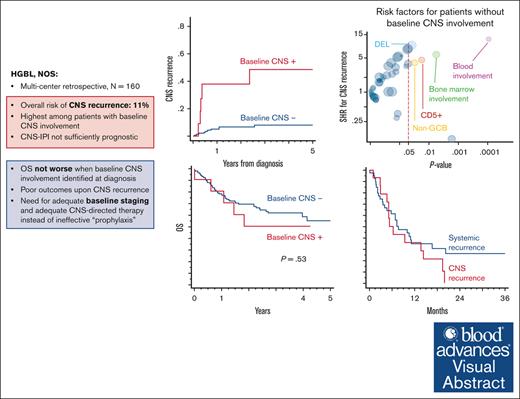
Key Points
CNS involvement is uncommon in HGBL NOS but highly prognostic for future CNS recurrence.
CNS recurrence was higher in patients with HGBL with blood or marrow involvement, CD5 expression, non–GCB cell of origin, and “DEL” phenotype.
Visual Abstract
Little is known about the central nervous system (CNS) risk in high-grade B-cell lymphoma, not otherwise specified (HGBL NOS). Hence, we sought to describe the rates of baseline CNS involvement, risk of CNS recurrence after primary therapy, and management strategies in HGBL NOS. In this multicenter retrospective study, we included 160 adults with newly diagnosed HGBL NOS treated between 2016 and 2021 at 20 US institutions. Eleven patients (7%) had baseline CNS involvement at diagnosis (leptomeningeal = 6, parenchymal = 4, and both = 1). Baseline CNS involvement was significantly associated only with MYC rearrangement (OR = 3.5) and testicular (in men) or female pelvic (in women) involvement (OR = 8.1). There was no significant difference in survival outcomes between patients with HGBL NOS with (median PFS = 4 years) or without (median PFS = 2.4 years) baseline CNS involvement (P = 0.45). The cumulative incidence of CNS recurrence at 3 years was 11%. Patients with baseline CNS involvement were at the highest risk (48.5% vs 8% for those without baseline CNS involvement) and were excluded from the risk factors analysis for CNS recurrence. The risk for CNS recurrence was significantly associated with blood or bone marrow involvement, CD5 expression, non–germinal center B-cell subtype, and “dual-expresser lymphoma” phenotype, however, high CNS IPI was not. The prognosis of relapsed HGBL NOS was poor, regardless of whether recurrence was systemic or limited to the CNS, and with currently available salvage strategies, including autologous transplantation and chimeric antigen receptor T-cell modalities, almost all patients with CNS recurrence ultimately succumbed to their disease.
Introduction
Central nervous system (CNS) involvement in patients with aggressive B-cell lymphomas poses a major therapeutic challenge. The site of CNS involvement may be parenchymal, leptomeningeal, or both.1,2 CNS progression or relapse in patients with aggressive B-cell lymphoma may be an isolated event or can occur synchronously with systemic relapse; both scenarios are associated with dismal prognosis.3-6 Several high-risk clinical (CNS International Prognostic Index [CNS-IPI]7 and multiple8 and specific9-15 extranodal sites) and biological (high-grade B-cell lymphoma [HGBL] with MYC and BCL2 or BCL6 rearrangements)16 factors associated with baseline CNS involvement and relapse have been well elucidated. However, little is known about the CNS recurrence risk in patients with HGBL not otherwise specified (NOS), a particularly aggressive but heterogeneous disease that involves morphologically high-grade tumors of variable biology.17-19 This entity encompasses mature B-cell tumors previously described as B-cell lymphomas, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma, those with blastoid morphology but a mature immunophenotype, as well as other rare entities that cannot be otherwise classified.20,21 The relevance of CNS-IPI has not been evaluated in the context of HGBL NOS, which typically presents with both high-risk clinical features (advanced stage, elevated lactate dehydrogenase [LDH], and involvement of multiple extranodal sites and bone marrow) and specific biology often resembling Burkitt or lymphoblastic lymphoma.22,23 Given the paucity of data, we sought to describe the rates of baseline CNS involvement, risk of CNS recurrence after primary therapy, and management strategies in patients with HGBL NOS. These neoplasms were diagnosed using the 2016 World Health Organization criteria and are derived from a large clinicopathologic data set of HGBL NOS cases diagnosed by experienced pathologists.22
Methods
Study design
This multicenter retrospective study included adults (age of ≥18 years at diagnosis) with newly diagnosed HGBL NOS treated between 2016 and 2021 at 20 US institutions. The study was approved by the institutional review boards at all participating sites and was conducted in compliance with the Declaration of Helsinki. Cases of HGBL NOS were selected locally by investigators (clinicians and pathologists), with a central review of all available pathology reports. Furthermore, expert hematopathologists from 10 participating institutions undertook a more detailed local review with direct inspection of biopsy slides to confirm fulfillment of World Health Organization criteria for their local HGBL NOS cases in 61% of cases, as described previously.22 We excluded any tumors consistent with DLBCL, Burkitt lymphoma, lymphoblastic lymphoma/leukemia, or blastoid mantle cell lymphoma by local or central determination. Additionally, cases not tested for MYC rearrangement or those with MYC and BCL2 and/or BCL6 rearrangements by local fluorescent in situ hybridization assay (double/triple-hit) were excluded. Cases of HGBL NOS presenting as a transformation from other histologies or as a posttransplant lymphoproliferative disorder were eligible. All staging, immunohistochemical, or molecular evaluations, including any baseline CNS imaging or cerebrospinal fluid examinations as well as treatments, were completed at the discretion of the treating physicians according to institutional practice.
Study variables and objectives
Investigators collected demographic, clinicopathologic, treatment, and outcome data using a standardized protocol. Serum LDH level was normalized to the local upper limit of normal and performance status was reported according to the Eastern Cooperative Group scale. Cell of origin (COO) was assigned using the immunohistochemical Hans algorithm.24 Cases with concurrent expression of B-cell lymphoma protein 2 (BCL2; ≥50%) and MYC (≥40%) determined by immunohistochemistry were designated as “dual-expresser lymphoma” (DEL). Cytomorphology was classified as Burkitt lymphoma-like, blastoid, or undetermined. When performed, we noted the presence of rearrangements of MYC (MYC-R, by definition only “single-hit”), BCL2 (BCL2-R), and BCL6 (BCL6-R), or extra copies of these genes shown by fluorescent in situ hybridization. Similar to prior observational studies of high-grade lymphomas,25,26 treatment regimens were classified as standard intensity, including R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone) with or without high-dose methotrexate (HDMTX) for CNS prophylaxis, or intensified (including DA-EPOCH-R [dose-adjusted cyclophosphamide, doxorubicin, vincristine, etoposide, with rituximab and prednisone],27 R-CODOX-M/IVAC [rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate, ifosfamide, etoposide, and cytarabine],28 and R-hyperCVAD/MA [rituximab, hyperfractionated cyclophosphamide, doxorubicin, vincristine, methotrexate, and cytarabine]).29 CNS-directed prophylactic therapy and risk of subsequent CNS recurrence were examined in a subset (n = 149) of patients without baseline CNS involvement.
Statistical analysis
Categorical variables were compared using the Fisher exact test, and continuous variables using rank-sum test. The association between patient characteristics and baseline CNS involvement was estimated using logistic regression model reporting odds ratios. Survival analysis was conducted using the Kaplan-Meier method; the analysis of cumulative incidence function for CNS recurrence accounted for competing risk (of systemic recurrence or death without CNS recurrence). Progression-free survival (PFS) was defined as the time from the start of first-line therapy until lymphoma relapse, progression, death from any cause, or censoring at the last clinical assessment. Overall survival (OS) was defined as the time from diagnosis until death from any cause or censoring at the last clinical assessment. All estimates are provided with 95% confidence intervals (95% CIs). In this exploratory study, we did not apply corrections for multiple testing, using P < .05 as indicator of statistical significance. Data analysis was conducted using Stata/SE 17.0 (College Station, TX).
Results
Patient characteristics
The study included 160 patients. The median age was 64 years (range, 49-72), 82% of tumors had germinal center B-cell like (GCB) immunophenotype, and 27% had (“single-hit”) MYC rearrangement. Most patients had advanced-stage disease (63%) and serum LDH greater than the upper limit of normal (74%). CNS-IPI could be calculated in n = 152 patients and was low in 34 (22%), intermediate in 70 (46%), and high in 48 (32%).
Eleven (7%) patients had baseline CNS involvement present clinically at diagnosis or discovered during staging evaluation (leptomeningeal = 6, parenchymal = 4, and both = 1; Table 1). When studied using univariate logistic regression, baseline CNS involvement was significantly associated with MYC rearrangement (6/11 cases; odds ratio, 3.5; 95% CI, 1.01-12.2; P = .048) and testicular or female pelvic (1 ovary, 2 unspecified extranodal pelvic site) involvement (2/11 cases; odds ratio, 8.1; 95% CI, 1.3-50.0; P = .02; supplemental Figure 1).
Patient characteristics of HGBL NOS based on CNS involvement at baseline
| Variable . | All patients N = 160 (%) . | Without CNS involvement, n = 149 . | With CNS involvement∗ n = 11 (%) . |
|---|---|---|---|
| Age (y), median, range | 64 (49-72) | 64 (51-72) | 54 (31-68) |
| Sex | |||
| Males | 108 (68) | 102 (69) | 6 (55) |
| Females | 52 (32) | 47 (31) | 5 (45) |
| Poor ECOG PS | 33 (21) | 31 (22) | 2 (20) |
| Advanced stage | 109 (63) | 99 (68) | 10 (91) |
| >1 extranodal site | 66 (41) | 60 (40) | 6 (55) |
| Kidney/adrenal involvement | 20 (13) | 17 (11) | 3 (27) |
| Testicular or female pelvis involvement | 6 (4) | 4 (3) | 2 (18) |
| BM involvement | 39 (24) | 35 (24) | 4 (36) |
| B symptoms | 59 (39) | 55 (39) | 4 (36) |
| LDH > ULN | 110 (74) | 100 (73) | 10 (91) |
| LDH > 3× ULN | 37 (25) | 34 (25) | 3 (27) |
| IPI | |||
| Low | 34 (23) | 33 (24) | 1 (10) |
| Intermediate low | 27 (18) | 26 (19) | 1 (10) |
| Intermediate high | 42 (29) | 35 (26) | 7 (70) |
| High | 44 (30) | 43 (31) | 1 (10) |
| CNS-IPI | |||
| Low | 34 (22) | 33 (23) | 1 (10) |
| Intermediate | 70 (46) | 64 (45) | 6 (60) |
| High | 48 (32) | 45 (32) | 3 (30) |
| Morphology | |||
| Burkitt lymphoma-like | 72 (45) | 65 (44) | 7 (64) |
| Blastoid | 38 (24) | 34 (23) | 4 (36) |
| Unreported | 50 (31) | 50 (34) | 0 |
| COO by IHC | |||
| GCB-like | 131 (82) | 122 (82) | 2 (10) |
| Non-GCB | 27 (17) | 25 (17) | 9 (90) |
| Undetermined | 2 (1) | 2 (1) | 0 |
| BCL6 expression | |||
| Positive | 120 (80) | 111 (80) | 9 (82) |
| Negative | 30 (20) | 28 (20) | 2 (18) |
| BCL2 expression | |||
| Positive | 83 (54) | 76 (53) | 7 (64) |
| Negative | 71 (46) | 67 (47) | 4 (36) |
| MYC expression | |||
| Positive | 102 (72) | 95 (72) | 7 (70) |
| Negative | 40 (28) | 37 (28) | 3 (30) |
| Dual MYC and BCL2 expresser | |||
| Yes | 52 (36) | 48 (36) | 4 (36) |
| No | 94 (64) | 87 (64) | 7 (64) |
| CD5 expression | |||
| Positive | 20 (14) | 19 (14) | 1 (11) |
| Negative | 122 (86) | 114 (86) | 8 (89) |
| MYC rearrangement | |||
| Yes | 44 (27) | 38 (25) | 6 (55) |
| No | 116 (73) | 111 (75) | 5 (45) |
| BCL2 rearrangement | |||
| Yes | 17 (13) | 17 (14) | 0 |
| No | 117 (87) | 108 (86) | 9 (100) |
| BCL6 rearrangement | |||
| Yes | 15 (11) | 15 (12) | 0 |
| No | 119 (89) | 111 (88) | 8 (100) |
| First-line therapy | |||
| DA-R-EPOCH | 68 (43) | 66 (45) | 2 (18) |
| R-CHOP | 53 (33) | 51 (35) | 2 (18) |
| R-CODOX-M/IVAC | 11 (7) | 8 (5) | 3 (27) |
| R-hyperCVAD/MA | 6 (4) | 4 (3) | 2 (18) |
| Other | 16 (10) | 14 (9) | 2 (18) |
| Untreated | 4 (2) | 4 (3) | 0 |
| HDMTX given | |||
| Yes | 38 (25) | 31 (22) | 7 (63) |
| No | 114 (75) | 110 (78) | 4 (36) |
| Variable . | All patients N = 160 (%) . | Without CNS involvement, n = 149 . | With CNS involvement∗ n = 11 (%) . |
|---|---|---|---|
| Age (y), median, range | 64 (49-72) | 64 (51-72) | 54 (31-68) |
| Sex | |||
| Males | 108 (68) | 102 (69) | 6 (55) |
| Females | 52 (32) | 47 (31) | 5 (45) |
| Poor ECOG PS | 33 (21) | 31 (22) | 2 (20) |
| Advanced stage | 109 (63) | 99 (68) | 10 (91) |
| >1 extranodal site | 66 (41) | 60 (40) | 6 (55) |
| Kidney/adrenal involvement | 20 (13) | 17 (11) | 3 (27) |
| Testicular or female pelvis involvement | 6 (4) | 4 (3) | 2 (18) |
| BM involvement | 39 (24) | 35 (24) | 4 (36) |
| B symptoms | 59 (39) | 55 (39) | 4 (36) |
| LDH > ULN | 110 (74) | 100 (73) | 10 (91) |
| LDH > 3× ULN | 37 (25) | 34 (25) | 3 (27) |
| IPI | |||
| Low | 34 (23) | 33 (24) | 1 (10) |
| Intermediate low | 27 (18) | 26 (19) | 1 (10) |
| Intermediate high | 42 (29) | 35 (26) | 7 (70) |
| High | 44 (30) | 43 (31) | 1 (10) |
| CNS-IPI | |||
| Low | 34 (22) | 33 (23) | 1 (10) |
| Intermediate | 70 (46) | 64 (45) | 6 (60) |
| High | 48 (32) | 45 (32) | 3 (30) |
| Morphology | |||
| Burkitt lymphoma-like | 72 (45) | 65 (44) | 7 (64) |
| Blastoid | 38 (24) | 34 (23) | 4 (36) |
| Unreported | 50 (31) | 50 (34) | 0 |
| COO by IHC | |||
| GCB-like | 131 (82) | 122 (82) | 2 (10) |
| Non-GCB | 27 (17) | 25 (17) | 9 (90) |
| Undetermined | 2 (1) | 2 (1) | 0 |
| BCL6 expression | |||
| Positive | 120 (80) | 111 (80) | 9 (82) |
| Negative | 30 (20) | 28 (20) | 2 (18) |
| BCL2 expression | |||
| Positive | 83 (54) | 76 (53) | 7 (64) |
| Negative | 71 (46) | 67 (47) | 4 (36) |
| MYC expression | |||
| Positive | 102 (72) | 95 (72) | 7 (70) |
| Negative | 40 (28) | 37 (28) | 3 (30) |
| Dual MYC and BCL2 expresser | |||
| Yes | 52 (36) | 48 (36) | 4 (36) |
| No | 94 (64) | 87 (64) | 7 (64) |
| CD5 expression | |||
| Positive | 20 (14) | 19 (14) | 1 (11) |
| Negative | 122 (86) | 114 (86) | 8 (89) |
| MYC rearrangement | |||
| Yes | 44 (27) | 38 (25) | 6 (55) |
| No | 116 (73) | 111 (75) | 5 (45) |
| BCL2 rearrangement | |||
| Yes | 17 (13) | 17 (14) | 0 |
| No | 117 (87) | 108 (86) | 9 (100) |
| BCL6 rearrangement | |||
| Yes | 15 (11) | 15 (12) | 0 |
| No | 119 (89) | 111 (88) | 8 (100) |
| First-line therapy | |||
| DA-R-EPOCH | 68 (43) | 66 (45) | 2 (18) |
| R-CHOP | 53 (33) | 51 (35) | 2 (18) |
| R-CODOX-M/IVAC | 11 (7) | 8 (5) | 3 (27) |
| R-hyperCVAD/MA | 6 (4) | 4 (3) | 2 (18) |
| Other | 16 (10) | 14 (9) | 2 (18) |
| Untreated | 4 (2) | 4 (3) | 0 |
| HDMTX given | |||
| Yes | 38 (25) | 31 (22) | 7 (63) |
| No | 114 (75) | 110 (78) | 4 (36) |
BM, bone marrow; DA-R-EPOCH, dose adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; ECOG, Eastern Cooperative Oncology Group; IHC, immunohistochemistry; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CODOX-M/IVAC, rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate, ifosfamide, etoposide, and cytarabine; PS, performance status; ULN, upper limit of normal.
Leptomeningeal = 6, parenchymal = 4, both = 1.
Survival of patients with HGBL NOS and baseline CNS involvement
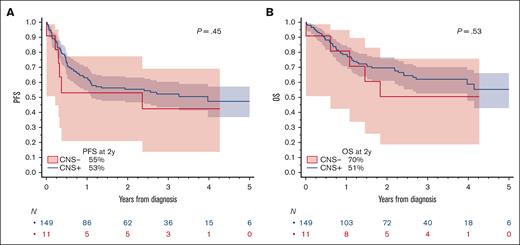
Median PFS was 4.0 years (95% CI, 1.1 to not reached) for patients without, and 2.4 years (95% CI, 0.2 to not reached) for patients with baseline CNS involvement (log-rank P = .45; Figure 1A). The corresponding 2-year PFS estimates were 55.4% (95% CI, 46.8-63.2) without and 53.0% (95% CI, 20.9-77.3) with baseline CNS involvement. Median OS was not reached for either group and 2-year OS estimates included 69.6% ([95% CI, 61.0-76.6] for without and 50.5% [18.7-75.7] with baseline CNS involvement; log-rank P = .53, Figure 1B). Considering this small sample size and uncertain treatment selection process, we did not investigate outcomes according to any specific first-line treatment strategy. Of 6 patients with baseline CNS involvement who remained alive at the end of the study, 3 received R-CODOX-M/IVAC regimen, 2 received DA-EPOCH-R, and 1 received HDMTX-based regimen.
PFS and OS in patients with HGBL NOS based on the presence of absence of baseline CNS involvement. (A) PFS and (B) OS. P values are from log-rank test.
PFS and OS in patients with HGBL NOS based on the presence of absence of baseline CNS involvement. (A) PFS and (B) OS. P values are from log-rank test.
CNS prophylaxis
Among 149 patients without baseline CNS involvement, data on the delivery of CNS prophylaxis were available in 140. Among this subgroup, 66 patients received CNS prophylaxis, with only intrathecal (IT) drugs in 29% (n = 40; median 4 doses; range, 1-10), only IV HDMTX in 6% (n = 9; median, 2 doses; range, 1-5), and both IT and IV MTX in 12% (n = 17). CNS prophylaxis was administered more often for patients with high or high-intermediate IPI (56%) than for others (36%, P = .026).
CNS recurrence
We observed 59 recurrences of HGBL after first-line therapy, with 15 recurrences involving the CNS; 7 were leptomeningeal, 4 were parenchymal, and 4 were both leptomeningeal and parenchymal. Among 8 patients with parenchymal CNS recurrence, 6 had received systemic HDMTX. Among the 11 patients with leptomeningeal CNS recurrence, 4 had received IT therapy. Five of 15 CNS recurrences (33%) occurred among patients with baseline CNS involvement at diagnosis. There was no significant difference in the median time to CNS-involving vs systemic-only recurrence (4.4 months vs 5.3 months, respectively; P = .41; supplemental Figure 2). Of 15 patients, 9 (60%) had a concurrent systemic recurrence of their HGBL.
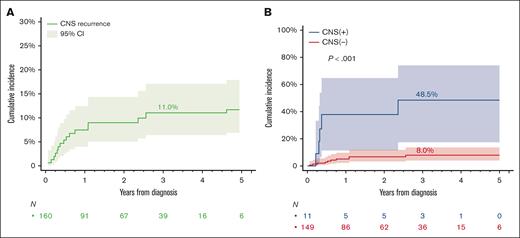
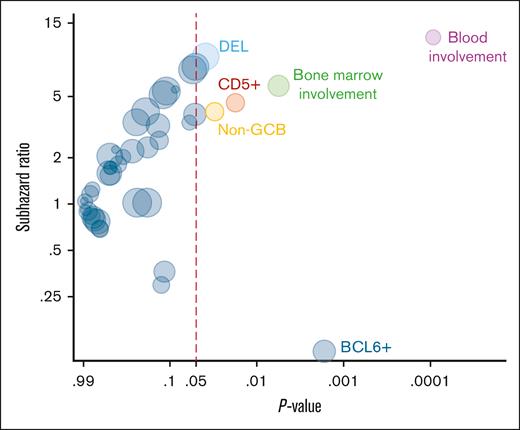
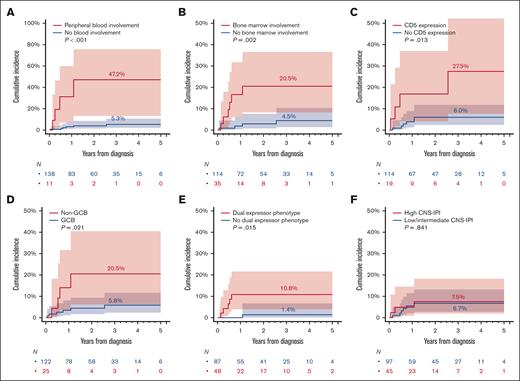
The cumulative incidence of CNS recurrence at 3 years was 11.0% (Figure 2A). Patients with baseline CNS involvement were at highest risk (48.5% [95% CI, 17.5-74.1] vs 8.0% [95% CI, 4.0-13.7]) for those without baseline CNS involvement (Gray test P < .001; Figure 2B). Therefore, we excluded the 11 patients with baseline CNS involvement from further analysis to investigate additional risk factors for CNS recurrence. Figure 3 shows the association of risk of CNS recurrence with clinical and pathologic factors. The risk for CNS recurrence was significantly associated with blood (47.2% [95% CI, 13.2-75.6] vs 5.3% [95% CI, 2.1-10.7]; P < .001) bone marrow involvement (20.5% [95% CI, 8.3-36.6] vs 4.5% [95% CI, 1.4-10.3]; P = .002; Figure 4A-B), CD5 expression (27.5% [95% CI, 7.7-52.2] vs 6.0% [95% CI, 2.4-11.8]; P = .013; Figure 4C), non-GCB COO (20.5% [95% CI, 6.2-40.5] vs 5.8 [95% CI, 2.3-11.7]; P = .021; Figure 4D), and DEL phenotype (10.8% [95% CI, 4.0-21.6] vs 1.4% [95% CI, 0.1-6.7]; P = .015; Figure 4E). We observed no prognostic value of high CNS-IPI for risk of CNS recurrence (P = .84; Figure 4F).
Cumulative incidence of CNS recurrence in patients with HGBL, NOS. (A) In all patients and (B) stratified by presence or absence of CNS involvement. P values are from a Gray test.
Cumulative incidence of CNS recurrence in patients with HGBL, NOS. (A) In all patients and (B) stratified by presence or absence of CNS involvement. P values are from a Gray test.
Factors associated with the risk of CNS recurrence. This is based on univariate Fine-Gray regression models. The size of the circles is proportional to the prevalence of the risk factor among patients with CNS recurrence.
Factors associated with the risk of CNS recurrence. This is based on univariate Fine-Gray regression models. The size of the circles is proportional to the prevalence of the risk factor among patients with CNS recurrence.
Risk of CNS recurrence in patients with HGBL, NOS based on blood or bone marrow involvement, CD5 expression, COO, and DEL phenotype and CNS-IPI. (A) Blood involvement; (B) bone marrow involvement; (C) CD5 expression; (D) non-GCB COO; (E) DEL phenotype; and (F) CNS-IPI. P values are from Gray’s test.
Risk of CNS recurrence in patients with HGBL, NOS based on blood or bone marrow involvement, CD5 expression, COO, and DEL phenotype and CNS-IPI. (A) Blood involvement; (B) bone marrow involvement; (C) CD5 expression; (D) non-GCB COO; (E) DEL phenotype; and (F) CNS-IPI. P values are from Gray’s test.
Supplemental Figure 3 shows the risk of CNS recurrence (or lack thereof) with additional HGBL NOS–specific factors. The risk for CNS recurrence was not associated with the presence or absence of MYC rearrangement (supplemental Figure 3A), morphology (supplemental Figure 3B-C), or whether the case underwent a confirmatory pathology review for the study. However, we noted that 5 of 15 patients with CNS recurrence had MYC-R, 3 of whom had a baseline CNS involvement. The risk of CNS recurrence was also higher in patients with high IPI and TP53 mutation, but the latter difference did not reach statistical significance (supplemental Figure 3D-E). Supplemental Figure 4 shows the risk of CNS recurrence with specific first-line therapy regimens and CNS prophylaxis modalities. There was no difference in the risk of CNS recurrence according to the type of first-line treatment (supplemental Figure 4A-B), receipt of any CNS prophylaxis (supplemental Figure 4C), or specific CNS prophylaxis modality using systemic HDMTX (supplemental Figure 4D) or IT therapy (supplemental Figure 4E-F).
Management of CNS recurrence
After a CNS recurrence of HGBL NOS, 15 patients received variable salvage strategies as outlined in supplemental Figure 5. Only 2 treated patients had a documented complete response (CR), and partial response to second-line therapy. Thirteen (87%) patients died after a CNS recurrence; 11 died from lymphoma, 1 from treatment-related toxicity, and 1 from a cause of uncertain attribution. The 2 surviving patients received platinum-based chemotherapy (R-DHAP or R-ICE), and 1 underwent autologous stem cell transplantation (ASCT) for consolidation. Three patients received chimeric antigen receptor T-cell (CAR-T) therapy with 1 CR, 1 partial response, and 1 progression, however, all 3 patients subsequently experienced a progression and death.
The median OS was not significantly different after CNS-involving and systemic-only recurrence (6.3 vs 7.7 months, respectively; P = .30; supplemental Figure 6), with 1-year OS estimates of 35.9% (95% CI, 13.1-59.6) and 35.0% (95% CI, 19.9-50.5), respectively.
Discussion
In this multi-institutional retrospective cohort study, we evaluated the prevalence of baseline CNS involvement, CNS prophylaxis strategies, and the risk of CNS recurrence in patients with HGBL NOS and made several observations. First, baseline CNS involvement is uncommon in HGBL NOS but highly prognostic for future CNS recurrence. Second, CNS-IPI may not predict a higher risk of CNS recurrence in patients with HGBL NOS. Third, CNS recurrence was higher in patients with HGBL with blood or marrow involvement, CD5 expression, non-GCB COO, and DEL phenotype. In 60% of cases CNS recurrence was part of a systemic recurrence of HGBL NOS, and both CNS-involving and systemic-only recurrences portend a poor prognosis.
Although HGBL NOS is generally a GCB disease, the risk of CNS recurrence was highest in the non-GCB phenotype. Involvement of testicles or female pelvis in HGBL NOS was associated with baseline CNS involvement, as observed in patients with DLBCL,30,31 in whom it correlates with the non-GCB phenotype and MCD molecular subtype. MYC-R was not prognostic for CNS recurrence, but this was partly because it was already strongly associated with baseline CNS involvement, highlighting the importance of adequate CNS-directed staging for patients with HGBL NOS and MYC-R. The higher risk of CNS recurrence in non-GCB tumors and in those with the associated DEL phenotype closely resemble findings from DLBCL and suggest that in clinical practice, the diagnosis of HGBL NOS is rendered for some non-GCB lymphomas that exhibit a high-grade morphology and have a propensity for extranodal and CNS dissemination.32,33 Further research will need to address whether this group overlaps molecularly with the MCD DLBCL or CD5+ DLBCL and whether at least some subgroup of MCD lymphomas (whether DLBCL or HGBL by morphology) could benefit from specific treatment strategies that could lower their risk of CNS recurrence.34-36
The overall risk of CNS recurrence in HGBL NOS was 11.0% at 3 years and 8.0% for those without baseline CNS involvement, which is similar to the risk of DLBCL with a high CNS-IPI score.7 Comparing HGBL NOS with the more common type of HGBL with MYC and BCL2 rearrangements (double-hit lymphoma), both the proportion of patients with baseline CNS involvement (7%) and with subsequent CNS recurrence (13% at 3 years, compared with 11% at 3 years in our cohort) are very similar.16,25 This suggests that HGBL NOS diagnosis should be included among other factors portending a high risk of CNS recurrence, together with testicular DLBCL, CD5+ DLBCL, and double-hit lymphomas, and considered for inclusion in trials of novel therapies aiming at reducing the risk of CNS invasion. Clinicians should consider adequate CNS-directed staging procedures (magnetic resonance imaging and lumbar puncture) in a more consistent manner to detect subclinical CNS invasion in this high-risk group.
In contrast to DLBCL, the CNS-IPI was not prognostic in our cohort, indicating that it does not capture the inherent biologic risk of CNS recurrence in HGBL NOS and aggressive disease, which is associated with many clinical high-risk features and a significant risk of early systemic progression.22 We found that peripheral blood involvement was the most important prognostic indicator, suggesting that hematogenous dissemination at presentation may facilitate subclinical CNS invasion in HGBL NOS. The fact that 7 of 15 CNS recurrences were leptomeningeal (rather than parenchymal, which is more often observed in DLBCL NOS1,2) indicates that HGBL NOS has lymphoblastic lymphoma/leukemia-like propensity to spread into the leptomeningeal compartment. Nevertheless, it appears that standard IT prophylaxis (as applied in clinical practice), although effective in acute lymphoblastic leukemia and Burkitt lymphoma, does not protect against CNS recurrence. Similarly, administration of HDMTX was not associated with any decrease in the risk of CNS recurrence, although as is inherent to retrospective studies, patients selected for HDMTX-based prophylaxis may have had a particularly high-risk profile. We note that several recent studies, both retrospective and prospective, have demonstrated a lack of efficacy of HDMTX for CNS prophylaxis in DLBCL,37-39 and this strategy is likely to be insufficient for HGBL NOS as well.
Patients with baseline CNS involvement by HGBL NOS are a particularly difficult group to manage, with a high risk of subsequent CNS progression or recurrence. Therapeutic options in this setting include intensive regimens that are known to control the CNS disease through the use of multiple CNS-penetrant agents (HDMTX, cytarabine, and ifosfamide) such as the MARIETTA strategy5 or R-CODOX-M/IVAC,40 typically with consolidative high-dose thiotepa-based chemotherapy with ASCT.41 Although our data reflect the application of these strategies in patients with HGBL NOS, we could not compare management strategies because of the small sample size and uncertain treatment selection process.
The advent of CAR-T therapy has revolutionized the therapeutic landscape of relapsed or refractory systemic DLBCL with US Food and Drug Administration approvals in the third-line14-16 and the second-line setting,17,18 including patients who are ineligible for ASCT. Although CAR-T has also been shown to be effective in secondary CNS lymphoma with high overall response rates and CR rates, the responses are not durable.42-47 We noted a similar trend in our study wherein 2 of 3 patients responded to CAR-T (overall response rates = 67%) but all subsequently experienced progressive disease.
The study is subjected to the inherent limitations of a retrospective cohort including the nonuniform selection of HGBL NOS patients and selection of treatment strategies. The diagnosis of HGBL NOS is often perceived to be arbitrary, and patterns of pathology evaluation and designation may differ between institutions and individual pathologists, especially considering the evolving nomenclature of high-grade lymphomas. Some specific entities, including HGBL with 11q aberration, may not be consistently recognized in routine practice. Recent studies raise hope of a possibility of more objective classification based on molecular features, although these at present rely on gene expression profiling technology which is not widely accessible for clinical purposes.17,18,21,23,48 Furthermore, the extent of CNS evaluation at diagnosis and at the time of relapse was not uniform, and our study supports more consistent CNS and cerebrospinal fluid evaluations as is practiced for high-risk DLBCL and double-hit lymphomas.
In conclusion, this case series describes in the largest cohort to date (per our knowledge) the rates of baseline CNS involvement, risk of CNS recurrence, and associated management strategies in patients with HGBL NOS. The prognosis of relapsed HGBL NOS is poor, regardless of whether recurrence is systemic or limited to the CNS, and with currently available salvage strategies, including ASCT and CAR-T modalities, almost all patients with CNS recurrence ultimately succumb to their disease. These patients represent a high unmet need and should be prioritized for experimental approaches.
Acknowledgment
A.J.O. is a clinical research scholar of the Leukemia & Lymphoma Society.
Authorship
Contribution: N.E. conceptualized and designed the study and prepared the first draft of the manuscript; N.E. and A.J.O. analyzed the data; and all authors collected, interpreted, and assembled data, provided critical and insightful comments, and approved the final manuscript.
Conflict-of-interest disclosure: N.E. reports research funding from BeiGene and Eli Lilly; serves on the speaker's bureau for BeiGene and Genentech; serves on advisory boards for Ipsen, Lilly, and ADC Therapeutics; and reports consultancy for Novartis. R.K. serves in a consultant role for Bristol Myers Squibb, Janssen, MorphoSys/Incyte, Epizyme, Genentech/Roche, GenMab, Lilly Oncology, AbbVie, and Miltenyi; reports grants/research support from Bristol Myers Squibb, Takeda, BeiGene, Gilead Sciences/Kite, and Miltenyi; and serves on the speakers' bureau for AstraZeneca, BeiGene, and Incyte. P.T. reports honoraria, consulting roles, and advisory board membership for TG Therapeutics, ADC Therapeutics, Genentech, GenMab, Seagen, AbbVie, and Lilly USA. A.J.O. reports research funding from Genmab, Precision Bio, Adaptive Biotechnologies, Celldex, Acrotech Biopharma, Schrodinger, TG Therapeutics, and Genentech. M.L.X. reports consulting roles for Pure Marrow and Treeline Biosciences. D.A.B. reports consulting roles for Novartis, Nurix Therapeutics, and ADC Therapeutics, and and received research funding from Novartis, Nurix Therapeutics, Incyte, Kite/Gilead, Bristol Myers Squibb, Genmab, and Accutar Biotechnology. A.M.B. reports honoraria and advisory board membership for AbbVie. M.K. reports research support/funding from Novartis; consultancy with AbbVie, AstraZeneca, Celgene/Bristol Myers Squibb, BeiGene, and Genentech; and serves on the speakers' bureau of Seagen, Celgene, and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Narendranath Epperla, Division of Hematology and Hematologic Malignancies, Department of Medicine, Huntsman Cancer Institute, 2000 Circle of Hope Dr, Salt Lake City, UT 84112; email: naren.epperla@hci.utah.edu.
References
Author notes
Data are available on request from the corresponding author, Narendranath Epperla (naren.epperla@hci.utah.edu).
The full-text version of this article contains a data supplement.