TO THE EDITOR:
In December 2023, the US Food and Drug Administration (FDA) approved 2 gene therapies for sickle cell disease (SCD). The data demonstrated substantial short-term clinical improvement.1,2 Approximately half of the 100 000 individuals in the United States with SCD are Medicaid enrollees.3 Allogeneic hematopoietic stem cell transplant (HSCT) is a well-described cure for the hemoglobinopathy, although its use is limited due to donor availability and toxicity.4
Prices for gene therapies have made headlines, whereas the payment timing and uncertainty in treatment durability compound the challenge for payers.5 The 2 new approvals for SCD apply to a population far larger than any existing commercial gene therapy.
We previously published a model exploring the budget impact of a gene therapy for SCD for 10 Medicaid plans, projecting an affordability challenge.6 Even cost-effective therapies with exceptional clinical benefit may prove unaffordable for payers balancing short-term budgets.
Since the initial publication, several key events occurred. First, Medicaid enrollment increased substantially. Second, 2 products are now available with higher launch prices than previously anticipated.7 Lastly, new data permit a more accurate estimation of the distribution of disease severity.8,9
Here, we update the model aiming to (1) estimate the potential short-term budget impact of a gene therapy for severe SCD from the perspective of 10 state Medicaid plans; and (2) describe how uncertainty in the input parameters influence budget impact.
This is an update of a prior analysis, and the original publication provides greater methodologic detail.6 The annual budget impact was calculated as the 1-time cost of the gene therapy minus the savings from patients having previously received the therapy. Only direct medical expenditures related to SCD were included. A static cohort entered the model receiving standard of care over a 5-year time horizon (supplemental Figure 1). Each year, a subset of the cohort received the therapy (defined by the market diffusion rate). The therapy yielded lifelong durability and averted all future SCD-related costs (intended to provide conservative estimate of the budget impact rather than reflect existing clinical data). The intervention was a theoretical therapy based upon the 2 commercially available gene therapies for SCD, using the lower price of exagamglogene autotemcel and requiring hospitalization with busulfan conditioning while deviating from the real world in assuming perfect effectiveness, durability, and safety (providing a more conservative estimate of the budget impact). The model was intended to represent utilization 3 to 5 years after market entry, assuming no supply-side constraints (ie, manufacturing or delivery center capacity).
The primary outcome was the incremental per-member per-month (PMPM) cost; the annual budget impact divided by the number of plan enrollees, divided by 12 months. All costs are adjusted to 2024 US dollars.
The 10 state Medicaid programs with the highest prevalence of SCD were included. State-level disease burden was updated using the rate of SCD among Medicaid enrollees in 2017 (supplemental Table 1).8 Enrollment data from 2022 were used to estimate the number of enrollees with SCD.8,10
The population eligible for the therapy was individuals with SCD aged 13 to 45 years with a severe phenotype (≥2 severe pain episodes annually). This age range is more stringent than the FDA labels (≥12 years) but conforms to Medicaid age strata. Individuals with hemoglobin SC were excluded.
Table 1 lists key model parameters. The annual market diffusion rate for the therapy was 7% (sensitivity analysis 2%-15%), informed by expert opinion alone and assuming no supply-side constraints. The gene therapy cost $2.29 million, derived from the launch price of exagamglogene autotemcel ($2.2 million)7 plus $90 000 for hospitalization for myeloablative conditioning. The hospitalization cost was derived from a commercially insured population undergoing autologous transplantation, and the cost was adjusted because Medicaid pays ∼57% of the commercial rate for inpatient care.11,12 The sensitivity analysis ($1.78-$3.19 million) represented a 23% discount from the base case (Medicaid “best-price” rebate), with the upper bound reflecting the launch price of lovotibeglogene autotemcel. List price overestimates cost but is widely used. No costs for gene therapy outpatient care or adverse effects were included. Univariate sensitivity analysis evaluated uncertainty with mean PMPM cost as the outcome. No institutional review board approval was obtained for this simulation.
Key model inputs
| Parameter . | Value . | Range considered in sensitivity analysis . | Original model inputs∗ . | References . |
|---|---|---|---|---|
| Percentage of patients with severe phenotype | 35% | 20%-60% | 25% (10%-40%) | 8,9 |
| Baseline annual expenditure for patient with SCD† | $51 700 | $21 830-$85 680 | $48 900 ($19 900-$78 100) | 13,14 |
| Cost of single gene therapy treatment per person (million) | $2.29 | $1.78-$3.19 | $2.267 ($1.769-$2.659) | 7,11,12 |
| Annual market diffusion rate for gene therapy | 7% | 2%-15% | 7% (2%-15%) | Assumed |
| Parameter . | Value . | Range considered in sensitivity analysis . | Original model inputs∗ . | References . |
|---|---|---|---|---|
| Percentage of patients with severe phenotype | 35% | 20%-60% | 25% (10%-40%) | 8,9 |
| Baseline annual expenditure for patient with SCD† | $51 700 | $21 830-$85 680 | $48 900 ($19 900-$78 100) | 13,14 |
| Cost of single gene therapy treatment per person (million) | $2.29 | $1.78-$3.19 | $2.267 ($1.769-$2.659) | 7,11,12 |
| Annual market diffusion rate for gene therapy | 7% | 2%-15% | 7% (2%-15%) | Assumed |
Costs adjusted from 2019 to 2024 US dollars.
Average of estimates in associated reference and adjusted to 2024 US dollars.
An estimated 7742 Medicaid enrollees with SCD would be eligible for the therapy nationally, with 4106 individuals in the 10 plans of interest (Table 2). This represents a substantial increase from our prior analysis estimating 2313 individuals eligible across the sample.
Budget impact analysis, Medicaid perspective for United States and selected state programs
| . | Total no. eligible for gene therapy . | No. eligible for therapy per 100 000 plan enrollees . | No. to receive therapy, year 1 . | Budget impact, year 1 (million USD) . | PMPM cost, year 1 . | % change in year 1 PMPM cost from original model∗6 . | PMPM cost, year 5 . |
|---|---|---|---|---|---|---|---|
| United States† | 7742 | 8.5 | 542 | $1241.0 | $1.13 | 18% | $0.75 |
| Alabama | 338 | 29.2 | 24 | $54.2 | $3.90 | 49% | $2.60 |
| Washington, DC | 69 | 23.6 | 5 | $11.0 | $3.15 | 45% | $2.10 |
| Florida | 904 | 18.7 | 63 | $144.9 | $2.50 | 41% | $1.67 |
| Georgia | 720 | 29.3 | 50 | $115.4 | $3.92 | 57% | $2.61 |
| Louisiana | 445 | 23.7 | 31 | $71.4 | $3.17 | 46% | $2.11 |
| Maryland | 329 | 19.7 | 23 | $52.8 | $2.63 | 59% | $1.75 |
| Mississippi | 203 | 26.5 | 14 | $32.5 | $3.55 | –4% | $2.36 |
| North Carolina | 409 | 18.1 | 29 | $65.5 | $2.41 | 28% | $1.60 |
| South Carolina | 350 | 27.1 | 25 | $56.1 | $3.62 | 43% | $2.42 |
| Virginia | 339 | 17.2 | 24 | $54.4 | $2.30 | 20% | $1.54 |
| Sample average | 411 | 21.9 | 29 | $65.8 | $3.11 | 40% | $2.08 |
| . | Total no. eligible for gene therapy . | No. eligible for therapy per 100 000 plan enrollees . | No. to receive therapy, year 1 . | Budget impact, year 1 (million USD) . | PMPM cost, year 1 . | % change in year 1 PMPM cost from original model∗6 . | PMPM cost, year 5 . |
|---|---|---|---|---|---|---|---|
| United States† | 7742 | 8.5 | 542 | $1241.0 | $1.13 | 18% | $0.75 |
| Alabama | 338 | 29.2 | 24 | $54.2 | $3.90 | 49% | $2.60 |
| Washington, DC | 69 | 23.6 | 5 | $11.0 | $3.15 | 45% | $2.10 |
| Florida | 904 | 18.7 | 63 | $144.9 | $2.50 | 41% | $1.67 |
| Georgia | 720 | 29.3 | 50 | $115.4 | $3.92 | 57% | $2.61 |
| Louisiana | 445 | 23.7 | 31 | $71.4 | $3.17 | 46% | $2.11 |
| Maryland | 329 | 19.7 | 23 | $52.8 | $2.63 | 59% | $1.75 |
| Mississippi | 203 | 26.5 | 14 | $32.5 | $3.55 | –4% | $2.36 |
| North Carolina | 409 | 18.1 | 29 | $65.5 | $2.41 | 28% | $1.60 |
| South Carolina | 350 | 27.1 | 25 | $56.1 | $3.62 | 43% | $2.42 |
| Virginia | 339 | 17.2 | 24 | $54.4 | $2.30 | 20% | $1.54 |
| Sample average | 411 | 21.9 | 29 | $65.8 | $3.11 | 40% | $2.08 |
USD, US dollar.
Percent change in the year 1 PMPM impact when comparing the original model with the current and updated results.6
The United States indicates all state Medicaid plans in aggregate.
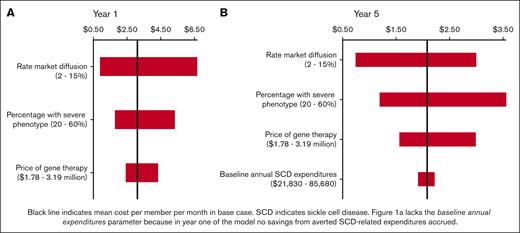
The updated model projected an average 1-year budget impact of $65.8 million per state program (Table 2), with an average of $3.11 PMPM (compared with $2.31 PMPM in the original analysis). The annual budget impact decreased over time, with a mean cost of $2.08 PMPM in year 5. Sensitivity analysis (Figure 1) demonstrated that the budget impact was most sensitive to the market diffusion rate. supplemental Table 3 details the primary model outcomes when using the lower bound of the diffusion rate (2% rather than 7%).
Univariate sensitivity analysis: average PMPM budget impact for 10 state sample. (A) Year 1 of the model, lacking baseline annual expenditures parameter because in year 1 no savings from averted SCD-related expenditures accrued. (B):Year 5 of the model.
Univariate sensitivity analysis: average PMPM budget impact for 10 state sample. (A) Year 1 of the model, lacking baseline annual expenditures parameter because in year 1 no savings from averted SCD-related expenditures accrued. (B):Year 5 of the model.
These updated results indicate that the gene therapy for SCD will likely produce a sizable short-term budget impact for many Medicaid plans. This analysis raises concern that the affordability challenge may jeopardize access.
Given payer idiosyncrasies, there are no thresholds for budgetary significance, although a budget impact >$2 PMPM is notable. The affordability challenge experienced in 2014 to 2015 with sofosbuvir for hepatitis C is a benchmark; the drug generated a mean PMPM cost of $1.89 in the 10 plans included in our analysis (2024 dollars).6 Sofosbuvir proved unaffordable for many Medicaid plans. A market diffusion rate <4% for the gene therapy for SCD would permit an average budget impact below this sofosbuvir benchmark.
This updated analysis demonstrates an increase in mean PMPM impact by 40% compared with the original study. This was driven by 2 factors. First, the new analysis assumed 35% with SCD have a severe phenotype (20% previously), supported by 2 recent studies.8,9 Second, the price of the products was higher than previously anticipated. The total number of Medicaid enrollees increased substantially in recent years, but this does not affect the PMPM cost, unless the rate of SCD among plan members changes.
Our original analysis demonstrated how an annuity payment model reduces the short-term budget impact. The innumerable proposed alternative payment models seek to address 2 key challenges: outcome uncertainty and payment timing. It is unclear what financing approaches states may consider; research is needed to explore the use and utility of novel payment strategies. The Center for Medicare and Medicaid Innovation is developing novel payment approaches for gene therapy for Medicaid plans.15 There is a social imperative to ensure access for those with SCD.16
Although we erred toward a conservative estimate of budget impact, the rate of market diffusion is uncertain and consequential. Uptake in the first year after FDA approval will be far <7% due to manufacturing, clinical, and financing challenges. Additionally, SCD affects groups with a history of exploitation within the health care system and ongoing inequity in access to care. We assumed gene therapy to be fully curative and durable with no SCD-related expenditures after gene therapy, an unlikely prospect but ensuring a conservative estimate of budget impact.
If a sizable proportion of gene therapy utilization replaces HSCT, this could lower the budget impact (although HSCT for SCD is relatively infrequent).4 The model’s eligibility criteria differ from the product label and align with the clinical trial criteria, ensuring a conservative cohort estimate. Lastly, the findings cannot be generalized to other payer perspectives.
The 2 new gene therapies for severe SCD will likely produce a considerable budget impact for some Medicaid plans, and the ensuing affordability challenge may jeopardize patient access.
Contribution: P.C.D., M.B.H., and J.A.R. designed the analysis; P.C.D. and M.B.H. collected parameter input data and constructed initial the model; P.C.D. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors contributed to model revisions and preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrick C. DeMartino, Division of Pediatric Hematology and Oncology, Department of Pediatrics, Oregon Health & Science University, 707 SW Gaines St, Portland, OR 97239; email: demartip@ohsu.edu.
References
Author notes
Original data are available on request from the corresponding author, Patrick C. DeMartino (demartip@ohsu.edu).
The full-text version of this article contains a data supplement.