Key Points
In the A.R.R.O.W.2 study, ORR was comparable between once-weekly KRd56 and twice-weekly KRd27 but not significant for noninferiority.
With numerically similar efficacy and safety, once-weekly KRd56 may be a convenient treatment option for RRMM.
Visual Abstract
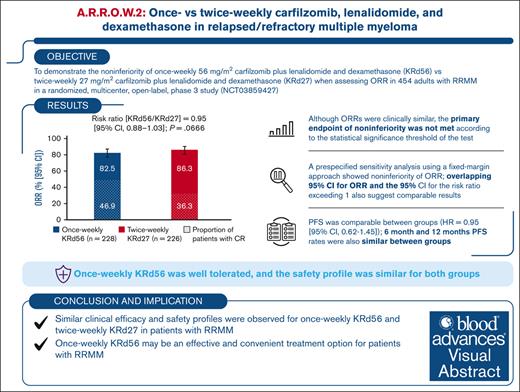
Twice-weekly carfilzomib (27 mg/m2) plus lenalidomide and dexamethasone (KRd27) is a standard of care in relapsed/refractory multiple myeloma (RRMM). Once-weekly carfilzomib regimens have shown clinical benefits with improved patient convenience. This open-label, phase 3, multicenter, randomized study aimed to demonstrate noninferiority of the overall response rate (ORR) for once-weekly carfilzomib (56 mg/m2) plus Rd (KRd56) vs twice-weekly KRd27 in RRMM. A total of 454 patients were randomized (1:1) to receive carfilzomib as once-weekly 30-minute infusions of 56 mg/m2 (KRd56; n = 228) or twice-weekly 10-minute infusions of 27 mg/m2 (KRd27; n = 226). Baseline characteristics were balanced between groups. ORR was 82.5% (95% confidence interval [CI], 76.9-87.2) in the once-weekly group vs 86.3% (95% CI, 81.1-90.5) in the twice-weekly group (risk ratio, 0.954 [95% CI, 0.882-1.032]) and did not meet the threshold for statistical significance of noninferiority (P = .0666). Complete response (CR) or better was obtained in 46.9% of patients in the once-weekly arm and 36.3% in the twice-weekly arm. The proportions of patients who achieved CR and were also assessed negative for minimal residual disease were 21.5% and 18.1%, respectively (odds ratio, 1.235 [95% CI, 0.775-1.970]). Progression-free survival was comparable between groups (hazard ratio, 0.945 [95% CI, 0.617-1.447]). The safety profile was similar for both groups. In conclusion, although statistical significance for noninferiority of ORR was not achieved, the efficacy and safety of once-weekly KRd56 were similar to those of twice-weekly KRd27, and once-weekly KRd56 may be an effective and convenient treatment option for patients with RRMM. This trial was registered at www.ClinicalTrials.gov as #NCT03859427.
Introduction
Multiple myeloma (MM) is a heterogeneous disease that remains incurable in most patients.1 Although treatment with immunomodulatory agents, proteasome inhibitors (PIs), and monoclonal antibodies has improved patient outcomes, most patients eventually relapse.2-4
Carfilzomib, an irreversible and specific second-generation PI, is approved for the treatment of adult patients with relapsed/refractory MM (RRMM), either as a single agent in patients who received ≥1 prior line of therapy or in combination with dexamethasone, lenalidomide-dexamethasone (Rd), daratumumab-dexamethasone, daratumumab-hyaluronidase fihj-dexamethasone, or isatuximab-dexamethasone in patients who received 1 to 3 prior lines of therapy.5 In the randomized phase 3 ASPIRE study, compared with treatment with Rd, treatment with twice-weekly carfilzomib (27 mg/m2) in combination with Rd (KRd27) demonstrated a significant improvement in the median progression-free survival (PFS; 26.3 months for KRd27 vs 17.6 months for Rd; hazard ratio [HR], 0.69 [95% confidence interval (CI), 0.57-0.83]; P < .0001) and median overall survival (48.3 months for KRd27 vs 40.4 months for Rd; HR, 0.79 [95% CI, 0.67-0.95]; P = .0045) in patients with RRMM.6,7
Approved twice-weekly carfilzomib regimens are effective and well tolerated, and once-weekly carfilzomib dosing in these regimens has shown comparable clinical benefits with potentially enhanced convenience for patients.8 The A.R.R.O.W. study demonstrated that once-weekly carfilzomib (70 mg/m2) in combination with dexamethasone (Kd70) was safe and more effective with a convenient dosing regimen than twice-weekly carfilzomib (27 mg/m2) and dexamethasone.9 Additionally, once-weekly carfilzomib (70 mg/m2) in combination with daratumumab and dexamethasone (DKd70) was clinically investigated and approved for the treatment of patients with RRMM, in addition to twice-weekly carfilzomib (56 mg/m2) in combination with daratumumab and dexamethasone (DKd56).5,10,11 Furthermore, a phase 1b study, which evaluated once-weekly carfilzomib (56 or 70 mg/m2) in combination with Rd (KRd56 or KRd70) in patients with RRMM, demonstrated that once-weekly KRd56 was efficacious and provides an acceptable safety profile.12,13 Given these results, the phase 3 A.R.R.O.W.2 study was initiated to evaluate the efficacy and safety of once-weekly KRd56 vs twice-weekly KRd27 in patients with RRMM.
Methods
Study design and participants
A.R.R.O.W.2 was a randomized, multicenter, open-label, phase 3 study (ClinicalTrials.gov identifier: NCT03859427)14 designed to evaluate noninferiority of the overall response rate (ORR) for once-weekly KRd56 vs twice-weekly KRd27 in patients with RRMM. Adults (age ≥18 years) with RRMM (1-3 prior lines of therapy) were eligible if they had achieved at least a partial response (PR) to ≥1 prior line of therapy. Patients with primary refractory MM were ineligible; refractory disease was defined as a disease that was nonresponsive or progressed within 60 days of the last therapy. Patients refractory to the most recent line of therapy were eligible, except in cases where the last treatment included a PI or a combination of Rd. Patients with prior therapy with a PI were included if they had at least a PR to the most recent treatment with the PI and if the disease had not relapsed within 60 days of discontinuation of the PI. Additionally, patients who had received single-agent maintenance therapy with lenalidomide within 60 days of enrollment were included. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2. Patients who were intolerant of study medications were excluded. Other exclusion criteria included New York Heart Association III or IV heart failure, symptomatic ischemia, uncontrolled arrhythmias, screening electrocardiogram with corrected QT interval of >470 millisecond, pericardial disease, myocardial infarction within 4 months before randomization, or uncontrolled hypertension (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥100 mmHg). Patients with significant neuropathy (grade 3-4 or grade 2 with pain) within 14 days before randomization were also excluded. All patients provided written informed consent. The study protocol was approved by the institutional review boards or ethics committees of all participating institutions.
Randomization
Eligible patients were randomly assigned (1:1) to receive either once-weekly KRd56 or twice-weekly KRd27 through an interactive voice/web response system. Patients were stratified according to the international staging system at study entry (stage 1 or 2 vs stage 3), prior lenalidomide treatment (yes vs no), prior PI treatment (yes vs no), and prior anti-CD38 exposure (yes vs no). No crossover between the treatment groups was allowed.
Procedures
The study design included a screening period of up to 28 days, treatment duration of up to 12 cycles of 28 days each, and safety follow-up period of 30 days. In the once-weekly group, carfilzomib was administered IV over 30 minutes on days 1, 8, and 15 (20 mg/m2 for cycle 1 day 1; 56 mg/m2 thereafter). In the twice-weekly group, carfilzomib was administered IV over 10 minutes on days 1, 2, 8, 9, 15, and 16 (20 mg/m2 for cycle 1 day 1 and cycle 1 day 2; 27 mg/m2 thereafter). Patients in both groups received 25 mg of lenalidomide orally on day 1 through day 21 and 40 mg of dexamethasone orally or IV on days 1, 8, and 15 of all cycles and on day 22 for cycles 1 to 9 only. Study treatment was administered in 28-day cycles, and the cycles were repeated until disease progression, occurrence of unacceptable toxicity, or withdrawal of consent. Patients could receive up to 12 cycles of the study treatment. Recommended concomitant medications included antiviral prophylaxis and venous thromboembolic prophylaxis. The study was initiated in 2019 and did not recommend the consideration of infectious prophylaxis, which is now recommended for patients with MM.15 Disease assessments were conducted within 21 days before randomization to determine eligibility, every 28 days after day 1 of cycle 1, at the end of treatment, and during long-term follow-up every 28 days until progressive disease, subsequent antimyeloma therapy, or both. After the discontinuation of study drug(s), patients had a safety follow-up visit 30 (+3) days after the last dose of all study drug(s). Safety was regularly reviewed by an independent data monitoring committee.
Outcomes
The primary end point was overall response, defined as the best overall response of PR, very good PR, complete response (CR), or stringent CR according to International Myeloma Working Group Uniform Response Criteria. The secondary end points were PFS, minimal residual disease–negative CR (MRD-negative CR), patient-reported convenience with the carfilzomib-dosing schedule after cycle 4 of treatment, and safety in patients from the treatment groups. PFS was defined as the time from the date of randomization until disease progression or death due to any cause, whichever occurred first. MRD was assessed using next-generation sequencing at a threshold of 10−5. Patient-reported convenience was measured using the Patient-reported Convenience with Carfilzomib-dosing Schedule Questionnaire. Patients who reported “very convenient” or “convenient” after cycle 4 were included in the “convenient” category, patients with a missing response of patient-reported convenience at all visits after cycle 4 were included in the “missing” category, and all other patients who reported “inconvenient” or “very inconvenient” were included in the “inconvenient” category.
Statistical analysis
The intention-to-treat population consisted of all randomly assigned patients and was the basis for the analysis of efficacy end points. Myeloma response and disease progression were determined by a masked independent review committee. ORR was calculated for each group, and the associated 95% CIs were estimated using the Clopper-Pearson method. The Mantel-Haenszel stratified risk ratio with 95% CI of ORR was estimated for the treatment effect. For the primary objective, the noninferiority test for ORR was performed using the synthesis approach (US Food and drug Administration, 2016)16 based on results of the phase 3 ASPIRE study6 (KRd vs Rd) to test whether once-weekly KRd56 preserves at least 60% of the treatment effect of twice-weekly KRd27 vs Rd. The reference value for this test was the stratified relative risk of ORR during the first 12 cycles of treatment in the ASPIRE study (relative risk, 0.755 [95% CI, 0.696-0.818]; twice-weekly Rd vs KRd27). The hypotheses for the primary and key secondary objectives (ORR, PFS, and convenience after cycle 4 of treatment) were tested using a fixed-sequence hierarchical testing procedure to control the familywise type I error rate at 1-sided level of 0.025. The testing order was as follows: noninferiority of ORR, noninferiority of PFS, and superiority of patient-reported convenience after cycle 4 of treatment. The sequential testing procedure required that the previous end point be achieved before testing for the next hierarchical end point.
Summary statistics for PFS were calculated using the Kaplan-Meier method. A stratified Cox proportional hazards model was used for HR and CI estimates. The restricted mean survival time (RMST) was evaluated as the area under the Kaplan-Meier curve up to 13 months. The Wald method was used to estimate the 95% CIs for RMST. CIs for the proportion of patients who achieved an MRD-negative CR and those who reported that the carfilzomib-dosing schedule was convenient were calculated using the Clopper-Pearson method. The Cochran-Mantel-Haenszel method was used to calculate the odds ratio and associated 95% CIs. The safety population was defined as all randomized patients who received at least 1 dose of any study treatment and were analyzed by safety groups corresponding to the actual treatment received. Any patient who received at least 1 dose of once-weekly carfilzomib (56 mg/m2) was included in the once-weekly KRd56 safety group, and the remaining patients were included in the twice-weekly KRd27 safety group. Adverse events were monitored for at least 30 days after the last dose of study treatment and graded per National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Medical Dictionary for Regulatory Activities (version 26.0) was used for the coding of adverse events.
Results
Patients and enrollment
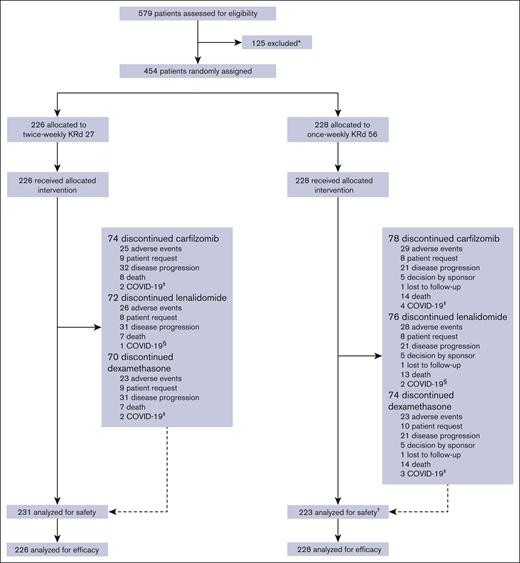
Between May 2019 and February 2022, 579 patients were screened for enrollment at 80 sites across Europe, Asia, and the United States. Of these patients, 454 were eligible and randomly assigned to receive either once-weekly KRd56 (n = 228) or twice-weekly KRd27 (n = 226; Figure 1) in the intent-to-treat population. Of the enrolled patients, 191 (83.8%) in the once-weekly KRd56 group and 197 (87.2%) in the twice-weekly KRd27 group completed the study. The cutoff date for the final analysis was 31 March 2023. Baseline demographics and disease characteristics were generally balanced between the treatment groups (Table 1). Most patients received 1 prior line of therapy (68.5%). The median duration of treatment with KRd was comparable between the treatment groups: 47.1 weeks for the once-weekly KRd56 group vs 47.0 weeks for the twice-weekly KRd27 group. The median duration of treatment with carfilzomib was 46.1 weeks for the once-weekly group vs 46.3 weeks for the twice-weekly group. The median duration of treatment with lenalidomide was 47.0 weeks and that of treatment with dexamethasone was 46.1 weeks for both once-weekly and twice-weekly groups.
Patient disposition. ∗Reasons for exclusion are listed in supplemental Table 5. †Five patients who were randomized to the once-weekly KRd56 group and did not receive a dose of 56 mg/m2 were included in the twice-weekly KRd27 group for safety analysis. ‡The primary reason for discontinuing carfilzomib/dexamethasone included adverse event, death, and patient request. §The primary reason for discontinuing lenalidomide included adverse event and patient request.
Patient disposition. ∗Reasons for exclusion are listed in supplemental Table 5. †Five patients who were randomized to the once-weekly KRd56 group and did not receive a dose of 56 mg/m2 were included in the twice-weekly KRd27 group for safety analysis. ‡The primary reason for discontinuing carfilzomib/dexamethasone included adverse event, death, and patient request. §The primary reason for discontinuing lenalidomide included adverse event and patient request.
Patient demographics and baseline disease characteristics
| . | Once-weekly KRd56 (N = 228) . | Twice-weekly KRd27 (N = 226) . |
|---|---|---|
| Age, n (%) | ||
| Median (range), y | 64.0 (40-83) | 65.0 (40-85) |
| 18 to <65 | 116 (50.9) | 107 (47.3) |
| 65 to <75 | 92 (40.4) | 102 (45.1) |
| ≥75 | 20 (8.8) | 17 (7.5) |
| Sex, n (%) | ||
| Male | 110 (48.2) | 126 (55.8) |
| Female | 118 (51.8) | 100 (44.2) |
| Race, n (%) | ||
| White | 209 (91.7) | 209 (92.5) |
| Asian | 12 (5.3) | 9 (4.0) |
| Black or African American | 1 (0.4) | 1 (0.4) |
| Other | 6 (2.6) | 7 (3.1) |
| ECOG performance status, n (%) | ||
| 0-1 (0 or 1) | 219 (96.1) | 215 (95.1) |
| 2 | 9 (3.9) | 11 (4.9) |
| ISS stage, n (%) | ||
| Stage I or II | 209 (91.7) | 209 (92.5) |
| Stage III | 19 (8.3) | 17 (7.5) |
| Cytogenetic risk by FISH, n (%)∗ | ||
| High risk | 44 (28.2) | 40 (27.4) |
| t(4; 14) | 25 (16.0) | 23 (15.8) |
| t(14; 16) | 3 (1.9) | 1 (0.7) |
| Deletion 17p | 21 (13.5) | 19 (13.0) |
| Standard risk† | 112 (71.8) | 106 (72.6) |
| Creatinine clearance, n (%) | ||
| Median (range) | 84.0 (28.8-220.2) | 84.6 (42.6-208.2) |
| <30 mL/min | 2 (0.9) | 0 (0.0) |
| ≥30 to <50 mL/min | 11 (4.8) | 4 (1.8) |
| ≥50 to <80 mL/min | 84 (36.8) | 95 (42.0) |
| ≥80 mL/min | 131 (57.5) | 127 (56.2) |
| β2 microglobulin, n (%) | ||
| Median (range) | 3.0 (1.4-21.6) | 2.9 (1.4-19.9) |
| <3.5 mg/L | 153 (67.1) | 150 (66.4) |
| ≥3.5 and <5.5 mg/L | 56 (24.6) | 59 (26.1) |
| ≥5.5 mg/L | 19 (8.3) | 17 (7.5) |
| Previous transplant, n (%) | 153 (67.1) | 148 (65.5) |
| Previous regimens, n (%) | ||
| 1 | 157 (68.9) | 154 (68.1) |
| 2 | 49 (21.5) | 43 (19.0) |
| >2 | 22 (9.6) | 29 (12.8) |
| Previous treatment, n (%) | ||
| Bortezomib | 210 (92.1) | 211 (93.4) |
| PI | 214 (93.9) | 218 (96.5) |
| Lenalidomide | 85 (37.3) | 79 (35.0) |
| Anti-CD38 therapy | 21 (9.2) | 17 (7.5) |
| Refractory to prior treatment‡, n (%) | ||
| Any previous bortezomib | 19 (8.3) | 16 (7.1) |
| Any previous PI | 22 (9.6) | 21 (9.3) |
| Any previous lenalidomide | 39 (17.1) | 40 (17.7) |
| Anti-CD38 therapy | 14 (6.1) | 11 (4.9) |
| Last prior line of therapy | 58 (25.4) | 52 (23.0) |
| . | Once-weekly KRd56 (N = 228) . | Twice-weekly KRd27 (N = 226) . |
|---|---|---|
| Age, n (%) | ||
| Median (range), y | 64.0 (40-83) | 65.0 (40-85) |
| 18 to <65 | 116 (50.9) | 107 (47.3) |
| 65 to <75 | 92 (40.4) | 102 (45.1) |
| ≥75 | 20 (8.8) | 17 (7.5) |
| Sex, n (%) | ||
| Male | 110 (48.2) | 126 (55.8) |
| Female | 118 (51.8) | 100 (44.2) |
| Race, n (%) | ||
| White | 209 (91.7) | 209 (92.5) |
| Asian | 12 (5.3) | 9 (4.0) |
| Black or African American | 1 (0.4) | 1 (0.4) |
| Other | 6 (2.6) | 7 (3.1) |
| ECOG performance status, n (%) | ||
| 0-1 (0 or 1) | 219 (96.1) | 215 (95.1) |
| 2 | 9 (3.9) | 11 (4.9) |
| ISS stage, n (%) | ||
| Stage I or II | 209 (91.7) | 209 (92.5) |
| Stage III | 19 (8.3) | 17 (7.5) |
| Cytogenetic risk by FISH, n (%)∗ | ||
| High risk | 44 (28.2) | 40 (27.4) |
| t(4; 14) | 25 (16.0) | 23 (15.8) |
| t(14; 16) | 3 (1.9) | 1 (0.7) |
| Deletion 17p | 21 (13.5) | 19 (13.0) |
| Standard risk† | 112 (71.8) | 106 (72.6) |
| Creatinine clearance, n (%) | ||
| Median (range) | 84.0 (28.8-220.2) | 84.6 (42.6-208.2) |
| <30 mL/min | 2 (0.9) | 0 (0.0) |
| ≥30 to <50 mL/min | 11 (4.8) | 4 (1.8) |
| ≥50 to <80 mL/min | 84 (36.8) | 95 (42.0) |
| ≥80 mL/min | 131 (57.5) | 127 (56.2) |
| β2 microglobulin, n (%) | ||
| Median (range) | 3.0 (1.4-21.6) | 2.9 (1.4-19.9) |
| <3.5 mg/L | 153 (67.1) | 150 (66.4) |
| ≥3.5 and <5.5 mg/L | 56 (24.6) | 59 (26.1) |
| ≥5.5 mg/L | 19 (8.3) | 17 (7.5) |
| Previous transplant, n (%) | 153 (67.1) | 148 (65.5) |
| Previous regimens, n (%) | ||
| 1 | 157 (68.9) | 154 (68.1) |
| 2 | 49 (21.5) | 43 (19.0) |
| >2 | 22 (9.6) | 29 (12.8) |
| Previous treatment, n (%) | ||
| Bortezomib | 210 (92.1) | 211 (93.4) |
| PI | 214 (93.9) | 218 (96.5) |
| Lenalidomide | 85 (37.3) | 79 (35.0) |
| Anti-CD38 therapy | 21 (9.2) | 17 (7.5) |
| Refractory to prior treatment‡, n (%) | ||
| Any previous bortezomib | 19 (8.3) | 16 (7.1) |
| Any previous PI | 22 (9.6) | 21 (9.3) |
| Any previous lenalidomide | 39 (17.1) | 40 (17.7) |
| Anti-CD38 therapy | 14 (6.1) | 11 (4.9) |
| Last prior line of therapy | 58 (25.4) | 52 (23.0) |
ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in-situ hybridization; ISS, International Staging System.
Cytogenetic risk data were missing for 72 patients (31.6%) in the once-weekly group and for 80 patients (35.4%) in the twice-weekly group. The % cytogenetic risk is calculated from patients with available data.
Patients with normal cytogenetics or chromosomal abnormalities other than t(4;14), t(14;16), and/or deletion 17p were included in the standard-risk group.
Patients were classified as refractory to prior treatment if the best response to prior treatment was stable or progressive disease, disease progression was the specific reason for treatment discontinuation, or disease progression occurred within 60 days of treatment discontinuation.
Efficacy
ORR was 82.5% (95% CI, 76.9-87.2) in the once-weekly KRd56 group vs 86.3% (95% CI, 81.1-90.5) in the twice-weekly KRd27 group (risk ratio [once-weekly KRd56/twice-weekly KRd27] = 0.954 [95% CI, 0.882-1.032]; P = .0666; Table 2), which did not reach statistical significance for noninferiority at the prespecified alpha threshold of P ≤ .025, 1-sided. An ORR of at least 84.2% in the once-weekly KRd56 group was required for statistically significant noninferiority. A prespecified sensitivity measure using the fixed-margin approach17 satisfied the criteria for noninferiority of ORR, as the lower limit of the 95% CI for the risk ratio (0.882) was greater than the prespecified noninferiority margin of 0.87 that was derived from comparison of ORR between KRd27 and Rd by cycle 12 in the placebo-controlled ASPIRE trial.6
Treatment responses per IMWG-URC in patients with RRMM treated with once-weekly KRd56 vs twice-weekly KRd27 over the study duration
| . | Once-weekly KRd56 (N = 228) . | Twice-weekly KRd27 (N = 226) . |
|---|---|---|
| ORR, % (95% CI) | 82.5 (76.9-87.2) | 86.3 (81.1-90.5) |
| 1-sided P∗ | 0.0666 | |
| Risk ratio (95% CI) | 0.954 (0.882-1.032) | |
| Best overall response, n (%) | ||
| Stringent CR | 46 (20.2) | 30 (13.3) |
| CR | 61 (26.8) | 52 (23.0) |
| VGPR | 59 (25.9) | 80 (35.4) |
| PR | 22 (9.6) | 33 (14.6) |
| Stable disease or progressive disease | 19 (8.3) | 20 (8.8) |
| Not evaluable | 21† (9.2) | 11‡ (4.9) |
| Median time to response§, mo (range) | 1.0 (1-10) | 1.0 (1-12) |
| . | Once-weekly KRd56 (N = 228) . | Twice-weekly KRd27 (N = 226) . |
|---|---|---|
| ORR, % (95% CI) | 82.5 (76.9-87.2) | 86.3 (81.1-90.5) |
| 1-sided P∗ | 0.0666 | |
| Risk ratio (95% CI) | 0.954 (0.882-1.032) | |
| Best overall response, n (%) | ||
| Stringent CR | 46 (20.2) | 30 (13.3) |
| CR | 61 (26.8) | 52 (23.0) |
| VGPR | 59 (25.9) | 80 (35.4) |
| PR | 22 (9.6) | 33 (14.6) |
| Stable disease or progressive disease | 19 (8.3) | 20 (8.8) |
| Not evaluable | 21† (9.2) | 11‡ (4.9) |
| Median time to response§, mo (range) | 1.0 (1-10) | 1.0 (1-12) |
IMWG-URC, International Myeloma Working Group Uniform Response Criteria; VGPR, very good partial response.
P was calculated via the synthesis approach (US Food and Drug Administration, 2016)17 for noninferiority comparison of ORR between treatment groups.
Seven patients had no postbaseline visit (including 5 deaths in cycle 1; 2 patients ended the study on days 46 and 62, respectively), 7 patients had 1 postbaseline visit, 4 patients had nonmeasurable disease, 1 patient had 2 baseline visits, 1 patient had no postbaseline plasmacytoma assessment, and 1 patient achieved a PR per investigator evaluation.
Four patients had no postbaseline visit (including 1 death each on days 17 and 76), 3 patients had 1 postbaseline visit, and 4 patients had nonmeasurable disease.
Time from the randomization date to the earliest date of confirmation of a PR or better.
ORR was assessed in both treatment groups according to several prespecified patient subgroups, with the 95% CIs of all risk ratios exceeding 1 (supplemental Figure 1). The proportion of patients who achieved a CR or better was 46.9% in the once-weekly group vs 36.3% in the twice-weekly group. The proportions of patients who achieved a CR and were MRD-negative were comparable between once-weekly KRd56 and twice-weekly KRd27 (21.5% vs 18.1%; odds ratio, 1.235 [95% CI, 0.775-1.970]; Table 3). Additionally, the proportion of patients who achieved a CR and were MRD-negative at 12 months was comparable between the groups (18.9% vs 18.1%; odds ratio, 1.060 [95% CI, 0.657-1.711]).
Patients who achieved a CR and who were MRD-negative following treatment with once-weekly KRd56 vs twice-weekly KRd27
| . | Once-weekly KRd56 (N = 228) . | Twice-weekly KRd27 (N = 226) . | Odds ratio (95% CI) . |
|---|---|---|---|
| No. of patients who were MRD-negative∗ | 49 | 41 | |
| MRD-negative rate, % (95% CI) | 21.5 (16.3-27.4) | 18.1 (13.3-23.8) | 1.235 (0.775-1.970) |
| No. of patients who were MRD-negative at 12 mo† | 43 | 41 | |
| MRD-negative rate, % (95% CI) | 18.9 (14.0-24.6) | 18.1 (13.3-23.8) | 1.060 (0.657-1.711) |
| . | Once-weekly KRd56 (N = 228) . | Twice-weekly KRd27 (N = 226) . | Odds ratio (95% CI) . |
|---|---|---|---|
| No. of patients who were MRD-negative∗ | 49 | 41 | |
| MRD-negative rate, % (95% CI) | 21.5 (16.3-27.4) | 18.1 (13.3-23.8) | 1.235 (0.775-1.970) |
| No. of patients who were MRD-negative at 12 mo† | 43 | 41 | |
| MRD-negative rate, % (95% CI) | 18.9 (14.0-24.6) | 18.1 (13.3-23.8) | 1.060 (0.657-1.711) |
MRD negativity was assessed using next-generation sequencing at a threshold of 10−5 over the duration of the study in the intention-to-treat population.
MRD-negative rate at 12 months was defined as the proportion of patients who achieved MRD negativity at 12 months (±4 weeks) from randomization, as assessed using next-generation sequencing at a threshold of 10−5, in the intention-to-treat population. MRD negativity results from bone marrow samples obtained at 8 to 13 months from randomization and before starting new antimyeloma therapy or disease progression were considered in the calculation.
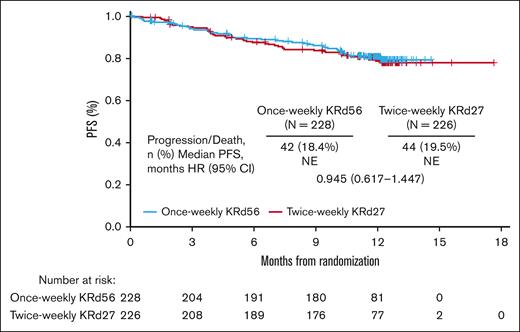
The overall PFS was similar for the treatment groups, with median PFS not reached in either group (HR, 0.945 [95% CI, 0.617-1.447]; Figure 2). The 6-month PFS rates were 89.5% in the once-weekly KRd56 group and 87.6% in the twice-weekly KRd27 group, whereas the 12-month PFS rates were 80.7% and 79.7%, respectively. The noninferiority test was not performed for PFS per the fixed-sequence hierarchical testing procedure since the noninferiority test for ORR did not demonstrate statistical significance. A prespecified RMST evaluation of PFS resulted in a difference of 0.11 months (∼3.3 days) between the once-weekly KRd56 and twice-weekly KRd27 groups.
Patient-reported convenience status after cycle 4 of treatment was comparable between the treatment groups. The proportion of patients in the convenient category was 81.6% (186/228) in once-weekly KRd56 and 80.5% (182/226) in twice-weekly KRd27 group, respectively (odds ratio [once-weekly KRd56 vs twice-weekly KRd27], 1.049 [95% CI, 0.653-1.683]; Table 4). In addition, 17.1% (39/228) patients responded to once-weekly KRd56 as “very convenient” compared with 10.6% (24/226) in the twice-weekly KRd27 regimen. Furthermore, 21 patients (9.3%) considered twice-weekly KRd27 regimen as “inconvenient” vs once-weekly KRd56 (7 patients [3.1%]).
Summary of patient-reported convenience status (intention-to-treat population)
| . | Twice-weekly KRd27 (N = 226) . | Once-weekly KRd56 (N = 228) . | Treatment difference . |
|---|---|---|---|
| Patient-reported convenience status at cycle 2 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 151 (66.8) (60.3-72.9) | 179 (78.5) (72.6-83.7) | |
| Inconvenient | 44 (19.5) | 10 (4.4) | |
| Missing | 31 (13.7) | 39 (17.1) | |
| All expected patients† | 222 | 217 | |
| Convenient | 151 (68.0) | 179 (82.5) | |
| Inconvenient | 44 (19.8) | 10 (4.6) | |
| Missing | 27 (12.2) | 28 (12.9) | |
| Patient-reported convenience status at cycle 5 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 160 (70.8) (64.4-76.6) | 172 (75.4) (69.3-80.9) | |
| Inconvenient | 31 (13.7) | 10 (4.4) | |
| Missing | 35 (15.5) | 46 (20.2) | |
| All expected patients† | 205 | 199 | |
| Convenient | 160 (78.0) | 172 (86.4) | |
| Inconvenient | 31 (15.1) | 10 (5.0) | |
| Missing | 14 (6.8) | 17 (8.5) | |
| Patient-reported convenience status at cycle 12 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 125 (55.3) (48.6-61.9) | 125 (54.8) (48.1-61.4) | |
| Inconvenient | 18 (8.0) | 8 (3.5) | |
| Missing | 83 (36.7) | 95 (41.7) | |
| All expected patients† | 161 | 157 | |
| Convenient | 125 (77.6) | 125 (79.6) | |
| Inconvenient | 18 (11.2) | 8 (5.1) | |
| Missing | 18 (11.2) | 24 (15.3) | |
| Patient-reported convenience status at safety follow-up‡ | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 129 (57.1) (50.3-63.6) | 142 (62.3) (55.6-68.6) | |
| Inconvenient | 24 (10.6) | 9 (3.9) | |
| Missing | 73 (32.3) | 77 (33.8) | |
| All expected patients† | 224 | 225 | |
| Convenient | 129 (57.6) | 142 (63.1) | |
| Inconvenient | 24 (10.7) | 9 (4.0) | |
| Missing | 71 (31.7) | 74 (32.9) | |
| Patient-reported convenience status after cycle 4 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 182 (80.5) (74.8-85.5) | 186 (81.6) (75.9-86.4) | |
| Very convenient, n (%) | 24 (10.6) | 39 (17.1) | |
| Inconvenient | 21 (9.3) | 7 (3.1) | |
| Missing | 23 (10.2) | 35 (15.4) | |
| Odds ratio (once-weekly KRd56 vs twice-weekly KRd27) (95% CI) | 1.049 (0.653-1.683) | ||
| . | Twice-weekly KRd27 (N = 226) . | Once-weekly KRd56 (N = 228) . | Treatment difference . |
|---|---|---|---|
| Patient-reported convenience status at cycle 2 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 151 (66.8) (60.3-72.9) | 179 (78.5) (72.6-83.7) | |
| Inconvenient | 44 (19.5) | 10 (4.4) | |
| Missing | 31 (13.7) | 39 (17.1) | |
| All expected patients† | 222 | 217 | |
| Convenient | 151 (68.0) | 179 (82.5) | |
| Inconvenient | 44 (19.8) | 10 (4.6) | |
| Missing | 27 (12.2) | 28 (12.9) | |
| Patient-reported convenience status at cycle 5 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 160 (70.8) (64.4-76.6) | 172 (75.4) (69.3-80.9) | |
| Inconvenient | 31 (13.7) | 10 (4.4) | |
| Missing | 35 (15.5) | 46 (20.2) | |
| All expected patients† | 205 | 199 | |
| Convenient | 160 (78.0) | 172 (86.4) | |
| Inconvenient | 31 (15.1) | 10 (5.0) | |
| Missing | 14 (6.8) | 17 (8.5) | |
| Patient-reported convenience status at cycle 12 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 125 (55.3) (48.6-61.9) | 125 (54.8) (48.1-61.4) | |
| Inconvenient | 18 (8.0) | 8 (3.5) | |
| Missing | 83 (36.7) | 95 (41.7) | |
| All expected patients† | 161 | 157 | |
| Convenient | 125 (77.6) | 125 (79.6) | |
| Inconvenient | 18 (11.2) | 8 (5.1) | |
| Missing | 18 (11.2) | 24 (15.3) | |
| Patient-reported convenience status at safety follow-up‡ | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 129 (57.1) (50.3-63.6) | 142 (62.3) (55.6-68.6) | |
| Inconvenient | 24 (10.6) | 9 (3.9) | |
| Missing | 73 (32.3) | 77 (33.8) | |
| All expected patients† | 224 | 225 | |
| Convenient | 129 (57.6) | 142 (63.1) | |
| Inconvenient | 24 (10.7) | 9 (4.0) | |
| Missing | 71 (31.7) | 74 (32.9) | |
| Patient-reported convenience status after cycle 4 | |||
| All randomized patients∗ | 226 | 228 | |
| Convenient, n (%) (95% CI) | 182 (80.5) (74.8-85.5) | 186 (81.6) (75.9-86.4) | |
| Very convenient, n (%) | 24 (10.6) | 39 (17.1) | |
| Inconvenient | 21 (9.3) | 7 (3.1) | |
| Missing | 23 (10.2) | 35 (15.4) | |
| Odds ratio (once-weekly KRd56 vs twice-weekly KRd27) (95% CI) | 1.049 (0.653-1.683) | ||
The number of all randomized patients was calculated as the number of randomized patients in each treatment group in the intention-to-treat population.
The number of all expected patients was calculated as the number of patients expected to have an assessment, that is, randomized patients who were still alive and remaining on carfilzomib treatment at the scheduled visit.
The number of patients at the safety follow-up visit was calculated as the number of randomized patients who had ended all study treatments or were remaining on treatment at the safety follow-up visit. Patients who had ended all study treatments included patients who discontinued treatment early or completed the 12-cycle treatment.
Safety
All 454 patients received ≥1 dose of carfilzomib and were included in the safety analysis set. Five patients in the once-weekly group did not receive 56 mg/m2 of carfilzomib and were assigned to the twice-weekly KRd27 safety group per protocol. Thus, the safety analysis set comprised 223 patients in the once-weekly safety group and 231 patients in the twice-weekly safety group. Any-grade adverse events occurred in 209 patients (93.7%) in the once-weekly KRd56 safety group and 219 patients (94.8%) in the twice-weekly KRd27 safety group (Table 5). Common any-grade treatment-emergent adverse events (TEAEs; ≥20% of patients in either group) were neutropenia, anemia, hypertension, and thrombocytopenia. TEAEs where the difference in incidence between the once-weekly KRd56 and the twice-weekly KRd27 group was ≥5% were thrombocytopenia (21.5% vs 14.3%), coronavirus disease 2019 (COVID-19; 12.6% vs 7.4%), asthenia (12.1% vs 5.6%), and peripheral neuropathy (9.9% vs 4.8%).
TEAEs in at least 10% of patients
| . | Once-weekly KRd56 (N = 223) . | Twice-weekly KRd27 (N = 231) . | ||
|---|---|---|---|---|
| Any-grade TEAEs . | Grade ≥3 TEAEs . | Any-grade TEAEs . | Grade ≥3 TEAEs . | |
| Overall, n (%) | 209 (93.7) | 141 (63.2) | 219 (94.8) | 144 (62.3) |
| Neutropenia | 65 (29.1) | 54 (24.2) | 74 (32.0) | 57 (24.7) |
| Anemia | 57 (25.6) | 26 (11.7) | 55 (23.8) | 20 (8.7) |
| Hypertension | 48 (21.5) | 21 (9.4) | 56 (24.2) | 23 (10.0) |
| Thrombocytopenia | 48 (21.5) | 27 (12.1) | 33 (14.3) | 24 (10.4) |
| Diarrhea | 42 (18.8) | 2 (0.9) | 44 (19.0) | 3 (1.3) |
| Upper respiratory tract infection | 32 (14.3) | 2 (0.9) | 33 (14.3) | 0 (0.0) |
| COVID-19 | 28 (12.6) | 7 (3.1) | 17 (7.4) | 4 (1.7) |
| Asthenia | 27 (12.1) | 5 (2.2) | 13 (5.6) | 3 (1.3) |
| Pyrexia | 25 (11.2) | 2 (0.9) | 28 (12.1) | 2 (0.9) |
| Fatigue | 24 (10.8) | 7 (3.1) | 29 (12.6) | 5 (2.2) |
| Constipation | 23 (10.3) | 2 (0.9) | 17 (7.4) | 0 (0.0) |
| . | Once-weekly KRd56 (N = 223) . | Twice-weekly KRd27 (N = 231) . | ||
|---|---|---|---|---|
| Any-grade TEAEs . | Grade ≥3 TEAEs . | Any-grade TEAEs . | Grade ≥3 TEAEs . | |
| Overall, n (%) | 209 (93.7) | 141 (63.2) | 219 (94.8) | 144 (62.3) |
| Neutropenia | 65 (29.1) | 54 (24.2) | 74 (32.0) | 57 (24.7) |
| Anemia | 57 (25.6) | 26 (11.7) | 55 (23.8) | 20 (8.7) |
| Hypertension | 48 (21.5) | 21 (9.4) | 56 (24.2) | 23 (10.0) |
| Thrombocytopenia | 48 (21.5) | 27 (12.1) | 33 (14.3) | 24 (10.4) |
| Diarrhea | 42 (18.8) | 2 (0.9) | 44 (19.0) | 3 (1.3) |
| Upper respiratory tract infection | 32 (14.3) | 2 (0.9) | 33 (14.3) | 0 (0.0) |
| COVID-19 | 28 (12.6) | 7 (3.1) | 17 (7.4) | 4 (1.7) |
| Asthenia | 27 (12.1) | 5 (2.2) | 13 (5.6) | 3 (1.3) |
| Pyrexia | 25 (11.2) | 2 (0.9) | 28 (12.1) | 2 (0.9) |
| Fatigue | 24 (10.8) | 7 (3.1) | 29 (12.6) | 5 (2.2) |
| Constipation | 23 (10.3) | 2 (0.9) | 17 (7.4) | 0 (0.0) |
Grade ≥3 TEAEs were reported in 141 patients (63.2%) in the once-weekly KRd56 safety group vs 144 patients (62.3%) in the twice-weekly KRd27 safety group (Table 4). The most frequently reported grade ≥3 adverse events (≥5% of patients in either group) were neutropenia (24.2% and 24.7% in the once-weekly KRd56 and twice-weekly KRd27 safety groups, respectively), thrombocytopenia (12.1% and 10.4%), anemia (11.7% and 8.7%), hypertension (9.4% and 10.0%), and pneumonia (5.4% and 3.0%; supplemental Table 1). Serious TEAEs occurred in 84 patients (37.7%) in the once-weekly KRd56 safety group and 75 patients (32.5%) in the twice-weekly KRd27 safety group (supplemental Table 2). The most frequently reported serious TEAEs (≥2% of patients in either group) were pneumonia (5.4% and 3.9% in the once-weekly and twice-weekly safety groups, respectively), COVID-19 pneumonia (3.6% and 4.8%), and COVID-19 (2.7% and 1.3%). TEAEs leading to carfilzomib discontinuation occurred in 38 patients (17.0%) in the once-weekly KRd56 safety group and 33 patients (14.3%) in the twice-weekly KRd27 safety group. The most frequently reported adverse events leading to carfilzomib discontinuation (≥1% of patients in either group) were COVID-19 pneumonia (2.2% and 1.7% in the once-weekly and twice-weekly safety groups, respectively), thrombocytopenia (1.3% and 0.0%), pneumonia (0.4% and 1.3%), and heart failure (0.0% and 1.3%). Supplemental Table 3 shows TEAEs of interest that occurred in ≥5% of patients in either group. Treatment-emergent deaths occurred in 12 patients (5.4%) in the once-weekly KRd56 safety group and 10 patients (4.3%) in the twice-weekly KRd27 safety group (supplemental Table 4). At the end of cycle 1, 3 deaths each were reported in the once-weekly and twice-weekly safety groups. The most frequently reported fatal events (≥1% of patients in either group) were COVID-19 pneumonia (1.8% and 0.4% in the once-weekly and twice-weekly safety groups, respectively), death (1.3% and 0.0%), and pneumonia (0.0% and 1.3%). One patient in the once-weekly KRd56 group had a fatal adverse event that was considered by the investigator to be treatment related. The patient was a 71-year-old male with a prior history of well-controlled hypertension and no other apparent cardiac risk factors who experienced unexpected death due to cardiac arrest 190 days after receiving the first dose, 10 days after receiving the last dose of carfilzomib (administered in combination with Rd).
Discussion
This study was designed to evaluate whether once-weekly KRd56 offered a convenient treatment option with efficacy comparable to the twice-weekly KRd27 standard of care in patients with RRMM. Although ORRs were numerically similar in both treatment groups, the primary end point of noninferiority of ORR was not achieved in patients treated with once-weekly KRd56 vs twice-weekly KRd27 according to the statistical significance threshold of the test. Given the number of patients enrolled and the observed ORR of 86.3% in the twice-weekly KRd27 group, the threshold for ORR in the once-weekly KRd56 group to achieve statistically significant noninferiority was at least 84.2% (at most a 2.1% difference between treatment arms). The observed ORR in this study for the once-weekly group was 82.5%, representing an estimated 3.8% difference between groups. However, a prespecified sensitivity analysis using the fixed-margin approach17 did show noninferiority of ORR, suggesting that although statistical significance for noninferiority was not reached using the synthesis method, the ORR for KRd56 and KRd27 in this study may be clinically comparable. In addition, the 95% CIs for ORR overlapped between the groups, and the risk ratio was 0.954 (95% CI, 0.882-1.032), with the 95% CIs exceeding 1, suggesting similar ORRs. The proportions of patients with a CR or better and those who achieved a CR who were MRD-negative were also comparable between the groups. Although PFS was not tested according to the hierarchical statistical analysis sequence, the reported 6- and 12-month PFS rates, HRs, and ∼3.3-day difference between the groups based on a prespecified RMST analysis suggested that PFS was similar between the once-weekly and twice-weekly groups.
Treatment with once-weekly KRd56 was safe and well tolerated. The incidence of grade ≥3 TEAEs was comparable between the safety groups (63.2% for the once-weekly KRd56 safety group vs 62.3% for the twice-weekly KRd27 safety group). Additionally, the incidence of grade ≥3 cardiac disorders was comparable between the groups (3.1% in the once-weekly KRd56 group vs 3.0% in the twice-weekly KRd27 group).
The efficacy and safety results for patients treated with once-weekly KRd56 reported in this study were consistent with those reported for the same dosing regimen in patients with RRMM in previous studies. ORR (82.5%) and the proportion of patients with a CR or better (46.9%) were similar to those reported in a previous study conducted in French patients with early RRMM treated with once-weekly KRd56 (n = 42, median of 1 prior line of therapy; ORR, 83.0%; proportion of patients with a CR or better, 45.0%).13 Additionally, ORR reported in this study was consistent with that (90.0%) reported for 10 patients treated with once-weekly KRd56 in a phase 1b study.12,13 The incidence of grade ≥3 TEAEs for patients treated with once-weekly KRd56 was 70.0% in the phase 1b study vs 63.2% in this study.12
Previous clinical trials have consistently shown that once-weekly carfilzomib-dosing regimens are feasible and effective for the treatment of patients with RRMM.9,10,18 In an interim analysis of the phase 3 A.R.R.O.W. study, ORR for patients treated with a once-weekly regimen of carfilzomib (70 mg/m2) and dexamethasone was 62.9%, proportion of patients with a CR or better was 7.0%, and incidence of grade ≥3 TEAEs was 68%.9 Results from the phase 1b EQUULEUS study, which evaluated a once-weekly regimen of daratumumab-carfilzomib (70 mg/m2) and dexamethasone in patients with RRMM, showed that ORR was 84.0%, proportion of patients with a CR or better was 33.0%, and incidence of grade ≥3 TEAEs was 77.0%.10 These results were consistent with the results from the current study (ORR, 82.5%; proportion of patients with a CR or better, 46.9%; incidence of grade ≥3 TEAEs, 63.2%) and support the treatment validity of once-weekly KRd56 in patients with RRMM.
Patient-reported convenience status after cycle 4 of treatment was comparable between the treatment groups. The patients who responded to the twice-weekly KRd27 regimen overcame the “inconvenience” of coming to the clinic twice a week. In addition, clinical trial participants may represent a different population that is more open to increased health care touchpoints and less affected by the 6 infusions/visits per month than typical nonclinical trial patients. Importantly, the increased convenience of the once-weekly dose schedule could be significant for patients who are working, those caring for family members or unable to attend frequent clinic visits such as older patients with limited mobility. Therefore, by improving convenience with the appropriate carfilzomib dose, patients may stay on once-weekly regimen longer and could derive additional benefit without incremental risk compared with twice-weekly KRd27 regimen.
A limitation of this study was the noninferiority trial design and use of a synthesis approach with a relatively small sample size rather than a fixed-margin approach requiring a larger sample size. The assessment of ORR alone is a singular measure of treatment efficacy that provides limited interpretability for clinical value, especially because subsequent end points are not evaluated for their statistical significance.
In conclusion, although statistical significance of the primary end point of noninferiority of ORR was not met, there were no clinically meaningful differences between the once-weekly KRd56 and twice-weekly KRd27 regimens. Consistent with other once-weekly carfilzomib regimens, once-weekly KRd56 may be considered as an effective, tolerable, and convenient dosing option for patients with RRMM.
Acknowledgments
The authors and sponsor (Amgen, Inc) participated in the conception and design of the study, and analysis and interpretation of data. Additionally, the authors thank the patients who participated in the study and their families.
This work was supported by research funding from Amgen Inc. Swapnil Kher and Manoj Kumar Goyal of Cactus Life Sciences (part of Cactus Communications) provided medical writing and editorial assistance, funded by Amgen Inc. Graphics support was provided by Bob Dawson of Cactus Communications and funded by Amgen Inc.
Authorship
Contribution: T.U., B.F., M.B., and R.T. were involved in the design of the trial, analysis, and interpretation of data; M.A.D., D.C., S.D., I.Š., E.F., and X.L. obtained data and participated in the analysis and interpretation of the data; and all authors participated in the drafting and revising of the manuscript and approved the final version before submission.
Conflict-of-interest disclosure: M.A.D. received honoraria from Amgen, Sanofi, Regeneron, Menarini, Takeda, GlaxoSmithKline (GSK), Bristol Myers Squibb (BMS), Janssen, and BeiGene, and has a consulting or advisory role for Amgen, Sanofi, Regeneron, Menarini, Takeda, GSK, BMS, Janssen, and BeiGene. S.D. received honoraria from Janssen, Takeda, Amgen, and Celgene. I.Š. received honoraria from and served on a speakers’ bureau for Celgene, Amgen, Janssen, Takeda, BMS, Novartis, and Sanofi. T.U., B.F., R.T., and M.B. are employed by Amgen. E.F. received honoraria while serving as a consultant and/or participating in advisory boards for Amgen, Celgene, Janssen, AbbVie, Adaptive Biotechnologies, CTI BioPharma, Cardinal Health, GSK, Karyopharm, Sanofi Genzyme, Takeda, and Kite/Gilead. X.L. received honoraria from Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptides, Karyopharm, Roche, AbbVie, CARsgen, GSK, and Harpoon Therapeutics. D.C. declares no competing financial interests.
Correspondence: Meletios A. Dimopoulos, School of Medicine, National and Kapodistrian University of Athens, Alexandra Hospital, 80 Vasilissis Sofias Ave, 115 28 Athens, Greece; email: mdimop@med.uoa.gr.
References
Author notes
Qualified researchers may request access to data through the Amgen clinical studies platform at https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
The full-text version of this article contains a data supplement.