Key Points
Multiassay strategy was developed to screen, quantify, and characterize neutralizing and nonneutralizing VWF antibodies in VWD plasma.
The prevalence of IgG and IgM anti-VWF antibodies among a cohort of 49 unrelated patients with type 3 VWD is 18%.
Visual Abstract
von Willebrand disease (VWD) is an inherited bleeding disorder caused by quantitative or qualitative defects in the von Willebrand factor (VWF) protein. Type 3 VWD has a severe bleeding phenotype caused by the absence of VWF, in which treatment usually involves replacement therapy with VWF-containing products. The immune system can react to the VWF product and form anti-VWF antibodies to neutralize or clear the VWF, which can compromise efficacy of treatment or lead to anaphylaxis. Current diagnostic testing is limited to the detection of anti-VWF antibodies that neutralize VWF binding to platelets by using a ristocetin cofactor assay. We set out to develop assays to identify both neutralizing and nonneutralizing antibodies to screen, quantify, and characterize anti-VWF antibodies in samples from the Zimmerman Program, a large multicenter study of patients with VWD. We detected anti-VWF immunoglobulin G (IgG) or IgM antibodies in 18% of 49 unrelated individuals with type 3 VWD. The antibodies ranged in concentration and consisted of 33% nonneutralizing and 67% neutralizing to factor VIII, collagen III, platelet glycoprotein Ib alpha (GPIbα), and/or collagen IV binding. Of the positive type 3 VWD samples, 8 of 9 were IgG, which were further subclassified into mostly IgG1 and IgG4 antibodies. Through a series of testing methods, we identified VWF-specific antibodies in 9 unrelated individuals with type 3 VWD with varying demographics, bleeding phenotypes, and genetic variants. This anti-VWF antibody testing strategy provides a useful tool to assess risk and better navigate treatment options for patients with type 3 VWD.
Introduction
von Willebrand disease (VWD) is an inherited disorder that is the result of a quantitative or qualitative defect in the von Willebrand factor (VWF) protein that can lead to mild to severe bleeding phenotypes. VWF, which circulates as a large multimeric glycoprotein, promotes hemostasis by tethering platelets to injured endothelium and serves as a carrier protein for factor VIII (FVIII). Laboratory testing for VWD can examine all these functions, including platelet binding, type III or IV collagen binding, and FVIII binding.1,2 Type 3 VWD is typically defined as having absent VWF.3 Treatment for patients with type 3 VWD requires replacement therapy with either plasma-derived or recombinant VWF (rVWF). Thus, patients with type 3 VWD are at risk of developing antibodies against VWF, which can lead to increased clearance or functional inhibition of VWF, thereby complicating treatment.
Antibodies are a response of the immune system, and VWF-specific antibodies form due to exposure to replacement therapies used to control bleeding. Current identification of VWF antibodies assesses the neutralization of VWF binding to platelets using the ristocetin cofactor (RCo) activity assay (VWF:RCo). Our goal was to develop assays capable of identifying a broader range of both neutralizing and nonneutralizing antibodies that disrupt VWF binding to FVIII, collagens III and IV, as well as platelets. The prevalence of antibodies and antibody type was examined using samples from the Zimmerman Program for the Molecular and Clinical Biology of VWD (Zimmerman Program), a large multicenter study of well-characterized patients with VWD with extensive laboratory and bleeding phenotypes as well as VWF genotype.
Methods
Patient population
This study was approved by each site’s institutional review board, and all participants gave informed consent in accordance with the declaration of Helsinki. Healthy control and patients with VWD were enrolled through the Zimmerman Program, which involved recruitment from 11 primary clinical hematology centers along with multiple secondary centers throughout North America, as listed in Appendix. Patients with a preexisting VWD diagnosis were consented at the time of enrollment and subsequently had confirmatory phenotypic assessment in the central laboratory at Versiti as previously described.4 Patient’s bleeding symptoms were quantified using the ISTH-BAT bleeding score.5 Genotyping involved full-length VWF exonic Sanger sequencing and array comparative genomic hybridization to detect deletions or duplications.4 Forty-nine phenotypically assigned type 3 VWD index cases along with 51 healthy controls were assessed for anti-VWF antibodies. Positive controls consisted of individual immunoglobulin G (IgG) and IgM human anti-VWF plasmas (positive control). Type 3 VWD anti-VWF negative control plasma (T3 Ab–) is a single plasmapheresis collection from 1 donor (HRF, Inc, Raleigh, NC).
VWF antibody screening ELISA
Anti-VWF IgG and IgM antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Within each 96-well Immulon 1B microtiter plate (ThermoFisher Scientific, Rochester, NY), an equal number of wells were coated with either rVWF (VONVENDI, Takeda Pharmaceuticals Inc, Lexington, MA) at 25 IU/dL (diluted in 0.05 M carbonate buffer; pH 9.6) or carbonate buffer alone to serve as individual sample blanks. Wells were washed (0.05% volume-to-volume ratio Tween-20 in phosphate-buffered saline; pH 7.2) between each subsequent step. After wells were blocked (1% phosphate-buffered saline/1% bovine serum albumin; pH 7.2), diluted control and test plasma (1:25 in blocking buffer), along with a blocking buffer control (blank), were added to both the VWF- and carbonate buffer–coated wells. Plasma was incubated for 1 hour at room temperature to allow VWF-specific antibodies present in the plasma to bind to the coated plate. Alkaline phosphatase–conjugated fragment goat anti-human IgG, Fcᵧ fragment–specific or anti-human IgM, Fc5μ fragment–specific antibodies (Jackson ImmunoResearch Inc, West Grove, PA) were then added to detect the presence of anti-VWF IgG or IgM in the plasma. P-nitrophenyl phosphate substrate (Life Technologies Corp, Frederick, MD) diluted in substrate buffer (1 M diethanolamine per 0.5 mM MgCl2; pH 9.8) was added to the wells and incubated in the dark, and the optical density (OD) was read on a SpectraMax Plus 384 microplate reader with SoftMax Pro 7 software (Molecular Devices, LLC; https://www.moleculardevices.com) at a wavelength of 405 to 650 nm. The average OD from carbonate buffer–coated wells was subtracted from the VWF-coated wells to account for any nonspecific binding.
A screening cut point (threshold) was established to classify samples into negative and positive by applying an upper-negative limit of 95% to the distribution of 51 Zimmerman Program healthy control participants.6,7 A specific cut point was calculated for both IgG and IgM detections (GraphPad, Prism 9 software, Boston, MA).
VWF antibody quantification
Confirmation and titer (semiquantitative)
For all plasma samples with calculated ODs >95th percentile cut point, titration of the plasma was performed to confirm and quantify VWF antibody binding. This was done by following the same methods as described in the screening ELISA with the following changes. Serial 2-fold dilutions of plasma into blocking buffer, beginning at 1:25, were made to create titration curves. The cut point used was calculated from the same population of healthy controls using the average OD, blank subtracted, from the 1:25 plasma dilution of VWF-coated wells. Titer was determined as the greatest dilution with OD more than cut point.
Concentration in units (quantitative)
Chimeric human monoclonal anti-VWF IgG and IgM antibodies (Absolute Antibody, Wilton, United Kingdom) were provided by Versiti’s product development team and served to establish calibration curves to further quantify the amount of anti-VWF antibody present in positive samples. In addition to following the same methods used in the screening ELISA, a 7-point human anti-VWF IgG or human anti-VWF IgM curve was added to VWF-coated wells at the time of plasma addition. The human IgG and IgM curves were serially diluted 1:2 in blocking buffer from 200 ng/mL to 3 ng/mL and 800 ng/mL to 12 ng/mL, respectively. Based on previously determined titer levels, plasma was run at 2 dilutions to fall within the curve’s parameters and ensure parallelism. VWF antibody plasma concentrations were calculated (SoftMax Pro 7) for each plasma dilution, subtracting the concentration of the noncoated wells from that of the VWF-coated wells, resulting in a final concentration in nanogram per milliliter. Based upon observed concentration ranges from control and test plasmas, we set 100 ng/mL to equal 1 arbitrary unit (A.U.) of IgG or IgM.
VWF antibody characterization
Functional VWF activity ELISAs
To characterize the VWF antibodies as neutralizing or nonneutralizing, we used competition assays, mixing patient plasma or control T3 Ab– plasma 1:1 with rVWF prediluted to 100 IU/dL in T3 Ab– plasma, incubated at 37°C for 1 hour, and tested on previously described VWF activity ELISAs consisting of VWF binding to platelet receptor glycoprotein Ibα (VWF:GPIbM), human type III collagen (VWF:CB3), human type IV collagen (VWF:CB4), and FVIII (VWF:F8B).8-11 Reduction in VWF detection when compared with the T3 Ab– control mixture indicated an inhibitory or neutralizing effect from the antibody present in the patient plasma.
IgG subclass determination
Further characterization of plasma containing VWF antibodies was done to determine IgG subclass. The same direct capture of rVWF, block, and addition of plasma steps from the screening ELISA methods were used. Additionally, diluted plasma was added to 4 sets of duplicate wells to detect antibodies for each of the subclasses. Sheep anti-human IgG1, IgG2, IgG3, and IgG4 kappa purified polyclonal antibodies conjugated with horseradish peroxidase (The Binding Site, Birmingham, United Kingdom; kindly provided by R.H. Aster, Versiti Blood Research Institute) were individually diluted in blocking buffer, added to the wells, and incubated at room temperature for 30 minutes. After washing, 0.5 mg/mL o-phenylenediamine dihydrochloride substrate in stable peroxide substrate buffer (Thermo Scientific, Rockford, IL) was added, incubated in the dark, and read at 450 nm with blank subtracted to determine average OD for each subclass.
Results
Detection and characterization of VWF antibodies
A multitiered assessment strategy (Figure 1) was applied to test the plasma of patients with type 3 VWD enrolled in the Zimmerman Program to determine the prevalence of and further characterize anti-VWF antibodies in this population. Detailed phenotypic and genetic characterization of the patients with VWF antibodies was available on these type 3 patients analyzed in the Zimmerman Program, as previously described.4 A summary of the VWF antibody qualities, demographics, bleeding scores, ABO blood group, and VWF genetic variants of each patient are outlined in Table 1. The patients with antibody titers >1:100 also had the highest bleeding scores (16-32). Fifty-six percent of the VWF antibody–positive population had type O blood group (5/9), similar to the overall type 3 VWD cohort (55%). Loss-of-function variants spanning the VWF gene, including 7 stop, 8 frameshift, 1 splice site, and 1 intronic variant, were linked to the pathogenesis of VWD in these patients.
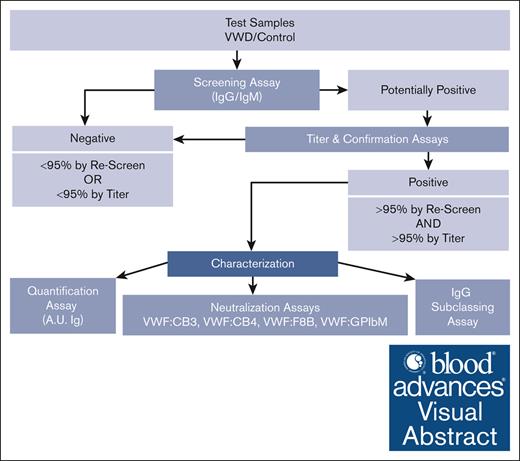
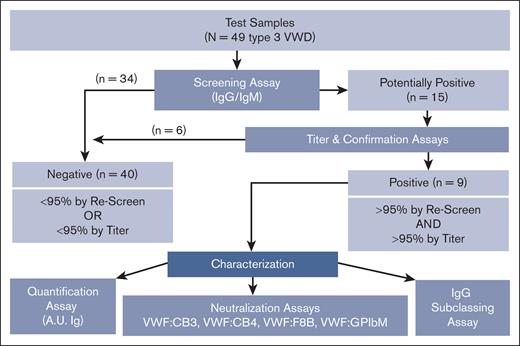
Flowchart describing how VWF antibodies are detected and characterized through multiple laboratory methods. VWF antibody assessment strategy used to detect, quantify, and characterize anti-VWF IgG and IgM antibodies in type 3 VWD plasma and control samples using a systematic ELISA-based approach. Plasma samples are screened and confirmed using 95th percentile thresholds (95%) as determined by the healthy control cohort. VWF antibodies in the positive samples are further assessed for concentration in A.U. of immunoglobulin (A.U. Ig), neutralization of VWF function as tested via mixing studies with VWF:CB3, VWF:CB4, VWF:F8B, and VWF:GPIbM activity ELISAs, and IgG subclass if applicable.
Flowchart describing how VWF antibodies are detected and characterized through multiple laboratory methods. VWF antibody assessment strategy used to detect, quantify, and characterize anti-VWF IgG and IgM antibodies in type 3 VWD plasma and control samples using a systematic ELISA-based approach. Plasma samples are screened and confirmed using 95th percentile thresholds (95%) as determined by the healthy control cohort. VWF antibodies in the positive samples are further assessed for concentration in A.U. of immunoglobulin (A.U. Ig), neutralization of VWF function as tested via mixing studies with VWF:CB3, VWF:CB4, VWF:F8B, and VWF:GPIbM activity ELISAs, and IgG subclass if applicable.
Summary of patients with type 3 VWD with anti-VWF antibodies
| Patient ID/color code . | Age . | Sex . | Race . | Ethnicity . | ABO . | ISTH BS . | VWF variant 1 . | VWF variant 2 . | VWF: GPIbM % inh . | VWF: CB3 % inh . | VWF: CB4 % inh . | VWF: F8B % inh . | VWF Ab class . | Ab titer . | Ab, A.U. . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 051 051 | 11 | F | Black | Not Hisp | O | 32 | p.Trp1144∗ | p.Leu1281Cysfs∗16 | 28 | ≥94 | 27 | 43 | IgG | 800 | 135 |
 393 393 | 41 | F | White | Not Hisp | A | 24 | p.Met814Hisfs∗5 | p.Arg1566∗ | < | 20 | < | 57 | IgG | 400 | 47 |
 101 101 | 38 | M | White | Unk | O | 16 | p.Cys689∗ | p.Pro812Argfs∗31 | < | 36 | < | < | IgG | 200 | 27 |
 171 171 | 8 | F | Asian | Not Hisp | Unk | 4 | c.6798 + 1G>A | c.6798 + 1G>A | < | < | < | 20 | IgG | 100 | 18 |
 020 020 | 48 | M | White | Not Hisp | O | 10 | p.Cys331∗ | c.324-14_-4delins∗ | 11 | < | < | 15 | IgG | 100 | 12 |
 004 004 | 27 | F | Asian | Not Hisp | B | 6 | p.Leu970Serfs∗9 | p.Leu970Serfs∗9 | < | < | < | < | IgG | 100 | 3 |
 146 146 | 1 | F | White | Not Hisp | O | 6 | p.Arg575Thrfs∗16 | p.Val1546∗ | < | < | < | < | IgG | 25 | 2.5 |
 008 008 | 34 | F | Asian | Not Hisp | O | 10 | p.Cys665Alafs∗3 | p.Gln1113∗ | < | < | < | < | IgG | 25 | 3 |
 646 646 | 17 | F | White | Hispanic | B | NA | p.Leu451Argfs∗19 | p.Arg1659∗ | < | < | < | 14 | IgM | 25 | 43 |
| Patient ID/color code . | Age . | Sex . | Race . | Ethnicity . | ABO . | ISTH BS . | VWF variant 1 . | VWF variant 2 . | VWF: GPIbM % inh . | VWF: CB3 % inh . | VWF: CB4 % inh . | VWF: F8B % inh . | VWF Ab class . | Ab titer . | Ab, A.U. . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 051 051 | 11 | F | Black | Not Hisp | O | 32 | p.Trp1144∗ | p.Leu1281Cysfs∗16 | 28 | ≥94 | 27 | 43 | IgG | 800 | 135 |
 393 393 | 41 | F | White | Not Hisp | A | 24 | p.Met814Hisfs∗5 | p.Arg1566∗ | < | 20 | < | 57 | IgG | 400 | 47 |
 101 101 | 38 | M | White | Unk | O | 16 | p.Cys689∗ | p.Pro812Argfs∗31 | < | 36 | < | < | IgG | 200 | 27 |
 171 171 | 8 | F | Asian | Not Hisp | Unk | 4 | c.6798 + 1G>A | c.6798 + 1G>A | < | < | < | 20 | IgG | 100 | 18 |
 020 020 | 48 | M | White | Not Hisp | O | 10 | p.Cys331∗ | c.324-14_-4delins∗ | 11 | < | < | 15 | IgG | 100 | 12 |
 004 004 | 27 | F | Asian | Not Hisp | B | 6 | p.Leu970Serfs∗9 | p.Leu970Serfs∗9 | < | < | < | < | IgG | 100 | 3 |
 146 146 | 1 | F | White | Not Hisp | O | 6 | p.Arg575Thrfs∗16 | p.Val1546∗ | < | < | < | < | IgG | 25 | 2.5 |
 008 008 | 34 | F | Asian | Not Hisp | O | 10 | p.Cys665Alafs∗3 | p.Gln1113∗ | < | < | < | < | IgG | 25 | 3 |
 646 646 | 17 | F | White | Hispanic | B | NA | p.Leu451Argfs∗19 | p.Arg1659∗ | < | < | < | 14 | IgM | 25 | 43 |
Symbol < represents value <10.
Ab, antibody; F, female; inh, inhibition; M, male; NA, not obtained; Not Hisp, not Hispanic; Unk, unknown.
c.324-14_-4delins, c.324-14_324-4delinsAAGTTCAGAGTCT.
Screening assay to detect VWF-specific IgG and IgM antibodies
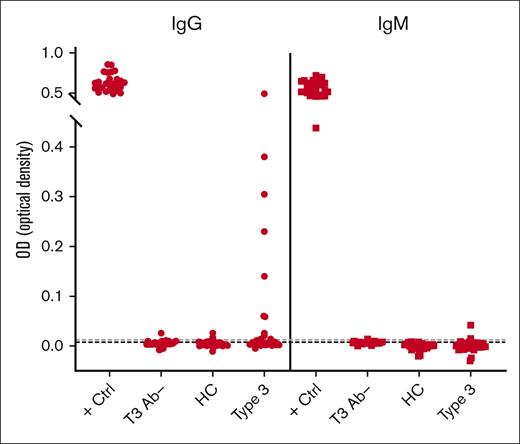
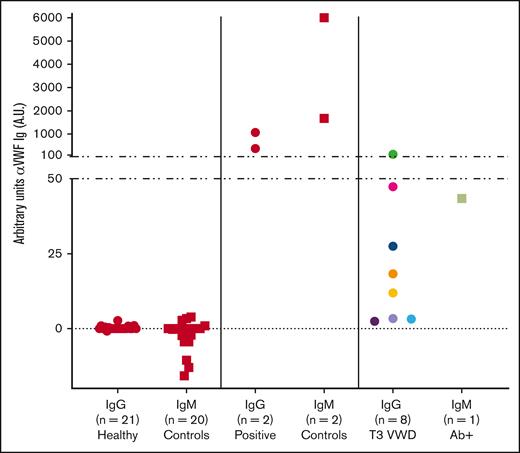
As an initial screen of human plasma for the presence of anti-VWF antibodies, we developed an ELISA method using rVWF to detect VWF-specific antibodies in patients with VWD and control cohorts. A known positive (positive control) and a negative control (T3 Ab–) for each of the IgG and IgM assays was included on every plate. The distribution of healthy control plasma (N = 51) established the cut points used to classify samples as potentially positive using an upper-negative limit at the 95th percentile (IgG = 0.0122 OD; IgM = 0.0077 OD), resulting in 2 of 51 healthy control plasmas (4%) with anti-VWF antibody levels above the cut point for both IgG and IgM. This initial screen detected VWF-specific antibodies in 15 of 49 patients with type 3 VWD (IgG, 11/49 [22%]; IgM, 4/49 [8%]; Figures 1 and 2).
Screening assay detects VWF-specific antibodies among type 3 VWD cohort. Patients enrolled in the Zimmerman Program were screened for IgG (●) and IgM (■) antibodies that bind to rVWF by ELISA. High-titer positive control (+ Ctrl) and negative control (T3 Ab–) plasma was run on every test plate. Fifty-one healthy controls (HCs) established a threshold for positivity (dotted lines). This initial screen detected 15 of 49 patients with type 3 VWD (Type 3) positive for either IgG (11/49) or IgM (4/49) anti-VWF antibodies.
Screening assay detects VWF-specific antibodies among type 3 VWD cohort. Patients enrolled in the Zimmerman Program were screened for IgG (●) and IgM (■) antibodies that bind to rVWF by ELISA. High-titer positive control (+ Ctrl) and negative control (T3 Ab–) plasma was run on every test plate. Fifty-one healthy controls (HCs) established a threshold for positivity (dotted lines). This initial screen detected 15 of 49 patients with type 3 VWD (Type 3) positive for either IgG (11/49) or IgM (4/49) anti-VWF antibodies.
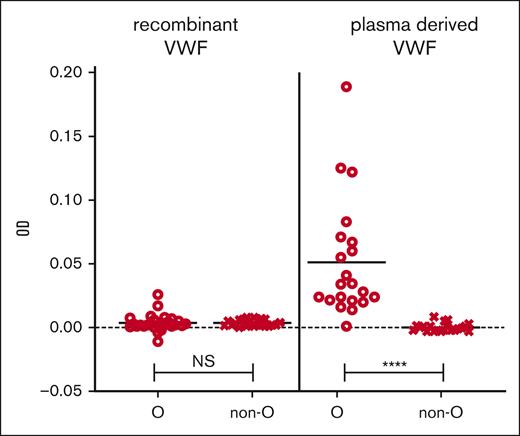
During assay development, we compared plasma-derived VWF and rVWF as potential sources for the VWF capture. When plasma-derived VWF was the source of antibody capture, we noted a significant increase in IgG OD in type O healthy controls compared with non-O; however, this was corrected for by using rVWF (Figure 3). Similarly, we investigated the binding of VWF to the microtiter plate and found that VWF was best bound directly to the plastic wells because the use of monoclonal mouse anti-human VWF antibodies to capture the VWF led to false positives in some healthy controls. Additionally, the plastic of the microtiter ELISA plates made a difference because we found that high binding hydrophilic polystyrene plastic (ThermoFisher Scientific) led to IgG/IgM detection even without bound VWF in healthy control and VWD plasma, but this observation went away with the use of medium binding hydrophobic polystyrene plates (data not shown).
Plasma-derived VWF captured IgG in blood type O HCs. rVWF (VONVENDI) was compared with plasma-derived VWF (Humate-P) in the screening assay for their abilities to capture and bind IgG in the plasma of 51 and 41 HCs, respectively. Mann-Whitney tests were performed on the ODs between bound and detected IgG in blood group O individuals and non-O (A, B, and AB) individuals. There was no significant difference (NS) between O and non-O IgG levels when captured by rVWF but significantly increased IgG detection in type O vs non-O plasma applied to the plasma-derived VWF. ∗∗∗∗P ≤ .0001.
Plasma-derived VWF captured IgG in blood type O HCs. rVWF (VONVENDI) was compared with plasma-derived VWF (Humate-P) in the screening assay for their abilities to capture and bind IgG in the plasma of 51 and 41 HCs, respectively. Mann-Whitney tests were performed on the ODs between bound and detected IgG in blood group O individuals and non-O (A, B, and AB) individuals. There was no significant difference (NS) between O and non-O IgG levels when captured by rVWF but significantly increased IgG detection in type O vs non-O plasma applied to the plasma-derived VWF. ∗∗∗∗P ≤ .0001.
The IgG- and IgM-positive samples identified in the initial screening assay were further investigated using a slightly modified ELISA that included titration curves of test plasma (Figure 4A). The OD of each plasma dilution >95th percentile cut point as determined by the same set of healthy controls indicated that those samples were confirmed positive for anti-VWF antibodies. This confirmatory process reassigned 6 of the 15 screened antibody-positive type 3 VWD samples into the antibody-negative group along with most healthy controls (Figure 1), resulting in a total of 9 of 49 patients with type 3 VWD (18%) with confirmed anti-VWF antibody (T3 VWD Ab+; Table 2).
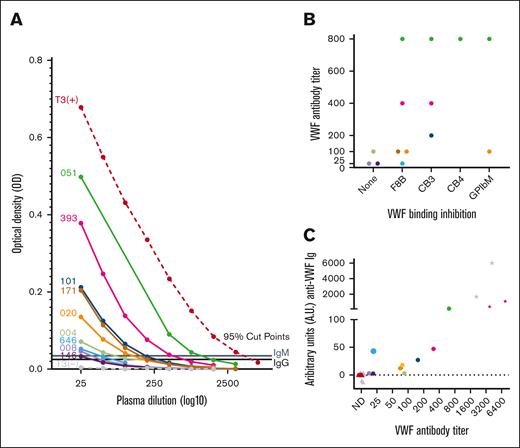
VWF antibody titer analysis. (A) Titration curves of antibody-positive type 3 VWD samples (patient IDs left of curves) confirm and semiquantify VWF antibody presence. Patient plasma was titrated in blocking buffer and screened by ELISA to determine maximum dilution where anti-VWF antibody OD remained above the 95th percentile cut points as determined by the HC cohort. (B) Distribution of antibodies that inhibited VWF binding to factor FVIII, collagens type III and IV, and platelet receptor GPIbα (F8B, CB3, CB4, and GPIbM accordingly) along the x-axis by antibody titer level on the y-axis (abbreviated to titer value without 1: prefix; eg, 1:25 = 25). (C) Correlation of VWF antibody titer from none detected (ND) to 1:6400 on x-axis (abbreviated as in panel B) with A.U. measured by ELISA using calibration curves. Symbols represent plasma samples and are differentiated into IgG (black) and IgM (gray) anti-VWF immunoglobulins (Igs), healthy control (▴), type 3 VWD antibody–positive (•), or positive control (★).
VWF antibody titer analysis. (A) Titration curves of antibody-positive type 3 VWD samples (patient IDs left of curves) confirm and semiquantify VWF antibody presence. Patient plasma was titrated in blocking buffer and screened by ELISA to determine maximum dilution where anti-VWF antibody OD remained above the 95th percentile cut points as determined by the HC cohort. (B) Distribution of antibodies that inhibited VWF binding to factor FVIII, collagens type III and IV, and platelet receptor GPIbα (F8B, CB3, CB4, and GPIbM accordingly) along the x-axis by antibody titer level on the y-axis (abbreviated to titer value without 1: prefix; eg, 1:25 = 25). (C) Correlation of VWF antibody titer from none detected (ND) to 1:6400 on x-axis (abbreviated as in panel B) with A.U. measured by ELISA using calibration curves. Symbols represent plasma samples and are differentiated into IgG (black) and IgM (gray) anti-VWF immunoglobulins (Igs), healthy control (▴), type 3 VWD antibody–positive (•), or positive control (★).
VWF antibody detection and inhibitor characterization within type 3 VWD cohort (N = 49)
| VWF Abs in Zimmerman type 3 VWD index cases . | No. of patients/total patients (%) . |
|---|---|
| VWF antibody positive | 9/49 (18%) |
| IgG positive | 8/9 (89%) |
| IgM positive | 1/9 (11%) |
| Nonneutralizing antibodies | 3/9 (33%) |
| Neutralizing/inhibitory antibodies | 6/9 (67%) |
| VWF:F8 inhibitor | 5/9 (56%) |
| VWF:CB3 inhibitor | 3/9 (33%) |
| VWF:GPIbM inhibitor | 2/9 (22%) |
| VWF:CB4 inhibitor | 1/9 (11%) |
| VWF Abs in Zimmerman type 3 VWD index cases . | No. of patients/total patients (%) . |
|---|---|
| VWF antibody positive | 9/49 (18%) |
| IgG positive | 8/9 (89%) |
| IgM positive | 1/9 (11%) |
| Nonneutralizing antibodies | 3/9 (33%) |
| Neutralizing/inhibitory antibodies | 6/9 (67%) |
| VWF:F8 inhibitor | 5/9 (56%) |
| VWF:CB3 inhibitor | 3/9 (33%) |
| VWF:GPIbM inhibitor | 2/9 (22%) |
| VWF:CB4 inhibitor | 1/9 (11%) |
VWF antibody inhibitor characterization
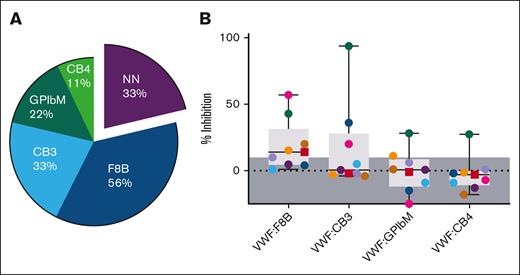
Mixing studies were performed to measure which VWF antibody–positive plasma samples inhibited the binding of rVWF to FVIII, collagens type III and IV, and the GPIbα platelet receptor. Compared with the T3 Ab– control mix, a reduction in VWF detection when tested by VWF:F8B, VWF:CB3, VWF:CB4, and VWF:GPIbM ELISAs suggested that the VWF antibody in the plasma had a neutralizing (inhibiting) effect to the normal function of VWF (Figure 5). Although 3 of 9 antibody-positive samples (33%) were nonneutralizing, 6 of 9 (67%) were found to possess inhibitory functions, resulting in 6 of 49 type 3 patients (12%) with neutralizing VWF antibodies. Inhibitors of FVIII binding to VWF (VWF:F8B) were the most prevalent (5/9 [56%]), with inhibition between 14% and 57%. VWF:CB3 was inhibited in 3 of 9 T3 VWD Ab+ patients (33%) and ranged even higher from 20% to ≥94% inhibition. VWF:GPIbM and VWF:CB4 functions were also disrupted but in fewer of the samples and at a lower percent inhibition (Figure 5B). The correlation between antibody titer and inhibitor presence is noted in Figure 4B. VWF inhibitor prevalence within the T3 VWD Ab+ group is summarized in Table 2.
Antibodies were characterized as inhibiting VWF binding interactions to FVIII, collagen, and platelet receptor GPIbα. Plasma from the 9 patients with type 3 VWD who are antibody positive, along with the T3 Ab– control for comparison, was mixed and preincubated with rVWF to allow for antibody/VWF complex formation, then measured for VWF binding function using VWF:F8B, VWF:CB3, VWF:CB4, and VWF:GPIbM ELISA methods. (A) CB3, CB4, GPIbM, and F8B indicate inhibited function of VWF binding to collagen type III, collagen type IV, platelet receptor GPIbα, and FVIII, respectively. Percent of the patients with each type of inhibitor revealed 33% of the samples contained nonneutralizing (NN) antibodies, whereas the majority of antibody samples inhibited VWF:F8B (56%) followed by VWF:CB3 (33%). (B) Percent of VWF binding inhibition when compared with the T3 Ab–/rVWF control ranged in severity between undetected (shaded, ≤10%) and ≥94% (VWF:CB3). Among the 8 IgG (●) and 1 IgM (■) anti-VWF samples, FVIII and collagen III to VWF inhibition were found to be most prevalent and had higher degrees of inhibitory function.
Antibodies were characterized as inhibiting VWF binding interactions to FVIII, collagen, and platelet receptor GPIbα. Plasma from the 9 patients with type 3 VWD who are antibody positive, along with the T3 Ab– control for comparison, was mixed and preincubated with rVWF to allow for antibody/VWF complex formation, then measured for VWF binding function using VWF:F8B, VWF:CB3, VWF:CB4, and VWF:GPIbM ELISA methods. (A) CB3, CB4, GPIbM, and F8B indicate inhibited function of VWF binding to collagen type III, collagen type IV, platelet receptor GPIbα, and FVIII, respectively. Percent of the patients with each type of inhibitor revealed 33% of the samples contained nonneutralizing (NN) antibodies, whereas the majority of antibody samples inhibited VWF:F8B (56%) followed by VWF:CB3 (33%). (B) Percent of VWF binding inhibition when compared with the T3 Ab–/rVWF control ranged in severity between undetected (shaded, ≤10%) and ≥94% (VWF:CB3). Among the 8 IgG (●) and 1 IgM (■) anti-VWF samples, FVIII and collagen III to VWF inhibition were found to be most prevalent and had higher degrees of inhibitory function.
Precise unit quantification of VWF antibody
Anti-VWF antibodies of positive controls, a subset of healthy controls, and the T3 VWD Ab+ samples were quantified in an ELISA using humanized anti-VWF IgG and IgM chimeric monoclonal antibodies as standard curves. Lower limits of detection (LODs) for the IgG (<1 A.U.) and IgM (<3 A.U.) were determined.12 The majority of healthy control values fell below the LODs with ranges from <1 to 2.7 for anti-VWF IgG and <3 to 3.8 for IgM. T3 VWD Ab+ IgG titers ranging from 1:25 to 1:800 showed values between 2 and 135 A.U., whereas the high-titer positive controls exceeded 1000 A.U. of anti-VWF IgG. The single IgM-positive type 3 VWD with a low titer of 1:25 measured 43 A.U. of anti-VWF IgM. (Figure 6). The correlation of VWF antibody titers and A.U.s is depicted in Figure 4C.
Quantification of VWF-specific antibodies. Humanized anti-VWF IgG and IgM chimeric monoclonal antibodies were used to establish standard curves to detect anti-VWF Ig antibodies. T3 VWD Ab+ IgG titers from 1:25 to 1:800 had a range of 2 to 135 A.U., whereas the higher titer positive controls reached over 1000 A.U. anti-VWF IgG. The single IgM-positive type 3 VWD with a low titer of 1:25 measured at 43 A.U. anti-VWF IgM. Symbols represent plasma samples and are differentiated into IgG (●) and IgM (■).
Quantification of VWF-specific antibodies. Humanized anti-VWF IgG and IgM chimeric monoclonal antibodies were used to establish standard curves to detect anti-VWF Ig antibodies. T3 VWD Ab+ IgG titers from 1:25 to 1:800 had a range of 2 to 135 A.U., whereas the higher titer positive controls reached over 1000 A.U. anti-VWF IgG. The single IgM-positive type 3 VWD with a low titer of 1:25 measured at 43 A.U. anti-VWF IgM. Symbols represent plasma samples and are differentiated into IgG (●) and IgM (■).
IgG subclass characterization in 8 positive patients with type 3 VWD
Plasma VWF antibodies from the 8 IgG-positive type 3 patients were captured with rVWF to determine IgG subclasses 1 to 4. Patients were categorized as neutralizing or nonneutralizing, and the ODs were totaled per each subclass. This revealed a prominence of IgG1 overall (48%) but particularly in the nonneutralizing category, with IgG4 and IgG3 notably elevated in the neutralizing class of antibodies (Figure 7A). Multiple subclasses per sample were detected in all but 1 patient, and the individual compositions within each sample were observed in order of overall antibody titer along with the type of inhibitor function detected (Figure 7B). The samples with VWF:CB3 inhibitors (also highest titers) were associated with >46% IgG4 composition.
IgG subclass distribution in type 3 VWD IgG-positive samples revealed increased IgG4 and IgG3 in the higher titer inhibitor samples. (A) Measured by ELISA, adding type 3 VWD IgG-positive plasma to rVWF and detecting specifically for each subclass, we found IgG1 as the prominent subclass in NN antibody samples, whereas similar amounts of both IgG1 and IgG4 were identified in the neutralizing antibodies. (B) Each pie chart represents 1 of the 8 IgG-positive antibody samples, arranged from highest titer antibody to lowest. If the antibody inhibited VWF binding to FVIII, collagen type III, collagen type IV, or platelet receptor GPIbα (F8B, CB3, CB4, and GPIbM accordingly), it is noted beneath the sample ID and ranked by degree of inhibition using the greater than carrot (>) if multiple activities were neutralized. We observed increased IgG4 composition in all patients associated with CB3 inhibition, which were also the highest titer samples.
IgG subclass distribution in type 3 VWD IgG-positive samples revealed increased IgG4 and IgG3 in the higher titer inhibitor samples. (A) Measured by ELISA, adding type 3 VWD IgG-positive plasma to rVWF and detecting specifically for each subclass, we found IgG1 as the prominent subclass in NN antibody samples, whereas similar amounts of both IgG1 and IgG4 were identified in the neutralizing antibodies. (B) Each pie chart represents 1 of the 8 IgG-positive antibody samples, arranged from highest titer antibody to lowest. If the antibody inhibited VWF binding to FVIII, collagen type III, collagen type IV, or platelet receptor GPIbα (F8B, CB3, CB4, and GPIbM accordingly), it is noted beneath the sample ID and ranked by degree of inhibition using the greater than carrot (>) if multiple activities were neutralized. We observed increased IgG4 composition in all patients associated with CB3 inhibition, which were also the highest titer samples.
Discussion
The ability to measure and characterize VWF-specific antibodies in type 3 VWD plasma is a valuable tool because their presence can lead to accelerated VWF clearance or competitive binding of infused VWF.13,14 By developing a multitiered testing approach and following several well-established guidelines to test immunogenicity of therapeutic proteins,12,15 we are now able to detect, quantify, and characterize neutralizing and nonneutralizing VWF antibodies in human plasma. Although some literature may use the terms interchangeably, it is crucial to emphasize that the anti-VWF antibodies reported here differ from VWF inhibitors. VWF inhibitor antibodies represent only a fraction of the total antibodies identified in our study. This comprehensive VWF antibody assessment strategy applied to a large, diverse, unrelated type 3 VWD population has enhanced our understanding of the prevalence and nature of anti-VWF antibodies within these patients.
Previously considered rare in type 3 and severe VWD studies, with estimated prevalence ranging from 5.8% to 9.5%,16-18 we detected VWF antibodies in 18% of our unrelated Zimmerman Program type 3 VWD index case cohort. A recent study of 99 Iranians with type 3 VWD reported a similar prevalence of 19.2% VWF inhibitors, although 88% were born to consanguineous parents.19 Another report involving 213 previously diagnosed patients with type 3 VWD, including related individuals, found only 8.4% with anti-VWF antibodies.20 In a summary of reported VWF antibody cases, many of the individuals who developed anti-VWF antibodies were related, suggesting a heritable risk for antibody development.21 However, heritability did not contribute to the prevalence in our study because our type 3 population is unrelated and spread throughout the United States and Canada.
Assessing for VWF antibodies is especially useful in clinical cases in which a patient is not responding well to VWF replacement therapy22 and could also be used to monitor antibody development with time and exposure to minimize the risk of anaphylactic response.21,23 A detailed description and characterization of the Zimmerman Program type 3 VWD cohort has previously been reported.4 Among the 9 patients with type 3 VWD with anti-VWF antibodies (Table 1), we observed variability in demographics including age, race, ethnicity, and blood type. The mean age of those with anti-VWF antibodies was 25 years (range, 1-48) compared with 22 years in the type 3 cohort. Although most positive type 3 antibody patients were White (55%), 33% self-identified as Asian (Far East, Southeast Asia, or India) compared with 12% in the overall type 3 cohort. Although the 49 type 3 VWD index cases were divided equally between male and female, 7 of 9 of those with VWF antibodies (78%) were female. Although 2 of 7 were too young for first menstruation, it raises the question whether females may be at a greater risk of antibody development due to increased therapeutic exposure to control bleeding during menses.
We observed that the highest concentrations of VWF antibody correlate with the highest bleeding scores. The greater the bleeding symptoms, the more likely frequent VWF replacement therapy has been involved. Although many of the first reported cases of VWF antibodies involved partial or complete VWF gene deletions, subsequent reports have also identified several nonsense and frameshift variants.20,21 Of the 18 alleles from the 9 patients with unrelated T3 VWD Ab+ in Zimmerman Program expected to contain pathogenic variants, 15 of 18 alleles were found to contain nonsense and frameshift pathogenic variants, and 1 allele contained an intronic pathogenic variant. One patient was found to have the same pathogenic splice donor variant on both alleles, which came from both parents, suggesting consanguinity.
Current clinical testing for VWF antibodies is rarely performed, and when it is, it is limited to assays that neutralize VWF’s ability to bind platelets using a RCo activity assay. However, the anti-VWF antibody IgG and IgM assay that stemmed from a collaboration between Versiti Blood Research Institute and Versiti Diagnostic Laboratories is now available commercially24 and incorporates the methods described here from the Zimmerman study. Due to the lack of testing availability, a modified Bethesda assay, traditionally used to measure FVIII inhibitors, has recently been introduced, but it is still limited to detecting neutralizing VWF antibodies.20,23 The methods described in this assessment strategy are inclusive of nonneutralizing antibodies, which accounted for 33% of our type 3 VWD Ab+ samples. The nonneutralizing antibodies were also low titer (≤1:100), which could account for the lack of inhibitory function measured.
Using similar mixing study approaches as described previously,14,23,25 this strategy implemented for the Zimmerman Program patients also contributes to the understanding of the specific inhibitory activities of VWF antibodies. Among the 67% T3 VWD Ab+ samples with neutralizing antibodies, over half showed VWF:F8B inhibition, and 33% had detectable VWF:CB3 inhibition. Fewer samples showed VWF:GPIbM (22%) and VWF:CB4 inhibitors (11%). If the RCo inhibitor assay were the only VWF diagnostic test used for these samples, at best a maximum of 4% (2/49) of Zimmerman Program type 3 VWD samples would have been found to contain VWF antibodies. Given the prevalence of VWF antibodies in 18% of the Zimmerman type 3 VWD index cases, consideration of formal periodic testing for antibodies to VWF should be considered, similar to the approach in hemophilia A.
Defining concentration of VWF antibody in plasma using titration or calibration curves is important to understand the potential risk the antibody poses. Higher concentrations will lead to greater clearance or neutralization of infused VWF, and even low titer antibodies could suggest a need to be closely monitored over time with increased VWF exposure. Although titers are universally recognized, they are only broad measurements dependent on different testing parameters at each laboratory. Using humanized anti-VWF IgG and IgM chimeric monoclonal antibody calibration curves, a more precise unit of measurement can be obtained and compared across laboratories more uniformly. For example, samples with an IgG antibody titer of 1:100 had concentrations ranging from 3 to 18 A.U.s, whereas the 1:800 titer sample concentration was 135 A.U. It is recommended that assay sensitivities measuring anti-drug antibody formation would be at least 250 to 500 ng/mL (2.5-5 A.U.s for our assay) IgG or IgM,12 and some data suggest clinical events associated with antibody concentrations as low as 100 ng/mL26,27; therefore, our LODs of 1 and 3 A.U.s are more than capable of detecting low-level antibodies with potential clinical risk. A limitation to a single monoclonal antibody standard is that it only represents 1 antibody formation/epitope binding site. The IgG subclass data presented here and previous studies that mapped and characterized VWF antibodies show that the anti-VWF antibodies in patient plasma are often a diverse composition of polyclonal IgG.28
Because 89% of detected VWF antibodies were IgG, further characterization allowed for a glimpse into which IgG subclasses were formed and whether there was any functional correlation observed. IgG1, the most abundant subclass induced by immune responses,29 comprised 48% of the cumulative IgG, and 76% of the nonneutralizing (lower titer) antibodies were IgG1 (Figure 7A). The most notable difference between neutralizing and nonneutralizing was the elevated IgG4 detected in inhibitor samples, especially those with VWF:CB3 inhibition (Figure 7B). This elevated IgG4 among the neutralizing antibodies is not surprising given its association in response to therapeutic proteins such as factors VIII and IX.29-33 Because the A3 domain of VWF is the primary binding site of type III collagen,34 exposure of this region may increase antibody development to epitopes in the A3 binding region.
Our data lay the groundwork for future investigations into understanding the development of VWF antibodies in individuals with type 3 VWD. Exploring the relationship between a patient’s treatment history (type of product, dose, frequency, and clinical response) and VWF antibody formation would be valuable. In the recent 3WINTERS-IPS study, basic exposure to replacement therapies, self-reported by the patients at the time of enrollment, revealed that all patients with VWF inhibitors and available therapeutic information had been previously exposed to replacement therapy.20 A limitation to our study is the lack of detailed treatment information or pharmacokinetic results. Pairing treatment information with antibody assessment among patients with type 3 VWD longitudinally would greatly strengthen our understanding of who is at at highest risk for development of VWF antibodies, how long they remain in the system, and assessing their clinical consequences.
In summary, we developed ELISA-based approaches to easily screen, quantify, and characterize neutralizing and nonneutralizing anti-VWF antibodies in type 3 VWD plasma. These assays provide valuable tools to assess and track VWF antibody development in patients requiring therapeutic product infusions to prevent severe bleeding, because these antibodies could render VWF less effective or even lead to anaphylaxis. In this report of 49 unrelated type 3 patients in the Zimmerman Program, 18% had anti-VWF IgG or IgM antibodies, and 12% had inhibitory antibodies. Most T3 VWD Ab+ patients had varying degrees and specificities of neutralization, and one-third of them had nonneutralizing antibodies, which suggests that these patients would benefit from continued testing for anti-VWF antibodies that could influence their clinical therapies in the future.
Acknowledgments
The authors thank the participating patients, hematology centers, and laboratory personnel who were all critical to this study, in addition to the resources generously gifted by Versiti Diagnostic and the Versiti Blood Research Institute laboratories.
Research reported in this publication was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (award numbers HL081588, HL144457, and HL126810) for the Zimmerman Program and multiple investigators.
Authorship
Contribution: R.R.M. conceived the original study, designed the research, analyzed the data, and edited the manuscript; C.L.P. designed and performed the research, analyzed the data, and wrote the manuscript; V.H.F. and P.A.C. designed the research, analyzed the data, and wrote the manuscript; and S.L.H. and T.A.A. served as the collaborators at Versiti Diagnostic Laboratories and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Zimmerman Program appears in “Appendix.”
Correspondence: Veronica H. Flood, Pediatric Hematology/Oncology/Blood and Marrow Transplant, Medical College of Wisconsin, Milwaukee, WI, 53226; email: vflood@mcw.edu.
Appendix
Zimmerman Program investigators
Principal investigators: R. Montgomery, V. Flood, S. Haberichter, T. Abshire, and H. Weiler, Versiti Blood Research Institute, Milwaukee, WI; D. Lillicrap and P. James, Queen's University, Kingston, ON, Canada; J. O’Donnell, Royal College of Surgeons in Ireland, Dublin, Ireland; C. Ng, University of Colorado, Denver, CO; and J. DiPaola and B. Sadler, Washington University in St. Louis, St. Louis, MO.
Directors of the primary centers: T. Abshire, C. Bennett, and R. Sidonio, Emory University School of Medicine, Atlanta, GA; M. Manco-Johnson, J. Di Paola, and C. Ng, Mountain States Regional Hemophilia and Thrombosis Center, Aurora, CO; J. Journeycake, and A. Zia, UT Southwestern, Dallas, TX; J. Lusher and M. Rajpurkar, Wayne State University, Detroit, MI; A. Shapiro, Indiana Hemophilia & Thrombosis Center, Indianapolis, IN; S. Lentz, University of Iowa, Iowa City, IA; J. Gill and V. Flood, Comprehensive Center for Bleeding Disorders, Milwaukee, WI; C. Leissinger, Tulane University Health Sciences Center, New Orleans, LA; M. Ragni and N. Machin, University of Pittsburgh, Pittsburgh, PA; M. Tarantino and J. Roberts, Bleeding & Clotting Disorders Institute, Peoria, IL; and P. James, Queen's University, Kingston, ON, Canada.
In addition, numerous secondary centers contributed to patient recruitment: J. Hord, Akron Children's Hospital, Akron, OH; J. Strouse, Johns Hopkins Children's Center, Baltimore, MD; A. Ma, The University of North Carolina at Chapel Hill, Chapel Hill, NC; L. Valentino and L. Boggio, Rush University Medical Center, Chicago, IL; A. Sharathkumar, Children's Memorial Hospital, Chicago, IL; R. Gruppo, Cincinnati Children's Hospital, Cincinnati, OH; B. Kerlin, Nationwide Children's Hospital, Columbus, OH; R. Kulkarni, Michigan State University, East Lansing, MI; D. Green, Northwestern University, Evanston, IL; K. Hoots and D. Brown, The University of Texas Health Science Center at Houston, Houston, TX; D. Mahoney, Baylor College of Medicine, Houston, TX; L. Mathias and A. Bedros, Loma Linda University Medical Center, Loma Linda, CA; C. Diamond, University of Wisconsin Madison, Madison, WI; A. Neff, Vanderbilt University, Nashville, TN; D. DiMichele and P. Giardina, Weill Cornell Medical College, New York, NY; A. Cohen, Newar Beth Israel Medical Center, Newark, NJ; M. Paidas, Yale School of Medicine, New Haven, CT; E. Werner, Children's Hospital of the King's Daughters, Norfolk, VA; A. Matsunaga, Children's Hospital & Research Center Oakland, Oakland, CA; F. Shafer, Drexel University College of Medicine, Philadelphia, PA; B. Konkle and A. Cuker, University of Pennsylvania, Philadelphia, PA; P. Kouides, Rochester General Hospital, Rochester, NY; and D. Stein, Toledo Children's Hospital, Toledo, OH.
References
Author notes
Data are available on request from the corresponding author, Veronica H. Flood (vflood@mcw.edu).