Key Points
New-onset CRS and ICANS are rare after 2 weeks for axi-cel, tisa-cel, and liso-cel, making flexible monitoring beyond 14 days seem safe.
NRM beyond day 28 is largely driven by infection and highlights the need to monitor for late effects and complications.
Visual Abstract
CD19–directed chimeric antigen receptor T-cell (CAR T) therapies, including axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel), have transformed the treatment landscape for B-cell non-Hodgkin lymphoma, showcasing significant efficacy but also highlighting toxicity risks such as cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). The US Food and Drug Administration has mandated patients remain close to the treatment center for 4 weeks as part of a Risk Evaluation and Mitigation Strategy to monitor and manage these toxicities, which, although cautious, may add to cost of care, be burdensome for patients and their families, and present challenges related to patient access and socioeconomic disparities. This retrospective study across 9 centers involving 475 patients infused with axi-cel, tisa-cel, and liso-cel from 2018 to 2023 aimed to assess CRS and ICANS onset and duration, as well as causes of nonrelapse mortality (NRM) in real-world CAR T recipients. Although differences were noted in the incidence and duration of CRS and ICANS between CAR T products, new-onset CRS and ICANS are exceedingly rare after 2 weeks after infusion (0% and 0.7% of patients, respectively). No new cases of CRS occurred after 2 weeks and a single case of new-onset ICANS occurred in the third week after infusion. NRM is driven by ICANS in the early follow-up period (1.1% until day 28) and then by infection through 3 months after infusion (1.2%). This study provides valuable insights into optimizing CAR T therapy monitoring, and our findings may provide a framework to reduce physical and financial constraints for patients.
Introduction
There are currently 3 CD19–directed chimeric antigen receptor T-cell (CAR T) products approved by the US Food and Drug Administration (FDA) for the treatment of relapsed/refractory (R/R) large B-cell lymphoma (LBCL) in the third and later line of therapy. These therapies, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel), have demonstrated clear improvements in patient outcomes compared with historical salvage regimens.1-6 More recently, axi-cel and liso-cel have gained regulatory approval as second-line therapy for R/R LBCL.7-9 The success of these products is tempered by the risk of unique toxicities including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), which require close monitoring and early intervention. The incidence and severity of these side effects vary by product.
In the pivotal trials leading to regulatory approval, rates of CRS and ICANS for axi-cel were reported in 93% (13% grade ≥3) and 64% (21% grade ≥3), respectively, in the ZUMA-1 trial, whereas JULIET reported a lower incidence of CRS and ICANS, reported in 58% (22% grade ≥3) and 21% (12% grade ≥3), respectively, for tisa-cel.10 Liso-cel had a similarly low incidence of CRS and ICANS, reported in 42% (2% grade ≥3) and 30% (10% grade ≥3), respectively, in the TRANSCEND trial.6 Although 2 of these pivotal trials used the Lee 2014 criteria to grade CRS, the JULIET trial used Penn criteria, which overestimates CRS severity compared with the Lee criteria.11,12 The trials used the National Cancer Institute Common Terminology Criteria for Adverse Events V4.03 criteria to grade ICANS.13,14 These grading systems differed from the American Society for Transplantation and Cellular Therapy consensus grading system used in our study, which was selected to minimize inconsistencies.12,15
To mitigate the risks of high-grade or fatal CRS and ICANS, which were unique to CAR T, the FDA implemented a Risk Evaluation and Mitigation Strategy (REMS), which mandates that patients must reside within 2-hour proximity of the authorized treatment center (ATC) for a period of 4 weeks after infusion, to allow for prompt evaluation and treatment of potentially life-threatening CRS and ICANS and to ensure that patients can receive tocilizumab within 2 hours of symptom onset.16-19 The REMS also advises refraining from driving for up to 8 weeks after CAR T infusion.16-19
Although the REMS requirements were established to promote patient safety, the current risk mitigation strategy may present significant barriers to CAR T access. There are already several barriers to CAR T therapy, including cost constraints. The requirement that patients be proximal to the ATC for a 4-week period after infusion may create an additional geographic barrier because of additional financial and physical burden for patients and caregivers who need to relocate, thereby making this therapy less accessible for patients in lower socioeconomic strata and minority populations.20-23 This also adds to the cost of care. Although industry and payers sometimes provide support for relocation, it is possible that frequent visits and laboratory testing for all 4 weeks after infusion may not be required for all patients. Similarly, the 8-week driving restriction may also pose an additional barrier for socioeconomically disadvantaged patients. The optimal duration of monitoring has not been comprehensively evaluated, highlighting the need to better delineate safety outcomes during the 4-week stringent CRS and ICANS monitoring period. Therefore, data from the real world are needed to examine the optimal frequency and level of monitoring after CAR T therapy in current era.
We conducted this retrospective study to investigate the onset and duration of CRS and ICANS and to investigate causes of nonrelapse mortality (NRM) in a large real-world population of adult patients receiving commercial axi-cel, tisa-cel, and liso-cel for R/R LBCL to better inform a transition from the current monitoring mandate to a flexible monitoring period after CAR T administration.
Patients and methods
Using the Cell Therapy Consortium registry, we identified commercial axi-cel, tisa-cel, and liso-cel recipients with R/R LBCL who underwent CAR T infusion between March 2018 and May 2023. The choice among commercial CAR T products, lymphodepletion regimen (fludarabine/cyclophosphamide or bendamustine), and treatment setting (inpatient/outpatient) was per institutional guidelines and physician preference. The American Society for Transplantation and Cellular Therapy consensus grading system was used for both CRS and ICANS.12 Cytopenia and infections were graded using the Common Terminology Criteria for Adverse Events version 5.24 NRM was death due to causes other than disease progression. New-onset CRS and ICANS were reported as a percentage of patients at risk.
Statistical analysis
Baseline patient and treatment characteristics and variables regarding toxicity and complications were summarized using descriptive statistics and frequency tables. Between-group comparisons were performed using the Pearson χ2or Fisher exact tests for categorical variables and the Wilcoxon rank-sum test for continuous variables. All statistical analyses were 2 sided and performed using SPSS version 28 (IBM, Armonk, NY), and P <.05 was considered significant.
This study was approved by the institutional review boards at individual institutions and conducted according to the Declaration of Helsinki.
Results
Among the 475 patients, 216 (45%) received axi-cel, 158 (33%) received tisa-cel, and 101 (21%) received liso-cel. Baseline characteristics of the patient population are presented in Table 1. There were 48.8% of patients aged ≥65 years (axi-cel 34%, tisa-cel 54%, and liso-cel 71%; P < .001). There were 28.2% primary refractory cases (axi-cel 37%, tisa-cel 23%, and liso-cel 15%; P = .0003). Most (69.8%) received CAR T as third or later line (axi-cel 70%, tisa-cel 80%, and liso-cel 53%; P < .001). Although most patients (80%) received fludarabine/cyclophosphamide lymphodepleting chemotherapy, and 20% (axi-cel 1%, tisa-cel 41%, and liso-cel 26%; P < .001) received bendamustine. Median follow-up for all patients was 11 months (interquartile range [IQR], 4-19.8).
Baseline and treatment characteristics
| Characteristic . | All patients (N = 475) . | Axi-cel (n = 216) . | Tisa-cel (n = 158) . | Liso-cel (n = 101) . | P value . |
|---|---|---|---|---|---|
| Age at leukapheresis | |||||
| Median (IQR), y | 65 (57-71) | 60 (53-68) | 66 (59-72) | 70 (63-77) | <.0001 |
| ≥65 y, n (%) | 232 (48.8) | 74 (34) | 86 (54) | 72 (71) | <.0001 |
| Male sex, n (%) | 307 (64.6) | 156 (72) | 90 (57) | 61 (60) | .0058 |
| ECOG score 0/1, n (%) | 395 (87.2) | 186 (88) | 137 (88) | 72 (84) | .5606 |
| Disease type, n (%) | |||||
| DLBCL/tFL | 426 (90.1) | 191 (89) | 143 (91) | 92 (91) | .2047 |
| HGBCL | 42 (8.9) | 22 (10) | 13 (8) | 7 (7) | |
| PMBCL | 5 (1.1) | 2 (1) | 1 (1) | 2 (2) | |
| Disease status at referral, n (%) | |||||
| Primary refractory | 125 (28.2) | 79 (37) | 32 (23) | 14 (15) | .0003 |
| Refractory | 164 (36.9) | 75 (36) | 51 (36) | 38 (42) | |
| Relapsed | 155 (34.9) | 57 (27) | 59 (42) | 39 (43) | |
| Previous lines of therapy, n (%) | |||||
| 1 | 16 (3.3) | 0 | 0 | 16 (15.8) | <.0001 |
| 2 | 126 (26.5) | 64 (30) | 31 (20) | 31 (30.7) | |
| >2 | 332 (69.8) | 151 (70) | 127 (80) | 54 (53) | |
| Lymphodepletion, n (%) | |||||
| Fludarabine/cyclophosphamide | 380 (80) | 213 (99) | 92 (58) | 75 (74) | <.0001 |
| Bendamustine | 93 (19.6) | 2 (1) | 65 (41) | 26 (26) | |
| Other/none | 2 (0.4) | 1 (<1) | 1 (1) | 0 | |
| Location of infusion, n (%) | |||||
| Inpatient | 355 (74.7) | 204 (94) | 65 (41) | 86 (85) | <.0001 |
| Outpatient | 120 (25.3) | 12 (6) | 93 (59) | 15 (15) | |
| CRS | |||||
| Incidence, all grade, n (%) | 285 (60) | 172 (79.6) | 64 (10.1) | 49 (48.5) | <.0001 |
| Grade ≥3, incidence, n (%) | 24 (5) | 16 (7) | 5 (3) | 3 (3) | .099 |
| Median days to onset (IQR) | 3 (1-4) | 3 (1-5) | 3 (1-4) | 3 (2-4) | .3792 |
| Median days to resolution (IQR) | 4 (2-6) | 5 (3-7) | 2 (1-4) | 3 (1-4) | <.0001 |
| Onset beyond day 14, n (%) | 0 | 0 | 0 | 0 | ND |
| ICANS | |||||
| Incidence, all grade, n (%) | 154 (32.4) | 112 (51.8) | 16 (10.1) | 26 (25.7) | <.0001 |
| Grade ≥3, incidence, n (%) | 85 (18) | 72 (33) | 3 (2) | 10 (10) | <.0001 |
| Median days to onset (IQR) | 5 (4-7) | 5 (4-7) | 4 (1-7) | 5 (2-8) | .0728 |
| Median days to resolution (IQR) | 5 (2-10) | 6 (3-10) | 3 (2-11) | 3 (0-13) | .1888 |
| Onset beyond day 14, n (%) | 1 (0.7) | 0 | 1 (6) | 0 | ND |
| Tocilizumab use | |||||
| Used at least 1 dose, n (%) | 206 (43.6) | 134 (62) | 41 (26) | 31 (31) | <.0001 |
| Median doses used (IQR) | 1 (1-2) | 2 (1-3) | 1 (1-2) | 1 (1-2) | .0405 |
| Steroids: used at least 1 dose, n (%) | 155 (32.6) | 108 (50) | 16 (10) | 31 (31) | <.0001 |
| Infections: incidence, n (%) | 69 (14.5) | 47 (29) | 22 (16) | 0 | .0050 |
| Mortality | |||||
| All-cause mortality, n (%) | 202 (42.8) | 99 (46) | 76 (48) | 27 (27) | .0011 |
| NRM, n (%) | 45 (9.5) | 25 (12) | 11 (7) | 9 (9) | .3150 |
| Median follow-up, mo (IQR) | 11 (4-19.8) | 13 (5-21) | 11 (5-22) | 6 (3-12) | <.0001 |
| Characteristic . | All patients (N = 475) . | Axi-cel (n = 216) . | Tisa-cel (n = 158) . | Liso-cel (n = 101) . | P value . |
|---|---|---|---|---|---|
| Age at leukapheresis | |||||
| Median (IQR), y | 65 (57-71) | 60 (53-68) | 66 (59-72) | 70 (63-77) | <.0001 |
| ≥65 y, n (%) | 232 (48.8) | 74 (34) | 86 (54) | 72 (71) | <.0001 |
| Male sex, n (%) | 307 (64.6) | 156 (72) | 90 (57) | 61 (60) | .0058 |
| ECOG score 0/1, n (%) | 395 (87.2) | 186 (88) | 137 (88) | 72 (84) | .5606 |
| Disease type, n (%) | |||||
| DLBCL/tFL | 426 (90.1) | 191 (89) | 143 (91) | 92 (91) | .2047 |
| HGBCL | 42 (8.9) | 22 (10) | 13 (8) | 7 (7) | |
| PMBCL | 5 (1.1) | 2 (1) | 1 (1) | 2 (2) | |
| Disease status at referral, n (%) | |||||
| Primary refractory | 125 (28.2) | 79 (37) | 32 (23) | 14 (15) | .0003 |
| Refractory | 164 (36.9) | 75 (36) | 51 (36) | 38 (42) | |
| Relapsed | 155 (34.9) | 57 (27) | 59 (42) | 39 (43) | |
| Previous lines of therapy, n (%) | |||||
| 1 | 16 (3.3) | 0 | 0 | 16 (15.8) | <.0001 |
| 2 | 126 (26.5) | 64 (30) | 31 (20) | 31 (30.7) | |
| >2 | 332 (69.8) | 151 (70) | 127 (80) | 54 (53) | |
| Lymphodepletion, n (%) | |||||
| Fludarabine/cyclophosphamide | 380 (80) | 213 (99) | 92 (58) | 75 (74) | <.0001 |
| Bendamustine | 93 (19.6) | 2 (1) | 65 (41) | 26 (26) | |
| Other/none | 2 (0.4) | 1 (<1) | 1 (1) | 0 | |
| Location of infusion, n (%) | |||||
| Inpatient | 355 (74.7) | 204 (94) | 65 (41) | 86 (85) | <.0001 |
| Outpatient | 120 (25.3) | 12 (6) | 93 (59) | 15 (15) | |
| CRS | |||||
| Incidence, all grade, n (%) | 285 (60) | 172 (79.6) | 64 (10.1) | 49 (48.5) | <.0001 |
| Grade ≥3, incidence, n (%) | 24 (5) | 16 (7) | 5 (3) | 3 (3) | .099 |
| Median days to onset (IQR) | 3 (1-4) | 3 (1-5) | 3 (1-4) | 3 (2-4) | .3792 |
| Median days to resolution (IQR) | 4 (2-6) | 5 (3-7) | 2 (1-4) | 3 (1-4) | <.0001 |
| Onset beyond day 14, n (%) | 0 | 0 | 0 | 0 | ND |
| ICANS | |||||
| Incidence, all grade, n (%) | 154 (32.4) | 112 (51.8) | 16 (10.1) | 26 (25.7) | <.0001 |
| Grade ≥3, incidence, n (%) | 85 (18) | 72 (33) | 3 (2) | 10 (10) | <.0001 |
| Median days to onset (IQR) | 5 (4-7) | 5 (4-7) | 4 (1-7) | 5 (2-8) | .0728 |
| Median days to resolution (IQR) | 5 (2-10) | 6 (3-10) | 3 (2-11) | 3 (0-13) | .1888 |
| Onset beyond day 14, n (%) | 1 (0.7) | 0 | 1 (6) | 0 | ND |
| Tocilizumab use | |||||
| Used at least 1 dose, n (%) | 206 (43.6) | 134 (62) | 41 (26) | 31 (31) | <.0001 |
| Median doses used (IQR) | 1 (1-2) | 2 (1-3) | 1 (1-2) | 1 (1-2) | .0405 |
| Steroids: used at least 1 dose, n (%) | 155 (32.6) | 108 (50) | 16 (10) | 31 (31) | <.0001 |
| Infections: incidence, n (%) | 69 (14.5) | 47 (29) | 22 (16) | 0 | .0050 |
| Mortality | |||||
| All-cause mortality, n (%) | 202 (42.8) | 99 (46) | 76 (48) | 27 (27) | .0011 |
| NRM, n (%) | 45 (9.5) | 25 (12) | 11 (7) | 9 (9) | .3150 |
| Median follow-up, mo (IQR) | 11 (4-19.8) | 13 (5-21) | 11 (5-22) | 6 (3-12) | <.0001 |
DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HGBL, high-grade B-cell lymphoma; PMBCL, primary mediastinal B-cell lymphoma; tFL, transformed follicular lymphoma. Bolded P values are significant.
Safety
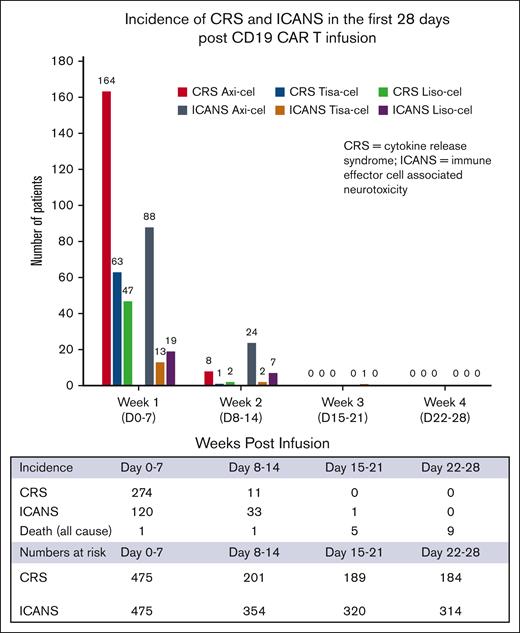
The incidence of any grade CRS was 60% in all patients, with a higher incidence in axi-cel recipients (79.6% [n = 172]) than tisa-cel recipients (40.5% [n = 64]) and liso-cel recipients (48.5% [n = 49]) (P < .0001). In the first 7 days after CAR T infusion, new-onset CRS occurred in 57.5% of the total population, with a higher incidence in axi-cel (75.9% [n = 164]) than tisa-cel (39.8% [n = 63]) and liso-cel recipients (46.5% [n = 47]) (P < .0001; Figure 1). New-onset CRS in days 8 to 14 after CAR T infusion was observed in 5.4% of patients at risk, with a higher incidence in axi-cel (15.4% [n = 8]) than tisa-cel (1% [n = 1]) and liso-cel recipients (3.4% [n = 2]) (P = .001). There were no cases of new-onset CRS beyond 14 days after CAR T infusion. The median time to CRS onset for all patients was 3 (IQR, 1-4) days, with no significant differences between products. The incidence of grade ≥3 CRS was 5% in all patients, similar between products, and median time to onset of grade ≥3 CRS for all patients was 6 days (IQR, 4-10), with no significant differences between products (Table 1). The median time to resolution of CRS for all patients was 4 days (IQR, 2-6) (axi-cel 5 days, tisa-cel 2 days, liso-cel 3 days; P < .001). CRS that remained unresolved beyond day 14 occurred in 7% of patients with a similar incidence in axi-cel (10% [n = 15]), tisa-cel (4% [n = 2]), and liso-cel recipients (4% [n = 2]) (P = .225). CRS that remained unresolved beyond day 28 occurred in 1.4% (n = 4) of patients, all of whom had received axi-cel. Greater than 98% of CRS resolved by day 28 after CAR T infusion.
The incidence of any grade ICANS was 32.4% in all patients, with a higher incidence in axi-cel (51.8% [n = 112]) than tisa-cel (10.1% [n = 16]) and liso-cel recipients (25.7% [n = 26]) (P < .0001). In the first 7 days after CAR T infusion, new-onset ICANS occurred in 25.3% of the total population, with a higher incidence in axi-cel (40.7% [n = 88]) than tisa-cel (8.2% [n = 13]) and liso-cel recipients (18.8%, [n = 19]) (P < .0001). New-onset ICANS in days 8 to 14 after CAR T infusion was observed in 9.3% of patients at risk, with a higher incidence in axi-cel (19% [n = 24]) than tisa-cel (1% [n = 2]) and liso-cel recipients (9% [n = 7]) (P < .0001). One tisa-cel recipient developed ICANS in the third week after CAR T infusion. This tisa-cel recipient who developed ICANS on day 21 was a 71-year-old male with diffuse LBCL, who had 4 previous lines of therapy, no central nervous system involvement, and a normal lactate dehydrogenase at infusion. He developed maximum grade 2 ICANS that lasted till day 34 after infusion. The median time to ICANS onset for all patients was 5 days (IQR, 4-7), with no significant differences between products (Table 1). The incidence of grade ≥3 ICANS was 17.9% in all patients, with a higher incidence in axi-cel (33% [n = 72]) than tisa-cel (2% [n = 3]) and liso-cel recipients (10% [n = 10]) (P < .0001). Median time to grade ≥3 ICANS for all patients was 7 days (IQR, 5-10). The median time to resolution of ICANS for all patients was 5 days (IQR, 2-10). ICANS that remained unresolved beyond day 14 occurred in 34% of patients with a similar prevalence in axi-cel (36% [n = 39]), tisa-cel (23% [n = 3]), and liso-cel recipients (31% [n = 8]) (P = .617). ICANS that remained unresolved beyond day 28 occurred in 6.5% (n = 10) of patients, with a similar prevalence between CAR T products. More than 93% of ICANS resolved by day 28 after CAR T infusion.
Toxicity management
Tocilizumab was used in 43.6% of patients, with a higher use in axi-cel (62% [n = 134]) than tisa-cel (26% [n = 41]) and liso-cel recipients (31% [n = 31]) (P < .0001). There was increased utilization of tocilizumab in axi-cel (median, 2 doses [IQR, 1-3]) compared with tisa-cel (median, 1 dose [IQR, 1-2]) and liso-cel recipients (median, 1 dose [IQR, 1-2]) (P = .040).
Steroids were used in 32.6% of patients, with a higher use in axi-cel (50% [n = 108]) than tisa-cel (10% [n = 16]) and liso-cel recipients (31% [n = 31]) (P < .0001).
Infectious complications and NRM
Infections of any grade within the 28 days after CAR T infusion were documented in 14.5% of patients, with a higher rate among axi-cel (29% [n = 47]) than tisa-cel (16% [n = 22]) and liso-cel recipients (0%, [n = 0]) (P = .005). There were no differences in the infectious source, whether bacterial, viral, or fungal, among products.
There were 7 deaths (1.6% [n = 431]) within the first 28 days after CAR T infusion attributed to causes other than lymphoma progression. Four of these deaths were related to isolated ICANS, 1 was related to concurrent CRS and ICANS, and 2 were related to infection (Figure 2). There was no NRM due to isolated CRS.
There were an additional 9 deaths (2.7% [n = 330]) not related to progression of lymphoma between day 29 and day 90 after CAR T infusion. Five of these deaths were related to infection, 1 was related to isolated ICANS, 1 was cardiac related, and the remaining 2 had unknown causes of death (supplemental Table 1). The patient who died of cardiac causes was an axi-cel recipient who had cardiomyopathy before CAR T infusion secondary to doxorubicin. Infectious complications were the most common cause of NRM days 0 to 90, accounting for 7 of the 16 NRM events, mostly occurring after day 28.
Discussion
Current REMS monitoring requirements for CRS and ICANS are focused on ensuring patient safety, although they were implemented with a relative paucity of real-world data on the onset, incidence, and duration of CRS and ICANS after CAR T administration. In addition, these requirements were implemented with little consideration of monitoring for other causes of NRM during the observation period.
Within the cellular therapy community, limited patient access to CAR T has been increasingly recognized as an important barrier to treatment.20 Previous analyses have demonstrated that only 20% to 30% of patients who are eligible for CAR T are eventually able to receive therapy.25,26 Disparities in access to CAR T are higher in vulnerable populations including minorities, those from lower socioeconomic groups, and those who live at a greater distance from the ATC.21,22,27 Of these patients, more than half must relocate to the ATC for the required REMS monitoring period.20 Insurance and industry may help defray some of the relocation costs, although much will fall directly to the patient.28-30 Multiple strategies to minimize barriers to access are being explored including the ability to receive CAR T on an outpatient basis, establishing improved collaboration between community oncologists and ATCs and decentralizing the manufacturing of CAR T.30-32 Nevertheless, the REMS mandate of a 4-week monitoring period for CRS and ICANS remains a factor contributing to the inequity in access.
Our report is the first to provide granular insights into the occurrence and severity of CRS and ICANS beyond 2 weeks after CAR T infusion and during extended follow-up. Our data suggest that, beyond 2 weeks, onset of CRS and ICANS after approved CD19 CAR T for LBCL is rare. Our median onset times for CRS and ICANS with axi-cel, tisa-cel, and liso-cel align with other select clinical trial and real-world reports, as shown in supplemental Table 2.4,6,8-10,33-36 Notably, in those reports, there was no new-onset CRS beyond 14 days, except in the Center for International Blood and Marrow Transplant axi-cel real-world report by Jacobson et al,34 in which the median time to CRS onset was 7 days, with a range of 1 to 36 days.
Although there are no published data on the incidence of new-onset ICANS by week, clinical trial and retrospective studies reveal that the median onset of ICANS for axi-cel and tisa-cel occurred in ≤7 days, whereas the median ranged from 6 to 11 days in liso-cel studies (supplemental Table 2).6,9,35 This suggests that the incidence of new-onset ICANS beyond 2 weeks is likely rare with these products in R/R LBCL. Furthermore, in the referenced studies, no new-onset ICANS occurred beyond 3 weeks of axi-cel, tisa-cel, or liso-cel infusion.4,8-10,33-35 One notable exception is the TRANSCEND study, in which the median time to ICANS onset was 9 days with a range up to 66 days. This potential delayed ICANS onset, even beyond the 4-week monitoring period and the 8-week driving restriction period, highlights the need for continued vigilance for late complications.6 The FDA mandate restricts CAR T recipients from driving for up to 8 weeks after infusion; however, our data highlight that new-onset ICANS beyond 2 weeks is rare and does not support a prolonged driving restriction mandate for all patients.
Evolution in prophylactic and preemptive management strategies may be associated with a difference in time to onset and maximum grade of CRS and ICANS in recent years.37 However, none of our member institutions routinely implemented dexamethasone prophylaxis for CRS or ICANS during this period. Moreover, half of the institutions implemented levetiracetam prophylaxis from day 0 to 30, whereas others only initiated levetiracetam at the onset of CRS/ICANS. All member institutions discontinued levetiracetam by day 30; therefore, a shortened period of seizure prophylaxis may be explored for these products in LBCL.
Of note, the onset of CAR T–related toxicities for other cellular therapy products, such as brexucabtagene autoleucel, BCMA–directed CAR T therapies (eg, ciltacabtagene autoleucel and idecabtagene vicleucel), and investigational products, may differ from our results.38-42
We observed that, beyond 2 weeks, infection emerged as a leading cause of mortality. Of note, we only report NRM up to day 90, and infection-related NRM may persist beyond this period. Although infection prophylaxis strategies were comparable across institutions, the risk of infection, primarily bacterial, is relatively high in the early CAR T infusion period.43-45 Neutropenia after CAR T can occur early but can also be persistent for many months after infusion, potentially resulting in a prolonged period of infection risk, lasting well beyond the current 4-week monitoring period mandate.46-50 Beyond the first 4 weeks after CAR T infusion, the risk of infection, especially viral infections, persists due to other CAR T–related effects such as lymphopenia, B-cell aplasia, and hypogammaglobulinemia.51,52 Our reported NRM rate of 1.8% due to infectious complications is comparable with other studies in which NRM ranged from 1% to 6%, and may be affected by product-, disease-, and patient-related characteristics.51,53,54
It is important to note that other fatal complications may occur beyond 2 weeks after infusion, such as IEC-HS (immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome).55,56 Although we did not note any deaths due to IEC-HS in our study, we acknowledge that IEC-HS may have been underreported given that its definition and pathobiology was only more recently codified.
An FDA mandate restricts CAR T recipients from driving for up to 8 weeks after infusion; however, our data highlight that new-onset ICANS beyond 4 weeks is rare and do not support a prolonged driving restriction mandate for all patients. Given the low incidence of new-onset CRS and ICANS beyond 2 weeks after CD19 CAR T infusion for LBCL, we propose a dynamic approach to CRS and ICANS monitoring that may alleviate some of the burden inherent with current REMS monitoring requirements. Although CRS and ICANS were the only considerations when implementing current REMS mandates, our data suggest that CAR T outcomes may be improved with vigilant prevention and prompt management of infectious complications. Given that the risk of infections and mortality due to infections persists beyond the stringent 4-week monitoring period, robust supportive care to minimize the risk of infections along with strategies to educate and collaborate with community oncologists to reduce the risk of infectious complications will be critical.57,58
A robust partnership with community oncologists is crucial to the successful transition of a CAR T patient back to local management.31 Education on recognizing infection and initiating appropriate and prompt therapy is of the utmost importance for the ongoing success of CAR T as a therapeutic modality. Developing a framework for late toxicity monitoring and management that includes community oncology centers, and not just ATCs, may be a consideration to facilitate this aim. Consequently, we propose a 2-week monitoring period in the vicinity of the ATC with the flexibility to extend monitoring up to 4 total weeks depending on patient stability, physician comfort, and perhaps, most importantly, strong local community oncology support.
Our study has limitations largely ascribed to the retrospective nature of the study. Although data were recorded and organized in a standardized database, each treating center had individual institutional guidelines that influenced patient eligibility, choice of lymphodepleting chemotherapy, and management of CRS and ICANS. We were not able to capture certain laboratory variables including late-onset neutropenia and hypogammaglobulinemia, along with other metrics such as patient-reported outcomes and caregiver education practices. Furthermore, this study was limited to LBCL and these results may not be generalizable to other disease states (eg, acute lymphoblastic leukemia, indolent lymphoma, mantle cell lymphoma, and multiple myeloma) or other cellular therapy products (eg, brexucabtagene autoleucel, approved BCMA CAR T therapies, or other investigational cellular therapies).
Conclusion
Our data suggest that the incidence of new-onset CRS and ICANS is rare beyond 2 weeks after CAR T infusion. Moreover, late NRM (more than day 28) does occur and is predominantly due to infectious complications. These data support further investigation into individualized monitoring strategies for stable patients. A flexible monitoring period may help to decrease financial and geographic limitations for patients and make CAR T more accessible and feasible. We propose reducing the monitoring period to 2 weeks with the flexibility of extending monitoring to a total of 4 weeks contingent on patient stability, physician comfort, and the availability of local community oncology support. Engagement and educational initiatives with community oncologists may facilitate such a monitoring strategy beyond the early observation period in patients with LBCL undergoing CAR T.
Acknowledgments
The authors thank the data managers at each Cell Therapy Consortium member institution who collected patient data and maintained the database.
This research was supported in part by funding from Bristol Myers Squibb and Novartis Pharmaceutical, Inc (D.L.P.), the National Institutes of Health, National Cancer Institute (grant P30 CA008748 [M.-A.P. and G.L.S.]), and the National Center for Advancing Translational Sciences of the National Institutes of Health (award number UL1-TR002494 [V.B.]).
Authorship
Contribution: N.A. was responsible for conception of the study, data analysis, and writing the first draft; W.W. was responsible for the analysis and initial drafting of the manuscript; P.A.R. was responsible for the analysis; and all authors made significant contributions to revisions and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.A. reports receiving advisory board fees from Bristol Myers Squibb (BMS) and Legend Biotech; and consultancy, institutional research support, and funding from Kite/Gilead. D.L.P. reports receiving honoraria from Novartis, Kite/Gilead, Janssen, BMS, bluebird bio, Angiocrine, Mirror Biologics, Capstan Therapeutics, Sana Biotechnology, and Verismo Therapeutics; research funding from Novartis and BMS; has stock and other ownership interests in Genentech, Roche (spouse former employment); and reports receiving patent and royalty payments from Novartis and Tmunity. V.B. serves on Data and Safety Monitoring Board (DSMB) for Miltenyi Biotec; is member of ad hoc advisory board for AstraZeneca, ADC, Allogene and BMS; and has received research funding from Incyte, Gamida Cell, CitiusTech. L.J.N. has received honoraria from AbbVie, Atarra Biotherapeutics, BMS, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, Janssen, Novartis, and Takeda; and research support from BMS, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. M.A.-P reports honoraria from Adicet Bio, Allogene, AlloVir, Caribou Biosciences, Celgene, BMS, Equilium, ExeVir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, Orca Bio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences; is a member of the scientific advisory board of NexImmune; has ownership interests in NexImmune, Omeros, and Orca Bio; has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. R.T.M. reports serving as a consultant for Autolous, Kite/Gilead, and Novartis; receiving research support from Gamida, Allovir, Orca Bio, and Novartis; participating in a DSMB for Athersys, Novartis, Century Therapeutics, and Vor Biopharma; and holding a patent with Athersys. G.L.S. reports research funding to the institution from Janssen, Amgen, BMS, and Beyond Spring; and serves on DSMB for Arcellx. A.I.C. reports involvement in clinical trials with Novartis, Fate Therapeutics, and BMS; and serving as consultant for Elsevier. O.O.O. reports consultancy and advisory board roles with Pfizer, Kite, Gilead, AbbVie, Janssen, TGR Therapeutics, ADC, Novartis, Epizyme, Curio Science, Nektar, Cargo, and Caribou; received institution funding from Kite, Pfizer, Daiichi Sankyo, and Allogene; and honoraria from Pfizer and Gilead. S.J.S. reports consultancy for Acerta, Celgene, Genentech, Novartis, and Pharmacyclics; research funding from Celgene, Gilead, Janssen Research & Development, Merck, Novartis, and Pharmacyclics; membership on scientific advisory committees for Nordic Nanovector and Caribou Biosciences; and holds patents for “Methods for Treating Progressive Multifocal Leukoencephalopathy (PML)” (US patent number 13/537,330, 2012) and “Combination Therapies of Chimeric Antigen Receptor and PD-1 Inhibitors” (US patent number 62/368100, 455,547, 482846, 482846, 2016). M.R.B. reports consultancy for Kite/Gilead, Sana Biotechnology, Chimeric Therapeutics, Iovance, WindMIL Therapeutics, Agio, CRISPR Therapeutics, bluebird bio, Arcellx, Autolus, Novartis, BMS/JUNO Therapeutics, Incyte; research funding from Tmunity, Triumvira, Kite/Gilead, CRISPR Therapeutics, Novartis, Arcellx, Immatics, and Autolus; honoraria from Kite/Gilead, Agio, Novartis, BMS, Sanofi, Incyte, and Celgene; participation in speakers bureaus for Kite/Gilead, Agio, BMS, Kite/Gilead, Servier, AstraZeneca, ADC Therapeutics, Incyte, Servier, and Sanofi; travel support (other) from Kite, a Gilead company, Agios, Novartis, BMS, and Incyte; and membership on the board of directors or advisory committees for Kite/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, BMS/Juno Therapeutics, Incyte, Sana Biotechnology, Iovance Biotherapeutics, In8bio, and Chimeric Therapeutics. J.P.M. reports consultancy roles for Kite, Novartis, Nektar, BMS, Envision, Sana, Legend Biotech, and CRISPR Therapeutics; and advisory board roles for AlloVir and Autolus. P.A.R. serves as consultant and/or advisory board member for AbbVie, Novartis, BMS, ADC Therapeutics, Kite/Gilead, Sana Biotechnology, Nektar Therapeutics, Nurix Therapeutics, Intellia Therapeutics, CVS Caremark, Genmab, BeiGene, Janssen, and Pharmacyclics; receives honoraria from Novartis; and has research support from BMS, Kite Pharma, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, AstraZeneca, Genentech, and Tessa Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Nausheen Ahmed, Division of Hematologic Malignancies and Cellular Therapeutics, The University of Kansas Cancer Center, Westwood, KS 66203; email: nahmed5@kumc.edu.
References
Author notes
N.A. and W.W. are joint first authors and contributed equally to this study.
The full-text version of this article contains a data supplement.