Key Points
Treatment with ruxolitinib and interferon alfa-2a is well tolerated with anemia as the main cause for dose reduction.
Treatment with ruxolitinib and interferon alfa-2a elicits high rates of hematologic and molecular response.
Visual Abstract
We report the 2-year end-of-study results from the phase 2 COMBI II clinical trial investigating the combination treatment of ruxolitinib and low-dose pegylated interferon alfa-2a in patients with newly diagnosed polycythemia vera (PV). The primary outcome was safety and key secondary endpoints were efficacy, based on hematologic parameters, quality-of-life measurements, and JAK2V617F variant allele frequency (VAF). We used the 2013 European LeukemiaNet and International Working Group-Myeloproliferative Neoplasms Research remission criteria. The remission criteria included remissions in symptoms, splenomegaly, peripheral blood counts, and bone marrow. We included 25 patients with PV with a median age of 70 years; 5 of those had prior thromboembolic events and 3 had computed tomography–verified splenomegaly. Two patients stopped both study drugs; 1 of these due to progression to post-PV myelofibrosis, the only one with a grade 3 infection. No events of herpes zoster infections were observed. None of the patients discontinued treatment due to psychiatric symptoms. The peripheral blood cell count remission rate was 92% at 24 months. Using the 2013 European LeukemiaNet and International Working Group-Myeloproliferative Neoplasms Research remission criteria, 14 (56%) achieved remission at 24 months; 3 (12%) achieved complete remission and 11 (44%) achieved partial remission. The following items from the Myeloproliferative Neoplasm Symptom Total Symptom Score were significantly reduced: abdominal discomfort, night sweats, itching, and bone pain. The median JAK2V617F VAF decreased from 47% (95% confidence interval [CI], 35-59) to 7% (95% CI, 3-15), and 60% of patients achieved molecular remission. In conclusion, combination treatment improved cell counts; bone marrow cellularity, and fibrosis; and decreased JAK2V617F VAF; with acceptable toxicity in patients with PV. The trial was registered at www.clinicaltrialsregister.eu as #EudraCT2018-004150-13.
Introduction
In the phase 2 COMBI I trial, we showed that the combination treatment of pegylated interferon alfa-2a (Peg-IFN-α2a) and ruxolitinib (RUX) is safe and efficacious in patients with polycythemia vera (PV) and myelofibrosis.1 The patients in COMBI I were mostly intolerant or refractory to Peg-IFN-α2a monotherapy, and the majority were also refractory or intolerant to hydroxyurea (HU). Most patients needed a reduction in RUX dose to 10 mg twice daily, from the 20 mg twice daily starting dose. In the COMBI II study, we investigated the safety and efficacy of the treatment in newly diagnosed patients with PV with a starting dose of 10 mg twice daily RUX.
IFN-α2 has been used for the treatment of Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) for decades.2 However, 20% to 40% of patients are intolerant or show limited response to Peg-IFN-α2a; moreover, it may not offer complete symptom control.2-6 A new formulation of IFN-α2, ropeginterferon alpha-2b (Ropeg-α2b) has been introduced and the latest follow-up of the randomized CONTINUATION-PV trial shows better event-free survival in the Ropeg-α2b group compared with HU in patients with PV.7 The JAK1/2 inhibitor, RUX is an anti-inflammatory agent used as a first-line treatment in patients with myelofibrosis and a second-line treatment in patients with PV.8,9 In studies in patients with PV who were refractory or intolerant to HU, RUX improves hematocrit control and reduces symptom burden and event-free survival compared with the best available treatment.8-10 Taken together, a combination of RUX and Peg-IFN-α2a seems an attractive option in the treatment of MPNs, and a lower dose of the 2 drugs needed may minimize the side effects and reduce MPN-related symptoms compared with monotherapy.11
Treatment of PV is considered to be lifelong. However, several reports suggest that Peg-IFN-α2a can induce a deep hematologic, molecular, and histological response.12,13 Some of these patients can safely discontinue treatment for several months to years, and restart of Peg-IFN-α2a is effective when cell counts begin to rise. Particularly, patients with a complete hematologic response, for >2 years, and a JAK2V617F variant allele frequency (VAF) <10% can be considered for treatment pause.12 Results from the COMB I trial showed a remarkably high rate of hematologic response and a fast reduction in the JAK2V617F VAF. Therefore, an initial higher-intensity regimen may help to control the disease allowing for treatment pause or low-dose Peg-IFN-α2a monotherapy for several years.14
Herein, we report the safety and efficacy data from the COMBI II trial in 25 patients with newly diagnosed PV followed for 2 years.
Methods
Study design
The COMBI II study trial identifier: EudraCT2018-004150-13 was an investigator-initiated, single-center, open-label, single-arm phase 2 study, which included 25 patients with newly diagnosed PV. The study was carried out from 2019 to 2023 at the Department of Hematology, Zealand University Hospital in Denmark, and approved by the Danish Regional Science Ethics Committee and the Danish Medicines Agency. It was carried out under the principles of the Declaration of Helsinki. Patients gave written informed consent.
Patient material
Patients aged ≥18 years with a PV diagnosis according to the 2016 World Health Organization criteria were considered eligible, if they had evidence of active disease, defined as 1 or more of the following: need for phlebotomy, white blood cell count ≥10 × 109/L, platelet count ≥400 × 109/L, constitutional symptoms, pruritus, symptomatic splenomegaly, or previous thrombosis. Patients fulfilling 1 or more of the following criteria were excluded: pregnancy, allergic hypersensitivity to study medications, Eastern Cooporative Oncology Group performance status ≥3, other active malignancy within 5 years (not including nonmelanoma skin cancer and prostate cancer without the need for treatment), impaired renal or hepatic function; psychiatric disease, severe neurological disease, uncontrolled metabolic disease, severe cardiac disease, white blood cell count <1.5 × 109/L, and platelet count <100 × 109/L. Patients would withdraw from the study if they withdrew consent or did not receive any study medication for 6 months.
Treatment schedule and dosing
Before initiation of treatment, patients were pretreated according to normal practice with phlebotomies. Moreover, high-risk patients, aged ≥60 years or those with prior thromboembolic events, were treated with HU at the investigator’s discretion.15 HU was withheld 1 week before the initiation of protocol treatment. Patients were initially treated with Peg-IFN-α2a (Pegasys; Genentech, Roche, South San Francisco, CA) 45 μg per week and RUX (Jakavi; Novartis, Basel, Switzerland) 10 mg twice daily orally. The medication was self-administered. Dosing was adjusted based on toxicity and efficacy (supplemental Table 1). Acetylsalicylic acid 75 mg per day was given to all patients who had no evidence of bleeding unless treated with another platelet inhibitor or anticoagulant.
Patient evaluations
Study visits were scheduled at baseline, 2 weeks, 1 month, 3 months, and every third month after that until 2 years of treatment. Each visit included documentation of adverse events (AEs), full-scale hematology, blood biochemistry investigations, MPN Symptom Total Symptom Score (TSS),16 and assessment of compliance and registration of AEs by research staff. Bone marrow biopsies were done after 1 and 2 years of treatment and compared with diagnostic biopsies. All patients had a bone marrow biopsy done at diagnosis. Bone marrow biopsies were analyzed by local specialized hematopathologists. Spleen size was assessed by computed tomography (CT) scans of the abdomen. JAK2V617F VAF were quantified using a high-sensitivity, real-time quantitative polymerase chain reaction on whole blood at baseline and after 3 months, 6 months, 1 year, and 2 years.17,18 An amendment has been filed to follow-up patients until 2043 without further intervention.
End points
The primary outcome was safety based on a recording of AEs during treatment and changes in quality-of-life measurements using the TSS questionnaire. The following AEs were routinely graded at every visit: dyspepsia, palpitations, nausea, constipation, abdominal pain, muscle pain, joint pain, bone pain, headache, influenza-like symptoms, fever, fatigue, local irritation at the injection site, itching, upper airway infections, pneumonia, urinary tract infections, weight increase, depressive symptoms, peripheral neuropathy, dizziness, shortness of breath, and night sweats. All AEs were graded according to the Common Terminology Criteria for Adverse Events; version 5.0.
The key secondary outcome was efficacy, based on hematologic parameters and the JAK2V617F VAF. Modified 2013 European LeukemiaNet and International Working Group-Myeloproliferative Neoplasms Research response criteria were used to assess the efficacy.19,20
In brief, complete remission (CR) required resolution of disease-related symptoms and palpable hepatosplenomegaly; peripheral blood count remission (PBCR), with a hematocrit <0.45 without need for phlebotomies for 12 weeks, platelet count <400 × 109/L and white blood cell count <10 × 109/L; no progression of the disease and no hemorrhagic or thrombotic events; and bone marrow histological remission (BMHR), with age-adjusted normocellularity and disappearance of trilinear hyperplasia, and fibrosis grade ≤1. A partial remission (PR) required all of the above except BMHR. A criterion for resolution of disease-related symptoms in patients with PV is large symptom improvement defined as a ≥10-point decrease in TSS. We modified this criterion for patients with a baseline TSS of ≤10 (n = 10). In these patients, durable TSS <10, and no disease-related symptoms reported at the study visit fulfilled the criteria. CT verified splenomegaly was defined as a longitudinal axis >13 cm. Molecular response (MR) was defined as either complete molecular response (CMR), defined as no detectable JAK2V617F mutation (VAF <0.1% in our assay), or partial molecular response, defined as a ≥50% relative decrease in JAK2V617F VAF from baseline in patients with a baseline VAF ≥20%. To compare with previous studies, we also performed an analysis with CMR, set at VAF ≤1%.
Statistical analyses
Statistical analyses were performed using R.3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio 1.0.136 (RStudio, Boston, MA). Remission rates were presented with descriptive statistics. All patients initiating the study treatment were evaluated for response. For analyses of numerical repeated measurements, we used unstructured covariance or unstructured correlation generalized linear mixed models. Non-normally distributed variables were transformed using either logit transformation or log transformation and transformed back accordingly. For analyses of individual symptom scores, we used generalized linear mixed models for ordinal outcomes. An external statistician supervised statistical methods. P values of < .05 were considered statistically significant.
Results
Patient characteristics
We included 25 patients in the study. Baseline characteristics are presented in Table 1. Of the 25 patients included, 5 (20%) had a prior thrombosis, and 19 (76%) were considered high risk based on prior thrombosis or age ≥60 years. The median time from diagnosis to the start of treatment was 67 days.
Baseline characteristics
| No. of patients . | N = 25 . |
|---|---|
| Age, median (IQR) | 70 (58-75) |
| Sex, male (%) | 14 (56) |
| Days from diagnosis to baseline, median (IQR) | 67 (46-90) |
| Phlebotomies from diagnosis to baseline, median (IQR) | 3 (2-5) |
| High risk, n (%) | 19 (76) |
| Prior thrombosis, n (%) | 5 (20) |
| Splenomegaly, n (%) | 3 (12) |
| Hemoglobin (g/dL), median (IQR) | 13.8 (13.4-15.3) |
| Hematocrit, median (IQR) | 0.44 (0.41-0.48) |
| White blood cell count (×109/L), median (IQR) | 9.9 (7.3-11.4) |
| Platelet count (×109/L), median (IQR) | 415 (275-570) |
| JAK2V617F VAF at diagnosis (%), median (IQR) | 50 (35-65) |
| JAK2V617F VAF at baseline (%), median (IQR) | 54 (21-66) |
| No. of patients . | N = 25 . |
|---|---|
| Age, median (IQR) | 70 (58-75) |
| Sex, male (%) | 14 (56) |
| Days from diagnosis to baseline, median (IQR) | 67 (46-90) |
| Phlebotomies from diagnosis to baseline, median (IQR) | 3 (2-5) |
| High risk, n (%) | 19 (76) |
| Prior thrombosis, n (%) | 5 (20) |
| Splenomegaly, n (%) | 3 (12) |
| Hemoglobin (g/dL), median (IQR) | 13.8 (13.4-15.3) |
| Hematocrit, median (IQR) | 0.44 (0.41-0.48) |
| White blood cell count (×109/L), median (IQR) | 9.9 (7.3-11.4) |
| Platelet count (×109/L), median (IQR) | 415 (275-570) |
| JAK2V617F VAF at diagnosis (%), median (IQR) | 50 (35-65) |
| JAK2V617F VAF at baseline (%), median (IQR) | 54 (21-66) |
IQR, interquartile range.
Dropout and toxicity
Of the 25 patients, 1 (4%) dropped out within 2 years. The patient progressed to post-PV myelofibrosis verified on a bone marrow biopsy ∼9 months after initiation of treatment and withdrew his consent to continue in the study. The patient had reticulin fibrosis grade 1 to 2 at baseline. The patient later died of progressive myelofibrosis. Moreover, 1 patient discontinued both drugs while 2 patients discontinued Peg-IFN-alpha2a and 2 patients discontinued RUX, and continued monotherapy with the other drug.
Patient 5 (supplemental Figure 2) discontinued both study drugs; RUX after 15 months due to anemia and Peg-IFN-α2a after 18 months due to side effects. The patient shifted to HU which reduced the symptoms. Patient 9 discontinued RUX due to a tumor in the parotid gland. Histology showed a localized follicular lymphoma which was treated with radiotherapy. Patient 14 paused RUX and Peg-IFN-α2a due to increased liver enzymes; Peg-IFN-α2a was restarted but not RUX, due to normalized cell counts on Peg-IFN-α2a monotherapy. Patient 17 paused Peg-IFN-α2a after 9 months due to unclear neurological symptoms that may have been exacerbated during Peg-IFN-α2a treatment. Peg-IFN-α2a was not restarted and the symptoms were less frequent with PBCR on RUX monotherapy. Patient 22 discontinued Peg-IFN-α2a after 15 months due to anemia and continued RUX monotherapy with PBCR. Doses of study drugs during treatment are presented in supplemental Figures 1 and 2.
The prevalence of all AEs, including disease-related and study drug-unrelated events, observed in ≥10% of the patients and all grade 3 to 4 AEs are presented in Table 2. The AEs routinely asked for are reported in “Methods.” Only a few grade 3 to 4 events were observed. Of note, 1 thromboembolic event, a non-ST elevation myocardial infarction, occurred during the study. The patient had PBCR at the time. Only 1 patient had a grade 3 infection, which included pneumonia and an upper airway infection which required intravenous antibiotics. Shortly thereafter the patient dropped out due to progression to post-PV myelofibrosis. Grade 1 depressive symptoms were reported in 9 (36%) and grade 2 in 4 (16%) of patients; however, no clinical depression was diagnosed, and no psychiatric treatment was needed. These symptoms were mostly intermittent. No patients had to discontinue treatment due to psychiatric symptoms. Grade 1 to 2 anemia was frequent and handled by dose adjustment (Table 2; Figure 1). Notably, 17 patients (68%) grade reported grade 1 to 2 sensory neuropathy at any time point; 9 of these reported the symptom at 3 or fewer time points without symptoms at the final evaluation.
AEs reported
| No. of patients . | N = 25 . |
|---|---|
| Drop-outs, n (%) | 1 (4) |
| Total discontinuation of PEG-IFN-α2a, n (%)∗ | 4 (16) |
| Total discontinuation of RUX, n (%)∗ | 4 (16) |
| No. of patients . | N = 25 . |
|---|---|
| Drop-outs, n (%) | 1 (4) |
| Total discontinuation of PEG-IFN-α2a, n (%)∗ | 4 (16) |
| Total discontinuation of RUX, n (%)∗ | 4 (16) |
| AE . | Grade 1, n (%) . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . |
|---|---|---|---|---|
| Hematologic, n (%) | ||||
| Anemia | 17 (68) | 5 (20) | 0 (0) | 0 (0) |
| Thrombocytopenia | 8 (32) | 1 (4) | 0 (0) | 0 (0) |
| Leukopenia | 12 (48) | 3 (12) | 0 (0) | 0 (0) |
| Nonhematological, n (%) | ||||
| Myocardial infarction | 0 (0.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Palpitations | 13 (52) | 1 (4) | 0 (0) | 0 (0) |
| Chest pain | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Arterial hypertension | 3 (12) | 1 (4) | 2 (8) | 0 (0) |
| Fatigue | 13 (52) | 8 (32) | 3 (12) | 1 (4) |
| Night sweats | 13 (52) | 1 (4) | 0 (0) | 0 (0) |
| Influenza-like symptoms† | 7 (28) | 3 (12) | 0 (0) | 0 (0) |
| Fever | 9 (36) | 0 (0) | 0 (0) | 0 (0) |
| Itching | 16 (64) | 3 (12) | 0 (0) | 0 (0) |
| Rash | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Cheilitis | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Laceration | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Local injection site reactions† | 9 (36) | 0 (0) | 0 (0) | 0 (0) |
| Dry mouth | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Dyspepsia | 10 (40) | 5 (20) | 0 (0) | 0 (0) |
| Abdominal pain | 4 (16) | 5 (20) | 0 (0) | 0 (0) |
| Nausea | 6 (24) | 2 (8) | 0 (0) | 0 (0) |
| Constipation | 10 (40) | 3 (12) | 0 (0) | 0 (0) |
| Increased appetite | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Upper airway infection | 0 (0) | 6 (24) | 1 (4) | 0 (0) |
| Pneumonia | 0 (0) | 1 (4) | 1 (4) | 0 (0) |
| Urinary tract infection | 0 (0) | 6 (24) | 0 (0) | 0 (0) |
| Frequent urination | 3 (12) | 0 (0) | 0 (0) | 0 (0) |
| Decreased renal function | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Kidney stone | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Flank pain | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Weight gain | 11 (44) | 3 (12) | 0 (0) | 1 (4) |
| Joint pain | 11 (44) | 7 (28) | 0 (0) | 0 (0) |
| Bone pain | 3 (12) | 6 (24) | 0 (0) | 0 (0) |
| Muscle pain | 9 (36) | 6 (24) | 0 (0) | 0 (0) |
| Muscle cramps | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Cold fingers | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Depressive symptoms | 9 (36) | 4 (16) | 0 (0) | 0 (0) |
| Insomnia | 3 (12) | 0 (0) | 0 (0) | 0 (0) |
| Neuropathy: sensory | 9 (36) | 8 (32) | 0 (0) | 0 (0) |
| Dizziness | 11 (44) | 6 (24) | 1 (4) | 0 (0) |
| Headache | 7 (28) | 3 (12) | 2 (8) | 0 (0) |
| Eye pain | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Hordeulum | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Increasing hyperopia | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Dyspnea | 8 (32) | 5 (20) | 1 (4) | 0 (0) |
| Follicular lymphoma | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
| Elevated alanine transaminase | 10 (40) | 5 (20) | 0 (0) | 0 (0) |
| Elevated lactate dehydrogenase | 16 (64) | 0 (0) | 0 (0) | 0 (0) |
| AE . | Grade 1, n (%) . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . |
|---|---|---|---|---|
| Hematologic, n (%) | ||||
| Anemia | 17 (68) | 5 (20) | 0 (0) | 0 (0) |
| Thrombocytopenia | 8 (32) | 1 (4) | 0 (0) | 0 (0) |
| Leukopenia | 12 (48) | 3 (12) | 0 (0) | 0 (0) |
| Nonhematological, n (%) | ||||
| Myocardial infarction | 0 (0.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Palpitations | 13 (52) | 1 (4) | 0 (0) | 0 (0) |
| Chest pain | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Arterial hypertension | 3 (12) | 1 (4) | 2 (8) | 0 (0) |
| Fatigue | 13 (52) | 8 (32) | 3 (12) | 1 (4) |
| Night sweats | 13 (52) | 1 (4) | 0 (0) | 0 (0) |
| Influenza-like symptoms† | 7 (28) | 3 (12) | 0 (0) | 0 (0) |
| Fever | 9 (36) | 0 (0) | 0 (0) | 0 (0) |
| Itching | 16 (64) | 3 (12) | 0 (0) | 0 (0) |
| Rash | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Cheilitis | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Laceration | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Local injection site reactions† | 9 (36) | 0 (0) | 0 (0) | 0 (0) |
| Dry mouth | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Dyspepsia | 10 (40) | 5 (20) | 0 (0) | 0 (0) |
| Abdominal pain | 4 (16) | 5 (20) | 0 (0) | 0 (0) |
| Nausea | 6 (24) | 2 (8) | 0 (0) | 0 (0) |
| Constipation | 10 (40) | 3 (12) | 0 (0) | 0 (0) |
| Increased appetite | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Upper airway infection | 0 (0) | 6 (24) | 1 (4) | 0 (0) |
| Pneumonia | 0 (0) | 1 (4) | 1 (4) | 0 (0) |
| Urinary tract infection | 0 (0) | 6 (24) | 0 (0) | 0 (0) |
| Frequent urination | 3 (12) | 0 (0) | 0 (0) | 0 (0) |
| Decreased renal function | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Kidney stone | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Flank pain | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Weight gain | 11 (44) | 3 (12) | 0 (0) | 1 (4) |
| Joint pain | 11 (44) | 7 (28) | 0 (0) | 0 (0) |
| Bone pain | 3 (12) | 6 (24) | 0 (0) | 0 (0) |
| Muscle pain | 9 (36) | 6 (24) | 0 (0) | 0 (0) |
| Muscle cramps | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Cold fingers | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Depressive symptoms | 9 (36) | 4 (16) | 0 (0) | 0 (0) |
| Insomnia | 3 (12) | 0 (0) | 0 (0) | 0 (0) |
| Neuropathy: sensory | 9 (36) | 8 (32) | 0 (0) | 0 (0) |
| Dizziness | 11 (44) | 6 (24) | 1 (4) | 0 (0) |
| Headache | 7 (28) | 3 (12) | 2 (8) | 0 (0) |
| Eye pain | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Hordeulum | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Increasing hyperopia | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Dyspnea | 8 (32) | 5 (20) | 1 (4) | 0 (0) |
| Follicular lymphoma | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
| Elevated alanine transaminase | 10 (40) | 5 (20) | 0 (0) | 0 (0) |
| Elevated lactate dehydrogenase | 16 (64) | 0 (0) | 0 (0) | 0 (0) |
AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs (version 5.0).
Including the patient that dropped out of the study.
Related to injection of PEG-IFN-α2a.
Anemia during treatment. Spaghetti plot of individual hemoglobin values. With lines representing the LLN for female (red dotted line) and male (blue dotted line) patients, along with lines representing CTC grade 2 anemia (black dotted line) and CTC grade 3 anemia (black full line). CTC, Common Terminology Criteria for Adverse Events; LLN, lower limit of normal.
Anemia during treatment. Spaghetti plot of individual hemoglobin values. With lines representing the LLN for female (red dotted line) and male (blue dotted line) patients, along with lines representing CTC grade 2 anemia (black dotted line) and CTC grade 3 anemia (black full line). CTC, Common Terminology Criteria for Adverse Events; LLN, lower limit of normal.
Patient–reported quality-of-life outcomes
During the 2 years of treatment, we observed a reduction in TSS from baseline; however, this was only significant at 2 of the visits: 14 days and 21 months (Figure 2). The following items of the TSS were significantly reduced at more than half of the time points, compared with baseline: abdominal discomfort (P < .001), night sweats (P < .001), itching (P < .05), and bone pain (P < .001). None of the items increased significantly during the study (supplemental Figure 3).
Change in MPN symptom TSS. Left panel shows median TSS with 95% CI. The dotted line represents median value at baseline. Right panel shows spaghetti plot of individual TSS.
Change in MPN symptom TSS. Left panel shows median TSS with 95% CI. The dotted line represents median value at baseline. Right panel shows spaghetti plot of individual TSS.
Response evaluations
At 12 months, the overall remission rate was 52% (13/25 patients); 3 (12%) had CR and 10 (40%) had PR (supplemental Figure 4). At 24 months, the overall remission rate was 56% (14/25 patients); 3 (12%) had CR and 11 (44%) had PR. Two additional patients achieved BMHR at 12 months. Both had complete PBCR but did not fulfill the criteria for symptom remission. Likewise, one additional patient achieved BMHR at 24 months and had PBCR, but did not fulfill the criteria for symptom remission. In total, 5 of 25 (20%) and 4 of 25 (16%) patients had a BMHR at 12 and 24 months, respectively. Moreover, 3 patients had a partial response at 24 months with a technically unsuccessful bone marrow biopsy. One of these had BMHR at 12 months.
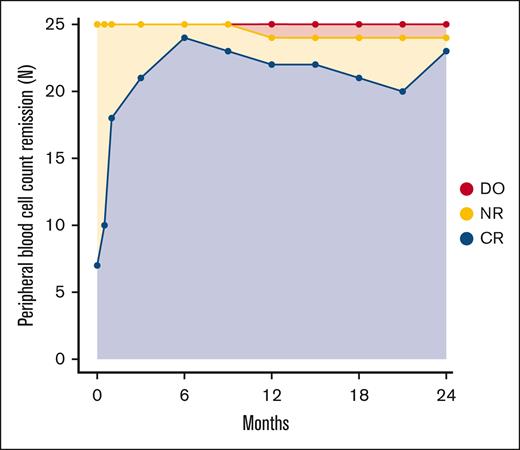
Peripheral blood cell count remission
Of 25 patients with PV, 6 fulfilled the criteria for PBCR at baseline. At 12 months, 22 of 25 patients had PBCR and at 24 months 23 had PBCR (Figure 3). The response over the study period for the individual hematologic parameters is shown in Figure 4. One patient developed severe leukocytosis and was diagnosed with secondary post-PV myelofibrosis (described above). Two patients required phlebotomies after the first 3 months of treatment (supplemental Figure 6). The first patient (patient 11) was administered a reduced dose initially due to anemia, and later required phlebotomies. After dose escalation the patient achieved PBCR. The second patient (patient 14) discontinued RUX as described above and continued Peg-IFN-α2a and later HU after developing a need for phlebotomies.
Rate of peripheral blood cell count remission during treatment. ∗One patient in CR at 24 months was receiving HU after discontinuation of study drugs at 18 months due to side effects. DO, dropout; NR, no remission.
Rate of peripheral blood cell count remission during treatment. ∗One patient in CR at 24 months was receiving HU after discontinuation of study drugs at 18 months due to side effects. DO, dropout; NR, no remission.
Change in hematologic parameters during treatment. Left panel shows absolute change in HCT with 95% CI. The baseline median HCT was 0.44. Middle panel shows relative change in WBC with 95% CI. Baseline WBC was 9.9. Right panel shows relative change in PLA with 95% CI. The baseline median PLA was 415. Note the different y-axis due to the non-normalized distribution of WBC count and PLA. HCT, hematocrit; PLA, platelet count; WBC, white blood cell count.
Change in hematologic parameters during treatment. Left panel shows absolute change in HCT with 95% CI. The baseline median HCT was 0.44. Middle panel shows relative change in WBC with 95% CI. Baseline WBC was 9.9. Right panel shows relative change in PLA with 95% CI. The baseline median PLA was 415. Note the different y-axis due to the non-normalized distribution of WBC count and PLA. HCT, hematocrit; PLA, platelet count; WBC, white blood cell count.
Spleen size
In 3 patients, CT verified splenomegaly was present at baseline. In 2 of these patients, the spleen was normal after 12 months and in all of these, the spleen was normal after 24 months. One patient, the 1 progressing to post-PV myelofibrosis developed splenomegaly during the study.
MR
The overall MR was 52% (13/25 patients) at 12 months and 68% (17/25 patients) at 24 months. No patient obtained complete molecular remission as defined as VAF <0.1 % (the detection limit in our assay). Of 22 evaluable (baseline JAK2V617F VAF ≥20) patients for PR, 13 (59%) and 17 (77%) patients had a partial response at 12 and 24 months, respectively. When using a cutoff of <1%, the overall response was 13 of 25 (52%) at 12 months and 18 of 25 (72%) at 24 months; 4 patients had a CMR at 24 months.
We observed a statistically significant reduction in the JAK2V617F VAF at all time points (P < .001) (Figure 5). The median JAK2V617F VAF after 2 years was 7% (95% confidence interval [CI], 3-15) compared with 47% (95% CI, 35-59) at baseline.
Change in JAK2V617F VAF during treatment. Left panel shows median and 95% CI of JAK2V617F VAF using generalized linear mixed models. Right panel shows spaghetti plot with individual JAK2V617F values. ∗∗∗P < .001.
Change in JAK2V617F VAF during treatment. Left panel shows median and 95% CI of JAK2V617F VAF using generalized linear mixed models. Right panel shows spaghetti plot with individual JAK2V617F values. ∗∗∗P < .001.
All patients with CR had a MR, except one with a baseline JAK2V617F VAF of 8.3% reduced to 0.51% at 24 months, and therefore, did not strictly qualify as having either partial molecular response or CMR.
Discussion
In this study, we have shown that the combination treatment with RUX and low-dose Peg-IFN-α2a has acceptable toxicity and is a highly efficacious treatment, for patients with newly diagnosed PV. This aligns with the findings from the COMBI I trial, which included Peg-IFN intolerant or refractory patients with PV or myelofibrosis.
In total, 4 patients (16%) discontinued Peg-IFN-α2a and 4 (16%) discontinued RUX. Similar, or higher, rates of Peg-IFN-α2a discontinuation were reported in previous studies on Peg-IFN-α2a treatment.4-6,21-24 The discontinuation rate of Peg-IFN-α2a was higher in COMBI I; however, this was expected since all but 1 patient in COMBI I had previously been exposed to Peg-IFN-α2a. In the PROUD-PV study, 20% of patients discontinued Ropeg-α2b, and 15% discontinued HU during the first year. In the second line, discontinuation rates of 15% to 23% have been observed with RUX monotherapy.8-10 Moreover, it is encouraging that the combination was tolerable in a relatively older population with a median age of 70 years. Taken together, combination treatment with RUX and Peg-IFN-α2a does not seem more toxic than the alternative treatment options.
RUX has been shown to increase the risk of infections, particularly in patients with myelofibrosis.8,9,25-27 In COMBI I, we observed a relatively high infection rate with 16% of patients with PV having grade 3 infections. However, in this study, only 1 patient had grade 3 infections, and this was a patient progressing to post-PV myelofibrosis. Moreover, no patients had herpes zoster infection, which has been problematic in prior RUX studies. Patients with PV are likely less vulnerable to infections than MF patients, due to severely impaired immune function in the advanced myelofibrosis stage. Indeed, it is reassuring that only a few severe infections were observed in the present study. The relatively low dose of RUX needed in this study may be associated with a lower risk of infections compared with higher doses needed in monotherapy.10
We observed a high rate of anemia; 68% and 20% had grade 1 or 2 anemia, respectively. Anemia was managed by reduction of doses and no grade 3 to 4 anemia was observed. In comparison, only 13% in the Ropeg-α2b group and 24% in the HU group of the CONTINUATION-PV trial had grade 1 to 2 anemia28; 7% of RUX treated in the MAJIC trial had grade 3 anemia, while grade 1 to 2 were not reported.10 In the end, most patients were treated with lower doses of the drugs than the starting point.
One case of follicular lymphoma was observed which led to discontinuation of RUX due to a report showing an increased risk of aggressive lymphoma in patients with MF being treated with RUX.29 However, later reports have not been able to cooperate with this finding and this event is most likely unrelated to the treatment.30
Surprisingly, 68% of our patients reported sensory neuropathy; however, more than half only reported this symptom at 3 or fewer time points and not at the final evaluation. Our data does not suggest that these symptoms worsened during the treatment. There are no prior studies to suggest that Peg-IFN-α2a or RUX induce neuropathy, including the COMBI I study.1,31,32 Indeed, chronic neuropathy may be a much-overlooked symptom in the elderly population, that is unraveled when patients are being asked regularly about it like in this study.33 However, this issue needs further investigation.
There was a difference in the frequencies of grade 1 to 2 AEs compared with COMBI I. In this study, in which safety was the primary objective, we routinely asked the patient to grade several AEs (described in “Methods”); this was not done in COMBI I study. This will inadvertently result in more AEs not necessarily related to the study drugs. Taken together, we do not believe that the toxicity profile of this treatment should raise concern.
The following items of the TSS were significantly reduced, compared with baseline: abdominal discomfort, night sweats, itching, and bone pain. Fatigue was a common symptom in the patients, and the score did not change during treatment. Reduction of MPN-related symptoms in PV has been reported with RUX in second-line treatment.8-10 Contrarily, in a study comparing HU with Peg-IFN-α2 and in PROUD-PV/CONTI-PV, no reduction in TSS was observed in either group,34 however, in a study in low-risk patients with PV, Ropeg-α2b was associated with better TSS than standard care.35 In our study, the TSS was reduced during treatment but not statistically significant like in COMBI I study. This may be due to the lower baseline symptom burden TSS 14 vs 21 or the fewer patients.
Normalization of blood cell counts may be critical in reducing the risk of thrombosis and improving survival.36-39 Indeed, in the MAJIC trial CR at 12 months was associated with a significantly better thrombosis-free survival than those not in CR.10 Therefore, a key finding of this study is the very fast and sustained normalization of elevated blood counts. Similarly, high rates of hematologic responses have been observed previously with a 90 μg per week starting dose of Peg-IFN-α2 in patients with PV.6,21,22 In the CONTINUATION-PV study, the rates of response after 2 years were ∼70% in the Ropeg-α2b arm and 50% in the HU arm, compared with 92% at 2 years in this study. The rates of response in the MAJIC trial and the RESPONSE 1 and 2 trials were considerably lower; however, these patients were intolerant or refractory to HU treatment and may represent a group of patients with a more aggressive phenotype than patients in our study.8,9
In total, 20% and 16% of patients fulfilled the criteria for BMHR at 1 year and 2 years, respectively. These findings suggest a disease-modifying effect of this treatment.19,20 These findings support similar findings from the COMBI I study. Profound effects of Peg-IFN-α2a on bone marrow histology have been observed before, but usually after longer treatment durations.13,40-42 The marked reduction in the JAK2V617F VAF and a 52% MR rate add further evidence of a selective effect of the treatment on the malignant clone. Similar findings were observed in patients treated with Peg-IFN-α2a, and in the RESPONSE I study where 34% achieved MR after a median of 25 months of RUX treatment.6,22,43,44 In the CONTINUATION-PV study, 68% of Ropeg-α2b–treated and 33% of HU-treated patients that rolled over from the PROUD-PV study had MR after 2 years of follow-up.
The clinical benefit of an MR is still unclear, but higher JAK2V617F VAF may be associated with a higher risk of thromboembolic events and progression to myelofibrosis.38,45 The latest data from the CONTINUATION-PV study and MAJIC-PV study show that patients with MR have a better event-free survival than patients not in MR.10,46 In this study, all patients with CR had MR. This aligns with findings from COMBI I showing an association between MR and both remission and PBCR. Similarly, during Ropeg-α2b treatment, an association between MR and hematologic response was observed.28,47 Likewise, in a phase 2 study of Peg-IFNα2, 94% of patients with MR also had a hematologic response, and 10 of 13 patients with complete bone marrow response, also achieved MR.5,41
The inclusion criteria used in this trial do not directly reflect the European LeukemiaNet guidelines for the initiation of cytoreductive treatment.48 Indeed, both high- and low-risk patients with PV were included. This is similar to most contemporary clinical trials in patients with PV where criteria like splenomegaly, need for frequent phlebotomies, vasomotor symptoms, diabetes or hypertension has been considered high-risk factors 6,10,22,47,49 In Denmark, Peg-IFN-α2 may be administered on wider indications than internationally, and a strategy of early intervention targeting the malignant clone has previously been described.50 Likewise, a recent study in low-risk PV supports the use of IFN in the early disease stage.35 Indeed, combination treatment as an early intervention strategy may induce deep clinical, histological, and molecular remission more frequently than with PEG-IFN-α2 monotherapy.6,13,22,41,43,51 Thus, the inclusion criteria used in our study should be considered when comparing our data, with studies using other inclusion criteria and studies of real-world populations. Achieving deep remission could lead to periods of observation without treatment or maintenance treatment with a low dose of PEG-IFN-α2a, which may improve the quality of life in patients with MPNs.12 This may be particularly important in younger patients with a need for treatment for decades.
A strength of our study is the investigation of bone marrow remissions, which is essential in assessing a possible disease-modifying effect of new treatments.19,20 In addition, we used the MPN TSS questionnaire to assess the symptom burden during the treatment. In patients with MPNs, balancing the efficacy with the toxicity of treatment is essential, and accordingly, measurement of the quality-of-life outcomes is a key clinical end point.19,20 The study was planned for 2 years of treatment, but hematologic and histologic responses have been observed after several years of interferon treatment, and the response after 2 years may underestimate the long-term clinical effect.13,40-42 Post hoc analyses with longer follow-up periods are being planned to assess the long-term response to combination treatment and rate of treatment discontinuation in patients, who have obtained deep MRs.
In conclusion, the COMBI II study shows a reassuring toxicity profile of combination therapy with RUX and Peg-IFN-α2a in newly diagnosed patients with PV while supporting results from COMBI I study showing a very high rate of hematologic response, and a swift reduction in the JAK2V617F VAF, along with histological responses and even normalization of bone marrow in a subset of patients within 2 years. This study supports the previously described theory that combination therapy with RUX and Peg-IFN-α2a may be 1 of the most promising treatment options in patients with MPNs.52 Further analysis of this study will show how many patients can achieve stable disease without the need for cytoreductive treatment.
Acknowledgments
M.E.B. has received funding for his PhD along with 2 travel grants from Novartis. C.E. is partly funded by the Laboratory Medicine Endowment Fund of Boston Children's Hospital. Novartis provided free ruxolitinib for the study.
Authorship
Contribution: A.L.S., H.H., and M.E.B. designed the study; H.H., C.L.A., and U.M.O. included patients in the trial; H.H. and M.E.B. treated patients in the trial; V.S., L.K., C.T., and L.M.R.G. gathered data for the trial for example, analysis of blood samples, bone marrow biopsies, and radiological analysis; A.L.S. did the statistical analysis and wrote the first draft of the manuscript; and all authors contributed to the analysis of data and final wording of the manuscript.
Conflict-of-interest disclosure: H.H. is on the advisory boards of Orphan Pharmaceuticals and Incyte. C.L.A. reports consultancy for GlaxoSmithKline, Orphan Pharmaceuticals, Pfizer, AstraZeneca, AbbVie, Novartis, Haleon, and Bristol Myers Squibb. T.A.K. has received fees from Global Healthcare Network Pharma Nordic. The remaining authors declare no competing financial interests.
Correspondence: Anders Lindholm Sørensen, Hæmatologisk Afdeling, Sjællands Universitetshospital, Roskilde, Sygehusvej 10, 4000 Roskilde, Denmark; email: anderslindholmsorensen@hotmail.com.
References
Author notes
Data are available on request from the corresponding author, Anders Lindholm Sørensen (anderslindholmsorensen@hotmail.com).
The full-text version of this article contains a data supplement.