Key Points
ADAMTS13 exome/genome data from 807 162 gnomAD population was analyzed to establish worldwide/within-population prevalence of hereditary TTP.
Hereditary TTP prevalence is substantially higher than currently estimated, suggesting that many patients are not diagnosed.
Visual Abstract
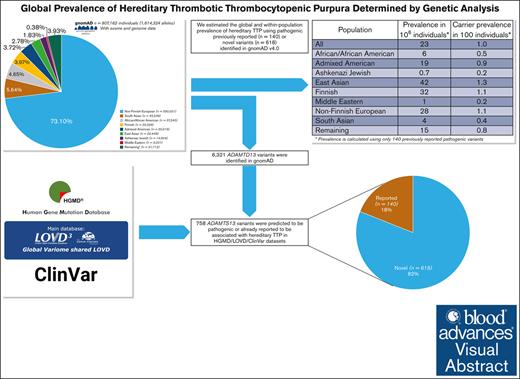
Hereditary thrombotic thrombocytopenic purpura (hTTP) is a rare autosomal recessive, life-threatening disorder caused by a severe deficiency of the plasma enzyme, ADAMTS13. The current estimated prevalence of hTTP in different regions of the world, 0.5 to 2.0 patients per million, is determined by the frequency of diagnosed patients. To evaluate more accurately the worldwide prevalence of hTTP, and also the prevalence within distinct ethnic groups, we used data available in exome and genome sequencing of 807 162 (730 947 exomes, 76 215 genomes) subjects reported recently by the Genome Aggregation Database (gnomAD-v4.1). Among 1 614 324 analyzed alleles in the gnomAD population we identified 6321 distinct ADAMTS13 variants. Of these, 758 were defined as pathogenic; 140 (18%) variants had been previously reported and 618 (82%) were novel (predicted as pathogenic). In total 10 154 alleles (0.6%) were carrying the reported or predicted pathogenic variants; 7759 (77%) with previously reported variants. Considering all 758 pathogenic variants and also only the 140 previously reported variants, we estimated a global hTTP prevalence of 40 and 23 cases per 106, respectively. Considering only the 140 previously reported variants, the highest estimated prevalence was in East Asians (42 per 106). The estimated prevalences of other populations were: Finnish, 32 per 106; non-Finnish Europeans, 28 per 106; Admixed Americans, 19 per 106; Africans/African Americans, 6 per 106; and South Asians, 4 per 106. The lowest prevalences were Middle Eastern, 1 per 106 and Ashkenazi Jews, 0.7 per 106. This population-based genetic epidemiology study reports that hTTP prevalence is substantially higher than the currently estimated prevalence based on diagnosed patients. Many patients with hTTP may not be diagnosed or may have died during the neonatal period.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening systemic disorder caused by severe deficiency of the plasma protein, ADAMTS13.1 ADAMTS13 is required for proteolysis of von Willebrand factor (VWF) when it is secreted from endothelial cells.1,2 Severe ADAMTS13 deficiency allows the circulation of ultralarge VWF multimers that can bind platelets and cause systemic microvascular thrombi, microangiopathic hemolysis, and thrombocytopenia.3 TTP may be an acquired disorder caused by autoantibodies reacting with ADAMTS13 or a hereditary disorder (hereditary TTP [hTTP]) caused by homozygous or compound heterozygous ADAMTS13 mutations. hTTP is much less common than acquired TTP; the current estimated prevalence of hTTP is 0.5 to 2.0 per 106 population.4
Three previous reports have estimated the prevalence of hTTP. A report from Japan in 2011 analyzed 3200 healthy individuals and suggested that 1.1 individuals per 106 should be homozygous or compound heterozygous for severe ADAMTS13 deficiency.5 The authors recognized that they may have underestimated the prevalence because of the small sample size.5 A report from the Central Norway Health Region in 2015 estimated the prevalence of hTTP to be remarkably higher, 16.7 per 106. Their data were based on 11 patients with hTTP in a region with a population of 659 621.6 This high prevalence was attributed to the significantly higher frequency of the pathogenic mutations, c.4143_4144dupA (c.4143dup) and p.Arg1060Trp, in central Norway compared with western and southeastern Norway.6 In another study, using genomic data, the prevalence and carrier frequency of hTTP were estimated to be 0.43 per 106 and 1.31 per 1000 people.7 The authors chose an allele frequency filtering (Whiffin equation), and they based their calculation on previous estimates. Thus, several pathogenic variants, in particular the 2 known frequent variants, p.Arg1060Trp and p.Asp187His, were excluded from their analysis. This resulted in a lower hTTP estimate.7
The prevalence of hTTP could be significantly underestimated because patients may be asymptomatic if a risk factor does not occur or because acute episodes of hTTP are commonly misdiagnosed.8 Birth and the few days after birth may be the time of greatest risk for death because of the turbulent circulation caused by the reversal of blood flow through the ductus arteriosus to provide blood for the newly functional lungs.9 The turbulent circulation that occurs at birth can unfold the ultralarge VWF multimers, exposing the platelet binding site that allows platelet aggregation and increases risk for microvascular thrombosis.10 Severe hemolysis and thrombocytopenia occur during the first hours after birth in 40% to 50% of newborn infants with hTTP; they are almost always misdiagnosed.6,11-13 These data are from infants with hTTP who were misdiagnosed as having red blood cell immunization14 and successfully treated with whole-blood exchange transfusion. However, many of the mothers of these surviving infants had previous neonatal deaths (for example, as previously reported by Stubbs et al15), contributing to the current underestimates of hTTP prevalence. Pregnancies in women with hTTP almost always cause severe complications.16 In pregnant women, acute episodes of hTTP are misdiagnosed as severe preeclampsia or HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, that are also characterized by microangiopathic hemolytic anemia and thrombocytopenia caused by the turbulent placental circulation.17-19 Delivery of the fetus to stop the presumed preeclampsia causes delay of consideration of hTTP. Ischemic strokes are common in children with hTTP,20 yet hTTP is not included among the many etiologies for embolic strokes of undetermined source.21 The frequency of missed diagnoses suggests that the prevalence of hTTP may be much greater than 0.5 to 2.0 per 106 population.
Recently, novel facilities, lower cost of genotyping, and availability of next-generation sequencing (NGS) are being used in a growing number of large-scale population-based studies.22 The estimate of disease prevalence can be obtained through allele frequency information from a large number of sequenced samples.23-25 To determine the global and within-population prevalence of hTTP, we analyzed ADAMTS13 sequencing data of 807 162 individuals from the Genome Aggregation Database (gnomAD version 4.1), the largest and most widely used publicly available genetic database.
Methods
ADAMTS13 variants in the population-based gnomAD
We extracted and analyzed all variants identified in ADAMTS13 gene from the gnomAD population (version 4.1), which comprises 730 947 whole exomes (416 555 individuals from the UK Biobank) and 76 215 genomes around the world. These sequencing data are from various population genetic studies, totaling 807 162 individuals and were aligned to GRCh38 of the human reference genome. A wide range of ethnicities around the globe is represented in this population-based database. The data set has undergone extensive quality control measures by the gnomAD investigators to remove poor-quality sequences and to flag those variants of questionable reliability.22 Accordingly, only those ADAMTS13 variants that had passed quality controls (gnomAD random forest filters) were considered for our evaluation.
Considering the technical limitations of NGS for detecting large insertions, duplications, and deletions, and also complex rearrangements, our analysis focused only on short insertions/deletions and single-nucleotide variants. Among ADAMTS13 variants identified in the 807 162 gnomAD population, we considered the following as pathogenic (abbreviations are described in supplemental Table 1):
all variants in the Human Gene Mutation Database (HGMD) and/or the Leiden Open Variation Database version 3 (LOVD) and/or ClinVar that were reported to cause hTTP;
null variants (ie, nonsense and frameshift);
inframe deletion or insertion variants predicted to be deleterious by all of the following 3 in silico programs: FATHMM-indel,26 MutPred-Indel,27 and SIFT-indel;
disruptive splice-site variants affecting the first or last 2 intronic nucleotides;
splicing variants located at the first or last 8 intronic positions and predicted to be deleterious by all of the following 4 in silico tools: SpliceAI,28 ESEFinder,29 BDGP,30 and CADD,31 with a CADD score of ≥20 considered deleterious; and
missense variants predicted to be deleterious by all of the following 7 in silico programs: CADD,31 SIFT,32 PolyPhen2,33 LRT,34 MutationTaster,35 MutationAssessor,36 and FATHMM.37
Calculation of hTTP prevalence
We applied 2 different approaches to calculate the worldwide and within-population prevalence of hTTP. First, we used variants identified in the gnomAD population that have been previously reported to cause hTTP in the available genetic databases (HGMD, LOVD, or ClinVar) or in the literature. In the second approach, we considered all variants identified in gnomAD that were classified as pathogenic according to 1 of 6 previously mentioned selection criteria. We calculated the estimated prevalence using the Hardy-Weinberg equation (p2 + 2pq + q2 = 1), in which p is the population frequency of the major allele and q is the population frequency of the minor allele. Because hTTP is recessively inherited, we estimated the prevalence of individuals homozygous or compound heterozygous for ADAMTS13 variants and heterozygous individuals.
Identification of previously reported variants associated with hTTP
We searched PubMed for articles describing hTTP to identify previously reported ADAMTS13 variants that cause hTTP. Furthermore, variants reported to cause hTTP in the HGMD (accessed 2023; abbreviations are described in supplemental Table 1) or the LOVD (accessed 2023) were included. Finally, we searched for ADAMTS13 variants that in ClinVar have been classified as “pathogenic” or “likely pathogenic.” Two independent authors checked all previously reported variants to select only those that had been associated with hTTP. Variants with unclear association with hTTP were excluded.
Results
High-quality sequencing data were collected from the gnomAD population of 807 162 individuals with different ethnicities (Table 1). The average depth of sequencing coverage per base in all 29 exons of ADAMTS13 gene was almost always >30× for both exome and genome sequencing (supplemental Figure 1), indicating adequate coverage.
gnomAD database composition according to populations
| Population . | Total . |
|---|---|
| All | 807 162 |
| African/African American | 37 545 |
| Admixed American | 30 019 |
| Ashkenazi Jewish | 14 804 |
| East Asian | 22 448 |
| Finnish | 32 026 |
| Middle Eastern | 3 031 |
| European (non-Finnish) | 590 031 |
| South Asian | 45 546 |
| Remaining∗ | 31 712 |
| Population . | Total . |
|---|---|
| All | 807 162 |
| African/African American | 37 545 |
| Admixed American | 30 019 |
| Ashkenazi Jewish | 14 804 |
| East Asian | 22 448 |
| Finnish | 32 026 |
| Middle Eastern | 3 031 |
| European (non-Finnish) | 590 031 |
| South Asian | 45 546 |
| Remaining∗ | 31 712 |
There were 31 712 people with no assigned ethnicity by gnomAD. The data of gnomAD are organized by these terms that are described as genetic ancestry. Of note, genetic ancestry is not comparable with race, ethnicity, or geographic identification. We have used the genetic ancestry groupings as was described by the gnomAD investigators. More information about the ancestry assignment and population labels provided by different sequencing projects is available on the gnomAD website.
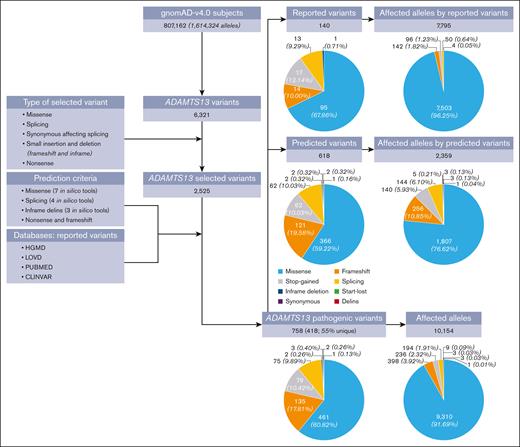
There were 6321 distinct ADAMTS13 variants among the 1 614 324 alleles analyzed in the gnomAD population (Figure 1). Using a conservative approach to classify variants as pathogenic (causing hTTP), we identified 758 distinct ADAMTS13 variants; 140 (18%) had been previously reported to cause hTTP (supplemental Table 3) and 618 (82%) were novel, predicted to be pathogenic. None of the 140 previously reported variants were classified as “benign” or “likely benign” in ClinVar, supporting their pathogenicity.
Flowchart of data analyses. The gnomAD data set includes 807 162 subjects (1 614 324 alleles). Among this population, 6321 distinct ADAMTS13 variants were identified, of which 2525 variants were short insertions/deletions and single-nucleotide variants. We applied our pathogenicity criteria (supplemental Table 2) to identify pathogenic variants among the 2525 variants. Overall, 758 distinct variants were classified as pathogenic; among them, 418 variants (55%) were identified in only 1 individual (ie, were unique). Of these 758 pathogenic ADAMTS13 variants, 140 had been previously reported to cause hTTP and 618 were newly predicted to be pathogenic. A total of 10 154 alleles were carrying these 758 pathogenic variants, 7795 (77%) reported variants and 2359 (23%) predicted variants. Missense variants were the most common form of the pathogenic variants (61%), and they were responsible for the majority of the 10 154 affected alleles by pathogenic variants (92%). A start-lost variant is a point mutation in the ATG start codon, which prevents the original start translation site from being used. A stop-gained is a DNA variant that changes at least 1 base of a codon, leading to a premature stop codon.
Flowchart of data analyses. The gnomAD data set includes 807 162 subjects (1 614 324 alleles). Among this population, 6321 distinct ADAMTS13 variants were identified, of which 2525 variants were short insertions/deletions and single-nucleotide variants. We applied our pathogenicity criteria (supplemental Table 2) to identify pathogenic variants among the 2525 variants. Overall, 758 distinct variants were classified as pathogenic; among them, 418 variants (55%) were identified in only 1 individual (ie, were unique). Of these 758 pathogenic ADAMTS13 variants, 140 had been previously reported to cause hTTP and 618 were newly predicted to be pathogenic. A total of 10 154 alleles were carrying these 758 pathogenic variants, 7795 (77%) reported variants and 2359 (23%) predicted variants. Missense variants were the most common form of the pathogenic variants (61%), and they were responsible for the majority of the 10 154 affected alleles by pathogenic variants (92%). A start-lost variant is a point mutation in the ATG start codon, which prevents the original start translation site from being used. A stop-gained is a DNA variant that changes at least 1 base of a codon, leading to a premature stop codon.
Population-based prevalence of hTTP
First, we used only the allele frequency of the 140 variants that were identified in gnomAD and were previously reported to cause hTTP (Table 2). The global prevalence of hTTP was 23 per 106 people, with a carrier frequency of 1 per 100 people. Three ethnicities showed the highest prevalence, including East Asians (42 per 106), Finnish (32 per 106), and European populations (28 per 106), followed by Admixed Americans (19 per 106) and Africans/African Americans (6 per 106). South Asians had a lower estimated prevalence of 4 per 106. The lowest prevalence was found in Middle Eastern (1 per 106) and Ashkenazi Jewish (0.7 per 106) populations.
Estimated prevalence of hTTP using only 140 pathogenic reported variants
| Population . | Total number of alleles . | Total number of homozygote . | Total number of affected alleles . | Collective frequency of variants∗ . | Heterozygote frequency . | Prevalence in 106 individuals . | Carrier prevalence in 100 individuals† . |
|---|---|---|---|---|---|---|---|
| All | 1 614 324 | 15 | 7795 | 0.005 | 0.010 | 23 | 1.0 |
| African/African American | 75 090 | 0 | 179 | 0.002 | 0.005 | 6 | 0.5 |
| Admixed American | 60 038 | 1 | 261 | 0.004 | 0.009 | 19 | 0.9 |
| Ashkenazi Jewish | 29 608 | 0 | 25 | 0.001 | 0.002 | 0.7 | 0.2 |
| East Asian | 44 896 | 1 | 294 | 0.006 | 0.013 | 42 | 1.3 |
| Finnish | 64 052 | 2 | 360 | 0.006 | 0.011 | 32 | 1.1 |
| Middle Eastern | 6 062 | 0 | 6 | 0.001 | 0.002 | 1 | 0.2 |
| European (non-Finnish) | 1 180 062 | 10 | 6240 | 0.005 | 0.011 | 28 | 1.1 |
| South Asian | 91 092 | 1 | 187 | 0.002 | 0.004 | 4 | 0.4 |
| Remaining | 63 424 | 0 | 243 | 0.004 | 0.008 | 15 | 0.8 |
| Population . | Total number of alleles . | Total number of homozygote . | Total number of affected alleles . | Collective frequency of variants∗ . | Heterozygote frequency . | Prevalence in 106 individuals . | Carrier prevalence in 100 individuals† . |
|---|---|---|---|---|---|---|---|
| All | 1 614 324 | 15 | 7795 | 0.005 | 0.010 | 23 | 1.0 |
| African/African American | 75 090 | 0 | 179 | 0.002 | 0.005 | 6 | 0.5 |
| Admixed American | 60 038 | 1 | 261 | 0.004 | 0.009 | 19 | 0.9 |
| Ashkenazi Jewish | 29 608 | 0 | 25 | 0.001 | 0.002 | 0.7 | 0.2 |
| East Asian | 44 896 | 1 | 294 | 0.006 | 0.013 | 42 | 1.3 |
| Finnish | 64 052 | 2 | 360 | 0.006 | 0.011 | 32 | 1.1 |
| Middle Eastern | 6 062 | 0 | 6 | 0.001 | 0.002 | 1 | 0.2 |
| European (non-Finnish) | 1 180 062 | 10 | 6240 | 0.005 | 0.011 | 28 | 1.1 |
| South Asian | 91 092 | 1 | 187 | 0.002 | 0.004 | 4 | 0.4 |
| Remaining | 63 424 | 0 | 243 | 0.004 | 0.008 | 15 | 0.8 |
Collective frequency of variant is calculated as the total number of affected alleles divided to total number of alleles.
Carrier prevalence is calculated from heterozygote frequency multiplied by 100. Prevalence is calculated according to Hardy-Weinberg principle.
We then expanded the estimation to include all 758 identified pathogenic variants (140 previously reported, 618 novel variants). We estimated a global prevalence of 40 per 106 people for hTTP, with a global carrier frequency of 1.3 per 100 people (Table 3). The estimated prevalence was 84 per 106 cases in East Asians and 47 per 106 in Admixed Americans. For non-Finnish European, Finnish, and African/African American populations the estimated prevalence was 42 per 106, 41 per 106, and 40 per 106 respectively; for the Middle Eastern population, 28 per 106 and for South Asians, 19 per 106. The lowest prevalence was observed in Ashkenazi Jews (1.5 per 106).
Estimated prevalence of hTTP using all the 758 pathogenic variants
| Population . | Total number of alleles . | Total number of homozygote . | Total number of affected alleles . | Collective frequency of variants∗ . | Heterozygote frequency . | Prevalence in 106 individuals . | Carrier prevalence in 100 individuals† . |
|---|---|---|---|---|---|---|---|
| All | 1 614 324 | 17 | 10 154 | 0.006 | 0.013 | 40 | 1.3 |
| African/African American | 75 090 | 0 | 395 | 0.005 | 0.010 | 28 | 1.1 |
| Admixed American | 60 038 | 1 | 410 | 0.007 | 0.014 | 47 | 1.4 |
| Ashkenazi Jewish | 29 608 | 0 | 36 | 0.001 | 0.002 | 1.5 | 0.2 |
| East Asian | 44 896 | 1 | 412 | 0.009 | 0.018 | 84 | 1.8 |
| Finnish | 64 052 | 2 | 409 | 0.006 | 0.013 | 41 | 1.3 |
| Middle Eastern | 6 062 | 1 | 32 | 0.005 | 0.011 | 28 | 1.1 |
| European (non-Finnish) | 1 180 062 | 11 | 7 685 | 0.007 | 0.013 | 42 | 1.3 |
| South Asian | 91 092 | 1 | 394 | 0.004 | 0.009 | 19 | 0.9 |
| Remaining | 63 424 | 0 | 381 | 0.006 | 0.012 | 36 | 1.2 |
| Population . | Total number of alleles . | Total number of homozygote . | Total number of affected alleles . | Collective frequency of variants∗ . | Heterozygote frequency . | Prevalence in 106 individuals . | Carrier prevalence in 100 individuals† . |
|---|---|---|---|---|---|---|---|
| All | 1 614 324 | 17 | 10 154 | 0.006 | 0.013 | 40 | 1.3 |
| African/African American | 75 090 | 0 | 395 | 0.005 | 0.010 | 28 | 1.1 |
| Admixed American | 60 038 | 1 | 410 | 0.007 | 0.014 | 47 | 1.4 |
| Ashkenazi Jewish | 29 608 | 0 | 36 | 0.001 | 0.002 | 1.5 | 0.2 |
| East Asian | 44 896 | 1 | 412 | 0.009 | 0.018 | 84 | 1.8 |
| Finnish | 64 052 | 2 | 409 | 0.006 | 0.013 | 41 | 1.3 |
| Middle Eastern | 6 062 | 1 | 32 | 0.005 | 0.011 | 28 | 1.1 |
| European (non-Finnish) | 1 180 062 | 11 | 7 685 | 0.007 | 0.013 | 42 | 1.3 |
| South Asian | 91 092 | 1 | 394 | 0.004 | 0.009 | 19 | 0.9 |
| Remaining | 63 424 | 0 | 381 | 0.006 | 0.012 | 36 | 1.2 |
Collective frequency of variant is calculated as the total number of affected alleles divided by total number of alleles.
Carrier prevalence is calculated from heterozygote frequency multiplied by 100. Prevalence is calculated according to Hardy-Weinberg principle.
Global mutational spectrum of ADAMTS13
Considering all 758 variants, missense accounted for the majority of them (461, 61%), followed by frameshift (135, 18%; Figure 1). Variants affecting stop codons including stop-gained (79, 10%) and stop-lost (n = 2) as well as splicing variants (77, 10%) were also identified. There were only 3 inframe deletions, 2 synonymous variants and 1 delins variant.
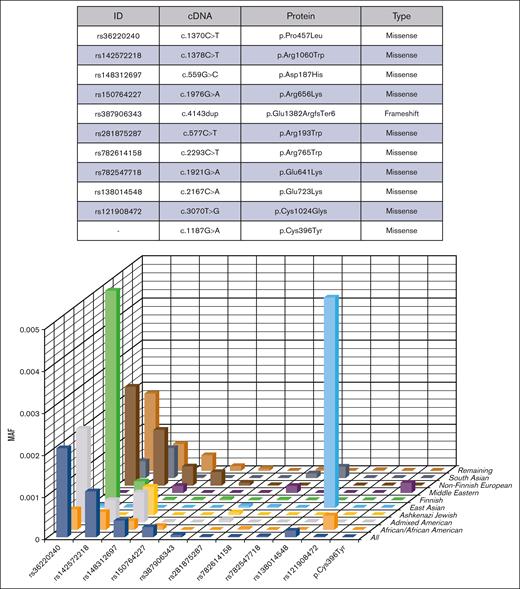
Of the 758 pathogenic variants, 418 (55%) were identified in only 1 individual; most were among the predicted variants (n = 376, 90%). Among all 1 614 324 analyzed alleles, 10 154 were carrying pathogenic variants, either previously reported (77%) or predicted pathogenic (23%) variants (supplemental Table 4). Of these 10 154 affected alleles, 92% were due to missense variants (n = 9310). The remaining 844 affected alleles were the result of frameshift (398, 4%), stop-gained (236, 2%), splicing (194, 2%), inframe deletion (n = 9), synonymous (n = 3), start-lost (n = 3), and delins (n = 1) variants (Figure 1). Among predicted variants (n = 618), p.Pro952Ser alone was found to affect 256 alleles and, among reported variants (n = 140), 5 variants affected 6410 alleles, including p.Pro457Leu (3363), p.Arg659Lys (394), p.Gln723Lys (252), p.Arg1060Trp (1761), and p.Asp187His (640); Figure 2).
Ethnicity-specific variants that determined the prevalence of hTTP. This figure shows the minor allele frequency (MAF) of the most frequent pathogenic variants identified in gnomAD population among different ethnicities. Variants p.Arg1060Trp, p.Pro457Leu, and p.Asp187His were shared by different populations. The high prevalences of hTTP found in the 3 ethnicities, including East Asians (42 per 106), Finns (32 per 106), and European populations (28 per106) were because of some pathogenic variants that were frequent in these ethnicities. Variant p.Gln723Lys was remarkably frequent among East Asians; p.Pro457Leu and p.Arg1060Trp in Finns; and variants p.Pro457Leu, p.Arg1060Trp, p.Asp187His, and c.4143dup in non-Finnish European population.
Ethnicity-specific variants that determined the prevalence of hTTP. This figure shows the minor allele frequency (MAF) of the most frequent pathogenic variants identified in gnomAD population among different ethnicities. Variants p.Arg1060Trp, p.Pro457Leu, and p.Asp187His were shared by different populations. The high prevalences of hTTP found in the 3 ethnicities, including East Asians (42 per 106), Finns (32 per 106), and European populations (28 per106) were because of some pathogenic variants that were frequent in these ethnicities. Variant p.Gln723Lys was remarkably frequent among East Asians; p.Pro457Leu and p.Arg1060Trp in Finns; and variants p.Pro457Leu, p.Arg1060Trp, p.Asp187His, and c.4143dup in non-Finnish European population.
Among the 807 162 gnomAD participants, 17 individuals (15 with previously documented hTTP-associated variants) were homozygotes for 5 different ADAMTS13 pathogenic variants (supplemental Table 5). They were p.Pro457Leu (1 Admixed American, and 2 Finnish and 7 non-Finnish Europeans), p.Gln723Lys (1 East Asian), p.Pro952Ser (1 Middle Eastern and 1 non-Finnish European), p.Arg1060Trp (2 non-Finnish European and 1 South Asian), and p.Asp187His (1 non-Finnish European). p.Pro952Ser is a novel variant; the other 4 variants were previously reported to cause hTTP.
Mutational spectrum of all previously reported ADAMTS13 variants
To identify all previously reported ADAMTS13 variants associated with hTTP we searched PubMed, HGMD, LOVD, and ClinVar. After removal of variants with no clear association with hTTP, we found 293 ADAMTS13 gene variants that have been reported to cause hTTP (supplemental Figure 2). For these variants, 142 (48%) were missense, 67 (23%) frameshift, 35 (12%) stop-gained, 29 (10%) splicing, 14 (5%) large deletion/insertions, and 6 (2%) inframe indels variants. Compared with those identified in gnomAD, there were fewer missense variants but more frameshift, stop-gained, and splicing variants reported to be associated with hTTP. Among these 293 ADAMTS13 variants, 140 had been included in the 758 variants identified in gnomAD, whereas 153 were not found in gnomAD.
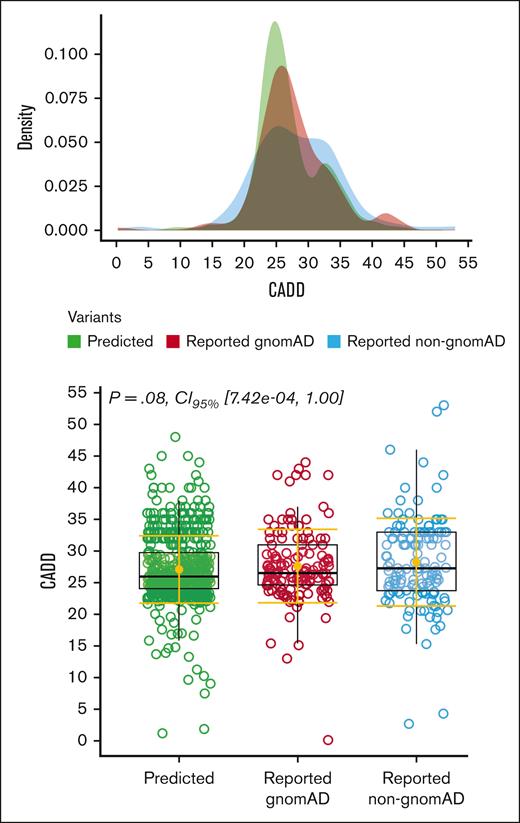
To compare the severity of the 758 pathogenic variants identified in gnomAD (140 reported and 618 novel variants) with the 153 variants that were not found in gnomAD, we used a CADD score distribution (Figure 3). No significant differences were observed between the 3 categories: novel variants (618), previously reported variants identified in gnomAD (140), and previously reported variants that were not found in gnomAD (153).
Evaluation of the severity of variants’ pathogenicity. We used a combined annotation dependent depletion score (CADD), a tool for scoring the deleteriousness of genetic variants, with cutoff of >2031 to determine the effect of variant severity, among the 3 genetic variants categories. They were including predicted variants (n = 618), previously reported variants that were identified in gnomAD (n = 140), and the 153 variants that have been reported to cause hTTP in the literature but were not found in gnomAD. This figure also shows the distribution of the CADD score among them, with no significant differences between these 3 groups.
Evaluation of the severity of variants’ pathogenicity. We used a combined annotation dependent depletion score (CADD), a tool for scoring the deleteriousness of genetic variants, with cutoff of >2031 to determine the effect of variant severity, among the 3 genetic variants categories. They were including predicted variants (n = 618), previously reported variants that were identified in gnomAD (n = 140), and the 153 variants that have been reported to cause hTTP in the literature but were not found in gnomAD. This figure also shows the distribution of the CADD score among them, with no significant differences between these 3 groups.
Most frequent pathogenic variants in the gnomAD population stratified by ethnicity
The 5 most frequent gnomAD pathogenic variants, considering only the 140 previously reported variants, identified in each ethnic group are shown in Table 4. Among them, p.Arg1060Trp, p.Pro457Leu, and p.Asp187His were shared by different populations (Figure 2). However, several other variants were ethnic group specific: p.Cys1024Gly and p.Ala606Pro in Africans/African Americans; p.Ile1217Thr and p.Arg1219Gln in Admixed Americans; p.Cys322Gly, p.Arg916Cys, and p.Arg1123His in Finns; p.Arg659Lys and p.Arg1274Cys in Europeans; and p.Glu641Lys and p.Gln436His in South Asians. East Asian and Middle Eastern populations exhibited unique mutational profiles, containing none or only 2 of the common pathogenic variants shared by other ethnicities, respectively (Table 4). Supplemental Table 6 presents the 5 most frequent pathogenic variants for each ethnicity, considering all 758 variants.
The most common previously reported pathogenic variants stratified by ethnicity
| Ethnic group . | c.DNA . | Protein . | rs ID . | Type of variant . | Domain . | MAF∗ . |
|---|---|---|---|---|---|---|
| African/African American | c.13y70C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00050 |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00043 | |
| c.3070T>G | p.Cys1024Gly | rs121908472 | Missense | TSP1_7 | 0.00035 | |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00021 | |
| c.1816G>C | p.Ala606Pro | rs281875290 | Missense | Spacer | 0.00009 | |
| Admixed American | c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00224 |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00072 | |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00053 | |
| c.3650T>C | p.Ile1217Thr | rs200847393 | Missense | CUB_1 | 0.00025 | |
| c.3656G>A | p.Arg1219Gln | rs782649881 | Missense | CUB_1 | 0.00010 | |
| Ashkenazi Jewish | c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00068 |
| c.577C>T | p.Arg193Trp | rs281875287 | Missense | Mp | 0.00007 | |
| c.1520G>A | p.Arg507Gln | rs281875296 | Missense | Cys_Rich | 0.00004 | |
| c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00003 | |
| c.2674C>T | p.Gln892Ter | rs1554792374 | Stop_gained | TSP1_4 | 0.00003 | |
| East Asian | c.2167C>A | p.Gln723Lys | rs138014548 | Missense | TSP1_2 | 0.00557 |
| c.2723G>A | p.Cys908Tyr | rs281875301 | Missense | TSP1_5 | 0.00039 | |
| c.1345C>T | p.Gln449Ter | rs121908476 | Stop_gained | Cys_Rich | 0.00020 | |
| c.530A>G | p.Tyr177Cys | rs200594025 | Missense | Mp | 0.00019 | |
| c.581G>T | p.Gly194Val | rs1554785242 | Missense | Mp | 0.00013 | |
| Middle Eastern | c.1187G>A | p.Cys396Tyr | – | Missense | TSP1_1 | 0.00024 |
| c.1922del | p.Glu641GlyfsTer57 | – | Frameshift | Spacer | 0.00024 | |
| c.3892+1G>A | – | rs1554796161 | Splicing | CUB_1 | 0.00018 | |
| c.2293C>T | p.Arg765Trp | rs782614158 | Missense | TSP1_3 | 0.00018 | |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00016 | |
| Finnish | c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00505 |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00046 | |
| c.964T>G | p.Cys322Gly | rs931645668 | Missense | Dis | 0.00016 | |
| c.2746C>T | p.Arg916Cys | rs374444423 | Missense | TSP1_5 | 0.00012 | |
| c.3368G>A | p.Arg1123His | rs782311861 | Missense | TSP1_8 | 0.00004 | |
| c.4143dup | p.Glu1382ArgfsTer6 | rs387906343 | Missense | CUB_2 | 0.00003 | |
| European | c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00235 |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00133 | |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00045 | |
| c.1976G>A | p.Arg659Lys | rs150764227 | Missense | Spacer | 0.00032 | |
| c.3820C>T | p.Arg1274Cys | rs371964138 | Missense | CUB_1 | 0.00014 | |
| South Asian | c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00071 |
| c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00040 | |
| c.1921G>A | p.Glu641Lys | rs782547718 | Missense | Spacer | 0.00027 | |
| c.1308G>C | p.Gln436His | rs587642546 | Missense | TSP1_1 | 0.00013 | |
| c.2293C>T | p.Arg765Trp | rs782614158 | Missense | TSP1_3 | 0.00011 |
| Ethnic group . | c.DNA . | Protein . | rs ID . | Type of variant . | Domain . | MAF∗ . |
|---|---|---|---|---|---|---|
| African/African American | c.13y70C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00050 |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00043 | |
| c.3070T>G | p.Cys1024Gly | rs121908472 | Missense | TSP1_7 | 0.00035 | |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00021 | |
| c.1816G>C | p.Ala606Pro | rs281875290 | Missense | Spacer | 0.00009 | |
| Admixed American | c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00224 |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00072 | |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00053 | |
| c.3650T>C | p.Ile1217Thr | rs200847393 | Missense | CUB_1 | 0.00025 | |
| c.3656G>A | p.Arg1219Gln | rs782649881 | Missense | CUB_1 | 0.00010 | |
| Ashkenazi Jewish | c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00068 |
| c.577C>T | p.Arg193Trp | rs281875287 | Missense | Mp | 0.00007 | |
| c.1520G>A | p.Arg507Gln | rs281875296 | Missense | Cys_Rich | 0.00004 | |
| c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00003 | |
| c.2674C>T | p.Gln892Ter | rs1554792374 | Stop_gained | TSP1_4 | 0.00003 | |
| East Asian | c.2167C>A | p.Gln723Lys | rs138014548 | Missense | TSP1_2 | 0.00557 |
| c.2723G>A | p.Cys908Tyr | rs281875301 | Missense | TSP1_5 | 0.00039 | |
| c.1345C>T | p.Gln449Ter | rs121908476 | Stop_gained | Cys_Rich | 0.00020 | |
| c.530A>G | p.Tyr177Cys | rs200594025 | Missense | Mp | 0.00019 | |
| c.581G>T | p.Gly194Val | rs1554785242 | Missense | Mp | 0.00013 | |
| Middle Eastern | c.1187G>A | p.Cys396Tyr | – | Missense | TSP1_1 | 0.00024 |
| c.1922del | p.Glu641GlyfsTer57 | – | Frameshift | Spacer | 0.00024 | |
| c.3892+1G>A | – | rs1554796161 | Splicing | CUB_1 | 0.00018 | |
| c.2293C>T | p.Arg765Trp | rs782614158 | Missense | TSP1_3 | 0.00018 | |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00016 | |
| Finnish | c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00505 |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00046 | |
| c.964T>G | p.Cys322Gly | rs931645668 | Missense | Dis | 0.00016 | |
| c.2746C>T | p.Arg916Cys | rs374444423 | Missense | TSP1_5 | 0.00012 | |
| c.3368G>A | p.Arg1123His | rs782311861 | Missense | TSP1_8 | 0.00004 | |
| c.4143dup | p.Glu1382ArgfsTer6 | rs387906343 | Missense | CUB_2 | 0.00003 | |
| European | c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00235 |
| c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00133 | |
| c.559G>C | p.Asp187His | rs148312697 | Missense | Mp | 0.00045 | |
| c.1976G>A | p.Arg659Lys | rs150764227 | Missense | Spacer | 0.00032 | |
| c.3820C>T | p.Arg1274Cys | rs371964138 | Missense | CUB_1 | 0.00014 | |
| South Asian | c.3178C>T | p.Arg1060Trp | rs142572218 | Missense | TSP1_7 | 0.00071 |
| c.1370C>T | p.Pro457Leu | rs36220240 | Missense | Cys_Rich | 0.00040 | |
| c.1921G>A | p.Glu641Lys | rs782547718 | Missense | Spacer | 0.00027 | |
| c.1308G>C | p.Gln436His | rs587642546 | Missense | TSP1_1 | 0.00013 | |
| c.2293C>T | p.Arg765Trp | rs782614158 | Missense | TSP1_3 | 0.00011 |
Number of previously reported pathogenic variants: n = 140.
c.DNA, coding DNA reference sequences; MAF, minor allele frequency; rs ID, the reference single nucleotide polymorphism (rs) ID.
MAF is the frequency at which the second most common allele occurs in a given population; it refers to the less frequent allele.
Discussion
hTTP is currently considered to be a rare disorder, with a prevalence of 0.5 to 2.0 per 106 people.4 Current genetic epidemiology methodologies that define valid prevalence estimations for population-based scales can more accurately estimate the prevalence of hTTP.23-25 The advent of NGS has revolutionized genetic epidemiology because data from large international consortia are now increasingly available.22 Our comprehensive genetic epidemiology investigation provides a global estimation of hTTP prevalence using genome and exome data from 807 162 individuals. Based on the frequency of 140 documented pathogenic variants, the worldwide prevalence of hTTP was estimated to be 23 per 106 people, with a carrier frequency of 1 per 100 people. This prevalence is significantly higher than the previously reported prevalence of 0.5 to 2.0 per 106.2,4 Because none of these 140 variants were classified as “benign” or “likely benign” in ClinVar, we believe that our findings reflect the actual hTTP prevalence. We identified 17 homozygous patients with 5 pathogenic variants (8 men and 9 women) in this gnomAD population, a prevalence of 17 per 807 162 people (or 21 per 106), similar to our estimated prevalence of 23 per 106 people. Of note, because of the extreme rarity of the predicted (novel) variants, homozygosity is less likely. The estimated prevalence of hTTP was even higher when we included the frequency of the additional 618 ADAMTS13 variants that were predicted to be pathogenic. Using all 758 variants, the global prevalence increased to 40 cases per 106 individuals, with a global carrier frequency of 1.3 per 100 people.
The prevalence of pathogenic variants causing hTTP that were established by this study indicates that the frequency of hTTP is much greater than previously reported. Many people carrying these biallelic variants are asymptomatic until a triggering event such as an infection or pregnancy occurs or, if they have symptoms, they are undiagnosed. Women with hTTP might remain undiagnosed because pregnancies in these women can lead to recurrent miscarriages or pregnancy losses.38 Consequently, these women may cease attempting to conceive further, thus never experiencing a pregnancy that would trigger the symptoms leading to a diagnosis of hTTP. The clinical variability of hTTP poses challenges for establishing the diagnosis. hTTP may not be recognized until adulthood,39 during pregnancy,17,18 or when stroke symptoms occur.20 The clinical features of hTTP are influenced by the ADAMTS13 variant(s) because different variants are associated with different levels of residual ADAMTS13 activity,40 although only few pathogenic ADAMTS13 variants give rise to reproducible residual activity. Considering that in many patients with hTTP the clinical manifestations require a stimulus, such as the sudden shift of pulmonary circulation in newborn infants,9,10 pregnancy,17,18 or infection,2,9,41 it can be expected that some patients with hTTP are not diagnosed.
Among the 140 reported variants, some were common in multiple ethnicities, but others were ethnicity specific. Prevalences among ethnicities ranged from 0.6 per 106 and 1 per 106 individuals in Ashkenazi Jews and Middle Eastern populations, respectively, to 42 per 106, 32 per 106, and 28 per 106 individuals in East Asians, Finnish, and non-Finnish Europeans, respectively. A partial explanation for this observation is that 5 common hTTP pathogenic variants (p.Pro457Leu, p.Arg1060Trp, p.Asp187His, p.Arg659Lys, and c.4143_4144dupA) were more frequent in the Finnish and non-Finnish Europeans (Figure 2; Table 4). p.Pro457Leu was frequently identified in all populations, however, globally, it has been reported in only few patients with hTTP.42-44 p.Gln723Lys was the driver of the high prevalence in East Asians, accounting for 85% of pathogenic alleles (Figure 2; Table 4). It is not known what advantage positively selected these 6 variants in the European and East Asian ancestries. It is also not known why Ashkenazi Jews and Middle Eastern people have such a low prevalence. We have 2 potential explanations for this observation: (i) it is likely that Middle Eastern patients with hTTP had fewer chances of survival in the past and thus had fewer chances of passing their genetic variants to the next generation; as consanguineous marriages are common there, homozygous phenotypes are more likely to occur, possibly resulting in higher mortality rates. Patients may also be grossly underdiagnosed or misdiagnosed and not properly treated. Notably, the hTTP prevalence in the Middle Eastern populations is substantially higher using both predicted and reported variants, suggesting a lack of genetic characterization of hTTP in these populations; (ii) the low prevalence of hTTP in Ashkenazi Jewish could be because of the closed community in which hTTP variants from other ethnicities did not enter in this population as much as in other populations.
p.Arg1060Trp is a widespread variant, being the predominant hTTP-associated variant in British and French patients, and is also reported in patients from Scandinavia, Central Europe, Italy, Turkey, and North Americans of European ancestry.2 c.4143_4144dupA (c.4143dup) was a frequent pathogenic variant in non-Finnish Europeans, 78 of 83 affected alleles by this variant were found in Europeans. Haplotype studies have suggested that c.4143dup is a founder mutation originating from a common ancestor in central Europe.45 The Hereditary TTP Registry46 reported that c.4143dup was the most common variant among patients with hTTP enrolled from Europe, Asia, the United States, and Africa; it was present in 60 (24%) of 246 mutated alleles. Other frequent hTTP-associated variants in the Hereditary TTP Registry patients were p.Arg1060Trp (13/246 alleles) and p.Arg193Trp (11/246 alleles). p.Gln723Lys is reported in a severe hTTP case from Japan,47 and we found it as the major pathogenic variant in East Asia.
A CADD score distribution was used to compare the severity of the pathogenic variants identified in the gnomAD (140 reported and 618 novel variants) with the 153 variants reported to be associated with hTTP but were not found in gnomAD. No significant differences were observed (Figure 3). This suggests that pathogenic variants identified in gnomAD have a similar severity effect on the ADAMTS13 protein as of those previously reported.
In addition to the 140 previously documented pathogenic variants in gnomAD, we identified 618 novel variants with pathogenic features. This emphasizes that many pathogenic ADAMTS13 variants remain unknown. Using PubMed and other genetic databases, we compiled an evidence-based data set of 293 pathogenic variants that are reportedly associated with hTTP. Among these 293 pathogenic variants, 140 were found in gnomAD and 153 were not.
A limitation of our identification of these 618 novel variants is the use of in silico algorithms to predict the pathogenicity of novel missense and splicing ADAMTS13 variants. To be conservative in determining pathogenic variants, we used a restricted approach that minimized false positives by using 7 different tools for missense and 4 for splicing variants. We anticipated that we underestimated the number of pathogenic variants, such as promoter and deep intronic variants. In addition, we would not have identified pathogenic gross deletions and gene rearrangements because they cannot be detected by exome sequencing. Because gnomAD provides only the aggregated allele frequencies without access to individual genomic sequences, we cannot directly estimate the number of compound heterozygotes for ADAMTS13 pathogenic variants. We assume that our conservative strategy led to the exclusion of some pathogenic variants, therefore, the prevalence of hTTP is probably greater than we have reported.
In conclusion, our study reports a higher than expected frequency of hTTP. This population-based genetic epidemiology analysis indicates that the prevalence of hTTP is 20- to 40-fold higher than previous estimates. Our data suggest that many patients with hTTP are undiagnosed, and many may have died during the neonatal period, emphasizing the importance of suspecting hTTP in patients with microangiopathic hemolysis and thrombocytopenia.
Acknowledgments
This work was partially supported by the Italian Ministry of Health (Bando Ricerca Corrente). The Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico is a member of the European Reference Network EuroBloodNet.
Authorship
Contribution: O.S. and F.P. designed the study; O.S. and A.C. collected and analyzed data; O.S. and J.N.G. wrote the manuscript; I.M., J.N.G., and F.P. critically revised the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: F.P. reports participation at educational meetings of Takeda and Spark; and reports participation on the advisory board of CSL Behring, Biomarin, Roche, Sanofi, and Sobi. I.M. reports participation at educational meetings organized by Sanofi and Werfen. The remaining authors declare no competing financial interests.
Correspondence: Flora Peyvandi, Fondazione IRCCS Ca'Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Via Pace 9, 20122 Milan, Italy; email: flora.peyvandi@unimi.it.
References
Author notes
Data are available on request from the corresponding author, Flora Peyvandi (flora.peyvandi@unimi.it).
The full-text version of this article contains a data supplement.