantly according to transplant type (allo HCT, 13%; auto HCT, 9%; P = .1). The 90-day and 3-year cumulative incidences of AF were 4.2% (95% CI, 3.1-5.5) and 7.2% (95% CI, 5.7-9.2) in recipients at low predicted AF risk compared with 16% (95% CI, 13-20) and 25% (95% CI, 20-30) in high predicted AF risk recipients (P < .001) in the entire cohort. The AI-ECG models performed similarly when tested separately in the allo and auto HCT subgroups (Figure 1B-C). In allo HCT, the 3-year cumulative risk of AF was 9.4% (95% CI, 6.7-13) in those recipients with low predicted AF risk compared with 27% (95% CI, 20-36) in high AF predicted risk recipients (P = .006). Similarly, in low-risk auto HCT recipients, the 3-year incidence of AF was 5.9% (95% CI, 4.1-8.2) compared with 24% (95% CI, 17-31) in high-risk patients (P < .001). Multivariable association with incidence of AF in each cohort are provided in Table 3 (full multivariable model results are provided in supplemental Table 4). The cumulative incidence of clinical AF according to AI-ECG–predicted AF risk in patients with or without prior history of AF is shown in supplemental Figure 2.
Cumulative incidence of clinical AF after HCT. (A) Cumulative incidence of clinical AF based on donor source. (B) Cumulative incidence of clinical AF in allo HCT recipients based on AI-ECG (low vs high predicted risk). (C) OS in the overall cohort based on AI-ECG prediction group. (D) Death without relapse in the overall cohort based on AI-ECG prediction.
Cumulative incidence of clinical AF after HCT. (A) Cumulative incidence of clinical AF based on donor source. (B) Cumulative incidence of clinical AF in allo HCT recipients based on AI-ECG (low vs high predicted risk). (C) OS in the overall cohort based on AI-ECG prediction group. (D) Death without relapse in the overall cohort based on AI-ECG prediction.
Multivariable regression model results for clinical AF, all-cause mortality, and NRM according to AI-ECG–predicted AF risk
| . | Combined HCT cohort . | Allo HCT cohort . | Auto HCT cohort . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Clinical AF after HCT | 7.4 (3.5-15.4) | <.001 | 6.0 (1.9-19.0) | .006 | 8.6 (3.3-22.3) | <.001 |
| All-cause mortality | 2.4 (1.3-4.5) | .004 | 2.9 (1.3-6.5) | .01 | 2.1 (0.8-5.5) | .13 |
| NRM | 3.4 (1.4-8.1) | .007 | 3.9 (1.3-12.2) | .03 | 2.5 (0.4-16.1) | .40 |
| . | Combined HCT cohort . | Allo HCT cohort . | Auto HCT cohort . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Clinical AF after HCT | 7.4 (3.5-15.4) | <.001 | 6.0 (1.9-19.0) | .006 | 8.6 (3.3-22.3) | <.001 |
| All-cause mortality | 2.4 (1.3-4.5) | .004 | 2.9 (1.3-6.5) | .01 | 2.1 (0.8-5.5) | .13 |
| NRM | 3.4 (1.4-8.1) | .007 | 3.9 (1.3-12.2) | .03 | 2.5 (0.4-16.1) | .40 |
Model evaluation in the whole cohort produced an area under the receiver operating curve of 0.70 (95% CI, 0.63-0.77); 0.68 (95% CI, 0.63-0.74) in the allo HCT subgroup and 0.73 (95% CI, 0.66-0.78) in the auto subgroup. The optimal dichotomization cut point defined using the maximal sum of the sensitivity and specificity in this study cohort was 0.07 (compared with 0.11 derived from model testing). Model diagnostics based on the threshold identified during model development are provided in supplemental Table 2 for each HCT subgroup. Receiver operating characteristic curves for the combined, allo, and auto HCT cohorts are provided in supplemental Figure 3.
AI-ECG prediction of AF and risk of mortality
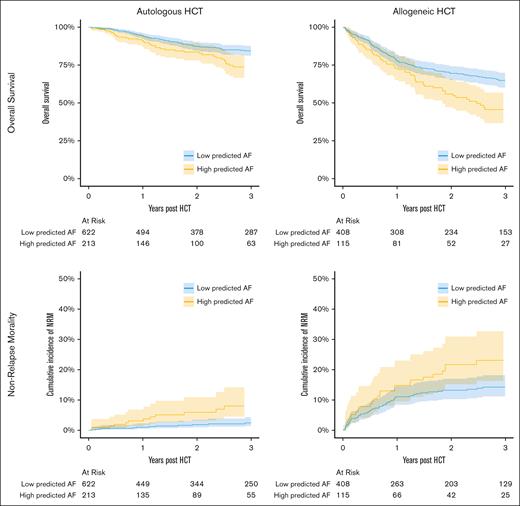
Development of an episode of clinical AF was associated with increased mortality both in recipients of auto HCT (multivariable hazard ratio [HR], 2.6; 95% CI, 1.7-4.1; P < .001) and allo HCT (multivariable HR, 2.4; 95% CI, 1.7-3.4; P < .001) in multivariable adjusted time-dependent regression models. We then sought to determine whether the AI-ECG–based prediction of AF also predicted mortality. In the entire cohort, AI-ECG AF prediction correlated with OS due to increased NRM using a multivariable Cox model (Table 3) stratified based on HCT type. We found a significant association between the risk of death and AI-ECG AF prediction in patients undergoing allo HCT (HR, 2.9; 95% CI, 1.3-6.5; P = .01; Table 3). In auto HCT recipients, there was not a statistically significant association between the risk of death and AI-ECG AF (HR, 2.1; 95% CI, 0.8-5.5; P = .1; Table 3). Full multivariable model results are provided in supplemental Table 4. Examination of subcauses of death demonstrated that the increased risk of mortality observed in allo HCT–treated patients was the result of increased NRM (HR, 3.9; 95% CI, 1.3-12.2; P = .03; Table 3), whereas AI-ECG AF prediction was not associated with NRM in auto HCT (HR, 2.5; 95% CI, 0.4-16.1; P = .4). There was no association between incidence of relapse and AI-ECG prediction of AF in either auto HCT (HR, 1.0; 95% CI, 0.36-2.79; P > .99) or allo HCT (HR, 1.6; 95% CI, 0.6-4.52; P = .4) recipients. Kaplan-Meier projections for OS and cumulative incidence estimates of NRM are provided in Figure 2.
Kaplan-Meier estimates for OS and cumulative incidence of NRM in the auto and allo HCT cohorts based on low- vs high-risk AI-ECG AF prediction.
Kaplan-Meier estimates for OS and cumulative incidence of NRM in the auto and allo HCT cohorts based on low- vs high-risk AI-ECG AF prediction.
Incidence of AF based on GVHD prophylaxis type in allo HCT recipients
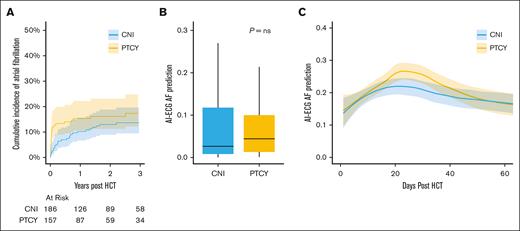
We then evaluated whether the use of cyclophosphamide–based GVHD prophylaxis resulted in variable patterns of AF after allo HCT. TCD recipients in this cohort received exclusively myeloablative conditioning and were therefore younger with lower underlying comorbidity (supplemental Table 1). Concordantly, the cumulative incidence of AF was lower after TCD than CNI or PTCY-based GVHD prophylaxis (supplemental Table 3). After adjustment for differences in baseline characteristics, there was no significant difference in the incidence of AF based on GVHD prophylaxis program: compared with recipients of CNI-based prophylaxis, multivariable competing risk regression hazards considering age and HCT-CI as covariables demonstrated an HR of 1.46 (95% CI, 0.85-2.52) in PTCY recipients and an HR of 0.97 (95% CI, 0.50-1.91) in TCD recipients. Given these differences in underlying baseline risk, we examined the patterns of AF development in CNI and PTCY recipients. Although the overall hazard of AF was similar between these 2 groups, temporal differences in the incidence of AF were evident (Figure 3A). The 90-day incidence of AF was 5% (3%-9%) in CNI patients vs 13% (95% CI, 9%-19%) in PTCY patients (P = .01); whereas the 1-year incidence of AF was 10% (95% CI, 6%-15%) in CNI-treated patients vs 15% (95% CI, 10%-21%) in PTCY-treated patients (P = .2). There was no difference in pre-HCT AI-ECG AF predicted risk (Figure 3B). Examination of AI-ECG model inference values of post-HCT ECGs that were in sinus rhythm demonstrates differential patterns based on cyclophosphamide exposure (Figure 3C). PTCY recipients exhibited a marked increase in AI-ECG prediction of AF compared with CNI-treated patients between days 15 and 30 after HCT (median prediction, 0.26 vs 0.12).
AI-ECG metrics in recipients of posttransplant cyclophosphamide (PTCY) or calcineurin inhibitor (CNI) based graft-vs-host-disease prophylaxis. (A) Cumulative incidence of clinical AF in recipients of PTCY-based prophylaxis and CNI-based prophylaxis. (B) Pre-HCT AI-ECG prediction of AF was similar between CNI and PTCY recipients. (C) Loess regression curves of post-HCT AI-ECGs in sinus rhythm. Post-HCT ECGs were subjected to AI-ECG inference, demonstrating transient increase in AF prediction in PTCY recipients relative to CNI recipients. ns, not significant.
AI-ECG metrics in recipients of posttransplant cyclophosphamide (PTCY) or calcineurin inhibitor (CNI) based graft-vs-host-disease prophylaxis. (A) Cumulative incidence of clinical AF in recipients of PTCY-based prophylaxis and CNI-based prophylaxis. (B) Pre-HCT AI-ECG prediction of AF was similar between CNI and PTCY recipients. (C) Loess regression curves of post-HCT AI-ECGs in sinus rhythm. Post-HCT ECGs were subjected to AI-ECG inference, demonstrating transient increase in AF prediction in PTCY recipients relative to CNI recipients. ns, not significant.
Correlation of clinical, electrocardiographic, and echocardiographic features with AI-ECG–predicted AF
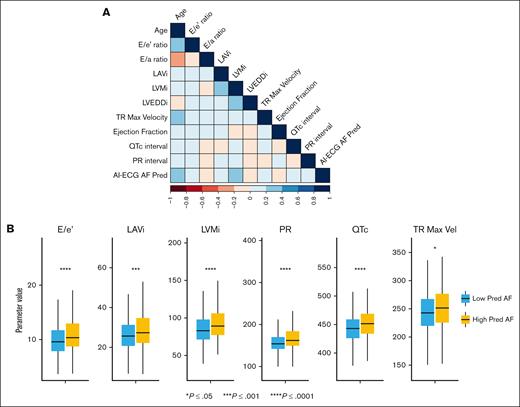
We finally sought to determine cardiac electromechanical patterns that correlated with the model to infer clinical findings that inform AI-ECG AF prediction. The Spearman correlation coefficient matrix of clinical, electrocardiographic, and echocardiographic parameters is given in Figure 4A. Advanced age was the strongest correlated clinical parameter with AI-ECG–predicted AF risk (Spearman correlation coefficient, 0.63; P < .001). Echocardiographic parameters including left ventricular hypertrophy (left ventricular mass index), left atrial enlargement (left atrial volume index), and diastolic dysfunction demonstrated a weak correlation (Spearman coefficient, <0.5) with AI-ECG model output (Figure 4B).
Correlation of AI-enabled ECG model output with clinical, routine ECG, and echocardiographic parameters. (A) Correlation plot between clinical and echocardiographic parameters and AI-ECG model output. (B) Plot of individual parameters correlated with AI-ECG model output, grouped by whether patients developed AF after HCT. ∗∗∗P < .001; ∗P < .05. LAVI, left atrial volume index; LVEDDi, left ventricular dimension at end diastole index; LVMI, left ventricular mass index; PR, PR interval; QTC, corrected QT interval; TR MAX Vel, tricuspid regurgitant maximum velocity.
Correlation of AI-enabled ECG model output with clinical, routine ECG, and echocardiographic parameters. (A) Correlation plot between clinical and echocardiographic parameters and AI-ECG model output. (B) Plot of individual parameters correlated with AI-ECG model output, grouped by whether patients developed AF after HCT. ∗∗∗P < .001; ∗P < .05. LAVI, left atrial volume index; LVEDDi, left ventricular dimension at end diastole index; LVMI, left ventricular mass index; PR, PR interval; QTC, corrected QT interval; TR MAX Vel, tricuspid regurgitant maximum velocity.
Discussion
In this study, we aimed to assess the predictive accuracy and clinical implications of an AI-enabled electrocardiography model for AF prediction applied in a well-defined population undergoing intensive treatment for hematologic malignancy. We demonstrated the following: (1) AI-ECG appraisal for AF risk was strongly correlated with development of clinically manifest AF after HCT in a multivariable, cause-specific hazards model; (2) AI-ECG–predicted AF risk was an independent predictor of increased mortality after HCT in allo HCT recipients; and (3) the AI-ECG–predicted AF risk model was an independent predictor of NRM. To our knowledge, this is the first study to assess the discriminatory capabilities and prognostic value of an AI-ECG tool applied to a large, well-defined cancer cohort with an appraised high risk for treatment-related AF, suggesting AI-appraised ECG data as a key biometric tool to define the risk of intensive therapy for hematologic malignancy. Interestingly, the optimal decision threshold for AF risk classification was 7% in this cohort vs 11% in the general population on which the model was trained, suggesting that patients with hematologic malignancies have a higher baseline risk for AF than the general population. This observation agrees with prior epidemiologic data suggesting a higher adjusted risk for AF in patients with cancer than the general population.2,4,20-22 As such, further optimization of the AI-ECG modeling decision thresholds in well-defined patient cohorts, such as cancer-specific populations, and incorporation of other clinical and oncologic parameters will improve the accuracy of this tool and may allow for further inference of the pathophysiological links between malignancy and AF.
In this study, the development of clinical AF was a strong prognostic indicator of mortality in this population, consistent with prior reports in patients with hematologic malignancy.4,20 We have now expanded on this observation to demonstrate that AI-ECG modeling for AF prediction based on pre-HCT ECGs in patients in sinus rhythm was an independent predictor for all-cause death and NRM. Whether there is a direct pathophysiologic link between AI-ECG and electromechanical cardiac changes that increase the risk for NRM cannot be readily discerned by this study because it is plausible that AI-ECG–predicted AF is an indirect marker of cardiovascular disease in this setting. The latter is supported by the higher frequency of cardiovascular conditions in patients with high AI-ECG–predicted AF risk. Interestingly, none of these conditions were predictive of future mortality or NRM when AI-ECG–predicted AF risk was included, suggesting that AI-ECG patterns partially reflect the structural impact of conditions such as hypertension or diabetes on the myocardium and thus may represent a more direct measurement of cardiac comorbidity. The AI-ECG metric associated to a greater degree with OS and NRM than HCT-CI in multivariable regression modeling, suggesting that AI-ECG may offer a more direct appraisal of cardiac vulnerability than HCT-CI, which is based only on reported patient medical history.23 This conclusion is certainly limited by a selection bias for transplant protocols based on known HCT-CI, whereas the AI-ECG metric was unknown at the time of HCT in this analysis. Indeed, the HCT-CI was associated with OS but did not associate with NRM in the allo HCT cohort, supporting some degree of selection bias in this group. Testing AI-ECG in a prospective fashion at multiple centers will be required to resolve the question of whether this tool may become an adjunct to HCT-CI in NRM risk approximation.
Subanalyses according to type of transplant (allo vs auto) were not powered for assessment of overall and NRM; nonetheless, in auto HCT, AI-ECG––predicted AF risk was not predictive of NRM. Whether this finding was related to the low NRM rate observed in this population (4%) or reflects a different cardiac physiologic response to the type of malignancy managed with auto HCT cannot be discerned from this study. It is also plausible that conditioning modalities and the utilization of GVHD prophylaxis in allo HCT may play a role in the heightened impact of AI-ECG utility. Indeed, early clinical AF occurring <90 days after allo HCT was more frequent after PTCY, whereas late AF was more frequent in CNI-based prophylaxis, suggesting differential contributions of cardiotoxic preparatory agents vs late inflammatory causes from GVHD may both contribute to AF risk. Whether ongoing need for GVHD prophylaxis increase the risk of clinical and AI-ECG–predicted AF and subsequently affect NRM in this subgroup requires further investigation.24,25
There are some limitations to this study. The conclusions here are based on retrospective analyses and can thus only be considered as hypothesis generating. AI-ECG analyses were performed on sinus rhythm ECGs; as such, the present study results are not applicable to patients with permanent or chronic AF and hematologic malignancies undergoing HCT. Similarly, episodes of very transient or paroxysmal AF were likely missed in the context of this study, because only AF acquired in a recorded ECG was used to derive the end point. Future study to deploy continuous telemetry monitoring in high-risk patients based on AI-ECG is planned. The optimal AI-ECG decision threshold applied in this analyses was based on previously reported cutoffs based on the general population and not a cancer patient population; whether different thresholds are applicable to well-defined populations at high risk for AF, such as patients with cancer, was not addressed in this study. AF incidence after HCT was based on ECG monitoring; as such, we cannot exclude a higher incidence of occult or asymptomatic AF.
Prior assessment of AI-ECG AF prediction models in patient cohorts largely derived from the general population have reported conflicting results in terms of predictive ability and clinical implications, likely due to a wide heterogeneity of underlying AF mechanisms and different pathophysiologic setting.26 In contrast, AI-ECG modeling in well-defined populations at high risk for AF, such as patients with hematologic malignancies, is an attractive screening tool with possible clinical risk stratification capabilities.5 As such, prediction of AF in this setting is essential and may provide insight on pre-HCT cardiovascular optimization and inform subsequent implementation of preventive and therapeutic strategies.27,28 Last, because novel oncologic therapies for hematologic malignancies are increasingly efficacious, multidisciplinary management of cardiovascular comorbidities must be incorporated into clinical practice, although specific pathways are yet underdeveloped. AI tools may provide an early cardiovascular screening mechanism for patients with hematologic or other malignancies scheduled for oncologic therapies.
Acknowledgments
This work was supported by National Cancer Institute grant P01 CA23766 and National Cancer Institute Cancer Center support grant P30 CA008748.
Authorship
Contribution: B.C.S. and I.K. conducted data collection and analysis and authored the manuscript; K.E.M., I.Z.A., and P.A.N. conducted artificial intelligence interpretation of electrocardiograms model inference; S.B. and S.D. conducted statistical analysis; and all other authors contributed to the data collection and data analytical plan, and reviewed and revised the final manuscript.
Conflict-of-interest disclosure: P.A.N., I.Z.A., F.L.-J., and P.A.F. have filed patents related to the application of AI-ECG for diagnosis and risk stratification and have licensed these AI-ECG algorithms to Anumana. B.C.S. reports consulting honoraria from Gamida Cell and Hansa BioPharma Inc, and research support from Merck. J.L. reports speaker honoraria from GE HealthCare and Philips Medical; research grant support from Johnson & Johnson; and data safety and monitoring board membership honoraria from Caelum Biosciences. M.-A.P. reports honoraria from Adicet Bio, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, ExeVir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, Orca Bio, Syncopation, VectivBio AG, and Vor Biopharma; serves on data safety and monitoring boards for Cidara Therapeutics, Medigene, and Sellas Life Sciences; serves on the scientific advisory board of NexImmune; has ownership interests in NexImmune, Omeros, and Orca Bio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. I.K. has received research support from Amgen; serves on advisory boards for Sanofi Inc and Pfizer Inc; and reports consulting for Edwards Inc. The remaining authors declare no competing financial interests.
Correspondence: Brian C. Shaffer, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: shaffeb1@mskcc.org.
References
Author notes
Reasonable requests for data sharing will be reviewed by the authors and should be directed to the corresponding author, Brian C. Shaffer (shaffeb1@mskcc.org).
The full-text version of this article contains a data supplement.