Key Points
The 10-year HGT cumulative incidence rate was 4.87% in FL and 2.95% in MZL.
Upfront treatment reduced the risk of transformation in FL but not in MZL.
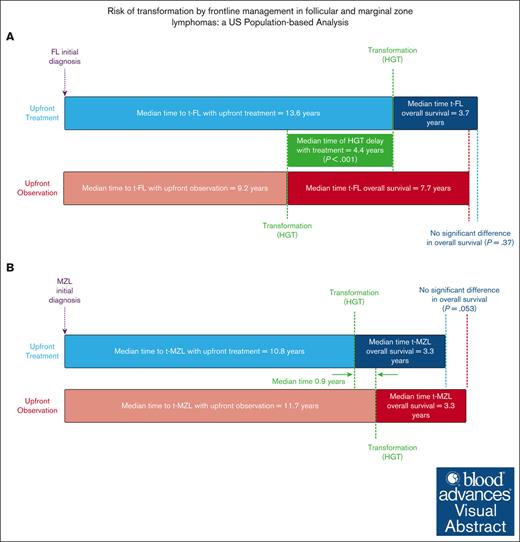
Visual Abstract
Follicular lymphoma (FL) and marginal zone lymphoma (MZL) often have long overall survival (OS), however, high-grade transformation (HGT) to diffuse large B-cell lymphoma markedly reduces survival. The roles of upfront treatment vs observation on the incidence and outcome of HGT remain unclear. Thus, we analyzed a Surveillance, Epidemiology, and End Results database to address this question. Patients diagnosed with FL grades 1 to 2 or MZL between 2000 and 2020 were included. Fine-Gray models estimated the impact of covariates on HGT cumulative incidence and lymphoma-specific survival (LSS) and Cox regression on OS. HGT occurred in 4.2% of 23 384 patients with FL and 2.5% of 20 530 patients with MZL. The 5- and 10-year HGT cumulative incidence rates were 2.80% and 4.87% for FL, and 1.74% and 2.95% for MZL, respectively, which are notably lower than in earlier studies. The annual HGT incidence rate peaked in the first 2 years, then steadily declined over 2 decades for FL and all MZL subtypes. In FL, upfront observation vs treatment increases HGT risk (sub-distribution hazard ratio [SHR], 1.23; 95% confidence interval [CI], 1.09-1.40; P < .001) and barely affects OS (hazard ratio [HR], 0.95; 95% CI, 0.90-0.99; P = .03). Conversely, upfront observation was associated with lower HGT risk in nodal (SHR, 0.71; 95% CI, 0.53-0.94; P = .01) and extranodal (SHR, 0.64; 95% CI, 0.48-0.86; P = .003) MZL and did not affect survival in extranodal disease (HR, 0.94; 95% CI, 0.97-1.02; P = .15). HGT was associated with decrease in LSS across all histologies. Upfront treatment reduced the risk of HGT only in FL but not MZL.
Introduction
Follicular lymphoma (FL) and marginal zone lymphoma (MZL) are subtypes of indolent non-Hodgkin lymphoma characterized by overall survival (OS) of >15 years.1,2 However, this relatively favorable prognosis can change dramatically after high-grade transformation (HGT) to a more aggressive histology, most commonly diffuse large B-cell lymphoma (DLBCL).3
The annual incidence of HGT of FL grades 1 to 3A to DLBCL ranges between 1% and 3%, depending on diagnosis methodologies, population heterogeneity, and the duration of follow-up.4-8 Notably, US and European studies reported a significant decrease in the risk of HGT with upfront treatment compared with observation.7-9 However, other studies do not support that early FL treatment initiation decreases the risk of HGT even in those with limited-stage FL treated with radiation therapy.6,10 Similar to FL, a large retrospective study estimated the overall annual transformation rate for MZL at 1%, with higher occurrence in nodal MZL (NMZL) and splenic MZL (SMZL) compared with extranodal MZL (EMZL).11
Management strategies for indolent lymphomas, encompassing FL grades 1 to 3A and MZL, span from observation to immunotherapy, immunochemotherapy, or radiotherapy. In patients with low tumor burden, historical data supported the recommendation of active observation as the primary management approach, given that there was no significant difference in survival outcomes compared with early treatment.12,13 However, treatments are used in patients presenting with symptoms or a high tumor burden. Some of the recent studies suggested a potential reduction in HGT rates among patients with FL grades 1, 2, and 3A treated with rituximab-containing regimens.9,14 In light of conflicting data on the role of initial treatment,5,6,8,15-17 it is imperative to examine how treatment with chemoimmunotherapy and radiation, in comparison with observation, influences the HGT rate and survival outcomes in a large population database of patients with FL and MZL.
Methods
Patients
We extracted data from the SEER (Surveillance, Epidemiology, and End Results) database maintained by the National Cancer Institute to identify patients diagnosed with FL or MZL between 2000 and 2020. Furthermore, we extracted a “de novo” DLBCL cohort, defined as DLBCL as first diagnosis, that was subsequently used for comparison with the identified transformed DLBCL (tDLBCL) group in our analysis. The SEER coding system does not differentiate between FL grade 3A and 3B so FL grade 3 was omitted from our study. To segregate EMZL from NMZL, primary site location codes indicating nodal involvement were used.18
Inclusion and exclusion criteria are shown in supplemental Figure 1.
DLBCL transformation was identified using the “person selection” option in SEER∗STAT, which identifies malignancies for each patient. In instances in which patients initially diagnosed with FL later developed DLBCL, we categorized these as cases of transformation. We only included DLBCL cases with histological confirmation in the SEER database.
For our analysis, we constructed an “upfront management” variable based on the SEER's “chemotherapy” and “radiotherapy” variables. A patient was categorized as “Yes” for treatment if they received either upfront chemotherapy and/or radiotherapy.
This study was exempt from institutional review board approval and informed consent because study participants were ascertained through a deidentified and publicly available database.
Statistical analysis
HGT time was calculated by subtracting the survival time (in months) of DLBCL from the survival time of the initial indolent lymphoma diagnosis (either FL 1-2 or MZL). Categorical clinical characteristics at diagnosis (age, sex, race, Ann Arbor stage, and management strategies) were compared between the 2 tumor types, FL 1-2 and MZL, using the χ2 tests.
The cumulative risk of HGT over time was estimated using competing risk methods. DLBCL was the event of interest, and death before transformation to DLBCL was the competing risk event. The effect of clinical characteristics at diagnosis (age, sex, race, and the staging Ann Arbor system) on the risk of HGT were estimated by multivariable Fine-Gray (FG) regression models.19 The annual incidence rates (AIRs) of HGT over time expressed per 1000 patient-years were calculated as AIR = (number of transformations at a given year divided by person-time at risk in years at a given year) × 1000. For better visualization, smooth curves estimated using the locally estimated scatterplot smoothing regression method were used to depict the pattern of the AIRs over time in plots.20
Survival analysis was also carried out within the HGT subgroup. In this context, OS, and lymphoma-specific survival (LSS) with causes of death unrelated to lymphoma regarded as competing risks, became the central focus. To evaluate predictors of OS and LSS, we used Cox proportional hazard models and Fine-Gray (FG) subdistribution hazard models, respectively. In these analyses, HGT was considered a time-dependent variable; therefore, we used the count process method whereby 2 records for each HGT case (before and after HGT) were used to incorporate time-dependent HGT in a Cox or FG model.21
To assess survival differences between transformed and de novo DLBCL, we assembled a de novo DLBCL cohort, meticulously matched by age, sex, year of diagnosis, and stage to the tDLBCL group. We used a Cox regression model to compare the DLBCL survival between these cohorts. Additionally, we used a separate Cox regression model to examine the influence of upfront management on posttransformation OS within each tDLBCL cohort.
We performed sensitivity analyses for the FL grade 3 cohort by calculating the 5- and 10-year cumulative incidence estimates for HGT. We also conducted multivariable analyses to assess predictors of the risk of HGT, LSS, and OS.
All statistical analyses were performed using R (version 34.3.1, R Foundation for Statistical Computing). Essential packages included “tidyverse” (version 2.0.0), “tidycmprsk” (version 0.2.0), and “ggsurvfit” (version 0.3.1).
Results
After eligibility criteria were met, 43 914 patients were identified for analysis (FL = 23 384, and MZL = 20 530). In the FL group, HGT occurred in 985 (4.2%) patients during the follow-up time. As for upfront management strategies, 9529 (40.8%) were under observation, whereas the remaining 13 855 (59.6%) received treatment (Table 1). In patients with FL, the median follow-up time was 6.4 years, with those under observation having a median follow-up of 5.9 years, compared with 6.8 years for those receiving treatment.
Characteristics of the entire indolent lymphoma cohort and of FL and MZL subsets
| . | Indolent lymphomas (N = 43 914) . | FL (n = 23 384) . | MZL (n = 20 530) . | P value . | |||
|---|---|---|---|---|---|---|---|
| n . | (%) . | n . | (%) . | n . | (%) . | ||
| Age (y) | |||||||
| <60 | 18 385 | 41.9 | 10 899 | 46.6 | 7 486 | 36.5 | <.001 |
| ≥60 | 25 529 | 58.1 | 12 485 | 53.4 | 13 044 | 63.5 | |
| Gender | |||||||
| Male | 21 010 | 47.8 | 11 719 | 50.1 | 9 291 | 45.3 | <.001 |
| Female | 22 904 | 52.2 | 11 665 | 49.9 | 11 239 | 54.7 | |
| Race | |||||||
| White | 37 823 | 86.1 | 20 878 | 89.3 | 16 945 | 82.5 | <.001 |
| Other | 6 091 | 13.9 | 2 506 | 10.7 | 3 585 | 17.5 | |
| Ann Arbor stage | |||||||
| I-II | 22 371 | 50.9 | 10 179 | 43.5 | 12 192 | 59.4 | <.001 |
| III-IV | 21 543 | 49.1 | 13 205 | 56.5 | 8 338 | 40.6 | |
| Lymphoma subtype | |||||||
| FL | 23 384 | 53.2 | 23 384 | 100 | - | - | |
| SMZL | 2 005 | 4.6 | - | - | 2 005 | 9.8 | |
| NMZL | 6 374 | 14.5 | - | - | 6 374 | 31 | |
| EMZL | 12 151 | 27.7 | - | - | 12 151 | 59.2 | |
| Management | |||||||
| Observation | 20 133 | 45.8 | 9 529 | 40.8 | 10 604 | 51.7 | <.001 |
| Treatment | 23 781 | 54.2 | 13 855 | 59.2 | 9 926 | 48.3 | |
| HGT | |||||||
| No | 42 423 | 96.6 | 22 399 | 95.8 | 20 024 | 97.5 | NA |
| Yes | 1 491 | 3.4 | 985 | 4.2 | 506 | 2.5 | |
| . | Indolent lymphomas (N = 43 914) . | FL (n = 23 384) . | MZL (n = 20 530) . | P value . | |||
|---|---|---|---|---|---|---|---|
| n . | (%) . | n . | (%) . | n . | (%) . | ||
| Age (y) | |||||||
| <60 | 18 385 | 41.9 | 10 899 | 46.6 | 7 486 | 36.5 | <.001 |
| ≥60 | 25 529 | 58.1 | 12 485 | 53.4 | 13 044 | 63.5 | |
| Gender | |||||||
| Male | 21 010 | 47.8 | 11 719 | 50.1 | 9 291 | 45.3 | <.001 |
| Female | 22 904 | 52.2 | 11 665 | 49.9 | 11 239 | 54.7 | |
| Race | |||||||
| White | 37 823 | 86.1 | 20 878 | 89.3 | 16 945 | 82.5 | <.001 |
| Other | 6 091 | 13.9 | 2 506 | 10.7 | 3 585 | 17.5 | |
| Ann Arbor stage | |||||||
| I-II | 22 371 | 50.9 | 10 179 | 43.5 | 12 192 | 59.4 | <.001 |
| III-IV | 21 543 | 49.1 | 13 205 | 56.5 | 8 338 | 40.6 | |
| Lymphoma subtype | |||||||
| FL | 23 384 | 53.2 | 23 384 | 100 | - | - | |
| SMZL | 2 005 | 4.6 | - | - | 2 005 | 9.8 | |
| NMZL | 6 374 | 14.5 | - | - | 6 374 | 31 | |
| EMZL | 12 151 | 27.7 | - | - | 12 151 | 59.2 | |
| Management | |||||||
| Observation | 20 133 | 45.8 | 9 529 | 40.8 | 10 604 | 51.7 | <.001 |
| Treatment | 23 781 | 54.2 | 13 855 | 59.2 | 9 926 | 48.3 | |
| HGT | |||||||
| No | 42 423 | 96.6 | 22 399 | 95.8 | 20 024 | 97.5 | NA |
| Yes | 1 491 | 3.4 | 985 | 4.2 | 506 | 2.5 | |
FL grade 1 to 2.
NA, not applicable.
The MZL group consisted of 2005 (9.8%) patients with SMZL, 6374 (31%) patients with NMZL, and 12 151 (59.2%) patients with EMZL. HGT was documented in 506 (2.5%) patients. As for upfront management strategies of patients with MZL, 10 604 (51.7%) were under observation, and 9926 (48.3%) were treated. The median follow-up time in this group was 6.1 years, with the observation subgroup averaging 5.6 years and the treatment subgroup, 6.8 years.
Significant differences were observed when comparing the demographic and clinical characteristics of the FL and MZL groups (Table 1).
FL
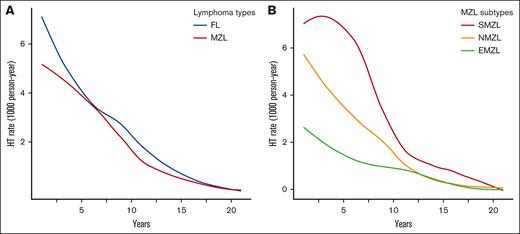
Among the 23 384 patients initially diagnosed with FL, 985 (4.2%) underwent HGT during the follow-up period, with a median time to HGT of 4.0 years. Considering 7699 deaths as competing risk events, the cumulative incidence rates of HGT at 5 and 10 years were 2.80% (95% confidence interval [CI], 2.58-3.03) and 4.87% (95% CI, 4.55-5.20), respectively. The estimated overall incidence rate of HGT was 5.7 events per 1000 patients per year. AIRs of HGT for FL demonstrated a consistent decline over time, with the highest rate in the first 2 years (Figure 1A).
AIR of HGT per 1000 person-years over a 20-year span, depicted using smooth curves estimated by locally estimated scatterplot smoothing regression. (A) By lymphoma types: FL vs MZL; and (B) by MZL subtypes, SMZL, NMZL, and EMZL.
AIR of HGT per 1000 person-years over a 20-year span, depicted using smooth curves estimated by locally estimated scatterplot smoothing regression. (A) By lymphoma types: FL vs MZL; and (B) by MZL subtypes, SMZL, NMZL, and EMZL.
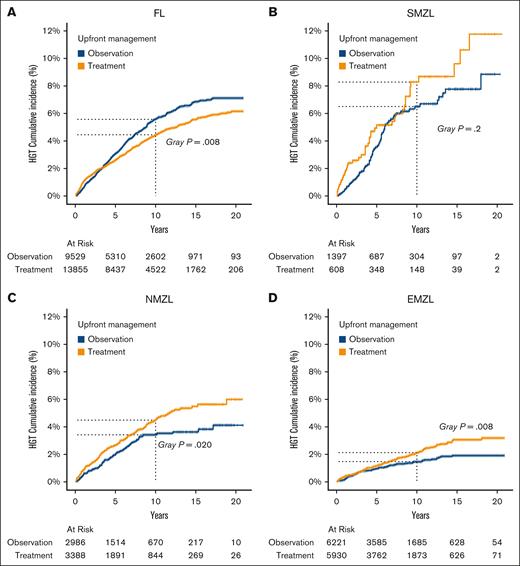
Analysis evaluating the risk of HGT in FL using a multivariable FG regression model, revealed several risk factors. Male patients were more likely to experience HGT than female patients (sub-distribution hazard ratio [SHR], 1.46; P < .01). Patients diagnosed with an advanced stage had a higher risk of HGT than patients presenting with an early stage (SHR, 1.60; P < .001). Notably, patients managed with upfront observation had an increased risk of HGT compared with patients who received treatment, in both univariable (SHR, 1.18; P = .008) and multivariable analyses (SHR, 1.23; P < .001; Table 2; Figure 2A).
Multivariable FG regression models assessing predictors of risk of HGT in different indolent lymphomas subtypes
| . | FL (n = 23 384) . | SMZL (n = 2005) . | NMZL (n = 6374) . | EMZL (n = 12 151) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | |
| Age (y) | ||||||||||||
| ≥60 vs <60 | 1.15 | 1.02-1.30 | .02 | 0.86 | 0.59-1.27 | .50 | 1.10 | 0.82-1.47 | .50 | 1.95 | 1.43-2.67 | <.001 |
| Gender | ||||||||||||
| Male vs female | 1.46 | 1.29-1.66 | <.001 | 1.13 | 0.78-1.65 | .50 | 0.97 | 0.73-1.28 | .80 | 1.11 | 0.84-1.47 | .50 |
| Ann Arbor stage | ||||||||||||
| III-IV vs I-II | 1.60 | 1.40-1.82 | <.001 | 0.84 | 0.55-1.29 | .40 | 1.25 | 0.91-1.70 | .20 | 1.23 | 0.88-1.71 | .20 |
| Management | ||||||||||||
| Observation vs treatment | 1.23 | 1.09-1.40 | .001 | 0.74 | 0.49-1.09 | .13 | 0.71 | 0.53-0.94 | .01 | 0.64 | 0.48-0.86 | .003 |
| . | FL (n = 23 384) . | SMZL (n = 2005) . | NMZL (n = 6374) . | EMZL (n = 12 151) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | |
| Age (y) | ||||||||||||
| ≥60 vs <60 | 1.15 | 1.02-1.30 | .02 | 0.86 | 0.59-1.27 | .50 | 1.10 | 0.82-1.47 | .50 | 1.95 | 1.43-2.67 | <.001 |
| Gender | ||||||||||||
| Male vs female | 1.46 | 1.29-1.66 | <.001 | 1.13 | 0.78-1.65 | .50 | 0.97 | 0.73-1.28 | .80 | 1.11 | 0.84-1.47 | .50 |
| Ann Arbor stage | ||||||||||||
| III-IV vs I-II | 1.60 | 1.40-1.82 | <.001 | 0.84 | 0.55-1.29 | .40 | 1.25 | 0.91-1.70 | .20 | 1.23 | 0.88-1.71 | .20 |
| Management | ||||||||||||
| Observation vs treatment | 1.23 | 1.09-1.40 | .001 | 0.74 | 0.49-1.09 | .13 | 0.71 | 0.53-0.94 | .01 | 0.64 | 0.48-0.86 | .003 |
FL grade 1 to 2.
HGT cumulative incidence by treatment status across different lymphoma subtypes diagnosed between 2000 to 2020. (A) FL, (B) SMZL, (C) NMZL, and (D) EMZL.
HGT cumulative incidence by treatment status across different lymphoma subtypes diagnosed between 2000 to 2020. (A) FL, (B) SMZL, (C) NMZL, and (D) EMZL.
Next, we evaluated risk factors associated with OS and LSS in patients with FL. In an univariable analysis, there was no significant difference in OS between patients with FL receiving upfront observation and those receiving treatment (hazard ratio [HR], 1.02; P = .5), whereas time-dependent HGT was associated with an elevated risk of death (HR, 4.15; P < .001). In the multivariable analysis of OS, age of ≥60 years, male sex, advanced stage at diagnosis, time-dependent HGT (HR, 4.22; P < .001), and upfront observation, as opposed to treatment (HR, 0.95; P = .03) were significantly associated with an increased risk of death (Table 3).
Multivariable Cox regression models assessing predictors of OS in different indolent lymphoma subtypes
| . | FL (n = 23 384) . | SMZL (n = 2005) . | NMZL (n = 6374) . | EMZL (n = 12 151) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age (y) | ||||||||||||
| ≥60 vs <60 | 3.67 | 3.47-3.87 | <.001 | 3.09 | 2.56-3.73 | <.001 | 3.85 | 3.45-4.29 | <.001 | 5.58 | 5.07-6.13 | <.001 |
| Gender | ||||||||||||
| Male vs female | 1.25 | 1.19-1.31 | <.001 | 1.14 | 0.99-1.32 | .07 | 1.20 | 1.10- 1.30 | <.001 | 1.23 | 1.15-1.31 | <.001 |
| Ann Arbor stage | ||||||||||||
| III-IV vs I-II | 1.25 | 1.19-1.31 | <.001 | 1.17 | 0.99-1.38 | .07 | 1.34 | 1.22-1.46 | <.001 | 1.49 | 1.38-1.60 | <.001 |
| HGT† | ||||||||||||
| Yes vs no | 4.22 | 3.83-4.65 | <.001 | 4.01 | 3.07-5.24 | <.001 | 3.74 | 3.08-4.54 | <.001 | 4.39 | 3.61-5.34 | <.001 |
| Management | ||||||||||||
| Observation vs treatment | 0.95 | 0.90-0.99 | .03 | 0.84 | 0.72-0.98 | .03 | 0.94 | 0.87-1.02 | .15 | 1.09 | 1.02-1.16 | .01 |
| . | FL (n = 23 384) . | SMZL (n = 2005) . | NMZL (n = 6374) . | EMZL (n = 12 151) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age (y) | ||||||||||||
| ≥60 vs <60 | 3.67 | 3.47-3.87 | <.001 | 3.09 | 2.56-3.73 | <.001 | 3.85 | 3.45-4.29 | <.001 | 5.58 | 5.07-6.13 | <.001 |
| Gender | ||||||||||||
| Male vs female | 1.25 | 1.19-1.31 | <.001 | 1.14 | 0.99-1.32 | .07 | 1.20 | 1.10- 1.30 | <.001 | 1.23 | 1.15-1.31 | <.001 |
| Ann Arbor stage | ||||||||||||
| III-IV vs I-II | 1.25 | 1.19-1.31 | <.001 | 1.17 | 0.99-1.38 | .07 | 1.34 | 1.22-1.46 | <.001 | 1.49 | 1.38-1.60 | <.001 |
| HGT† | ||||||||||||
| Yes vs no | 4.22 | 3.83-4.65 | <.001 | 4.01 | 3.07-5.24 | <.001 | 3.74 | 3.08-4.54 | <.001 | 4.39 | 3.61-5.34 | <.001 |
| Management | ||||||||||||
| Observation vs treatment | 0.95 | 0.90-0.99 | .03 | 0.84 | 0.72-0.98 | .03 | 0.94 | 0.87-1.02 | .15 | 1.09 | 1.02-1.16 | .01 |
FL grade 1 to 2.
Time-dependent variable.
In assessing factors that affect LSS, we found that age of ≥60 years, male gender, advanced stage at diagnosis, upfront treatment, and time-dependent HGT were all associated with an increased risk of lymphoma-specific deaths. In particular, time-dependent HGT was associated with a 4.6-times higher risk of lymphoma deaths (SHR, 4.61; 95% CI, 4.07-5.23; P < .001; supplemental Table 1).
MZL
Of 20 530 patients diagnosed with MZL, 506 (2.5%) underwent HGT over a 20-year follow-up period. The median time to HGT was 3.75 years. Cumulative incidence rates for HGT were 1.74% (95% CI, 1.55-1.94) and 2.95% (95% CI, 2.68-3.24) at 5 and 10 years, respectively. The computed incidence rate for HGT was 3.5 events per 1000 patient-years. The trend in AIR was notable for a surge in the initial 2 years, followed by a consistent decrease over the monitoring period (Figure 1A).
SMZL
A total of 109 (5.4%) patients with SMZL experienced HGT. Considering deaths before HGT as competing events (656 cases), SMZL exhibited a notably higher cumulative incidence of HGT than FL and other MZL subtypes. The 5-year cumulative incidence was 4.03% (95% CI, 3.15-5.08), and by the 10-year mark, this incidence rose to 7.05% (95% CI, 5.75-8.51). The overall incidence rate of HGT in SMZL subtype was 8.6 events per 1000 patients per year. With respect to the pattern over time, initially, SMZL had a high AIR of HGT, but this steadily declined, reaching lower AIRs beyond 12 years of follow-up. Although it began with a much higher HGT rate compared with other MZL subtypes, by the end of the 2-decade timeframe, its AIR was similar to AIRs in other MZL subtypes (Figure 1B). We used a univariable and multivariable FG regression model to evaluate factors associated with HGT, accounting for deaths before transformation as competing events. Surprisingly, none of the clinical variables, including age, sex, stage, and upfront treatment showed a significant association with HGT (Table 2; Figure 2B). Next, we conducted survival analyses to identify clinical characteristics associated with OS and LSS. In the univariable analysis, time-dependent HGT was significantly associated with shorter OS whereas in the multivariable analysis, factors associated with shorter OS were age of ≥60 years and time-dependent HGT (HR, 4.01; P < .001), whereas upfront observation was associated with longer OS for SMZL (HR, 0.84; P = .03; Table 3). Clinical characteristics associated with shorter LSS in the multivariable FG model include age of ≥60 years, advanced stage, and time-dependent HGT (SHR, 4.50; P < .001), whereas upfront observation was associated to longer LSS (SHR, 0.66; P < .001; supplemental Table 1).
NMZL
NMZL constituted nearly one-third of all MZL cases (31%). Of these, 201 (3.15%) underwent HGT. Considering the 2085 cases of deaths as a competing risk factor for HGT, the 5-year and 10-year cumulative incidences of HGT were 2.35% (95% CI, 1.97-2.78) and 4.01% (95% CI, 3.45-4.62), respectively, with an overall incidence rate of 4.8 events per 1000 patient-years. Examining the AIR, we observed a pronounced reduction during the initial decade, decreasing from 5.2 in the first year to <0.1 by the tenth year (Figure 1B).
Upon evaluating the potential factors associated with HGT in NMZL and factoring in deaths as competing events, we found that none of the clinical variables (age, sex, stage at diagnosis, or upfront management strategies) significantly affected HGT; however, upfront treatment significantly increased HGT risk in the unadjusted and adjusted analysis (Table 2). Next, we examined the clinical characteristics affecting OS and LSS. Notably, older age, male gender, advanced stage at diagnosis, and time-dependent HGT (SHR, 3.74; P < .001) were all associated with shorter OS, whereas upfront observation vs treatment was not associated with OS advantage (Table 3). In contrast, older age, advanced stage at diagnosis, and time-dependent HGT (SHR, 4.05; P < .001) were all associated with shorter LSS, whereas upfront observation vs treatment was associated with longer LSS (SHR, 0.79; P < .001; supplemental Table 1).
EMZL
EMZL was the most common subtype of MZL in the SEER database, accounting for 59.2% of cases, with HGT observed in 1.61% of patients. When considering deaths before HGT as competing events, 5-year and 10-year cumulative incidences of HGT were 1.07% (95% CI, 0.88-1.28) and 1.80% (95% CI, 1.53-2.09), respectively, with an overall incidence HGT rate of 2.2 events per 1000 patients per year. The AIR for EMZL began at relatively modest levels, notably lower than those observed for other MZL subtypes. Over the course of the 20-year observation period, there was a consistent and steady decrease in the AIR of EMZL (Figure 1B). In analyzing clinical characteristics associated with HGT in univariate and multivariable analyses, age of ≥60 years and upfront observation (multivariable SHR, 0.64; P = .003) reached statistical significance (Figure 2D). In an univariable analysis, age of ≥60 years, male gender, advanced stage, HGT, and upfront treatment were all associated with shorter OS. In subsequent multivariable evaluations, age of ≥60 years, male gender, advanced stage at diagnosis, and time-dependent HGT (HR, 4.39; P < .001), and upfront observation (HR, 1.09; P = .01) were associated with shorter OS (Table 3). When analyzing association with shorter LSS, age of ≥60 years, male gender, advanced stage at diagnosis, time-dependent HGT (SHR, 6.43; P < .001), and upfront observation (SHR, 0.71; P < .001) all reached statistical significance (supplemental Table 1).
Analysis of tDLBCL
We identified 8736 and 5059 de novo DLBCL cases, matched by age, sex, year of diagnosis, and stage with tDLBCL originating from FL (transformed FL [t-FL]) and MZL (transformed MZL [t-MZL]) cohorts, respectively. For the t-FL and matched de novo DLBCL cohort, the median DLBCL OS was 5.3 years (95% CI, 3.8-6.4) for the t-FL group and 10.5 years (95% CI, 10.0-11.1) for the de novo DLBCL group. The t-FL exhibited inferior DLBCL OS compared with "De novo" DLBCL (HR, 1.60; P < .001) (supplemental Figure 2A; supplemental Table 2). For the t-MZL and matched de novo DLBCL cohort, the median DLBCL OS for t-MZL was 3.33 years (95% CI, 2.5-4.5), whereas the median DLBCL OS for de novo DLBCL was 8.58 years (95% CI, 8.0-9.1). In this cohort, the t-MZL demonstrated shorter DLBCL OS than de novo DLBCL (HR, 1.65; P < .001; supplemental Figure 2B; supplemental Table 2).
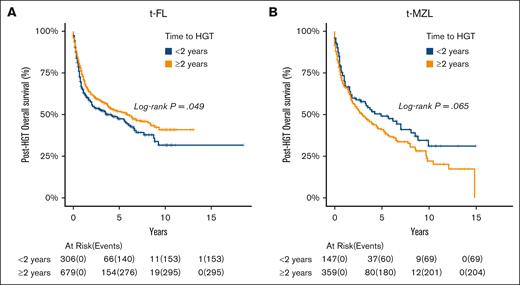
Subsequently, we assessed whether time to HGT affected posttransformation OS within the tDLBCL cohorts. When considering t-FL, time to HGT of <2 years was associated with shorter posttransformation OS compared with time to HGT of ≥2 years (HR, 0.82; P = .049; Figure 3A). However, this association was not observed in t-MZL (HR, 1.29; P = .065; Figure 3B).
OS based on time to HGT within the tDLBCL cohort, categorized by preceding indolent lymphoma subtype. (A) FL and (B) MZL.
OS based on time to HGT within the tDLBCL cohort, categorized by preceding indolent lymphoma subtype. (A) FL and (B) MZL.
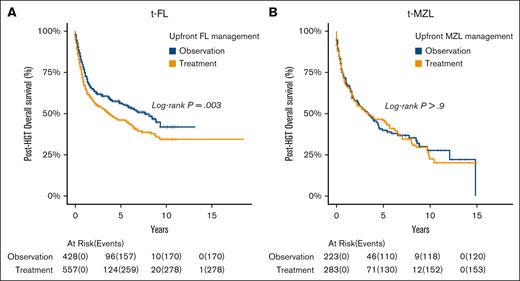
Using Cox regression models, we observed that patients with t-FL treated before transformation had shorter posttransformation OS compared with treatment-naïve t-FL (HR, 1.33; P = .003; Figure 4A). Conversely, for t-MZL, the upfront treatment before HGT did not affect posttransformation OS (HR, 1.01; P = .9; Figure 4B).
Posttransformation OS based on initial indolent lymphoma management strategies. (A) FL and (B) MZL.
Posttransformation OS based on initial indolent lymphoma management strategies. (A) FL and (B) MZL.
In an effort to analyze survival outcomes in DLBCL cohorts that were either treatment naïve or minimally treated, we compared a de novo DLBCL cohort with a t-FL cohort managed with upfront observation. Our analysis included 3928 patients with de novo DLBCL matched by age, sex, year of diagnosis, and stage to 428 patients in the t-FL observation cohort. Using a Cox regression model, we found that the OS in the t-FL cohort managed with observation was significantly better than that of the de novo DLBCL cohort (HR, 0.67; P < .001; supplemental Figure 3A). Similarly, when comparing a de novo DLBCL cohort with a t-MZL cohort managed with upfront observation, we included 2165 patients with de novo DLBCL matched by age, sex, year of diagnosis, and stage with 223 patients in the t-MZL observation cohort. In this analysis, we observed no significant difference in OS between the 2 cohorts (HR, 0.84; P = .06; supplemental Figure 3B).
Sensitivity analyses
Considering the absence of distinct separation between FL grade 3A and 3B in the SEER database, we analyzed the FL 3 cohort. The 5- and 10-year HGT cumulative incidences were 2.03% (95% CI, 1.67-2.45) and 3.75% (95% CI, 3.22-4.34), respectively, for FL grade 3, not exceeding the results estimated for FL 1-2. FL grade cohort revealed that an advanced stage was associated with an increased risk of HGT, whereas treatment was linked to a decreased risk, paralleling the findings for FL grade 1 to 2. Additionally, similar risk factors associated with OS and LSS were identified, particularly noting that HGT was correlated with inferior OS and LSS in the FL grade 3 cohort.
Discussion
This study presents, to our knowledge, the largest cohort of patients with FL and MZL, spanning a total 20-year follow-up, with a distinctive focus on HGT. This real-world data set, gathered from diverse centers across the United States, is discrepant from previous large series primarily originating from tertiary-academic cancer centers.5,7-9 The SEER database's limitation in differentiating between FL grades 3A and 3B dictated our exclusive focus on FL grades 1 and 2. Our projected cumulative incidences for HGT in FL at 5 and 10 years were 2.80% and 4.87%, respectively, considerably lower than 5.8% to 22% at 5 years and 7.7% to 31% at 10 years reported in earlier studies.4-7,9 Several factors may explain these discrepancies. The distinctiveness of our research lies in the methodology we adopted for calculating cumulative incidence. By factoring in deaths before transformation as competing risk events, we contend that our approach provides a more authentic representation of the data,22 diverging from earlier studies that did not incorporate competing events in their cumulative incidence estimations.4,6,8,9 Our data set recorded 7699 (32.9%) deaths treated as competing events. However, even without considering these competing risks, our calculated cumulative incidence rates at 5 and 10 years would stand at 3.01% and 5.70%, respectively, and both rates are still lower than those cited in prior studies.4-9 Factors potentially affecting our reported rates may be the temporal span of our data set, covering new FL diagnosed from 2000 to 2020, compared with former studies that encompassed the period 1972 to 2009. It is pivotal to emphasize that many of these prior studies recorded higher HGT rates, especially those observed in earlier data sets, as compared with later studies.4-6 Another distinguishing feature of our approach is its strict inclusion criterion. We only incorporated patients with a pathology-confirmed diagnosis, in contrast to some studies that permitted clinical diagnoses for HGT identification.4,6-8 Interestingly, studies that included clinically suspected but not biopsy-proven HGT diagnoses reported similar OS outcomes to those of nontransformed cases.23 Furthermore, we excluded patients with grade 3A disease, that may be associated with higher risk of HGT; however, in our sensitivity analysis conducted in patients with FL grade 3, the 5- and 10-year cumulative incidences are similar to those of the FL 1 to 2 cohort.
Furthermore, the expansive scope of our study, retrieving data from a myriad of centers across the United States, offers a viewpoint arguably more reflective of the general patient demographic than studies that originated from academic and tertiary centers. However, it is also possible that in contrast to academic centers, in nonacademic settings, many patients with suspected HGT receive treatment without HGT confirmation by biopsy and are thus not captured in the SEER database. This may contribute to lower HGT incidence, which is also observed in other data sets reflective of real-world experience outside of academic centers. A study by Federico et al, using data from the Aristotle study spanning 1997 to 2013, indicated a 5-year HGT cumulative incidence rate of 5.8% and a 10-year rate of 7.7%. These estimations align most closely with our findings. However, it is worth noting that their methodology did not incorporate competing risks in their cumulative incidence estimation, and their patient sample included FL grades 1 through 3A.9
We observed that the AIR per 1000 persons was at its peak in the initial 2 years and gradually declined over the subsequent 20 years of follow-up. Remarkably, although no prior studies in FL have reported AIR trends, a study by Montoto et al observed no HGT cases after 16 years.5
In our study, using competing risk analysis, we identified that male patients diagnosed at an advanced stage and those managed with upfront observation exhibited an elevated risk of HGT. Retrospective studies have previously highlighted an association between advanced stage and the development of HGT.5,15 Furthermore, the effect of chemoimmunotherapy on the risk of HGT was examined in previous investigations. Although rituximab-based immunotherapy has been shown to mitigate the risk of transformation,7-9 chemotherapy, especially alkylating-based regimens, has not significantly affected HGT risk.12 In our analysis, both chemoimmunotherapy and radiotherapy were categorized as treatment modalities. However, SEER was unable to distinguish between rituximab-based vs nonrituximab–based chemoimmunotherapy in comparison to the Aristotle study.9 Among patients receiving treatment, we documented a 15% reduction in the risk of HGT without adjustment for age, gender, and stage at diagnosis. When adjusted for these clinical variables, the decline was 19%. Our study observed that 40.2% of patients with FL were managed with upfront observation, a percentage that is higher than those reported in previous studies6,8,9,23 but more closely aligned with the findings of Link et al who reported 33%.7
We identified several characteristics that affect OS and LSS. Consistent with prior studies, older age, male gender, and advanced stage at diagnosis were all determinants of poorer outcomes in both OS and LSS.5-7 Although we detected a 6% increase in deaths in patients managed with upfront treatment, this is likely a selection bias rather than a true effect of treatment. Therefore, congruent with existing literature, our findings reinforce the assertion that the decision to administer upfront treatment to patients with FL, or alternatively to adopt an observation approach, does not exert a significant influence on OS.12 Moreover, aligning with prior studies, our data spotlight time-dependent HGT as a significant prognostic variable, manifesting a 322% augmentation in the hazard associated with shorter survival. Pertaining to LSS, both upfront treatment and HGT independently correlated with poorer survival outcomes. The intriguing association of upfront treatment with worse OS and LSS warrants cautious interpretation, particularly considering the potential of inherent indication bias commonly observed in retrospective studies. In such cases, patients with high-risk features might be more likely to undergo treatment, potentially obscuring the actual impact of treatment on survival outcomes.
We researched the clinical outcomes associated with t-FL. Our findings indicate that t-FL is associated with a poorer OS compared with de novo DLBCL, presenting a 60% higher risk of death. However, this poorer outcome is primarily attributed to t-FL managed with initial treatment, because t-FL managed with initial observation exhibited better OS compared with de novo DLBCL, highlighting a novel finding in our study. The median DLBCL OS observed for t-FL was 5.3 years, which is in line with the 5-year duration cited in the LymphoCare Study.8 Further analysis of the t-FL cohort demonstrated that early HGT, defined as time to HGT of <2 years, had shorter posttransformation OS than late HGT (HGT time of ≥2 years), a finding that aligns with a previous study that identified a HGT time of >18 months as being associated with improved posttransformation OS.7 Notably, we observed a higher mortality rate in patients with t-FL whose upfront FL management was treatment based rather than observation based. This finding is consistent with another study, which found better posttransformation survival outcomes in patients with FL who had not undergone treatment before transformation.24 This suggests that patients with t-FL managed with upfront observation may have a less aggressive disease or that the absence of prior treatment avoids selecting for resistant clones.25
Switching our focus to MZL, it is worth noting that, to our knowledge, this study represents the most extensive collection of patients with MZL to date compared with other large population-based studies.26,27 The majority of patients in our cohort were diagnosed with EMZL (59%), followed by NMZL (31.5%) and SMZL (9.5%). The proportion of patients who underwent HGT (2.5%) varied across subtypes. Notably, the incidence was significantly higher in SMZL (5.87%) compared with other subtypes and was also higher than the incidence observed in FL, consistent with prior studies.11,26 This real-world MZL data set identified a 2.6% HGT over 20 years, and a 5- and 10-year cumulative incidence of 1.74% and 2.95%, respectively. These estimates are lower than those from previous single–academic center studies2,11,28,29 but aligned more closely with the results of other European population–based studies. Specifically, a study using the Dutch cancer registry estimated a HGT of 4% at 7 years27; in contrast, a study using the Finnish cancer registry reported a HGT of 3.3% and 5- and 10-year cumulative incidences of 2.5% and 4.7%, respectively.7,9 Interestingly, the 5- and 10-year HGT cumulative incidence and the first 5 years AIR were higher for SMZL compared with the other MZL subtypes; this observation suggests that monitoring and counseling for HGT, especially during the first 5 years after diagnosis is important in patients with SMZL. In an approach similar to our FL analysis, we observed an incidence rate of 3.3 HGT events per 1000 person-years, which is notably less than the 11 HGT events per 1000 person-years reported by the Dutch study. It is essential to note that our study has a longer, 20-year follow-up, whereas the Dutch study spans a 5-year follow-up.27 This extended duration could influence the overall number at risk. Moreover, our analysis highlighted a peak in AIR during the initial 2 years, followed by a consistent decline, reaching its lowest levels by the end of our 20-year observation period. When examining MZL subtypes, we found that SMZL exhibited a higher AIR, whereas EMZL had a lower rate in the early years. In alignment with our FL results, older age and an advanced disease stage correlated with shorter OS and LSS. Importantly, across all MZL subtypes, both time-dependent HGT and upfront treatment were associated with shorter LSS.
In our examination of the t-MZL cohort, we observed a higher mortality rate than a matched de novo DLBCL group, a finding, to our knowledge, not previously reported. However, prior studies have indicated a poorer OS in MZL cases that underwent HGT.11,26,27 Unlike the results observed in FL, there was no discernible link between the upfront MZL management or HGT time and posttransformation OS.
Studies based on the SEER database have inherent limitations, including the absence of detailed data on specific chemoimmunotherapy regimens or the time window between diagnosis and therapy. Although SEER relies on pathological reports for lymphoma diagnoses, not all these reports are reviewed by expert hematopathologists in lymphoma. Additionally, we could not evaluate progression-free survival because the SEER database does not collect information on lymphoma progression or recurrence. Furthermore, we could not include patients with FL grade 3A, which are usually included in HGT studies7,9; however, in our sensitivity analysis conducted in patients with FL grade 3, the 5- and 10-year cumulative incidence was lower than in FL 1-2. Moreover, the SEER does not include information on prognostic scores such as the International Prognostic Index, which could have enabled the evaluation of its possible association with HGT. Despite these constraints, our study provided valuable insights and conclusions from a sizable cohort of patients experiencing HGT. Such insights might be challenging to achieve in retrospective studies from single or multiple institutions.
In conclusion, our study suggests a decreased cumulative incidence of HGT in FL compared with earlier, smaller, single-center studies. However, this discrepancy narrows when considering more recent single-center research. Male patients diagnosed at an advanced stage showed a higher risk of HGT. Conversely, upfront therapy with either chemoimmunotherapy or radiotherapy in FL decreased the risk of transformation compared with observation but did not affect OS. Consequently, although upfront therapy prolonged the time to transformation, it also resulted in shorter posttransformation survival, leading to similar OS outcomes compared with observation. Among the MZL subtypes, SMZL exhibited the highest cumulative HGT incidence, whereas EMZL showed the lowest, aligning with previous studies.
Acknowledgments
I.S.L. and J.P.A. are supported by the Sylvester Comprehensive Cancer Center National Cancer Institute Core grant (P30CA240139). J.P.A. is additionally supported by the Peykoff Initiative from the Lymphoma Research Foundation, the Dwoskin Family Foundation, and the US Department of Defense (grant CA220385). I.S.L. was supported by National Cancer Institute grants R01CA233945 and U01 CA195568, the Dwoskin and Anthony Rizzo Families Foundations, and the Jaime Erin Follicular Lymphoma Research Consortium.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the US Department of Defense.
Authorship
Contribution: J.A.F., I.S.L., and J.P.A. designed and performed the research, analyzed the data, were involved in data acquisition, and wrote the manuscript with input and approval of the final version from all coauthors; D.C. and I.M.R. designed and performed the research, analyzed the data, were involved in data acquisition, and critically reviewed and approved the manuscript; and all authors approved the manuscript for publication.
Conflict-of-interest disclosure: J.P.A. reports research support from ADC Therapeutics, Genmab, AbbVie, and BeiGene, and consultancy with ADC Therapeutics, Genentech, AbbVie, and Regeneron. I.S.L. received compensation for teaching from Kyowa Kirin Pharmaceutical Development Inc; reports a consulting role with Adaptive Biotechnology; and is an IND holder with ADC Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Juan P. Alderuccio, Division of Hematology, Department of Medicine, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, 1475 NW 12th Ave, Miami, FL 33136; email: jalderuccio@med.miami.edu.
References
Author notes
I.S.L. and J.P.A. contributed equally to this study.
Presented partially as an oral presentation at the 65th annual meeting of American Society of Hematology, San Diego, CA, 9 December 2023.
The data that support the findings of this study are available in the Surveillance, Epidemiology, and End Results database.
The full-text version of this article contains a data supplement.