TO THE EDITOR:
Recurrent somatic ethanolamine-kinase-1 (ETNK1; located on 12p12.1) mutations were originally discovered almost exclusively in patients with atypical chronic myeloid leukemia (aCML), also known as myelodysplastic syndrome/myeloproliferative neoplasm with neutrophilia (MDS/MPN-N).1-4 Contrarily, Shuai et al demonstrated that these mutations occur in a wide variety of myeloid neoplasms (MNs).5 Loss-of-function mutations in ETNK1 may promote the development of neoplasia by increasing mitochondrial activity, ultimately resulting in DNA double-strand breaks due to damage inflicted by reactive oxygen species.6 Herein, we lend further molecular and clinical characterization of ETNK1-mutated MN through a retrospective review of Mayo Clinic patients and our next-generation sequencing (NGS) database.
Adult patients (with/without MN) who underwent a 47 gene MN-targeting NGS panel at the Mayo Clinic Comprehensive Cancer Center (Minnesota, Arizona, and Florida) between 2018 and 2023 were included in this study. Additionally, a cohort of 98 patients with ETNK1 wild-type MDS was assessed for survival analysis. Exons 2 to 5 of the ETNK1 gene were sequenced by our NGS panel (supplemental Table 1). A clone was deemed dominant if it had the highest variant allele frequency (VAF) or a VAF ≤5% of the highest VAF comutation.7 Approval by the Mayo Clinic Institutional Review Board was obtained, and the study abided by the Declaration of Helsinki of 1975. The fifth edition of the World Health Organization and the 2022 European LeukemiaNet recommendations for acute myeloid leukemia (AML) were used as diagnostic criteria.3,8 GraphPad Prism 10 was used for data and statistical analysis.
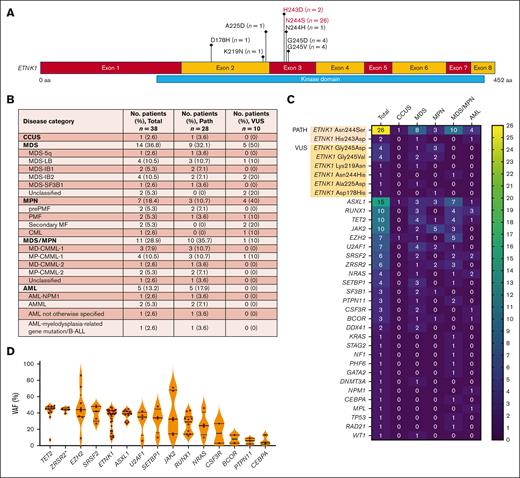
Of 5442 patient samples, 40 (0.7%) carried ETNK1 mutations, 28 of which had known pathogenic mutations (Asn244Ser and His243Asp) in exon 3 of the kinase domain (Figure 1A,C).1 The other 12 patients had variants of unknown significance (VUS) in exons 2 and 3 of the kinase domain (Figure 1A,C). These included Gly245Asp (n = 4), Gly245Val (n = 4), Arg244His (n = 1), Ala225Asp (n = 1), Lys219Asn (n = 1), and Asp178His (n = 1). Other research groups have reported the occurrence of Gly245Asp and Asn244His, as well as Gly245Ala, Asn244Ile, Asn244Tyr, Asn244Thr, and Asn244Lys, none of which have been functionally validated.2,5,9
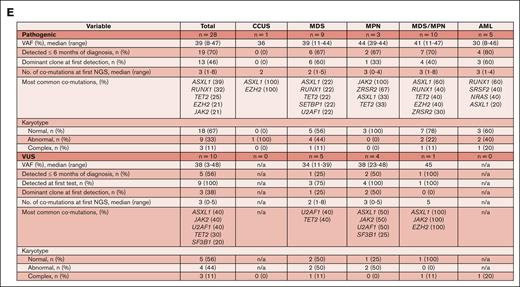
Molecular-genetic characterization of ETNK1-mutated MNs. (A) Schematic showing the location of identified mutations within the ETNK1 gene. The pathogenic mutations are highlighted in red; the VUS are shown in black. (B) Table summarizing the distribution of ETNK1 mutations among MN categories in 38 patients (the clinical data for 2 patients was not available). (C) Heat map showing the frequency of ETNK1 mutations and comutations among various MNs in 40 patients. (D) Violin plot demonstrating the comutations with the highest VAF values. ∗ZRSR2 VAF values were divided by 2 due to the gene’s location on the X chromosome. (E) Table summarizing the molecular-genetic features of 38 patients with either pathogenic mutations or VUS. Abnormal karyotypes had 1 or 2 chromosomal aberrations; complex karyotypes had ≥3 chromosomal aberrations. AMML, acute myelomonocytic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; LB, low blast; MF, myelofibrosis; PATH, pathogenic; PMF, primary myelofibrosis.
Molecular-genetic characterization of ETNK1-mutated MNs. (A) Schematic showing the location of identified mutations within the ETNK1 gene. The pathogenic mutations are highlighted in red; the VUS are shown in black. (B) Table summarizing the distribution of ETNK1 mutations among MN categories in 38 patients (the clinical data for 2 patients was not available). (C) Heat map showing the frequency of ETNK1 mutations and comutations among various MNs in 40 patients. (D) Violin plot demonstrating the comutations with the highest VAF values. ∗ZRSR2 VAF values were divided by 2 due to the gene’s location on the X chromosome. (E) Table summarizing the molecular-genetic features of 38 patients with either pathogenic mutations or VUS. Abnormal karyotypes had 1 or 2 chromosomal aberrations; complex karyotypes had ≥3 chromosomal aberrations. AMML, acute myelomonocytic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; LB, low blast; MF, myelofibrosis; PATH, pathogenic; PMF, primary myelofibrosis.
The distribution of ETNK1 mutations across MN categories is shown in Figure 1B-C. Of ETNK1-mutated MNs, 36.8% had MDS, 28.9% had MDS/MPN overlap (of which 90.9% were CMML), 18.4% had MPN (of which 86% were myelofibrosis), and 13.2% had AML. There were no patients with aCML. Similarly, only 2.5% of patients had aCML in a similar retrospective study by Shuai et al.5 Our findings reinforce their observation that ETNK1 mutations are not exclusive to aCML. Additionally, a recent study of patients with aCML from the Netherlands Cancer Registry found only 1 patient with an ETNK1 mutation among 49 patients with available molecular data.10
The identified ETNK1 comutations are shown in Figure 1C and summarized by disease category in Figure 1E. The most common comutations were ASXL1 (37.5%), RUNX1 (25%), TET2 (25%), JAK2 (25%), EZH2 (17.5%), U2AF1 (17.5%), SRSF2 (15%), and ZRSR2 (15%). These comutations are likely important in clonal evolution because they occurred with high VAFs (Figure 1D). ASXL1 was the most common comutation overall, an observation also made by Shuai et al,5 although its occurrence was not greater than expected (CMML, 43%-49%; MDS, 15%-21%; MPN, 8%-10%; AML, 3%-10%).11 The most common ASXL1 mutation was Gly646TrpfsX12 (53%); no unique hot spot or domain was identified. JAK2 and RUNX1 were the most common mutations in MPN and AML, respectively.
Our data may corroborate the proposed view that ETNK1 mutations are early occurring events in clonal evolution.5,9,12 The majority of ETNK1 mutations (70% pathogenic cases) were found within 6 months of diagnosis, and approximately half (46% pathogenic cases) were dominant clones at first detection (Figure 1E). Although the mutations appear to have occurred early, it should be noted that the time of diagnosis does not necessarily reflect the time of disease onset. The majority of pathogenic cases (67%) had normal karyotypes at first detection (Figure 1E). Thirty-three percent of karyotypes were not normal (no correlation with MN subtype). Of these, 33% were complex. Trisomy 8 was the most common abnormality (33%). Serial cytogenetic analysis by future studies will be required to test the hypothesis that ETNK1 mutations induce a mutator phenotype over time.6
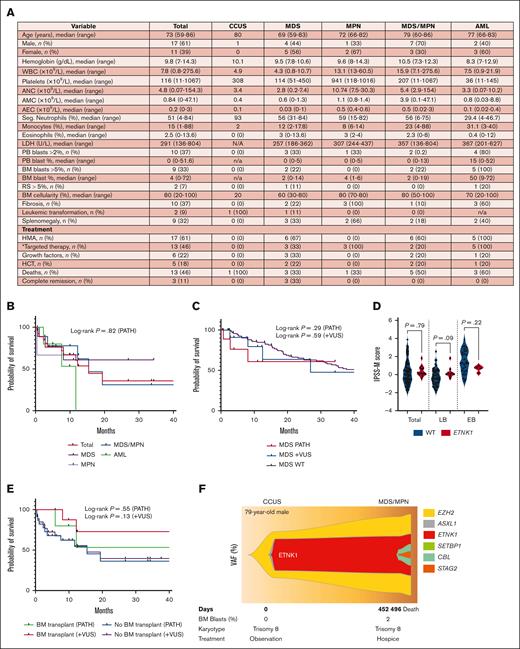
The median age of patients was 73 years (range, 59-86 years), with a male predominance of 61%. Evaluation of hemoglobin, white blood cells, platelets, neutrophils, eosinophils, lactate dehydrogenase, peripheral and bone marrow (BM) blasts, ring sideroblasts, BM cellularity, and splenomegaly did not reveal unique patterns (Figure 2A). Approximately half of patients with MDS had monocytosis (median absolute count, 0.6), reflecting a possible trajectory toward CMML. Additionally, a high proportion of AML cases had BM fibrosis (60%; median grade 2/3). Although an association between eosinophilia and ETNK1 mutations in systemic mastocytosis has previously been reported by our group,2 only 3 patients (12.7%; MD-CMML-1, MDS-SF3B1, and secondary myelofibrosis) had eosinophilia (median level of eosinophilia, 9.4%) in this myeloid cohort.
Clinical characterization of ETNK1-mutated MNs. (A) Table summarizing the clinicopathologic features of 28 patients with pathogenic mutations at the time of NGS (patients with VUS not included). (B) Kaplan-Meier survival curves of patients grouped by disease category. (C) Kaplan-Meier survival curves comparing patients with ETNK1-mutated MDS (n = 10) vs ETNK1 wild-type MDS (n = 98). (D) IPSS-M scores of patients with wild-type (n = 85) vs ETNK1-mutated MDS (n = 13; LB = 9 and EB = 4) at time of initial NGS. (E) Kaplan-Meier survival curves comparing patients that underwent HCT vs patients who did not. (F) Fishplot showing the clonal evolution of ETNK1 over serial NGS samples. ∗The targeted therapies in our cohort included venetoclax, ruxolitinib, and imatinib. AEC, absolute eosinophil count; AMC, absolute monocyte count; ANC, absolute neutrophil count; EB, excess blast; HMA, hypomethylating agent; LB, low blasts; LDH, lactate dehydrogenase; PATH, pathogenic; PB, peripheral blood; RS, ring sideroblast; WBC, white blood cell.
Clinical characterization of ETNK1-mutated MNs. (A) Table summarizing the clinicopathologic features of 28 patients with pathogenic mutations at the time of NGS (patients with VUS not included). (B) Kaplan-Meier survival curves of patients grouped by disease category. (C) Kaplan-Meier survival curves comparing patients with ETNK1-mutated MDS (n = 10) vs ETNK1 wild-type MDS (n = 98). (D) IPSS-M scores of patients with wild-type (n = 85) vs ETNK1-mutated MDS (n = 13; LB = 9 and EB = 4) at time of initial NGS. (E) Kaplan-Meier survival curves comparing patients that underwent HCT vs patients who did not. (F) Fishplot showing the clonal evolution of ETNK1 over serial NGS samples. ∗The targeted therapies in our cohort included venetoclax, ruxolitinib, and imatinib. AEC, absolute eosinophil count; AMC, absolute monocyte count; ANC, absolute neutrophil count; EB, excess blast; HMA, hypomethylating agent; LB, low blasts; LDH, lactate dehydrogenase; PATH, pathogenic; PB, peripheral blood; RS, ring sideroblast; WBC, white blood cell.
The prognostic effect of ETNK1 mutations is currently unknown. Among 25 patients with aCML, our group found no impact by ETNK1 mutations (2 patients) on overall survival.13 Among 43 patients with aCML parsed by good/bad prognosis, Fontana et al demonstrated more frequent ETNK1 mutations in the good prognosis group (22.7%) than in the bad prognosis group (6.7%).9 The available data are too limited for reliable conclusions. Survival curves of our study cohort from the time of NGS are shown in Figure 2B-C,E. Survival curves from the time of initial diagnosis, risk scores (IPSS-R, IPSS-M, DIPSS-PLUS, MIPSS-PLUS, and CPSS-Mol), and percent BM fibrosis are shown in supplemental Figures 1 and 2. No patients with ETNK1-mutated AML achieved complete remission as defined by the 2022 European LeukemiaNet criteria (treatment included hypomethylating agents plus venetoclax; 1 patient underwent hematopoietic cell transplant [HCT]).8 Comparison of ETNK1-mutated and ETNK1 wild-type MDS did not reveal differences in overall survival or IPSS-M scores, potentially due to the small sample size (Figure 2C-D). Similarly, significance was not attained between overall survival of patients who underwent HCT vs no HCT (Figure 2E). Our analysis of risk scores (supplemental Figure 2A-C) and BM fibrosis (supplemental Figure 2D) did not reveal an apparent prognostic impact. Unfortunately, bona fide survival analysis and risk assessment are precluded by the rarity of ETNK1 mutations and their heterogeneity across the MN subtypes.
There are many unanswered questions that remain. First, many VUS classified by the American College of Medical Genetics and Association of Medical Pathology guidelines using currently available information may be pathogenic, suggested by their location within the ETNK1 kinase domain and frequency of their occurrence in patients with MNs.14 These VUS should be evaluated by functional studies to be clinically useful. Second, a large multicenter study will be required to study survival and prognosis. Third, future studies should analyze serial NGS and karyotype data, which could shed light on specific molecular pathways of evolution and other genetic patterns. Serial assessment of 1 patient revealed an early acquisition and persistence of ETNK1 mutation throughout disease progression from CCUS to MDS/MPN (Figure 2F). Lastly, although a few, albeit encouraging, in vitro studies have suggested phosphoethanolamine as a promising potential targeted treatment, there are no suitable mouse models or studies that have attempted to target ETNK1 mutants in vivo. There is an intriguing avenue for the investigation of phosphoethanolamine as a treatment of ETNK1-mutated MN.6,9,15
Contribution: S.T. and A.A.-K. designed and performed the research; S.T. collected data and performed statistical analysis; S.T., R.H., P.G., M.P., and A.A.-K. wrote the manuscript; and all authors analyzed and interpreted data and read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aref Al-Kali, Division of Hematology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: al-kali.aref@mayo.edu.
References
Author notes
Original data are available on request from the author, Steven Tessier (tessier.steven@mayo.edu).
The full-text version of this article contains a data supplement.