Key Points
MCL and infiltrating T cells show enhanced MAPK in the presence of CD163+ macrophages.
CD163+ macrophages promote immune suppression in regions proximal to the tumor.
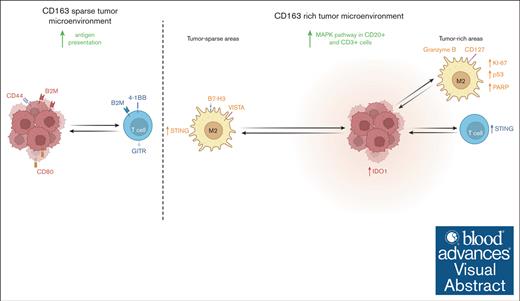
Visual Abstract
Mantle cell lymphoma (MCL) is dependent on a supportive tumor immune microenvironment (TIME) in which infiltration of CD163+ macrophages has a negative prognostic impact. This study explores how abundance and spatial localization of CD163+ cells are associated with the biology of MCL, using spatial multiomic investigations of tumor and infiltrating CD163+ and CD3+ cells. A total of 63 proteins were measured using GeoMx digital spatial profiling in tissue microarrays from 100 diagnostic MCL tissues. Regions of interest were selected in tumor-rich and tumor-sparse tissue regions. Molecular profiling of CD163+ macrophages, CD20+ MCL cells, and CD3+ T-cells was performed. To validate protein profiles, 1811 messenger RNAs were measured in CD20+ cells and 2 subsets of T cells. Image analysis was used to extract the phenotype and position of each targeted cell, thereby allowing the exploration of cell frequencies and cellular neighborhoods. Proteomic investigations revealed that CD163+ cells modulate their immune profile depending on their localization and that the immune inhibitory molecules, V-domain immunoglobulin suppressor of T-cell activation and B7 homolog 3, have higher expression in tumor-sparse than in tumor-rich tissue regions and that targeting should be explored. We showed that MCL tissues with more abundant infiltration of CD163+ cells have a higher proteomic and transcriptional expression of key components of the MAPK pathway. Thus, the MAPK pathway may be a feasible therapeutic target in patients with MCL with CD163+ cell infiltration. We further showed the independent and combined prognostic values of CD11c and CD163 beyond established risk factors.
Introduction
Mantle cell lymphoma (MCL) is an aggressive subtype of B-cell lymphoma.1,2 Intrinsic MCL properties have been studied extensively, for example, the constitutive activation of MAPK signaling.3,4 However, the relation between intrinsic and extrinsic MCL features, such as composition of the tumor immune microenvironment (TIME), remains unexplored.
Treatment with CD20-targeting antibodies and Bruton tyrosine kinase (BTK) inhibitors have led to major improvements in MCL outcome.5 However, late relapses are still common, emphasizing the need for stratified treatment strategies.6,7 The clinical success of BTK inhibitors suggests that disrupting the cross talk between MCL and its surroundings is therapeutically effective.8-11 Variation in the TIME, such as differences in the frequencies and functionality of T-cell and macrophage subsets, impacts the outcome and thus supports immunotherapy as an important treatment strategy in MCL.9,11-13
CD163+ macrophages (often referred to as M2 macrophages or tumor-associated macrophages, in contrast with M1 macrophages that have a more functional immune response toward tumor cells) have an anti-inflammatory profile commonly associated with tissue remodeling and the promotion of angiogenesis14 and are prioritized targets in immunotherapy.15,16
The potential of targeting CD163+ macrophages has been demonstrated in preclinical MCL studies. Papin et al showed that MCL cells can recruit and polarize CD163+ macrophages through the secretion of cytokines, which leads to tumor proliferation. Treatment with BTK inhibitors reduce interleukin-10 (IL-10) and colony stimulating factor 1 (CSF1) secretion, which mediate the protumorigenic effect of CD163+ macrophages.11 An additive treatment effect was seen when the CSF1 receptor was blocked, emphasizing that cotargeting of complementary pathways can circumvent treatment resistance. The capacity of MCL cells to polarize macrophages toward an M2 subtype was validated by Le et al,9 who showed that malignant, but not normal B cells, induce IL-10 and CD163 expression on macrophages.9 We have further recently shown that increased infiltration of CD163+ macrophages, measured both in tissue12 and as a soluble protein in serum,17 has a negative prognostic impact on the outcome of MCL.
Spatial localization of macrophages in relation to tumor cells and the composition of the microenvironment can affect the polarity of cells and induce distinct molecular activation states.18 Investigations of the cellular composition and organization of the MCL TIME can further advance our understanding of cell-to-cell interactions and the role of CD163+ macrophages. This has been made possible by the recent developments in high-plex spatial omics technologies and image analysis methods for cell segmentation and classification, thereby allowing the exploration of cellular neighborhoods that enable the understanding of the cellular composition of tissue.19,20
In this study, we aimed to understand how the molecular profile of CD163+ macrophages differs depending on their spatial localization in the TIME and how their presence impacts the molecular profile of MCL tumor cells and infiltrating T cells. Furthermore, we explored the cellular organization of the MCL TIME by analyzing cell frequencies, cell-to-cell distances, and spatial point metrics. To achieve this, state-of-the-art spatial omics and artificial intelligence–based image analysis were applied to a large population-based cohort of patients diagnosed with MCL.
Methods
Patient cohort
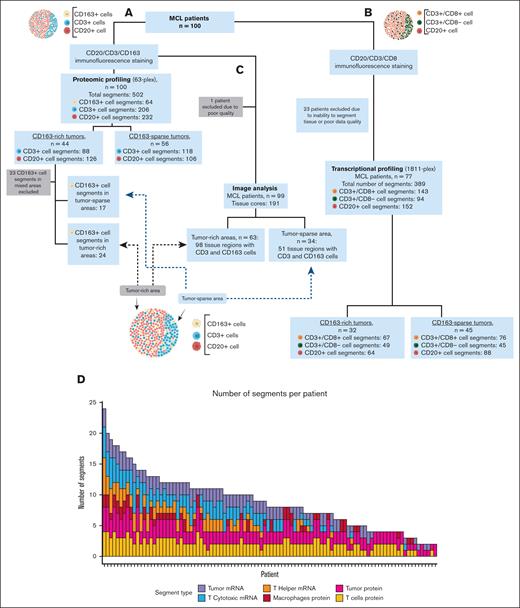
MCL tissue was obtained from the population-based cohort entitled “Biobank of Lymphomas in Southern Sweden.” A total of 100 patient samples, mostly derived from lymph nodes, were collected between 2000 and 2014 (supplemental Table 1). A schematic overview of the study is shown in Figure 1. The number of patients and corresponding tissue cores used in each part of the 3 workflows are shown in Figure 2A-C. All cases were reviewed by a hematopathologist, and relevant duplicate regions from formalin-fixed paraffin-embedded tissues were mounted in tissue microarrays, as previously described.21 Clinicopathologic information was available22,23 and is summarized in supplemental Table 1. The study protocol was approved by the Ethical Regional Committee in Lund (Dnr 2011/593). All subjects gave their informed consent for inclusion before they participated in the study. This study was conducted in accordance with the Declaration of Helsinki.
Schematic overview of the 3 workflows and the digital spatial technology. (A) Biopsies from patients diagnosed with MCL were embedded in formalin-fixed and paraffin-embedded blocks. Duplicate cores were sampled and transferred to a tissue microarray block. The full cohort was divided across 3 recipient blocks. Consecutive tissue sections were taken and used for the subsequent omics and image workflows. (B) For protein profiling, tissue was stained with fluorescent-labeled antibodies that targeted CD163, CD3, and CD20, together with barcoded antibodies that targeted 63 unique proteins. ROIs were selected in tumor-rich regions, and, when available, in tumor-sparse regions. Tumor-rich regions were defined as large carpets of mostly MCL cells, whereas tumor-sparse regions were dominated by T cells. Thresholding of Syto13, CD163, CD3, and CD20 allowed selection of MCL cells, CD163+ M2 macrophages, and T cells separately. Only a subset of the patients was CD163-rich (see “Methods” for definition), and the remaining patients were defined as CD163-sparse. (C) For mRNA profiling, tissue was stained with fluorescent-labeled antibodies targeting CD8, CD3, and CD20 together with barcoded-RNA probes targeting 1811 mRNAs. After technical filtering, 1482 mRNAs remained for biologic explorations. The threshold for Syto13, CD8, CD3, and CD20 allowed selection of MCL cells, cytotoxic T cells, and T-helper cells separately. (D) DSP was performed, and UV light was applied to each cell type segment in each ROI, thereby allowing aspiration of barcodes associated with the bound probes in each separate cell type and ROI. nCounter was used to count probes from the protein analysis, whereas Illumina sequencing was performed after library preparation of probes from the RNA profiling. (E) Image analysis using artificial intelligence–based software (Aiforia) was applied to the CD163/CD3/CD20 stained tissue sections. Tumor-rich and tumor-sparse regions were defined, and cells were segmented, classified according to their phenotype. The x/y coordinates were determined. Based on these data, cell frequencies and spatial metrics were determined. (F) Multiplex IF was used to validate the prognostic value of CD11c and CD163 combined.
Schematic overview of the 3 workflows and the digital spatial technology. (A) Biopsies from patients diagnosed with MCL were embedded in formalin-fixed and paraffin-embedded blocks. Duplicate cores were sampled and transferred to a tissue microarray block. The full cohort was divided across 3 recipient blocks. Consecutive tissue sections were taken and used for the subsequent omics and image workflows. (B) For protein profiling, tissue was stained with fluorescent-labeled antibodies that targeted CD163, CD3, and CD20, together with barcoded antibodies that targeted 63 unique proteins. ROIs were selected in tumor-rich regions, and, when available, in tumor-sparse regions. Tumor-rich regions were defined as large carpets of mostly MCL cells, whereas tumor-sparse regions were dominated by T cells. Thresholding of Syto13, CD163, CD3, and CD20 allowed selection of MCL cells, CD163+ M2 macrophages, and T cells separately. Only a subset of the patients was CD163-rich (see “Methods” for definition), and the remaining patients were defined as CD163-sparse. (C) For mRNA profiling, tissue was stained with fluorescent-labeled antibodies targeting CD8, CD3, and CD20 together with barcoded-RNA probes targeting 1811 mRNAs. After technical filtering, 1482 mRNAs remained for biologic explorations. The threshold for Syto13, CD8, CD3, and CD20 allowed selection of MCL cells, cytotoxic T cells, and T-helper cells separately. (D) DSP was performed, and UV light was applied to each cell type segment in each ROI, thereby allowing aspiration of barcodes associated with the bound probes in each separate cell type and ROI. nCounter was used to count probes from the protein analysis, whereas Illumina sequencing was performed after library preparation of probes from the RNA profiling. (E) Image analysis using artificial intelligence–based software (Aiforia) was applied to the CD163/CD3/CD20 stained tissue sections. Tumor-rich and tumor-sparse regions were defined, and cells were segmented, classified according to their phenotype. The x/y coordinates were determined. Based on these data, cell frequencies and spatial metrics were determined. (F) Multiplex IF was used to validate the prognostic value of CD11c and CD163 combined.
Study overview. Number of patients, number of cell specific segments for (A) protein or (B) mRNA analysis, and number of patients and tissue cores used for (C) image analysis and downstream extraction of spatial metrics. (D) The number of segments per patient is shown in the plot. The nuclear count per segment (area of illumination/area of interest) is shown for the protein workflow (E) and for the RNA workflow (F).
Study overview. Number of patients, number of cell specific segments for (A) protein or (B) mRNA analysis, and number of patients and tissue cores used for (C) image analysis and downstream extraction of spatial metrics. (D) The number of segments per patient is shown in the plot. The nuclear count per segment (area of illumination/area of interest) is shown for the protein workflow (E) and for the RNA workflow (F).
Selection of cell segments for proteomic and transcriptomic analyses using GeoMx DSP
Immunohistochemistry data on cyclin D1 and SOX11 were used to identify relevant tumor regions in each tissue core.
Patient and segment grouping
Samples from patients with MCL were categorized into CD163-rich and CD163-sparse based on the presence of at least 20 CD163+ cells within a ∼0.4 mm2 area of MCL tissue. To explore the plasticity of CD163+ cells in relation to spatial localization, the CD163+ segments were sampled either in tumor-rich regions, as infiltrates in large carpets of MCL cells, or in tumor-sparse regions located outside the immediate tumor niche in proximal surrounding lymphoid tissue. In some cases, regions of interest (ROIs) were selected in that both tumor-rich and tumor-sparse regions were collected (mixed regions). Such segments were excluded when tumor-rich and tumor-sparse segments were compared (supplemental Figure 1).
Proteomic profiling
ROIs were selected and segmented in the GeoMx digital spatial profiling (DSP) instrument into 3 separate areas of illumination, from now on referred to as segments. For protein analysis, 502 ROIs were selected from the 100 patients’ duplicate tissue cores and 3 types of segments were profiled, namely CD20+ (tumor cells), CD3+ (T cells), CD163+ (M2-like macrophages) (Figure 2A). GeoMx DSP was performed according to the manufacturer’s recommendations, as previously described (Figure 1).24 Target proteins (n = 63) and fluorochrome-labeled antibodies are listed in supplemental Tables 2 and 3, respectively. In brief, in each segment, oligos conjugated to bound reagents were cleaved off by ultraviolet light and collected into individual wells on a microtiter plate for separate profiling of each of the 3 types of collected segments. The collected oligos were quantified using nCounter (Nanostring) according to the manufacture’s protocol.
Transcriptomic profiling
For messenger RNA (mRNA) analyses, segments of CD20+ (tumor cells), CD3+CD8– (T-helper cells), and CD3+CD8+ (T-cytotoxic cells) were profiled from 77 patients (Figure 2B contains details on the number of segments). Expression of 1811 cancer-related genes in the GeoMx Cancer Transcriptome Atlas was evaluated. The presence of double-negative T cells (CD4– and CD8–) is rare (<3%), justifying the definition of T-helper cells by the absence of CD8 on CD3+ cells.25
A sequencing library of the collected oligos was prepared using Seqcode reagents (Nanostring) and was sequenced on a NextSeq550 instrument (Illumina, San Diego, CA) using the High Output Kit v2.5 (75 Cycles) (Illumina). Reads were passed through the GeoMx NGS pipeline (Nanostring) to generate counts according to the manufacturer’s protocol.
Preprocessing of GeoMx–based targeted proteomic and transcriptomic data
Proteomic data from each specific segment were scaled by respective segment area and separately normalized using cyclic loess to account for differences in cell sizes and segment areas. The same workflow was applied to mRNA data, but the 3 types of segments were normalized together because the area per cell was in the same range. For the mRNA analysis, transcripts were filtered and removed if >25% of the signal-to-noise ratio across patients was <1.05 in all 3 segment types, leaving 1482 transcripts for biologic explorations. The number of segments per patient and nuclei count per segment type in the proteomic and transcriptional analyses are shown in Figure 2D-F.
Image-based analysis of cell frequencies and neighborhoods of CD20+, CD3+, CD11c, and CD163+ cells
The multiplex immunofluorescence (IF) images from the GeoMx DSP instrument were uploaded to a software program for deep learning image analysis, namely Aiforia Create, version 5.3 (Aiforia Technologies Plc, Helsinki, Finland), as previously described.26 Briefly, the detection was split into 3 hierarchical layers representing (1) all detected tissue, (2) tumor-rich or tumor-sparse tissue regions, and (3) within each tissue region, measurement of the frequency and position of the different immune cells. Tumor regions were defined as tissue regions with >60% CD20+ cells when considering a field of view with ∼100 cells. An overview of the number of patients included in the image-based analysis of cell frequencies and cellular neighborhood can be found in Figure 2C. For validation, multiplex IF staining of CD20, CD11c, and CD163 (supplemental Table 3) was performed and analyzed using Cellpose27 and QuPath (v.0.5.0).28 A fine-tuned cell segmentation model was trained by applying the nuclei stain and the average signal from CD20, CD11, and CD163 using the Cellpose “cyto” model on 32 representative images. Classification of each marker was performed in QuPath with the Cellpose extension by training separate object classifiers for each channel using the random trees classifier.27 A manual check of each core was performed to exclude cores with a lack of tissue, insufficient segmentation, or high background signal, leading to a total of 183 cores and 93 patients.
Data and statistical analysis
All hierarchical clustering and heat map visualization were performed using the R package ComplexHeatmap.29 Differential expression analysis was performed using a linear mixed-effects model (LMM) to account for multiple observations within a given sample using the R package lmerTest.30 To account for multiple hypothesis testing, P values were adjusted based on false discovery rate using the Benjamini-Hochberg test. A false discovery rate of <0.1 was considered statistically significant. Pearson and Spearman correlations were used to assess CD3+ to CD163+ cell correlations. The Wilcoxon signed-rank test was applied to evaluate differences in the mean expression of proteins of interest. For KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment, the clusterProfiler package was used.31,32 A P value <.05 was considered statistically significant. To dichotomize patients based on abundance of CD163 mRNA in the CD20+ cell segment, maximally rank statistics (survminer:survcutpoint) against overall survival (OS) were used with a minimum group distribution set at 0.1.
Cellular neighborhood analyses, such as spatial point pattern and distance-based analysis, were performed to understand the cellular composition of tissue. Spatial point pattern analysis was performed using the L function (Besag transformation of Ripley K function33) in the R package spatstat.34 For distance-based analysis, Euclidean distance was calculated between any 2 spatial objects using their coordinates.
A Kaplan-Meier analysis with log-rank statistics to tests the differences between the curves was conducted using the R package survival (3.5-8). To estimate hazard ratios for the association between the markers (CD11 and CD163) and 5-year OS, univariable and multivariable Cox proportional hazards models were performed in R using the maxstat (0.7-25) package. Adjustments for age, sex, and cellular tumor antigen protein 53 (p53) expression were performed in the multivariable models.
Results
Exploring the plasticity of CD163+ cells in spatially distinct parts of the MCL TIME
To explore the plasticity of CD163+ cells, the differential expression of phenotypic and functional proteins in different spatial locations was evaluated in a subset of patients for whom both types of tissue regions were available. Patients who had tumor-sparse regions had no statistically significant difference in the mean OS when compared with patients who lacked such regions in the sampled tissue core (data not shown). Furthermore, the frequency of occurrence and cell neighborhood of the CD20+ tumor, CD163+, and CD3+ cells were analyzed.
Cell organization and immune regulation differ across spatially defined MCL regions
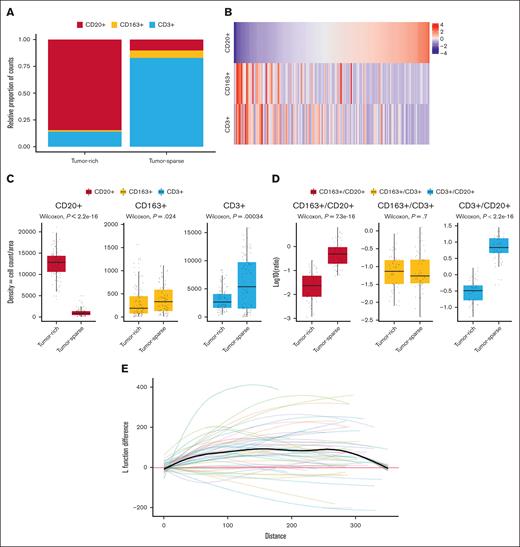
Nontumor cells, such as CD163+ cells, and T cells were, per definition, more frequent in tumor-sparse than in tumor-rich regions (Figure 3A,C). CD163+ and T-cell numbers were positively correlated across patients with a Pearson and Spearman correlation of 0.37 and 0.39, respectively (both P values <.001). The inverse relationship between the abundance of CD3+ and CD163+ cells and that of CD20+ cells is visualized in Figure 3B. Of note, the CD163+ to CD3+ cell ratio across the 2 spatially defined regions was constant (P = .7), indicating a positive correlation between the frequency of CD163+ cells and T cells irrespective of the spatial localization (Figure 3D).
Analysis of the MCL TIME composition using 4-plex IF–based image analysis shows that the distribution of cells varies between spatial regions, but CD3+ to CD163+ cell ratios remain constant. (A) CD20+ cells (tumor), CD163+ cells (macrophages), and CD3+ cells (T cells) in tumor-rich and tumor-sparse ROIs (99 patients, 191 tissue cores). (B) Heat map showing the distribution of cell density (cell counts per area) for the 3 cell types in the tumor-rich region. (C) Box plot comparison of the cell densities (cell counts per area) in tumor-rich and tumor-sparse regions for each of the 3 cell subtypes (63 patients, 98 tissue cores). (D) Box plot comparison of the cell-to-cell ratios in paired samples in which both tumor-rich and tumor-sparse regions were collected in the same patients (34 patients, 51 tissue cores), showing identical CD163+ macrophage to T-cell ratios between the 2 regions. The y-axis is log10 transformed for better visualization. Note that all analyses that compared the distributions and ratios were performed per tissue core but were validated per patient using the mean patient aggregate values (data not shown). (E) A spatial point pattern analysis33 plot that shows the difference in L function value for CD163+ macrophages in tumor-sparse and tumor-rich regions using paired samples (34 patients, 51 tissue cores). The colored lines show tissue-specific L function differences between the 2 spatial regions, and the bold black line shows the average L function difference across all tissue. A clustered pattern is suggested when the average L function value is above 0 with the range of 0 to 50 μm being most important.
Analysis of the MCL TIME composition using 4-plex IF–based image analysis shows that the distribution of cells varies between spatial regions, but CD3+ to CD163+ cell ratios remain constant. (A) CD20+ cells (tumor), CD163+ cells (macrophages), and CD3+ cells (T cells) in tumor-rich and tumor-sparse ROIs (99 patients, 191 tissue cores). (B) Heat map showing the distribution of cell density (cell counts per area) for the 3 cell types in the tumor-rich region. (C) Box plot comparison of the cell densities (cell counts per area) in tumor-rich and tumor-sparse regions for each of the 3 cell subtypes (63 patients, 98 tissue cores). (D) Box plot comparison of the cell-to-cell ratios in paired samples in which both tumor-rich and tumor-sparse regions were collected in the same patients (34 patients, 51 tissue cores), showing identical CD163+ macrophage to T-cell ratios between the 2 regions. The y-axis is log10 transformed for better visualization. Note that all analyses that compared the distributions and ratios were performed per tissue core but were validated per patient using the mean patient aggregate values (data not shown). (E) A spatial point pattern analysis33 plot that shows the difference in L function value for CD163+ macrophages in tumor-sparse and tumor-rich regions using paired samples (34 patients, 51 tissue cores). The colored lines show tissue-specific L function differences between the 2 spatial regions, and the bold black line shows the average L function difference across all tissue. A clustered pattern is suggested when the average L function value is above 0 with the range of 0 to 50 μm being most important.
The minimal cell-to-cell distance between CD163+ and CD3+ cells was highly variable (supplemental Figure 2A), but the median value was significantly shorter in the tumor-sparse than in tumor-rich regions (7.4 vs 8.5 μm) (supplemental Figure 2B), which is in line with the higher cellular abundance of CD163+ and CD3+ cells. The altered cellular neighborhood in the 2 spatial compartments and/or the closer proximity of T cells and macrophages in tumor-sparse regions was also reflected in the proteomic data in which higher levels of CD3, CD4, and CD163 were detected simultaneously in tumor-sparse regions than in tumor-rich regions (Figure 4; supplemental Table 4).
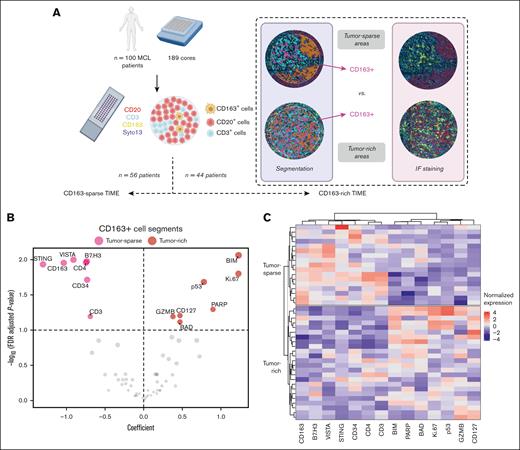
Spatial phenotypic profile of CD163+ cells in tumor-rich and tumor-sparse regions. (A) Tissue biopsies from 100 patients were evaluated with the GeoMx DSP technology and stained for CD20 (red), CD3 (light blue), CD163 (yellow), and Syto13 (dark blue) to identify MCL cells, T cells, and macrophages, respectively, and to measure the expression of 63 target proteins. Segmented and selected cells for which the protein quantification was measured are indicated as follows: CD20+ cells are marked in orange, CD3+ cells are marked in turquoise, and CD163+ cells are marked in pink. The biopsies from patients with MCL were then categorized into 2 groups, namely CD163-rich regions (n = 56) in which CD163+ cells could be sampled (at least 20 cells) and CD163-sparse region (n = 44) in which low or no CD163 infiltration was detected. The molecular comparisons of CD20+ cells and CD3+ cells using these groups are referred to as CD163-sparse vs CD163-rich TIMEs. Patients with CD163-rich MCL TIMEs were subsequently stratified based on the sampling of CD163+ cells in tumor-sparse regions (n = 17) or in tumor-rich regions (n = 24). The molecular comparison of CD163+ cells in these 2 tissue regions is referred to as tumor-sparse vs tumor-rich. (B) Significantly deregulated proteins among the 63 targeted proteins are visualized by plotting the LMM coefficient (x-axis) vs the false discovery rate (FDR) values (y-axis) in which proteins (marked in red) in the right part of the plot are higher in CD163+ segments collected in tumor-rich regions, and proteins (marked in pink) higher in CD163+ cells in tumor-sparse regions are shown to the left. To account for different numbers of segments collected per patient, a LMM with patient ID and tissue included as random effects were used to assess both the significance and relevance of each marker. Significantly differentially expressed proteins (FDR < 0.1) are indicated by name. (C) Heat map representation of the significant differential protein expression between CD163+ cells in tumor-rich and tumor-sparse ROIs. Data are normalized by column and protein intensities are displayed as colors ranging from red to blue as shown in the key. Rows are clustered based on spatial localization and columns are clustered using Ward error sum of squares method. Coefficients and FDR values are listed in supplemental Table 4.
Spatial phenotypic profile of CD163+ cells in tumor-rich and tumor-sparse regions. (A) Tissue biopsies from 100 patients were evaluated with the GeoMx DSP technology and stained for CD20 (red), CD3 (light blue), CD163 (yellow), and Syto13 (dark blue) to identify MCL cells, T cells, and macrophages, respectively, and to measure the expression of 63 target proteins. Segmented and selected cells for which the protein quantification was measured are indicated as follows: CD20+ cells are marked in orange, CD3+ cells are marked in turquoise, and CD163+ cells are marked in pink. The biopsies from patients with MCL were then categorized into 2 groups, namely CD163-rich regions (n = 56) in which CD163+ cells could be sampled (at least 20 cells) and CD163-sparse region (n = 44) in which low or no CD163 infiltration was detected. The molecular comparisons of CD20+ cells and CD3+ cells using these groups are referred to as CD163-sparse vs CD163-rich TIMEs. Patients with CD163-rich MCL TIMEs were subsequently stratified based on the sampling of CD163+ cells in tumor-sparse regions (n = 17) or in tumor-rich regions (n = 24). The molecular comparison of CD163+ cells in these 2 tissue regions is referred to as tumor-sparse vs tumor-rich. (B) Significantly deregulated proteins among the 63 targeted proteins are visualized by plotting the LMM coefficient (x-axis) vs the false discovery rate (FDR) values (y-axis) in which proteins (marked in red) in the right part of the plot are higher in CD163+ segments collected in tumor-rich regions, and proteins (marked in pink) higher in CD163+ cells in tumor-sparse regions are shown to the left. To account for different numbers of segments collected per patient, a LMM with patient ID and tissue included as random effects were used to assess both the significance and relevance of each marker. Significantly differentially expressed proteins (FDR < 0.1) are indicated by name. (C) Heat map representation of the significant differential protein expression between CD163+ cells in tumor-rich and tumor-sparse ROIs. Data are normalized by column and protein intensities are displayed as colors ranging from red to blue as shown in the key. Rows are clustered based on spatial localization and columns are clustered using Ward error sum of squares method. Coefficients and FDR values are listed in supplemental Table 4.
Visual inspection indicated that CD163+ cells were located close to vessels in the tumor-sparse regions (data not shown). This is supported by higher expression of CD34 in CD163+ cell segments in tumor-sparse regions (Figure 4; supplemental Table 4). In addition, the image analysis showed a more clustered pattern of CD163+ cells in tumor-sparse than in tumor-rich regions (Figure 3E), which is indicative of more structured immune cell interactions.
Proteomic analysis of the CD163+ cell segments in the tumor-sparse regions showed higher levels of the immune checkpoint regulators V-domain immunoglobulin suppressor of T-cell activation (VISTA) and B7 homolog 3 (B7-H3) and of immune-regulatory proteins, such as stimulator of interferon genes (STING) (Figure 4; supplemental Table 4). VISTA is a B7 family member associated with M2 reprogramming and induces production of the anti-inflammatory cytokine IL-10.35,36 B7-H3 is associated with the inhibition of antitumor response,37 whereas STING has been shown to drive cytokine production required for robust antitumor T-cell responses.38
CD163+ cells in tumor-rich regions upregulate proteins of cell survival
CD163+ cells in tumor-rich regions (CD163+ cell segments in close contact with tumor cells) showed upregulation of proteins involved in apoptosis, DNA-repair, and proliferation when compared with CD163+ cells outside the immediate tumor niche (CD163+ cells in tumor-sparse regions). In brief, higher levels of BIM (BCL2 like 11), BAD (BCL2 associated agonist of cell death), p53, GZMB (granzyme B), IL7R/CD127, and PARP (poly(ADP-ribose) polymerase 1) (Figure 4B; supplemental Table 4; supplemental Figure 3) were detected in CD163+ cells in tumor-rich regions. Of interest, it has been shown that GZMB39 and IL7R40 can be expressed by subsets of macrophages, in addition to being expressed by cytolytic lymphocytes. Hierarchical clustering was used to visualize the expression and relationship of each of the 14 genes that were differentially expressed between tumor-rich and tumor-sparse locations across patients (Figure 4C).
To investigate whether p53 expression was derived from CD163+ cell segments and not from closely adjacent MCL cells, patient samples in which p53 was overexpressed, based on previous immunohistochemistry staining of p53,23 were excluded. Higher p53 in CD163+ cell segments in tumor-rich regions was supported by analysis using only p53 low MCL cases (supplemental Figure 4), but further validation using multiplex staining is needed. The relatively higher level of Ki-67 (Antigen Kiel 67) suggests that closer contact with tumor cells leads to tumor-related proteins also being detected in the CD163+ cell segments.
Impact of the presence of CD163+ cells on tumor and immune cells in the MCL microenvironments
To explore how the presence of CD163+ cells is associated with variation in the proteomic and transcriptomic features of the MCL TIME, we performed comparative protein and mRNA analyses of segments collected from patients with MCL with different frequencies of CD163+ cells in their tumors. Approximately 50% of the patients included were classified as either CD163-rich or CD163-sparse, providing even groups for comparison (Figure 5A). Significantly deregulated proteins in CD20+ and CD3+ cells were observed when CD163-rich and CD163-sparse MCL TIMEs were compared as shown in Figure 5B-C. More details are found in the supplemental information section with P values and LMM coefficients listed in supplemental Tables 5 and 6 and box plots of differentially expressed genes listed in supplemental Figures 5 and 6.
Characterization of the phenotypic profile in tumor microenvironments in CD163-rich and CD163-sparse segments. (A) CD20+ MCL cells and infiltrating CD3+ cells were sampled in 56 patients with CD163-sparse (left) and 44 patients with CD163-rich (right) MCL TIMEs. IF staining of CD20+ cells are shown in red, CD3+ cells are shown in light blue, CD163+ cells are shown in yellow, and nuclear staining is in dark blue. Marked segments to be collected are shown in orange for CD20+ cells and turquoise for CD3+ cells. (B,C) Significantly deregulated proteins were visualized by plotting the LMM coefficients (x-axis) against the FDR–adjusted P values (y-axis), and proteins (shown in blue) in the right part of the plot are higher in CD163-rich MCL TIMEs and proteins (shown in red) in the left part of the plot are higher in CD163-sparse MCL TIMEs. Expression of the 63 targeted proteins in either (B) CD20+ cells or (C) CD3+ cell segments are compared. The LMM model included patient as a random effect, and proteins that were significantly differentially expressed (FDR < 0.1) are indicated by name. Coefficients and FDR values are listed in supplemental Tables 5 and 6. (D) Schematic representation of the MAPK pathway. Highlighted in red are proteins that showed increased levels in CD20+ cell segments in CD163-rich MCL. Highlighted in light blue are proteins that show increased levels in CD3+ cell segments in CD163-rich MCL TIMEs.
Characterization of the phenotypic profile in tumor microenvironments in CD163-rich and CD163-sparse segments. (A) CD20+ MCL cells and infiltrating CD3+ cells were sampled in 56 patients with CD163-sparse (left) and 44 patients with CD163-rich (right) MCL TIMEs. IF staining of CD20+ cells are shown in red, CD3+ cells are shown in light blue, CD163+ cells are shown in yellow, and nuclear staining is in dark blue. Marked segments to be collected are shown in orange for CD20+ cells and turquoise for CD3+ cells. (B,C) Significantly deregulated proteins were visualized by plotting the LMM coefficients (x-axis) against the FDR–adjusted P values (y-axis), and proteins (shown in blue) in the right part of the plot are higher in CD163-rich MCL TIMEs and proteins (shown in red) in the left part of the plot are higher in CD163-sparse MCL TIMEs. Expression of the 63 targeted proteins in either (B) CD20+ cells or (C) CD3+ cell segments are compared. The LMM model included patient as a random effect, and proteins that were significantly differentially expressed (FDR < 0.1) are indicated by name. Coefficients and FDR values are listed in supplemental Tables 5 and 6. (D) Schematic representation of the MAPK pathway. Highlighted in red are proteins that showed increased levels in CD20+ cell segments in CD163-rich MCL. Highlighted in light blue are proteins that show increased levels in CD3+ cell segments in CD163-rich MCL TIMEs.
The CD163-rich MCL microenvironment shows increased MAPK signaling
Both CD20+ and CD3+ cell segments showed consistent upregulation of multiple proteins associated with MAPK signaling in the CD163-rich MCL microenvironment (Figure 5B-C; supplemental Tables 5 and 6). In both CD20+ and CD3+ cell segments, increased levels of p90 ribosomal S6 kinase and p38 were detected (Figure 5B-C). In CD3+ cell segments, c-Raf (Raf-1 proto-oncogene, serine/threonine kinase), p-JNK (phosphorylated Jun N-terminal kinase), and extracellular signal-regulated kinase (ERK)1/2 were also higher in the CD163-rich TIME.
MAPK activation can occur through multiple cascades, and 3 of them were shown to be deregulated in our analyses (Figure 5D). In CD163-rich MCL, we observed higher expression of ERK1/2, which is activated mainly through the binding of growth factors to the respective receptors, whereas the p38-MAPK and p-JNK cascades are often activated following a stress response.41
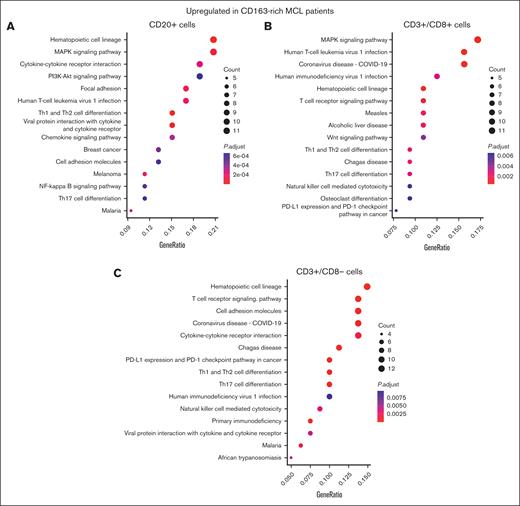
To validate the upregulation of the MAPK pathway, we explored a separate and complementary GeoMx data set in which 1482 targeted mRNAs were explored in CD20+ cell segments and subsets of CD3+ cell segments. LMM analyses, in which the patient was set as a random effect, were performed to compare the mRNA expression of CD20+ cell segments, CD3+/CD8– segments (T-helper cells), and CD3+/CD8+ segments (T-cytotoxic cells) between patients with CD163-rich (n = 32) and those with CD163-sparse (n = 45) MCL microenvironments (supplemental Tables 6-9). In CD20+ cell segments, the increased activity of MAPK-related pathways was confirmed with MAP3K5 being the top upregulated mRNA in the CD163-rich MCL TIMEs (supplemental Table 7). Enriched pathway analyses of differentially expressed genes (upregulation) and P values confirmed MAPK deregulation in CD20+ cell segments (Figure 6A) and CD3+/CD8+ cell segments (Figure 6B) but not in CD3+/CD8– cell segments (Figure 6C).
Enriched KEGG pathway analyses of mRNA data in CD20+ and T-cell subsets, respectively, confirm the association between CD163+ cell abundance and MAPK-upregulation in MCL TIMEs. To visualize the difference in gene expression between patients with MCL with CD163-rich and those with CD163-sparse TIMEs, enriched KEGG pathway analyses were performed based on differentially overexpressed genes in CD163-rich TIMEs in CD20+ cell segments (A), in CD3+/CD8+ cell segments (B), and CD3+/CD8– cell segments (C).
Enriched KEGG pathway analyses of mRNA data in CD20+ and T-cell subsets, respectively, confirm the association between CD163+ cell abundance and MAPK-upregulation in MCL TIMEs. To visualize the difference in gene expression between patients with MCL with CD163-rich and those with CD163-sparse TIMEs, enriched KEGG pathway analyses were performed based on differentially overexpressed genes in CD163-rich TIMEs in CD20+ cell segments (A), in CD3+/CD8+ cell segments (B), and CD3+/CD8– cell segments (C).
In CD20+ cell segments, CD163-rich TIMEs were also associated with the upregulation of markers involved in the induction of M2 macrophages, such as TGFB3 and IL10RA (supplemental Table 7).
The CD163-rich MCL microenvironment shows increased levels of immune-regulatory proteins
Both CD20+ and CD3+ cell segments showed differential regulation of key immune-regulatory proteins when CD163-rich MCL TIMEs were compared with CD163-sparse MCL TIMEs (Figure 5A-B; supplemental Tables 5 and 6; supplemental Figures 5 and 6).
In CD3+ cell segments, STING was higher in CD163-rich TIMEs, whereas GITR (glucocorticoid-induced tumor necrosis factor receptor-related protein) and 4-1BB (CD137; TNFRS9) were higher in CD163-sparse TIMEs. Activation of STING has been proposed to be a relevant costrategy to PD1 inhibition to boost T-cell response in tumors. GITR regulates cell survival through MAPK and NFKB signaling,42 whereas 4-1BB promotes the survival of T cells by inhibiting the activation of induced cell death and can enhance CD28 signaling.43
Interestingly, β2-microglobulin and CD80 expression were lower in the CD163-rich TIME in both the CD20 and CD3 cell segments. Together, the deregulation of these proteins indicates reduced antigen presentation in the CD163-rich TIME, contributing to a less efficient T-cell response. In CD20 cell segments, IDO1 (indoleamine 2,3-dioxygenase 1), an immunosuppressive enzyme known to block antigen-driven T-cell proliferation,44 was increased in the CD163-rich TIME.
Differential cell composition, organization, and functionality of the CD163-rich vs CD163-sparse MCL TIME
LMM-based analyses of the proteomic profiles showed that CD11c was the most significantly differentially expressed protein with higher levels in the CD163-sparse microenvironment than in the CD163-rich microenvironment in both the CD3+ and CD20+ cell segments (Figure 5B-C; supplemental Tables 5 and 6). CD11c is a common marker of myeloid cells, such as macrophages and dendritic cells, but can be expressed by several subsets of lymphoid cells, including normal and malignant B cells. However, CD11c is rarely found on MCL cells.45 Of interest, it is known that CD163+ and CD11c+ lymphoma microenvironments tend to be dominated by either 1 of the 2 cell types46 as also indicated by our results. Additional differences included higher levels of CD25, CD3, and CD56 in the CD163-sparse MCL microenvironments. Thus, the results indicate that CD11c+ myeloid cells, regulatory T cells, and natural killer cells are more abundant and/or closer to CD20+ and CD3+ cells in the CD163-sparse TIME.
To validate and explore the impact of myeloid markers, multiplex IF staining of CD11c, CD163, and CD20 was performed. CD11c is a pan-myeloid marker often associated with M1 macrophages, and CD163 is used as a surrogate marker for M2 macrophages, Cellpose-based segmentation and classification using QuPath was used to quantify the individual cell types (Figure 7A). Patients were divided into subgroups with high or low expression of each marker using maximally ranked statistics with cutoffs of 0.104% and 4.474% for CD163 and CD11c, respectively. For p53, the clinical routine cutoff of 30% was used. Cox regression was used to define the prognostic role in univariate and multivariate analyses (Figure 7B). We showed that CD163, as previously reported using immunohistochemistry,12 is a poor prognostic marker with a hazard ratio of 2.397 (P value = .0175). In contrast, CD11c was a good prognostic marker with a hazard ratio of 0.388 (P value = .0138). In addition, in a multivariate analysis in which age, sex, and p53 were included, both markers remained statistically significant. To estimate the combined effect, a Kaplan-Meier curve analysis using the 5-year OS to focus on outcome events close to the diagnosis was performed for each marker individually (Figure 7C-D) and in combination and emphasized that the combined use of CD11c and CD163 increased the predictive power of the poor prognostic subgroup of MCL TIME (Figure 7E).
CD163 and CD11c are independent prognostic markers in MCL. Multiplex IF staining of CD20, CD11c and CD163 was performed. (A) Examples of MCL TIMEs with either CD11clow/CD163high, CD11clow/CD163high, CD163high/CD11high, or CD11chigh/CD163low (from left to right) are shown. (B) The prognostic value of CD11c, CD163, and p53 was investigated in univariate and multivariate Cox regression analyses. The Kaplan-Meier curve analysis and log-rank test showed the prognostic value of CD11c (C) and CD163 (D) as individual markers and the combined effect of CD11c and CD163 (E).
CD163 and CD11c are independent prognostic markers in MCL. Multiplex IF staining of CD20, CD11c and CD163 was performed. (A) Examples of MCL TIMEs with either CD11clow/CD163high, CD11clow/CD163high, CD163high/CD11high, or CD11chigh/CD163low (from left to right) are shown. (B) The prognostic value of CD11c, CD163, and p53 was investigated in univariate and multivariate Cox regression analyses. The Kaplan-Meier curve analysis and log-rank test showed the prognostic value of CD11c (C) and CD163 (D) as individual markers and the combined effect of CD11c and CD163 (E).
In CD163-rich TIMEs, increased levels of α-SMA (alpha spinal muscular atrophy), fibronectin, and CD34 were detected in CD3 cell segments (Figure 5C). These 3 proteins are associated with the formation of new vessels and indicate a higher degree of angiogenesis and/or neovascularization47,48 in CD163-rich TIMEs.
Deregulation of cell survival–related proteins was also identified when the 2 types of MCL TIMEs were compared. CD95/FAS (Fas cell surface death receptor) was higher in CD3+ cell segments collected from CD163-sparse TIMEs (Figure 5C; supplemental Table 6). A higher expression of GZMB and cleaved caspase-9 in CD20+ cell segments indicates more active cytolytic activity in the CD163-rich TIMEs than in the CD163-sparse TIMEs (Figure 5B). In contrast, CD3+ cell segments showed lower levels of GMZA in the CD163-rich vs CD163-sparse TIMEs.
To assess whether there was a correlation between the abundance of CD163+ cells and the distance between CD20+ and CD3+ cells, the minimal distance between each CD20+ cell and the closest CD163+ and CD3+ cell, respectively, was determined in tumor regions. The presence of 1 or more CD163+ cells (within a 30-μm radius) was associated with a shorter CD20+ and CD3+ minimal cell distance (median value, 10.5 and 12.4 μm, respectively; P < 0.001) (supplemental Figure 2C), indicating that the presence of CD163+ cells is associated with a closer interaction between tumor cells and T cells. This is in line with the previously reported correlation between CD163+ and CD3+ cell numbers that was independent of the spatial compartment.
Discussion
Targeting immunosuppressive macrophages has become a promising strategy in cancer,15 and in this study, we have explored the spatial distribution and functional roles of macrophages in a large cohort of primary MCL. Because macrophages are highly susceptible to external stimuli, we hypothesized that their spatial localization, either in direct contact with MCL cells (tumor-rich) or adjacent to tumor-sparse lymphoid tissue, can have an impact on their functionality. To our knowledge, the limited number of previous studies on MCL TIMEs have solely focused on describing the abundance of cell subtypes in relation to the prognosis rather than on the cellular neighborhood metrics and molecular impact.12,49
We show that CD163+ macrophages in tumor-sparse regions had higher levels of the immune-regulatory proteins VISTA50 and B7-H3, suggesting active immunosuppression. VISTA, which is postulated to have a coinhibitory role,35 is highly expressed in myeloid cells.36 In pancreatic cancer, VISTA and PD-L1 (CD274) are expressed by different subsets of macrophages, suggesting that T cells have separate inhibitory mechanisms and placing emphasis on VISTA as a possible immunotherapeutic target beyond PD-L1.51 Albeit promising, solely targeting immune checkpoint molecules is not sufficient for many patients,52 and combinatorial treatments that drive more effective immune responses are needed.
One possible strategy to boost immune recognition is the activation of STING.53 In our study, STING was higher in CD163+ cell segments in tumor-sparse regions. STING drives type I interferon responses and leads to the production of inflammatory cytokines, thereby promoting antigen recognition by effector T cells and triggering apoptosis in cancer cells.54 Therapeutic strategies that include small molecules that activate STING in combination with anti-PD-1 treatment are being explored in clinical trials for advanced solid tumors and lymphomas.55
B7-H3/CD276 is an immune checkpoint molecule with dual effects in the regulation of the immune response. Expression of B7-H3 in cancer has been associated with a worse prognosis, suggesting an inhibitory role.56 A previous study showed that B7-H3 was overexpressed in MCL tumor tissue,57 whereas silencing of B7-H3 increased the sensitivity to rituximab and bendamustine by enhancing apoptosis in xenograft MCL models.58 Thus, higher expression of B7-H3 in CD163+ cell segments in tumor-sparse regions may contribute to the inhibition of T-cell proliferation, potentially preventing T cells from interacting with cancer cells.
The image analysis showed that the ratio of CD163+ macrophages to T cells was constant across spatial regions, but the subtype of interacting T cells seems to be different. Of interest, image-based analysis of cell frequencies and neighborhoods showed that the infiltration of CD163+ macrophages was associated with closer contact between T cells and MCL cells, supporting the correlation between CD163+ and CD3+ cell numbers.
When combined, our results indicate that immune system suppression by CD163+ macrophages hamper an effective immune response and that targeting of, for example, VISTA and/or B7-H3 might be relevant strategies to boost immune system activation outside the immediate tumor niche. The expression of STING in tumor-sparse regions might enhance the response to such checkpoint blockade. The fact that immune regulation of CD163+ macrophages is different in distinct spatial localizations strengthens the rationale of considering spatial metrics when biomarkers are measured.
To assess the effect of the presence of CD163+ cells, tumor cells and T cells were explored in MCL TIMEs with different frequencies of CD163+ cells. T cells in CD163-sparse TIMEs displayed higher levels of checkpoint inhibitors GITR and 4-1BB. GITR regulates cell survival through MAPK and NFKB signaling and is considered a clinically interesting target.42 4-1BB is a member of the tumor necrosis factor receptor superfamily T-cell costimulatory receptor and is expressed following antigen-specific signaling.43 The molecule is expressed by both T-helper and T-cytotoxic cells but favors expansion of T-cytotoxic cells, and is thus of therapeutic interest for agonistic stimulation.59
We further showed that MCL cells express enhanced levels of IDO1 in CD163-rich TIMEs when compared with CD163-sparse TIMEs. IDO1 is an enzyme involved in the rate-limiting step of tryptophan metabolism, which leads to impaired T-cytotoxic cell function, for example, by reducing antigen-specific proliferation of T cells.60 IDO1 is commonly expressed in several cancer types, for example, diffuse large B-cell lymphoma, and it is associated with aggressive disease and inferior survival.61,62 IDO1 is further associated with enhanced expression of the anti-inflammatory IL-10.63 Targeting IDO1 is an alternative approach to checkpoint inhibitors and is being evaluated in clinics with compounds granted orphan-drug designation for the treatment of high-stage melanoma.
One of the main results in our study is the association between CD163+ macrophages and MAPK signaling, which was seen in both the proteomic and transcriptional analyses. Frequent activation of the MAPK pathway in MCL is well characterized and occurs as a consequence of FAK (protein tyrosine kinase 2) and CXCR4 upregulation, which are directly influenced by SOX11, a key diagnostic antigen in MCL.8,64 Upregulation of FAK leads to activation of ERK1/2, NF-κB, and AKT, which is often associated with treatment resistance in MCL.65-67 MAPK pathway activation contributes to several cellular functions including apoptosis, migration, invasion, proliferation, and angiogenesis68 and has been associated with stem-like properties in MCL.69,70 Both p-ERK and p38, part of MAPK signaling, are associated with inferior outcomes in MCL.71 Our findings support that MAPK pathway activation is involved in macrophage polarization, promoting an M2 phenotype, and activation of ERKs, JNK, and p38 molecules upon CSF1 treatment.72-74 CD11c was upregulated in CD163-sparse TIMEs, and to investigate the tentative opposite prognostic role, multiplex IF staining was performed and showed that CD11c+ cell frequency is positively associated with OS. The combined use of CD11c and CD163 further stratified patients with MCL into risk groups and supports the importance of investigations into the TIME to enable risk-adapted therapy in MCL.
Studies have reported that MAPK is also involved in the direct interaction between cancer and T cells. MAPK family members have been shown to promote downregulation of the costimulatory molecule 4-1BB and to decrease T-cell activation and proliferation.68 In this study, we showed that T cells sampled in CD163-rich TIMEs have lower 4-1BB expression. Thus, MAPK inhibition is a promising strategy for increasing the levels of costimulatory molecules in the MCL microenvironment. We conclude that the consistent upregulation of MAPK, detected in both MCL and T-cell segments, is likely associated with the previously reported aggressive behavior of MCL with CD163+ cell infiltration.12
As exemplified by our study, recent technical advances in spatial omics and image analyses can advance our understanding of the MCL TIME. The use of intact tissue, in a format available in biobanks, allows for detailed molecular analyses in a retained spatial context of large patient cohorts. The technology comes with technical limitations, and because segments are selected based on IF staining, tissues with high autofluorescence, such as bone marrow samples, were excluded from the study. In addition, segmentation of individual cells is not perfect and expression from directly adjacent cells may be collected, which provides an opportunity to assess the differences in cellular neighbors but also hampers the possibility to firmly conclude whether a single protein is expressed by the phenotypically targeted cells or by neighboring cells.
Overall, this comprehensive study allowed us to reveal molecular features that are associated with the organization of the MCL TIME. We showed that increased MAPK signaling is connected to an abundance of CD163+ macrophages and likely explain part of the poor prognostic effect of such cell infiltration. Thus, targeting MAPK may particularly benefit patients with abundant CD163+ macrophage infiltration. In addition, targeting VISTA and B7-H3 might be a feasible approach in MCL to dampen the immunosuppressive effect of CD163+ macrophages in close vicinity to MCL and to promote increased T-cell infiltration.
Acknowledgments
The authors acknowledge that the study was performed by the SpatialOmics@LU facility and with preparative assistance by the HistoCore at Lund University. Sequencing was performed at clinical translational genomics, Lund University. The authors thank all technical staff involved in the collection and assembly of the Biobank of Lymphomas in Southern Sweden tissue cohort and for providing the associated clinical information, including Kristina Lövgren.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement number N754299, the Cancerfonden (under grant numbers 190309Pj and 211561Pj), the Cancera Foundation, Mats Paulssons Stiftelse för forskning, innovation och samhällsbyggande, Stiftelsen Stefan Paulssons cancerfond, and CREATE Health. All financial support was granted to S.E.
Authorship
Contribution: J.d.M.R. and L.L. were responsible for project organization and were a major part of the GeoMx DSP workflow; L.L. developed the necessary bioinformatic workflows for mRNA, protein and topology analyses and wrote part of the manuscript; J.d.M.R. performed data analyses and wrote parts of the manuscript; L.M.O. was responsible for the technical planning of the GeoMx DSP related workflows, including quality control of the data, and performed part of the data analysis; M.H. performed morphological staining and the GeoMx DSP workflow downstream of segment collection; A. Johansson and A. Janská were responsible for the staining of CD11c and CD163 and the subsequent image and data analysis; D.K., together with L.S. and J.d.M.R., were responsible for the Aiforia–based image analyses; A.S.G. was responsible for the development of the LMM models and general analyses workflows; P.H., A.N., and I.G. were involved in the discussion on CD163 staining; A.P. was responsible for the pathology review; M.J. was responsible for the collection of Biobank of Lymphomas in Southern Sweden and was involved in the conceptual design; S.E. was responsible for the conceptual design, data analyses, and manuscript writing; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sara Ek, Department of Immunotechnology, Lund University, Medicon Village, Scheelevägen 8, 223 87 Lund, Sweden; email: sara.ek@immun.lth.se.
References
Author notes
Data supporting the results in this study involve patient data and are available on reasonable request from the corresponding author, Sara Ek (sara.ek@immun.lth.se).
The full-text version of this article contains a data supplement.