Key Points
TAFA combined with LEN showed limited efficacy after CAR T-cell failure in a high-risk population.
Outcomes were comparable between the TAFA-LEN combination and other treatments for the first progression after CAR T-cell therapy.
Visual Abstract
Tafasitamab plus lenalidomide (TAFA-LEN) treatment relevance pre- or post-anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is debated. We analyzed patients with large B-cell lymphoma in the DESCAR-T registry treated with axi[1]cel or tisa-cel in ≥3rd line and TAFA-LEN before (n = 15, “TL-pre-CAR-T” set) or directly after (n = 52, “TL-post-CAR-T” set) CAR T-cell therapy. We compared TAFA-LEN v. other treatments using inverse probability weighting in the TL-post-CAR[1]T set. In the TL-post-CAR-T set, the median progression-free survival (mPFS), overall survival (mOS), and duration of response (mDOR) since the first treatment for progression (mPFS2/mOS2/mDOR2) were 3, 4.7, and 8.1 months, respectively. The best overall response rate (bORR) and best complete response rate (bCRR) after TAFA-LEN were 13.5% and 7.7%, respectively. Outcomes were better for patients who relapsed >6 months after CAR T-cell therapy (mPFS2: 5.6 vs 2 months, P = .0138; mOS2: not reached vs 3.8 months, P = .0034). The bORR and bCRR between TAFA-LEN and other treatments were 20.6% vs 24.9% and 11.6% vs 15.6%, respectively. Outcomes were similar between TAFA-LEN and other treatments (mPFS2: 2.9 vs 2.4 months, P = .91; mOS2: 3.3 vs 5.5 months, P = .06). In an exploratory analysis of the TL-pre-CAR-T set, the median TAFA-LEN treatment duration before CAR-T was 3.7 months with no patient becoming CD19 negative. The bORR, bCRR, 6- month PFS, and OS rates after CAR T-cell infusion were 45.5%, 36.4%, 20.1%, and 58.2%, respectively. Neither TAFA-LEN nor comparative salvage treatment improved outcomes for patients relapsing after CAR T-cell therapy.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has been the standard of care for relapsed/refractory large B-cell lymphomas (R/R LBCLs) since 2018.1-3 Despite the efficacy of these treatments, durable responses and curative effects have been reported in <40% of patients.4-7 The most common mechanism of resistance to CAR T cells is the loss of the target antigen, which is observed in ∼30% of patients with LBCL due to negative selection pressure.1,4,8-10 Commercial CAR T-cell therapies all target CD19, a 95-kDa transmembrane glycoprotein belonging to the immunoglobulin superfamily, which is found on the surface of both normal and tumor B lymphocytes.11,12 CD19 expression is commonly measured via immunohistochemistry (IHC) of formalin-fixed paraffin-embedded tumor biopsies or flow cytometry of live suspended cells. The most widely used clone is LE-CD19, which contains a cytosolic epitope (the intracellular domain of the protein). However, this technique does not determine whether CD19 integrity is maintained after anti-CD19 treatment.9,13 Commercially available CAR T-cell therapies currently used for the treatment of R/R LBCL include a single-chain variable fragment derived from a murine monoclonal antibody (mAb) against CD19 known as the FMC63 clone.14,15 However, despite the high cost of CAR T-cell therapy and the existence of simple techniques to detect the expression of CD19, health authorities and official labels do not require evidence of CD19 positivity before the administration of anti-CD19 CAR T-cell therapies. Responses were observed in CD19-negative patients after anti-CD19 CAR T-cell therapy in the ZUMA-1 clinical trial1 but the overall efficacy of these therapies in CD19-negative patients remains largely uncertain.
Additionally, tafasitamab (TAFA) is a cytolytic anti-CD19 mAb approved by the US Food and Drug Administration in 2020 and by the European Medical Agency in 2021 for the treatment of adult patients with R/R LBCL in combination with lenalidomide (LEN). TAFA is produced from a humanized murine anti-CD19 antibody, specifically the 4G7 clone,16 and has a terminal elimination half-life of 17 days.17 Emerging data have suggested that administering TAFA before anti-CD19 CAR T-cell therapy could lead to a delay in CD19 re-expression or demasking in pre-CAR T-cell biopsy samples, potentially interfering with patient treatment plans in centers that restrict CD19-targeted CAR T-cell therapy to patients with preserved CD19 expression.18 Furthermore, using a TAFA-competing antibody to detect CD19 expression in samples from patients exposed to TAFA may result in a reduction in signal intensity and confusion between CD19 epitope masking and antigen loss.19 In vitro data have revealed direct competition for CD19 binding between TAFAs and CD19-targeted CAR T-cell therapy in mantle cell lymphoma, LBCL, and B-cell acute lymphoblastic leukemia cell lines. There is a partial overlap between the 4G7 and FMC63 clones at an epitope centered on residue R144, without compromising CAR T-cell activity in terms of antigen-specific killing, degranulation, cytokine production, or proliferation.20 However, there is a scarcity of clinical data regarding the sequence of the 2 anti CD19 therapies in patients with R/R LBCL. Initial data on a limited number of patients or xenograft models seem to indicate that the use of an anti-CD19 mAb followed by an anti-CD19 CAR T-cell therapy does not appear to be detrimental and does not seem to cause the CD19 target antigen to disappear.21-23 Consequently, it remains uncertain whether exposure to one form of anti-CD19 therapy might compromise the effectiveness or safety of the other. Therefore, to better explore this topic, we aimed to assess outcomes in patients with R/R LBCL treated with TAFA-LEN as a pre- or post-anti-CD19 CAR T-cell therapy in real-life settings in France.
Patients and methods
Patients and data collection
This study is a retrospective analysis of data from adult patients with R/R LBCL collected before 1 September 2023 in the DESCAR-T registry who received TAFA-LEN as a pre- (previous treatment line or bridge: “TL-pre-CAR-T set”) or post-anti-CD19 CAR T-cell therapy (to treat the first progression after CAR T cell: “TL-post-CAR-T set”) between 2018 and 2023 (supplemental Methods). The analysis was restricted to patients who received CAR T-cell therapy with marketing authorization in France for treating LBCL in the third line or later (L3+). To contextualize the results observed in patients who received TAFA-LEN directly after CAR T-cell therapy, we performed an indirect comparison with data from other patients with LBCL from the DESCAR-T registry who were refractory to treatment or relapsed after CAR T-cell therapy was administered in L3+ and who did not receive TAFA-LEN for their first TL-post-CAR T-cell therapy (supplemental Methods). This study was approved by the Lymphoma Study Association (LYSA) (10 January 2023) and DESCAR-T (17 January 2023) Scientific Committees. Patients’ nonopposition to participation in this study was obtained through a written information note provided before inclusion in the registry. DESCAR-T is registered under the ClinicalTrials.gov identifier NCT04328298, and the study was conducted in accordance with the Declaration of Helsinki.
Study end points
The coprimary end points were progression-free survival (PFS), overall survival (OS), and duration of response (DOR) after treatment for the first progression post-CAR T-cell therapy, defined as PFS2, OS2, and DOR2, respectively, in the TL-post-CAR-T set. Secondary end points in the TL-post-CAR-T set were CAR T-cell-related safety events, response rates, PFS, and OS after CAR T-cell infusion, outcome according to CD19 status on biopsy if available, and indirect comparisons of outcomes between TAFA-LEN and other treatments as the first treatment for progression after CAR T-cell therapy. PFS2 and OS2 were compared between patients treated with TAFA-LEN and LEN (single agent) post hoc.
In an exploratory analysis, we evaluated the duration of the response to TAFA-LEN, washout time between the last TAFA-LEN administration and CAR T-cell infusion, CD19 status before TAFA-LEN, response rate, CAR T-cell-related safety events, and PFS/OS after CAR T-cell infusion in the TL-pre-CAR-T set.
Indirect comparison procedures
We conducted an inverse probability weighting (IPW) comparison of TAFA-LEN v. other treatments (chemotherapy, bispecific anti-CD3/anti-CD20 antibody, transplant, radiotherapy, kinase inhibitors, and other anticancer therapies, excluding anti-CD19 antibodies) and v. LEN (single agent) in the TL-post-CAR-T set. The patients included in the comparison were identified in the DESCAR-T registry, which included ∼1500 patients treated with CAR T-cell therapy for LBCL in L3+ (details provided in supplemental Methods).
Statistical considerations
Quantitative variables are displayed as means, standard deviations, medians, ranges, and quartiles. Qualitative variables are expressed as frequencies and percentages. Response rates, including complete response (CR) and partial response rates, are expressed as percentages with 95% exact Clopper-Pearson confidence intervals (supplemental Methods). Survival curves were obtained using the Kaplan-Meier method. P values for survival analysis were calculated using the log-rank test unless otherwise specified (supplemental Methods). The level of significance for each test was 5%. Statistical analyses were performed using SAS software version 9.4 and AdClin version 3.4.0.
Results
TL-post-CAR-T set
Patient characteristics and outcomes after CAR T-cell infusion and before TAFA-LEN initiation
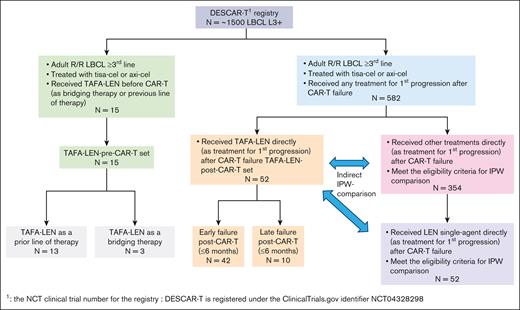
Within the DESCAR-T registry, we identified 52 patients treated with TAFA-LEN for first progression after CAR T-cell infusion in 18 different French centers (Figure 1).
Study flowchart. DESCAR-T, Dispositif d'Enregistrement et Suivi des patients traités par CAR T cells.
Study flowchart. DESCAR-T, Dispositif d'Enregistrement et Suivi des patients traités par CAR T cells.
The patient characteristics are summarized in Table 1. Briefly, these characteristics were as follows: median age, 65 years; Eastern Cooperative Oncology Group (ECOG) score 0 to 1, 88%; age-adjusted international prognostic index (aaIPI) 2 to 3, 61.7%; median prior lines of therapy (PLT) at the time of CAR T-cell treatment, 2; and refractory disease (defined as no response to the last line of therapy before enrollment), 85.4%. For some patients, only 1 PLT was recorded within the DESCAR-T registry by investigators, but 31 (60%) and 21 (40%) patients received CAR T-cell therapy in the third line and fourth line, respectively.
Patient characteristics in the TL-post-CAR-T set at the time of enrollment in the DESCAR-T registry
| . | First progression after CAR T-cell therapy . | |
|---|---|---|
| N = 52 . | ||
| Age (y) | ||
| Median (min; max) | 65.5 (20; 81) | |
| Age ≥65 y | 29 | (55.8%) |
| Sex, male | 30 | (57.7%) |
| ECOG | ||
| 0-1 | 44 | (88.0%) |
| >2 | 6 | (12.0%) |
| Missing | 2 | |
| LDH > normal | ||
| No | 21 | (41.2%) |
| Yes | 30 | (58.8%) |
| Ann Arbor stage | ||
| I-II | 6 | (12.0%) |
| III-IV | 44 | (88.0%) |
| Missing | 2 | |
| aaIPI | ||
| 0 | 1 | (2.1%) |
| 1 | 17 | (36.2%) |
| 2 | 28 | (59.6%) |
| 3 | 1 | (2.1%) |
| Missing | 5 | |
| Histology | ||
| LBCL | 39 | (75.0%) |
| Transformed FL or other indolent | 12 | (23.1%) |
| PMBL | 1 | (1.9%) |
| HCT-CI-comorbidity evaluated | 52 | (100.0%) |
| At least 1 HCT-CI-comorbidity | 25 | (48.1%) |
| Nb of PLT before CAR T-cell therapy∗ | ||
| Median (min; max) | 2.0 (1; 6)† | |
| Prior autologous transplant | 5 | (9.6%) |
| Time from initial diagnosis to CAR T-cell therapy eligibility (y) | ||
| Median (min; max) | 1.46 (0.2; 21.0) | |
| Disease status before leukapheresis | ||
| Refractory | 41 | (85.4%) |
| Relapse | 7 | (14.6%) |
| Missing | 4 | |
| BT | 44 | (84.6%) |
| Disease status before CAR T-cell infusion | ||
| CR | 3 | (6.8%) |
| Partial response (PR) | 11 | (25.0%) |
| Stable disease | 4 | (9.1%) |
| Progressive disease | 25 | (56.8%) |
| Not evaluated | 1 | (2.3%) |
| CAR T product administered | ||
| Tisa-cel | 21 | (40.4%) |
| Axi-cel | 31 | (59.6%) |
| . | First progression after CAR T-cell therapy . | |
|---|---|---|
| N = 52 . | ||
| Age (y) | ||
| Median (min; max) | 65.5 (20; 81) | |
| Age ≥65 y | 29 | (55.8%) |
| Sex, male | 30 | (57.7%) |
| ECOG | ||
| 0-1 | 44 | (88.0%) |
| >2 | 6 | (12.0%) |
| Missing | 2 | |
| LDH > normal | ||
| No | 21 | (41.2%) |
| Yes | 30 | (58.8%) |
| Ann Arbor stage | ||
| I-II | 6 | (12.0%) |
| III-IV | 44 | (88.0%) |
| Missing | 2 | |
| aaIPI | ||
| 0 | 1 | (2.1%) |
| 1 | 17 | (36.2%) |
| 2 | 28 | (59.6%) |
| 3 | 1 | (2.1%) |
| Missing | 5 | |
| Histology | ||
| LBCL | 39 | (75.0%) |
| Transformed FL or other indolent | 12 | (23.1%) |
| PMBL | 1 | (1.9%) |
| HCT-CI-comorbidity evaluated | 52 | (100.0%) |
| At least 1 HCT-CI-comorbidity | 25 | (48.1%) |
| Nb of PLT before CAR T-cell therapy∗ | ||
| Median (min; max) | 2.0 (1; 6)† | |
| Prior autologous transplant | 5 | (9.6%) |
| Time from initial diagnosis to CAR T-cell therapy eligibility (y) | ||
| Median (min; max) | 1.46 (0.2; 21.0) | |
| Disease status before leukapheresis | ||
| Refractory | 41 | (85.4%) |
| Relapse | 7 | (14.6%) |
| Missing | 4 | |
| BT | 44 | (84.6%) |
| Disease status before CAR T-cell infusion | ||
| CR | 3 | (6.8%) |
| Partial response (PR) | 11 | (25.0%) |
| Stable disease | 4 | (9.1%) |
| Progressive disease | 25 | (56.8%) |
| Not evaluated | 1 | (2.3%) |
| CAR T product administered | ||
| Tisa-cel | 21 | (40.4%) |
| Axi-cel | 31 | (59.6%) |
ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; HCT-CI, hematopoietic stem cell transplant comorbidity index; LDH, lactate dehydrogenase; max, maximum; min, Minimum; PMBL, primary mediastinal B-cell lymphoma.
Up to 10 treatment lines can be collected from the register.
Ongoing query within the DESCAR-T registry for patients with only 1 line of prior treatment.
The best overall response (bORR) rate, best complete response rate (bCRR), 6-month PFS, and OS after the first CAR T-cell infusion (Tisagenlecleucel [tisa-cel]: n = 21; Axicabtagene ciloleucel [axi-cel]: n = 31) were 63.5%, 34.6%, 21.2%, and 62.3%, respectively (supplemental Table 1; supplemental Figure 1). Failure after CAR T-cell therapy occurred after a median of 2.8 months (range, 0.4-34.7). Overall, 42 patients experienced early (ie, ≤6 months) failure after CAR T-cell therapy, and 10 experienced late (>6 months) failure.
Safety was evaluated in all patients. The all-grade cytokine release syndrome (CRS) and neurotoxicity rates were 82.7% (grade 3-4: n = 2, 3.8%) and 40.4% (grade 3-4: n = 7, 13.5%), respectively. Prolonged cytopenia and grade 3 to 5 infections occurred in 38 (73.1%) and 24 (46.2%) patients, respectively. In patients with CAR T-cell-specific toxicity (n = 45/52), the rates of tocilizumab use and intensive care unit admission were 71.1% and 17.8%, respectively.
Response rates and outcomes after TAFA-LEN initiation for first progression after CAR T-cell therapy
The median (Q1; Q3) time between CAR T-cell infusion and TAFA-LEN initiation was 2.84 (1.1; 5.7) months. The outcomes of 2 patients who received TAFA without LEN according to the DESCAR-T registry were reported by the investigators but were not excluded from the present intention-to-treat analysis.
After a median follow-up duration of 7 (5.9-13) months, the median PFS and OS since the first treatment for progression (mPFS2/mOS2) were 3 (95% confidence interval, 1.9-4.2) and 4.7 (3-5.6) months, respectively; the median (Q1; Q3) DOR2 was 8.1 (1; NA) months (supplemental Figure 2). The mPFS2 increased when TAFA-LEN was initiated >6 months after CAR T-cell infusion (late CAR T-cell failure subgroup): 5.6 (3.2-NA) v. 2 (1.6-3.1), P = .0138. In addition, mOS2 was not reached (5.6-NA) when TAFA-LEN was introduced later than the 6-month cutoff (v. 3.8 [2.2-5] months) for patients in the ≤6-month subset (P = .0034) (Figure 2). The causes of death after the first treatment for progression were lymphoma (91.4%), concurrent illness (5.7%), and late toxicity (2.9%) (Table 2). Failure after TAFA-LEN occurred after a median of 3.35 months (0.5-9.9). Three late-relapsing patients remained in CR at the last contact.
Outcomes Based on Timing of Progression After CAR T-Cell Therapy. PFS (A) and OS (B) since treatment for the first progression (PFS2 and OS2) in the TL-post-CAR-T set according to early (≤6 months) vs late (>6 months) TAFA-LEN introduction. CI, confidence interval; NA, not available.
Outcomes Based on Timing of Progression After CAR T-Cell Therapy. PFS (A) and OS (B) since treatment for the first progression (PFS2 and OS2) in the TL-post-CAR-T set according to early (≤6 months) vs late (>6 months) TAFA-LEN introduction. CI, confidence interval; NA, not available.
Causes of death since the first treatment for progression in the TL-post-CAR-T set
| . | TL-post-CAR-T set . | Period of first progression ≤6 mo . | Period of first progression >6 mo . | |||
|---|---|---|---|---|---|---|
| N = 52 . | n = 42 . | n = 10 . | ||||
| Event according to OS definition since first treatment for progression | ||||||
| No | 17 | (32.7%) | 8 | (19.0%) | 9 | (90.0%) |
| Yes | 35 | (67.3%) | 34 | (81.0%) | 1 | (10.0%) |
| If yes, cause of death | ||||||
| Progression | 32 | (91.4%) | 31 | (91.2%) | 1 | (100.0%) |
| Concurrent illness | 2 | (5.7%) | 2 | (5.9%) | 0 | (0.0%) |
| Late toxicity | 1 | (2.9%) | 1 | (2.9%) | 0 | (0.0%) |
| . | TL-post-CAR-T set . | Period of first progression ≤6 mo . | Period of first progression >6 mo . | |||
|---|---|---|---|---|---|---|
| N = 52 . | n = 42 . | n = 10 . | ||||
| Event according to OS definition since first treatment for progression | ||||||
| No | 17 | (32.7%) | 8 | (19.0%) | 9 | (90.0%) |
| Yes | 35 | (67.3%) | 34 | (81.0%) | 1 | (10.0%) |
| If yes, cause of death | ||||||
| Progression | 32 | (91.4%) | 31 | (91.2%) | 1 | (100.0%) |
| Concurrent illness | 2 | (5.7%) | 2 | (5.9%) | 0 | (0.0%) |
| Late toxicity | 1 | (2.9%) | 1 | (2.9%) | 0 | (0.0%) |
Outcomes according to CD19 status
CD19 expression before CAR T-cell infusion, after CAR T-cell infusion, and after TAFA-LEN treatment is summarized in supplemental Table 3. In most patients, CD19 expression was not routinely assessed. The bCRR after CAR T-cell therapy was numerically lower in patients who were negative for CD19 before CAR T-cell therapy than in patients who were positive for CD19 (n = 2/7, 28.6% v. n = 5/12, 41.7%; supplemental Table 4). The mOS after CAR T-cell infusion was lower in patients with negative vs positive CD19 expression before CAR T-cell infusion (6 [1.5; NA] vs 8 [2.9; NA] months), but the median PFS was similar (2.8 [0.9; 3.2] vs 2.7 [1; 7.8] months; supplemental Figure 3). mPFS2 and OS2 did not differ according to the CD19 status before CAR T-cell therapy (supplemental Figure 4). mPFS2 and OS2, according to the CD19 status after CAR T-cell therapy were also similar (supplemental Figure 5).
Comparison between TAFA-LEN and other treatments as initial therapies for progression after CAR T-cell therapy
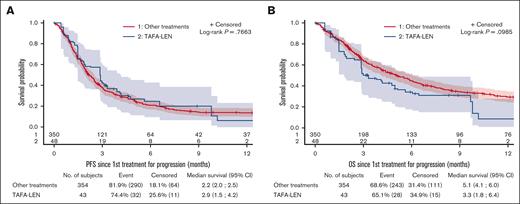
After IPW, outcomes were compared between 43 patients treated with TAFA-LEN (including 2 patients with TAFA only) and 354 control patients treated with other agents (immunomodulatory drug, 47.2%; chemotherapy, 25.7%; CD3/CD20 bispecific antibodies, 9.9%; and others, 17.2%; supplemental Table 5). A diagram illustrating the constitution of the efficacy comparison cohort is provided in supplemental Figure 6, including justification for the exclusion of patients due to missing values for weighting variables or other reasons. Patient characteristics were well balanced between the groups, with standardized mean differences <0.15 for all weighting variables (data not shown), but the median (Q1; Q3) follow-up was longer in the control group (19.1 [10.9; 30.1] vs 13 [4.6; 16.2] months). The bORR and bCRR between the TAFA-LEN and control groups were 20.6% v. 22.1% and 11.6% v. 13.8%, respectively (supplemental Table 6). PFS and OS were not significantly different between the 2 groups (median PFS, 2.9 [2-2.6] vs 2.2 [2-2.5] months, P = .77; median OS, 3.3 [1.8-6.4] vs 5.1 [4.1-6] months, P = .1; Figure 3). All the sensitivity analyses led to the same conclusions (supplemental Figure 8).
Comparison between TAFA-LEN and other treatments as initial therapies for progression after CAR T-cell therapy. PFS (A) and OS (B) since the first treatment for progression after CAR T-cell therapy according to IPW using stabilized weight (SW) method between TAFA-LEN and other treatments.
Comparison between TAFA-LEN and other treatments as initial therapies for progression after CAR T-cell therapy. PFS (A) and OS (B) since the first treatment for progression after CAR T-cell therapy according to IPW using stabilized weight (SW) method between TAFA-LEN and other treatments.
Comparison between TAFA-LEN and LEN as initial therapies for progression after CAR T-cell therapy
After IPW, outcomes were compared between 43 patients treated with TAFA-LEN (including 2 patients treated with TAFA only) and 52 control patients treated with LEN (supplemental Figure 7). Patient characteristics were well balanced between groups with standardized mean differences <0.15 for all weighting variables (data not shown), but the median follow-up was longer in the control group: 22.8 (8.5; 30.2) v. 6.2 (3.3; 13) months. The bORR and bCRR between the TAFA-LEN and LEN groups were 13.9% v. 16.4% (P = .74) and 6% v. 13.4% (P = .2), respectively (supplemental Table 7). PFS and OS were similar between the TAFA-LEN and LEN groups (median PFS: 3 [1.5-4.6] vs 1.8 [1-2.4] months, hazard ratio = 0.71 [0.44-1.13]; median OS 4.2 [2.4-6] vs 3.3 [2.3-3.9] months, hazard ratio = 0.88 [0.54-1.44]; Figure 4).
Comparison between TAFA-LEN and LEN as initial therapies for progression after CAR T-cell therapy. PFS (A) and OS (B) since the first treatment for progression after CAR T-cell therapy according to IPW using SW between TAFA-LEN and LEN (single agent).
Comparison between TAFA-LEN and LEN as initial therapies for progression after CAR T-cell therapy. PFS (A) and OS (B) since the first treatment for progression after CAR T-cell therapy according to IPW using SW between TAFA-LEN and LEN (single agent).
TL-pre-CAR-T set
Patient characteristics
Within the DESCAR-T registry, we identified 15 patients who received TAFA-LEN at any time before CAR T-cell infusion, as a PLT or bridging therapy (BT), in 12 different French centers (Figure 1). The patients’ characteristics were as follows: median (range) age, 71 (47-80) years; male sex, 66.7%; ECOG score 0 to 1, 86.7%; elevated lactate dehydrogenase, 50%; stage III to IV, 84.6%; aaIPI 2 to 3, 41.7%; median PLT, 3 (2-7); refractory disease, 100%; and prior autologous transplant, 6.7% (Table 3). TAFA-LEN was given as a PLT before CAR T-cell therapy in 13 patients. Among them, only 2 patients received no treatment between TAFA-LEN and CAR T-cell infusion, and all the other patients received a different BT. In addition, TAFA-LEN was given as a bridge before CAR T-cell therapy in 3 patients (1 patient received TAFA-LEN for both PLT and BT) (supplemental Table 8).
Patient characteristics in the TL-pre-CAR-T set at the time of enrollment in the DESCAR-T registry
| . | TL-pre-CAR-T set . | |
|---|---|---|
| N = 15 . | ||
| Age (y) | ||
| Median (min; max) | 71.0 (47; 80) | |
| Age ≥65 y | 10 | (66.7%) |
| Sex, male | 10 | (66.7%) |
| ECOG | ||
| 0-1 | 13 | (86.7%) |
| >2 | 2 | (13.3%) |
| LDH > normal | ||
| No | 7 | (50.0%) |
| Yes | 7 | (50.0%) |
| Missing | 1 | |
| Ann Arbor stage | ||
| I-II | 2 | (15.4%) |
| III-IV | 11 | (84.6%) |
| Missing | 2 | |
| aaIPI | ||
| 1 | 7 | (58.3%) |
| 2 | 4 | (33.3%) |
| 3 | 1 | (8.3%) |
| Missing | 3 | |
| Histology | ||
| LBCL | 11 | (73.3%) |
| Transformed FL | 3 | (20.0%) |
| Transformed Hodgkin | 1 | (6.7%) |
| HCT-CI-comorbidity evaluated | 15 | (100.0%) |
| At least 1 HCT-CI-comorbidity | 7 | (46.7%) |
| Nb of PLT before CAR-T∗ | ||
| Median (min; max) | 3.0 (2; 7) | |
| Prior autologous transplant | 1 | (6.7%) |
| Time from the initial diagnosis to CAR T-cell eligibility (y) | ||
| Median (min; max) | 1.62 (0.5; 18.2) | |
| Disease status before leukapheresis | ||
| Refractory | 14 | (100.0%) |
| Missing | 1 | |
| Disease status before CAR T-cell infusion | ||
| CR | 2 | (13.3%) |
| PR | 3 | (20.0%) |
| Progressive disease | 10 | (66.7%) |
| CAR T product administered | ||
| Tisa-cel | 3 | (20.0%) |
| Axi-cel | 12 | (80.0%) |
| . | TL-pre-CAR-T set . | |
|---|---|---|
| N = 15 . | ||
| Age (y) | ||
| Median (min; max) | 71.0 (47; 80) | |
| Age ≥65 y | 10 | (66.7%) |
| Sex, male | 10 | (66.7%) |
| ECOG | ||
| 0-1 | 13 | (86.7%) |
| >2 | 2 | (13.3%) |
| LDH > normal | ||
| No | 7 | (50.0%) |
| Yes | 7 | (50.0%) |
| Missing | 1 | |
| Ann Arbor stage | ||
| I-II | 2 | (15.4%) |
| III-IV | 11 | (84.6%) |
| Missing | 2 | |
| aaIPI | ||
| 1 | 7 | (58.3%) |
| 2 | 4 | (33.3%) |
| 3 | 1 | (8.3%) |
| Missing | 3 | |
| Histology | ||
| LBCL | 11 | (73.3%) |
| Transformed FL | 3 | (20.0%) |
| Transformed Hodgkin | 1 | (6.7%) |
| HCT-CI-comorbidity evaluated | 15 | (100.0%) |
| At least 1 HCT-CI-comorbidity | 7 | (46.7%) |
| Nb of PLT before CAR-T∗ | ||
| Median (min; max) | 3.0 (2; 7) | |
| Prior autologous transplant | 1 | (6.7%) |
| Time from the initial diagnosis to CAR T-cell eligibility (y) | ||
| Median (min; max) | 1.62 (0.5; 18.2) | |
| Disease status before leukapheresis | ||
| Refractory | 14 | (100.0%) |
| Missing | 1 | |
| Disease status before CAR T-cell infusion | ||
| CR | 2 | (13.3%) |
| PR | 3 | (20.0%) |
| Progressive disease | 10 | (66.7%) |
| CAR T product administered | ||
| Tisa-cel | 3 | (20.0%) |
| Axi-cel | 12 | (80.0%) |
Abbreviations are explained in Table 1.
Up to 10 treatment lines may be collected from the DESCAR-T registry.
For patients receiving TAFA-LEN before treatment (n = 13), the median (Q1; Q3) duration of treatment with TAFA-LEN was 3.7 (2; 7.4) months, and the median (Q1; Q3) washout time between the last TAFA-LEN administration and CAR T-cell infusion was 2.7 (2.1; 3.4) months (supplemental Table 9). The bORR after TAFA-LEN as a PLT was not collected in the registry.
For patients receiving TAFA-LEN as BT (n = 3), the median (Q1; Q3) duration of bridging with TAFA-LEN was 23 (14; 58) days, with a median washout time of 20 (3; 31) days. The responses after bridging with TAFA-LEN were partial response (n = 2) and progressive disease (n = 1) (supplemental Table 10).
Description of CD19 status
CD19 status (IHC) before TAFA-LEN was positive in 4 patients (26.7%), negative in 1 (6.7%), and not tested/not done in 10 (66.7%). On subsequent biopsy (if available) after TAFA-LEN and before CAR T-cell infusion, CD19 status was re-evaluated as follows: positive (n = 3, 20%), negative (n = 1, 6.7%), or not tested/not done (n = 11, 73.3%). No patients were reported to become CD19 negative after TAFA-LEN. After CAR T-cell infusion, 1 additional patient became negative for CD19 (supplemental Table 3).
Outcomes after CAR T-cell infusion
With a median (Q1; Q3) follow-up duration of 2.8 (1; 6.3) months, the bORR, bCRR, 6-month PFS rate, and OS rate after CAR T-cell infusion (tisa-cel: n = 3; axi-cel: n = 12) were 45.5%, 36.4%, 20.1%, and 58.2%, respectively (Table 4; Figure 5; supplemental Figure 9). Notably, we did not analyze treatments administered in the event of progression after CAR T-cell infusion in this patient set (2 patients received and responded to subsequent treatment for progression after CAR T-cell infusion).
Best response after CAR T-cell infusion in the TL-pre-CAR-T set
| . | Evaluable patients in the TL-pre-CAR-T set . | |
|---|---|---|
| n = 11/15 . | ||
| Best response | ||
| CR | 4 | (36.4%) |
| PR | 1 | (9.1%) |
| Stable disease | 1 | (9.1%) |
| Progressive disease | 4 | (36.4%) |
| Not evaluated | 1 | (9.1%) |
| bORR∗ | ||
| bORR | 5 | (45.5%) |
| 95% confidence interval | (16.7%-76.6%) | |
| Time to best response (months) | ||
| N | 5 | |
| Missing | 0 | |
| Mean (SD) | 0.97 (0.055) | |
| Median | 0.99 | |
| Q1; Q3 | 1.0; 1.0 | |
| P1; P99 | 0.9; 1.0 | |
| . | Evaluable patients in the TL-pre-CAR-T set . | |
|---|---|---|
| n = 11/15 . | ||
| Best response | ||
| CR | 4 | (36.4%) |
| PR | 1 | (9.1%) |
| Stable disease | 1 | (9.1%) |
| Progressive disease | 4 | (36.4%) |
| Not evaluated | 1 | (9.1%) |
| bORR∗ | ||
| bORR | 5 | (45.5%) |
| 95% confidence interval | (16.7%-76.6%) | |
| Time to best response (months) | ||
| N | 5 | |
| Missing | 0 | |
| Mean (SD) | 0.97 (0.055) | |
| Median | 0.99 | |
| Q1; Q3 | 1.0; 1.0 | |
| P1; P99 | 0.9; 1.0 | |
SD, standard deviation.
Defined as (CR + PR).
Outcomes after CAR T-cell infusion. PFS (A) and OS (B) after CAR T-cell infusion in the TL-pre-CAR-T set.
Outcomes after CAR T-cell infusion. PFS (A) and OS (B) after CAR T-cell infusion in the TL-pre-CAR-T set.
The safety was evaluated in 14 patients. The all-grade CRS and neurotoxicity rates were 64.3% and 14.3%, respectively (supplemental Figure 9). No grade 3 to 4 CRS was observed. In patients with CAR T-cell-specific toxicity (n = 10/14), the rates of tocilizumab use and intensive care unit admission were 30% and 10%, respectively. The rates of prolonged cytopenia and infection were 50% and 14.3%, respectively. Causes of death were lymphoma (n = 4, 66.7%), concurrent illness (n = 1, 16.7%), and acute toxicity (n = 1, 16.7%; data not shown).
Discussion
In this retrospective study of the French DESCAR-T registry, we reported the largest cohort to date of patients treated sequentially (either before or directly after CAR T-cell infusion) with 2 therapies targeting CD19: commercial anti-CD19 CAR T-cell therapy and the TAFA-LEN combination.
In the TL-post-CAR T-cell cohort, our results confirmed the poor prognosis of patients who relapsed after CAR T-cell therapy regardless of the treatment received. The median PFS was 3 months and the median OS was 4.7 months. This population displayed high-risk features, with 57.6% having aaIPI 2 to 3 disease, and we observed a slight overrepresentation of patients treated with tisa-cel (40.4%), compared with 27% in the study by Tomas et al and 36% in the study by Di Blasi et al.24,25 Our results are consistent with those for other cohorts in the literature regarding the outcomes of post-CAR T-cell-treated patients who subsequently benefited from subsequent lines of treatment. Indeed, the median PFS and OS after the first CAR T-cell treatment were 1.9 months and 8.5 months, respectively, in the study by Tomas A et al, and 2.8 months and 5.2 months, respectively, in the study by Di Blasi et al24,25 In the cohort of the US lymphoma CAR-T consortium in L3+, the outcome was also poor, with a mPFS2 of just 55 days.26 L2+ patients who received treatment for the first progression after CAR T in the experimental arm of ZUMA-7 displayed also a poor outcome, with a median PFS2 and OS2 of 1.7 and 8.1 months, respectively, after salvage chemotherapy.27 Checkpoint inhibitors were also not an effective salvage strategy for most patients after CAR T-cell failure, with median PFS2 and OS2 of 54 and 159 days, respectively, as reported by Major et al.28 Notably, Sesques et al recently described a cohort of 67 patients treated with glofitamab, a CD20 × CD3 bispecific antibody, for first progression after CAR T-cell therapy in a recent clinical trial (NCT04703686). Although the mPFS was short (∼4.5 months), the bORR (65.9%) and bCRR (36.4%) indicated antitumor efficacy, and the mOS was not reached with a follow-up of ∼10 months.29 Epcoritamab, another CD20 × CD3 bispecific antibody, also demonstrated antitumor efficacy (bORR, 54.1%; bCRR, 34.4%; median PFS, 4.4 months; and median OS, not reached) in patients who relapsed after CAR T-cell therapy (n = 61) in a recent clinical trial (NCT03625037).30 Therefore, targeting an epitope other than CD19 after CAR T-cell failure using a CD3 × CD20 bispecific antibody seems to be an interesting approach. However, more in-depth subgroup analyses are needed to identify the patients who could benefit the most from this strategy.
In a recent study reported in the form of an abstract at the American Society of Hematology (ASH) conference in 2023, the response and survival rates after TAFA-LEN in a real-life setting appeared lower than those reported in the pivotal Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND) study.31 In that study, the median PFS was 2.9 months (including all causes of death in progression events) and the median OS was 8.9 months, which is quite similar to the results observed in the TL-post-CAR T-cell cohort. In patients who received TAFA-LEN after CAR T-cell therapy in the study by Ruckdeschel et al (n = 24), the median PFS was 7.9 months, and the median OS was 9.2 months. Post-TAFA-LEN bCRR was lower in patients who were already exposed to CAR T-cell therapy (9.7% v. 21%), similar to our observation (bCRR TL-post-CAR-T set, 7.7%). However, outcomes after anti-CD19 mAb given post-CD19 CAR T-cell therapy were superior in another recently published real-life study involving a cohort of 53 patients treated with loncastuximab tesirin or TAFA-LEN.32 In that study, the bORR and bCRR were 27% and 10%, respectively (compared with 13.5% and 7.7%, respectively, in our TL-post-CAR T set), and the median PFS and OS were 8 and 20.9 months, respectively (compared with 3 and 4.7 months, respectively, in our study). Nevertheless, in that study, patients received a median of 1 line of treatment (range 0-5) between CAR T-cell and anti-CD19 mAb, with a median time of 7.3 (1.2-38.2) months between the 2 rounds of anti-CD19 immunotherapy, whereas in our cohort, patients received TAFA-LEN immediately after the first post-CAR T-cell therapy (median time between CAR T-cell therapy and the start of TAFA-LEN: 2.84 months). In addition, our exploratory analysis revealed that the outcome was better in the late CAR T-cell failure subgroup than that in the early failure subgroup (>6 months after CAR T-cell infusion). We speculate that a washout period of several months, probably >6 months, is associated with a better efficacy of subsequent anti-CD19 treatment. However, this interpretation could be biased because patients who relapse <3 months after CAR T-cell therapy have a well-known worse prognosis24,25 and because we were not able to make an indirect comparison between TAFA-LEN and other treatments in the subset of patients with late CAR T-cell failure (for whom the sample size was too low). We acknowledge the survival analysis because CAR T-cell infusion requires careful analysis because patients administered TAFA-LEN were alive before TAFA-LEN administration, creating an immortality bias. However, our main analyses focused on efficacy since the first progression/TAFA-LEN initiation, and not after CAR T-cell therapy.
Regarding the TL-pre-CAR-T set (n = 15), the limited sample size and short follow-up period prevented definitive conclusions from being drawn and hindered the creation of a relevant matched control cohort; therefore, we could not determine the specific impact of TAFA-LEN on post-CAR T-cell outcomes. In our opinion, TAFA-LEN is poorly represented in the DESCAR-T registry as a PLT because physicians fear CD19 expression loss by providing patients with anti-CD19 therapy when they can potentially benefit from anti-CD19 CAR T-cell therapy at subsequent lines. Patients in the TL-pre-CAR T-cell cohort presented high-risk characteristics, with a median age of 71 years, refractory disease in all patients, and a median of 3 previous lines of treatment, probably partly explaining the poor outcomes of these patients after CAR T-cell therapy (median OS, 8.1 months; median PFS, 3 months). The DESCAR-T registry did not include data on the best response to treatment with TAFA-LEN as a PLT (n = 13) or other treatments given before treatment with CAR T-cell therapy. However, the median duration of treatment with TAFA-LEN as a PLT was short (2.7 months), and all patients were reported to have refractory disease before leukapheresis; therefore, we speculate that TAFA-LEN was not effective in this selected subgroup. Notably, by definition, the TAFA-LEN efficacy assessment was biased in this subgroup because the patients were selected because they received CAR T-cell therapy after TAFA-LEN failure. It was recently reported that CAR T-cell therapies remained effective in patients with R/R LBCL after prior exposure to bispecific antibodies targeting different antigens33 but there is a scarcity of data in the literature regarding the outcomes of patients who are followed by CAR T-cell therapy. In a case series of 8 patients treated with TAFA-LEN before CAR T-cell therapy, 4 patients achieved subsequent CR after CAR T-cell therapy.34 Another study reported the use of anti-CD19 treatment, but unlike TAFA, loncastuximab tesirine (antibody-drug conjugated) was administered to 14 patients who subsequently received (median 120 days later) anti-CD19 CAR T-cell therapy. The response rate was 50%, and no survival data were available, with a median follow-up of only 6 months.22 Preclinical data recently demonstrated that prior treatment with TAFA increased the antitumor activity of CAR T cells in vitro and reduced the severity of CRS.23 These data have not been confirmed in clinical trials or real-life studies of the TAFA-LEN association. We could not compare adverse event rates in the TL-pre-CAR-T set with those in the TL-post-CAR-T cohort because the 2 populations were different, and we could not identify a cohort of comparable control patients (who would have received treatments other than TAFA-LEN before CAR T-cell therapy) in the DESCAR-T registry. In addition, TAFA efficacy in treating B-cell malignancies involves several mechanisms beyond its interaction with CD19, including antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and apoptosis induction in B cells through signaling pathways that do not involve immune cell mediation.17,35
Concerning the role of CD19 (IHC) as a potential biomarker of response to treatment with TAFA or CAR T-cell therapy, we were not able to study it with sufficient power, given that this staining is largely not performed in practice (in almost 3-quarters of patients) in a real-life setting. In France, neither histologically proving relapse/progression using rebiopsy nor checking CD19 positivity is required to prescribe CAR T-cell therapy, which certainly contributed to the low amount of data concerning CD19 status that we were able to collect. Additionally, it is important to note that the DESCAR-T registry does not collect histological reports or material. There is also no consensus on the specific IHC antibody to use for quantifying CD19 expression, nor on the cutoff percentage of stained cells necessary to consider a result positive. Therefore, for all these reasons, providing data on CD19 status, including retrospectively, is challenging. However, we observed that 7 of 52 (13.5%) patients in the TL-post-CAR-T cohort were negative for CD19 before CAR T-cell therapy, which did not prevent them from receiving this therapy, and that this status was not checked by another biopsy before the introduction of TAFA-LEN as the next line of therapy. Overall, CD19 status has rarely been studied in patients receiving anti-CD19 treatment, and this marker is rarely correlated with the response to treatment.31,34 In a study in which loncastuximab-tesirin was used before CAR-T cell therapy, 10 of 14 patients were CD19-positive after loncastuximab-tesirin.22 Further studies are required to better decipher the role of CD19 as a biomarker to tailor anti-CD19 therapies and to better understand the clinical relevance of targeting CD19 at different lines of therapy in patients with LBCL. We will strongly encourage the biopsy of patients and prospective evaluation of CD19 antigen loss in future studies, given its relevance to many available and investigational anti-CD19 therapies.
To conclude, this analysis confirmed the poor outcome of patients who relapsed after CAR T-cell therapy in L3+. The efficacy of TAFA-LEN administered directly after CAR T-cell therapy in this high-risk population was modest and similar to that of other salvage treatments, but the outcome improved when progression occurred later than 6 months after CAR T-cell therapy.
Acknowledgments
The authors thank the patients whose data were collected from the DESCAR-T registry and their families, and all the investigators and their staff involved in the data collection and analyses. The authors thank everyone from the Lymphoma Academic Research Organization (LYSARC) DESCAR-T study group who actively participated in the study, especially D. Laurenceau, C. Joubert, and E. Gat from the biostatistics department. The authors thank Christophe Bonnet, who reviewed this manuscript for the LYSA Scientific Committee.
This work was supported by Incyte Corporation and the LYSARC. The DESCAR-T registry was partly funded by Gilead and Novartis; however, they did not participate in the study design, data collection, statistical analysis, or interpretation, and did not provide assistance for manuscript writing or editorial support.
Authorship
Contribution: V.C., F.J., V.D., and A.M. contributed to the study design; V.C., R.H., G.B., B.T., S.B., P.S., J.D., D.K., L.O., F.L., K.B., A.C., L.T., J.A., C.H., F.M., G.L.D., S.G., S. Carras., L.-M.F., S. Choquet., O.H., J.P., A.C., O.C., L.D.L.R., C.C.-L., M.J., and S.L.G. enrolled and treated patients; V.D. performed the statistical analysis; V.C. wrote the manuscript; and all authors edited the manuscript, provided final approval for the article, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: V.C. has received honoraria from Incyte, AbbVie, AstraZeneca, Bristol Myers Squibb, Ideogen, Janssen, Kyowa Kirin, Kite/Gilead, Lilly, Novartis, Octapharma, Pfizer, Pierre Fabre, Sanofi, and Takeda. P.S. has received honoraria from Chugai, BMS, Novartis, Janssen, Kite/Gilead, AbbVie, and Roche. J.P. has received honoraria from Incyte. L.D.L.R. has received honoraria from Novartis and Kite/Gilead. The remaining authors declare no competing financial interests.
Correspondence: Vincent Camus, Department of Hematology, Centre Henri Becquerel, 1 rue d'Amiens, 76000 Rouen, France; email: vincent.camus@chb.unicancer.fr.
References
Author notes
Data from the DESCAR-T registry are subject to controlled access by the Lymphoma Academic Research Organization owing to privacy and legal requirements and proprietary reasons. Anonymized individual patient (or participant) data (IPD) requests will be promptly reviewed by the corresponding author, Vincent Camus (vincent.camus@chb.unicancer.fr) and the scientific committee of the DESCAR-T registry.
The full-text version of this article contains a data supplement.