Key Points
This was a prospective long-term follow-up study including 14 530 PBSC and 7123 BM volunteer URDs from NMDP.
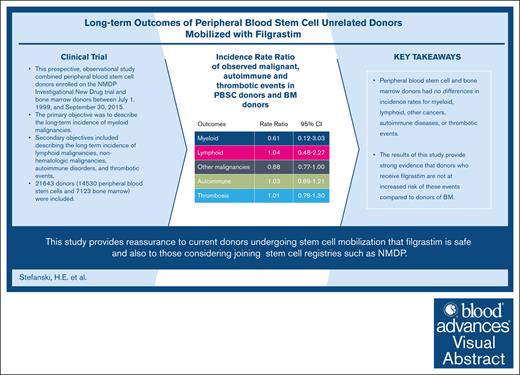
PBSC and BM donors had no differences in incidence rates for myeloid, lymphoid, other cancers, autoimmune diseases, or thrombotic events.
Visual Abstract
Allogeneic hematopoietic cell transplantation is a life-saving procedure used to treat a variety of devastating diseases. It requires hematopoietic stem cells collected via filgrastim-mobilized peripheral blood stem cells (PBSCs) or bone marrow (BM) harvest from volunteer unrelated donors (URDs). There is a paucity of safety data regarding donors’ long-term adverse events. This prospective, observational study combined PBSC donors enrolled in the NMDP Investigational New Drug trial and BM donors between 1 July 1999 and 30 September 2015. The primary objective was to describe the long-term incidence of myeloid malignancies. The secondary objectives included describing the long-term incidence of lymphoid malignancies, nonhematologic malignancies, autoimmune disorders, and thrombotic events. A total of 21 643 donors (14 530 PBSCs and 7123 BM) were included. The incidence rate of myeloid disorders per 100 000 person-years in donors of PBSCs was 2.53 (95% confidence interval [CI], 0.82-7.84) and in donors of BM, it was 4.13 (95% CI, 1.33-12.8). The incidence rate ratio of PBSCs/BM donors was 0.61 (95% CI, 0.12-3.03; P = .55). The incidence of other malignancies, autoimmunity, and thrombosis did not differ between the donor types. This comprehensive study of the long-term effects of filgrastim in URDs of PBSCs provides strong evidence that donors who receive filgrastim are not at an increased risk of these events compared with BM donors. It also provides reassurance to current donors undergoing stem cell mobilization as well as individuals considering joining stem cell registries, such as NMDP.
Introduction
Allogeneic hematopoietic cell transplantation is a life-saving procedure for the treatment of devastating diseases. The NMDP was established in 1987 to facilitate allogeneic hematopoietic cell transplantation from anonymous, volunteer adult unrelated donors (URDs) to patients in need. Hematopoietic stem cells (HSCs) are typically collected from URDs in 2 ways: either via bone marrow (BM) harvest or by recombinant human granulocyte colony-stimulating factor (filgrastim) mobilized peripheral blood stem cell (PBSC) collection. BM has been used as a source of HSC since the 1960s1; however, since the recognition that HSC can be mobilized from the BM by filgrastim treatment and collected via apheresis in the 1990s, PBSCs have been increasingly used.2,3 The data collected to date have primarily focused on short-term side effects for both BM and PBSC donors.4-22
Previous reports have suggested that filgrastim increases the risk of myelodysplastic syndrome or acute myeloid leukemia (AML) in patients with severe chronic neutropenia,23 breast cancer,24,25 and lung cancer.25 There have also been reports of URDs that developed myeloid malignancies after receiving filgrastim;26,27 however, additional studies with longer follow-up provided reassurance that filgrastim does not increase the risk of cancer in URDs.11,28-32
It has been documented that autoimmune diseases such as iritis can rarely be exacerbated after filgrastim administration in normal donors.33 Worsening autoimmune diseases can also be seen in patients with multiple sclerosis (MS),34,35 systemic lupus erythematosus,36-38 rheumatoid arthritis,39 and other immune-mediated vasculidities40-42 when treated for neutropenia with filgrastim.
Thrombosis is of concern as a side effect of filgrastim. Administration of filgrastim leads to a potentially prothrombotic state via stimulation of tissue factors and increased endothelial markers.43 Additionally, there have been reports of healthy donors having acute arterial thrombosis related to filgrastim.44
Since 1997, NMDP has maintained an Investigational New Drug (IND) application, accepted by the Food and Drug Administration for the manufacture of PBSC products from URDs. To address whether filgrastim administration in URDs has significant long-term consequences, we evaluated the incidence of malignant, autoimmune, and thrombotic disorders after HSC donation from PBSC donors. The results of this study, the largest and most comprehensive, are presented in this manuscript.
Design and methods
The NMDP initiated an IND to collect adverse events (AEs) for donors who underwent PBSC donation in 1997. All donors were mobilized with subcutaneous filgrastim (Neupogen; Amgen, Thousand Oaks, CA) at an approximate dose of 10 μg/kg per day for 5 days. The study population presented in this article was comprised of 2 cohorts of URDs from the United States and was activated on 1 October 2010, with all follow-up being completed by 30 September 2020. There was an early group, registered either prospectively on the NMDP PBSC IND trial or, if a BM donor, on the NMDP Registry BM follow-up trial between 1 July 1999 and 30 September 2010, and a later donor group who underwent collection from 1 October 2010 to 30 September 2015. The trials were approved by the NMDP Institutional Review Board.
The primary objective of this study was to describe the long-term incidence of malignant myeloid disorders (AML, myelodysplastic syndrome, chronic myelogenous leukemia, or chronic myeloproliferative disorders) in PBSC and BM donors and to compare the incidences between the donor cohorts, with BM donors serving as the control. The secondary objectives were to describe malignant lymphoid disorders (acute lymphoblastic leukemia, chronic lymphocytic leukemia, Hodgkin lymphoma, or non-Hodgkin lymphoma), nonhematologic malignant disorders (as defined by the Surveillance, Epidemiology, and End Results [SEER] Program database45), autoimmune diseases, and thrombotic events (venous and arterial) in PBSC and BM donors.
Donors completed biennial surveys to report new diagnoses of malignancy, autoimmune, or thrombotic (MAT) events over ∼10 years, until the study conclusion. All donor follow-up assessments were administered by trained staff members of the Center for International Blood and Marrow Transplant Research Survey Research Group (SRG) and Donor Center staff. Donors were asked if they had developed any cancers or autoimmune disorders, or had a blood clot anywhere other than where they had an IV placed because their donation or previous follow-up, and if yes, when the diagnosis occurred.
When donors reported a MAT event, the SRG requested a medical records release form and contacted donors’ clinics to obtain relevant medical records. NMDP medical staff reviewed and then documented whether the reported MAT event was correct and occurred after collection, correct but occurred before collection, incorrect or not a MAT event, duplicative of a previously reported event, or unverified with existing records. If a donor did not return a medical records release form or SRG was unable to obtain records from their clinic, the reported MAT event was coded as unverified.
Subjects
Inclusion criteria included: URDs who donated either BM or PBSC between 1 July 1999 and 30 September 2015; URDs who received at least 1 injection of filgrastim or more but did not donate PBSC between 1 July 1999 and 30 September 2015; donation was managed by a participating US donor center; and URDs provided informed consent for participation in this study. Exclusion criteria included URD who donated filgrastim-mobilized BM, donation managed by a non-US donor center, or donor was unable to verbally communicate in any of the following languages: English, Spanish, Mandarin Chinese, Cantonese Chinese, Vietnamese, Korean, or Portuguese. This study was registered as EUPAS19126 and as NCT01362179.
Statistical methods
Counts and percentages were used to describe the frequencies of donor characteristics. Donors in the BM group included those who later underwent filgrastim mobilization, and outcomes from BM donation were censored at the time of mobilization. Donors in the PBSC group who had previously donated BM were followed for outcomes as part of the PBSC group. A descriptive analysis was used to estimate the overall and age-specific incidence rates of MAT events. Rate ratios for MAT events were estimated for donors of PBSC vs BM. To determine if there were differences in myeloid, lymphoid, and other cancer events in donors who donated PBSC or BM compared with the general population, we used the SEER Program database,45 which provides incidence rates on cancer statistics among the US population, as a post hoc analysis. Age-adjusted rates were calculated using the 2000 US Standard Population. P values and 95% confidence intervals (CIs) for all rate ratios were calculated using Poisson regression methods. A P value of <.05 was considered significant. Statistical analyses were done using SAS version 9.4 (Cary, NC).
Results
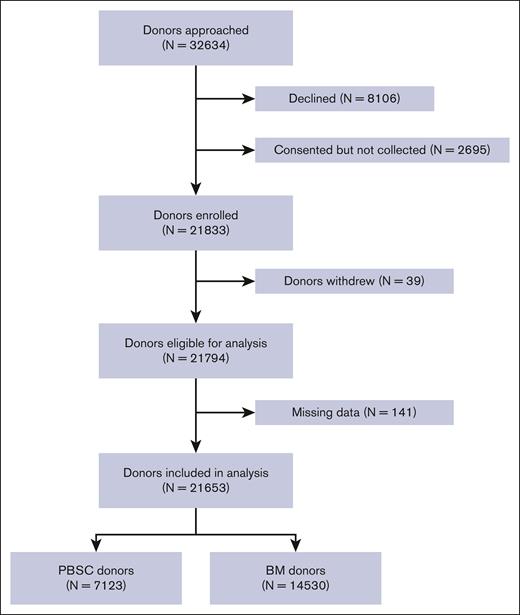
A total of 32 634 donors were contacted to participate in this study (Figure 1) and 21 833 (66.9%) donors were enrolled. Of the subjects enrolled, 39 donors withdrew and 141 subjects with missing data were removed, leaving 21 653 donors in the final analyses. The total donor years were 118 720 and 72 643 for donors of PBSC and BM, respectively.
The demographics of the donors are described in Table 1. There were 14 530 donors in the PBSC cohort, of whom 3.6% had previously donated BM. Most PBSC donors were male (59%) and white (76%). The median age (range) at the first filgrastim injection was 33 (18-62) years and the median time to follow-up was 7 years (0.6-20.2) (Table 1). There were 7123 donors in the BM donor cohort, of whom 6.3% were later exposed to filgrastim. Most BM donors were male (57%) and White (71%). The median age (range) at the first BM collection was 35 years (19-61), and the median time of follow-up after collection was 9 years (0-29.2).
Demographic characteristics of donors
| Characteristic . | PBSC donors n (%) . | BM donors n (%) . | P value . |
|---|---|---|---|
| No. of donors | 14 530 | 7123 | |
| No. of filgrastim exposures, n (%) | |||
| 1 | 13 579 (93) | N/A | |
| 2 | 416 (3) | N/A | |
| 3 | 6 (<1) | N/A | |
| No. of BM donations, n (%) | |||
| 1 | N/A | 6496 (91) | |
| 2 | N/A | 169 (2) | |
| 3 | N/A | 12 (<1) | |
| No. of both BM donations and filgrastim exposures∗, n (%) | .846 | ||
| 1 BM donation,1 filgrastim exposure | 498 (3) | 416 (6) | |
| 1 BM donation, 2 filgrastim exposures | 13 (<1) | 12 (<1) | |
| 2 BM donations, 1 filgrastim exposure | 18 (<1) | 18 (<1) | |
| Sex, n (%) | .019 | ||
| Female | 5975 (41) | 3048 (43) | |
| Male | 8 555 (59) | 4075 (57) | |
| Race, n (%) | <.001 | ||
| White | 10 980 (76) | 5070 (71) | |
| Hispanic | 1 252 (9) | 798 (11) | |
| Black | 498 (3) | 396 (6) | |
| Asian/Pacific Islander | 800 (6) | 407 (6) | |
| Native American | 103 (1) | 68 (1) | |
| Multiple races/other | 848 (6) | 360 (5) | |
| Unknown/decline | 49 (<1) | 24 (<1) | |
| Age at first BM collection or first filgrastim injection, y, n (%) | <.001 | ||
| Median (min-max) | 33 (18-62) | 35 (19-61) | <.001 |
| 18-29 | 5 896 (41) | 2542 (36) | |
| 30-39 | 3 871 (27) | 2186 (31) | |
| 40-49 | 3 231 (22) | 1804 (25) | |
| 50+ | 1 532 (11) | 591 (8) | |
| Year of first BM collection or first filgrastim injection, n (%) | <.001 | ||
| 1989 | 0 | 1 (<1) | |
| 1990 | 0 | 4 (<1) | |
| 1991 | 0 | 3 (<1) | |
| 1992 | 0 | 7 (<1) | |
| 1993 | 0 | 19 (<1) | |
| 1994 | 0 | 9 (<1) | |
| 1995 | 0 | 10 (<1) | |
| 1996 | 0 | 25 (<1) | |
| 1997 | 0 | 30 <1) | |
| 1998 | 0 | 36 (1) | |
| 1999 | 15 (<1) | 281 (4) | |
| 2000 | 137 (1) | 492 (7) | |
| 2001 | 195 (1) | 435 (6) | |
| 2002 | 317 (2) | 457 (6) | |
| 2003 | 466 (3) | 375 (5) | |
| 2004 | 453 (3) | 307 (4) | |
| 2005 | 537 (4) | 243 (3) | |
| 2006 | 587 (4) | 272 (4) | |
| 2007 | 652 (4) | 276 (4) | |
| 2008 | 777 (5) | 280 (4) | |
| 2009 | 850 (6) | 297 (4) | |
| 2010 | 1 001 (7) | 329 (5) | |
| 2011 | 1 450 (10) | 510 (7) | |
| 2012 | 1 772 (12) | 656 (9) | |
| 2013 | 2 010 (14) | 656 (9) | |
| 2014 | 1 817 (13) | 625 (9) | |
| 2015 | 1 479 (10) | 484 (7) | |
| 2016 | 15 (<1) | 4 (<1) | |
| Median (min-max) follow-up, y | 7.0 (0.6-20.2) | 9.0 (0.0-29.2) | <.001 |
| Characteristic . | PBSC donors n (%) . | BM donors n (%) . | P value . |
|---|---|---|---|
| No. of donors | 14 530 | 7123 | |
| No. of filgrastim exposures, n (%) | |||
| 1 | 13 579 (93) | N/A | |
| 2 | 416 (3) | N/A | |
| 3 | 6 (<1) | N/A | |
| No. of BM donations, n (%) | |||
| 1 | N/A | 6496 (91) | |
| 2 | N/A | 169 (2) | |
| 3 | N/A | 12 (<1) | |
| No. of both BM donations and filgrastim exposures∗, n (%) | .846 | ||
| 1 BM donation,1 filgrastim exposure | 498 (3) | 416 (6) | |
| 1 BM donation, 2 filgrastim exposures | 13 (<1) | 12 (<1) | |
| 2 BM donations, 1 filgrastim exposure | 18 (<1) | 18 (<1) | |
| Sex, n (%) | .019 | ||
| Female | 5975 (41) | 3048 (43) | |
| Male | 8 555 (59) | 4075 (57) | |
| Race, n (%) | <.001 | ||
| White | 10 980 (76) | 5070 (71) | |
| Hispanic | 1 252 (9) | 798 (11) | |
| Black | 498 (3) | 396 (6) | |
| Asian/Pacific Islander | 800 (6) | 407 (6) | |
| Native American | 103 (1) | 68 (1) | |
| Multiple races/other | 848 (6) | 360 (5) | |
| Unknown/decline | 49 (<1) | 24 (<1) | |
| Age at first BM collection or first filgrastim injection, y, n (%) | <.001 | ||
| Median (min-max) | 33 (18-62) | 35 (19-61) | <.001 |
| 18-29 | 5 896 (41) | 2542 (36) | |
| 30-39 | 3 871 (27) | 2186 (31) | |
| 40-49 | 3 231 (22) | 1804 (25) | |
| 50+ | 1 532 (11) | 591 (8) | |
| Year of first BM collection or first filgrastim injection, n (%) | <.001 | ||
| 1989 | 0 | 1 (<1) | |
| 1990 | 0 | 4 (<1) | |
| 1991 | 0 | 3 (<1) | |
| 1992 | 0 | 7 (<1) | |
| 1993 | 0 | 19 (<1) | |
| 1994 | 0 | 9 (<1) | |
| 1995 | 0 | 10 (<1) | |
| 1996 | 0 | 25 (<1) | |
| 1997 | 0 | 30 <1) | |
| 1998 | 0 | 36 (1) | |
| 1999 | 15 (<1) | 281 (4) | |
| 2000 | 137 (1) | 492 (7) | |
| 2001 | 195 (1) | 435 (6) | |
| 2002 | 317 (2) | 457 (6) | |
| 2003 | 466 (3) | 375 (5) | |
| 2004 | 453 (3) | 307 (4) | |
| 2005 | 537 (4) | 243 (3) | |
| 2006 | 587 (4) | 272 (4) | |
| 2007 | 652 (4) | 276 (4) | |
| 2008 | 777 (5) | 280 (4) | |
| 2009 | 850 (6) | 297 (4) | |
| 2010 | 1 001 (7) | 329 (5) | |
| 2011 | 1 450 (10) | 510 (7) | |
| 2012 | 1 772 (12) | 656 (9) | |
| 2013 | 2 010 (14) | 656 (9) | |
| 2014 | 1 817 (13) | 625 (9) | |
| 2015 | 1 479 (10) | 484 (7) | |
| 2016 | 15 (<1) | 4 (<1) | |
| Median (min-max) follow-up, y | 7.0 (0.6-20.2) | 9.0 (0.0-29.2) | <.001 |
N/A, not applicable; min, minimum; max, maximum; y, years.
Donors in the BM group who later had exposure to filgrastim were censored at the time of filgrastim exposure.
Myeloid malignancies
The incidence rate of myeloid disorders in PBSC donors was 2.53 (95% CI, 0.82-7.84) and in BM donors was 4.13 (95% CI, 1.33-12.8) per 100 000 person-years (Table 2). The incidence rate ratio of PBSC/BM donors was 0.61 (95% CI, 0.12-3.03; P = .55) (Table 2). The incidence rates by age range are shown in Tables 3-5 but as shown, there were very few events in both groups (3 each).
Incidence rates of MAT events
| Outcomes . | PBSC donors . | BM donors . | Rate ratio (95% CI) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|
| Person-years . | No. of events . | Incidence rate∗ (95% CI) . | Person-years . | No. of events . | Incidence rate∗ (95% CI) . | |||
| Myeloid | 118 712 | 3 | 2.53 (0.82-7.84) | 72 641 | 3 | 4.13 (1.33-12.8) | 0.61 (0.12-3.03) | .55 |
| Lymphoid | 118 649 | 17 | 14.33 (8.91-23.05) | 72 572 | 10 | 13.78 (7.41-25.61) | 1.04 (0.48-2.27) | .92 |
| Other malignancies | 116 213 | 535 | 460.36 (423.0-501.0) | 70 553 | 368 | 521.59 (471.1-577.6) | 0.88 (0.77-1.00) | .05 |
| Autoimmune | 116 566 | 449 | 385.19 (351.2-422.4) | 71 269 | 262 | 367.62 (325.8-414.9) | 1.03 (0.89-1.21) | .66 |
| Thrombosis | 117 977 | 156 | 132.23 (113.0-154.7) | 72 172 | 95 | 131.63 (107.7-160.9) | 1.01 (0.78-1.30) | .95 |
| Outcomes . | PBSC donors . | BM donors . | Rate ratio (95% CI) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|
| Person-years . | No. of events . | Incidence rate∗ (95% CI) . | Person-years . | No. of events . | Incidence rate∗ (95% CI) . | |||
| Myeloid | 118 712 | 3 | 2.53 (0.82-7.84) | 72 641 | 3 | 4.13 (1.33-12.8) | 0.61 (0.12-3.03) | .55 |
| Lymphoid | 118 649 | 17 | 14.33 (8.91-23.05) | 72 572 | 10 | 13.78 (7.41-25.61) | 1.04 (0.48-2.27) | .92 |
| Other malignancies | 116 213 | 535 | 460.36 (423.0-501.0) | 70 553 | 368 | 521.59 (471.1-577.6) | 0.88 (0.77-1.00) | .05 |
| Autoimmune | 116 566 | 449 | 385.19 (351.2-422.4) | 71 269 | 262 | 367.62 (325.8-414.9) | 1.03 (0.89-1.21) | .66 |
| Thrombosis | 117 977 | 156 | 132.23 (113.0-154.7) | 72 172 | 95 | 131.63 (107.7-160.9) | 1.01 (0.78-1.30) | .95 |
CI, Confidence interval
Incidence rate per 100 000 person-years.
Incidence rate of MAT events, stratified by age: crude incidence rate of MAT events in PBSC donors
| Age, years . | 18-29 . | 30-39 . | 40-49 . | 50+ . |
|---|---|---|---|---|
| No. of donors | 5896 | 3871 | 3231 | 1532 |
| Donor-years | 41 450 | 33 548 | 29 717 | 13 969 |
| Myeloid | ||||
| Donor-years | 41 444 | 33 584 | 29 715 | 13 968 |
| Events | 1 | 0 | 1 | 1 |
| Crude rate∗ | 2.41 | 0.00 | 3.37 | 7.16 |
| 95% CI | (0.341-7.13) | N/A | (0.47-23.89) | (1.01-50.82) |
| Lymphoid | ||||
| Donor-years | 41 432 | 33 558 | 29 704 | 13 956 |
| Events | 4 | 5 | 5 | 3 |
| Crude rate∗ | 9.65 | 14.90 | 16.83 | 21.50 |
| 95% CI | (3.62-25.72) | (6.20-35.79) | (7.01-40.44) | (6.93-66.64) |
| Other malignancies | ||||
| Donor-years | 41 249 | 33 099 | 28 644 | 13 221 |
| Events | 49 | 105 | 225 | 156 |
| Crude rate∗ | 118.79 | 317.23 | 785.50 | 1179.97 |
| 95% CI | (89.80-157.2) | (262.1-384.0) | (689.6-894.7) | (1009.5-1379.1) |
| Autoimmune | ||||
| Donor-years | 40 855 | 32 915 | 29 105 | 13 690 |
| Events | 137 | 140 | 115 | 57 |
| Crude rate∗ | 335.33 | 425.34 | 395.12 | 416.35 |
| 95% CI | (238.7-396.4) | (360.5-501.8) | (329.2-474.2) | (321.3-539.5) |
| Thrombosis | ||||
| Donor-years | 41 345 | 33 401 | 29 409 | 13 821 |
| Events | 25 | 40 | 60 | 31 |
| Crude rate∗ | 60.47 | 119.76 | 204.02 | 224.29 |
| 95% CI | (40.86-89.48) | (87.86-163.2) | (158.5-262.7) | (157.8-318.8) |
| Age, years . | 18-29 . | 30-39 . | 40-49 . | 50+ . |
|---|---|---|---|---|
| No. of donors | 5896 | 3871 | 3231 | 1532 |
| Donor-years | 41 450 | 33 548 | 29 717 | 13 969 |
| Myeloid | ||||
| Donor-years | 41 444 | 33 584 | 29 715 | 13 968 |
| Events | 1 | 0 | 1 | 1 |
| Crude rate∗ | 2.41 | 0.00 | 3.37 | 7.16 |
| 95% CI | (0.341-7.13) | N/A | (0.47-23.89) | (1.01-50.82) |
| Lymphoid | ||||
| Donor-years | 41 432 | 33 558 | 29 704 | 13 956 |
| Events | 4 | 5 | 5 | 3 |
| Crude rate∗ | 9.65 | 14.90 | 16.83 | 21.50 |
| 95% CI | (3.62-25.72) | (6.20-35.79) | (7.01-40.44) | (6.93-66.64) |
| Other malignancies | ||||
| Donor-years | 41 249 | 33 099 | 28 644 | 13 221 |
| Events | 49 | 105 | 225 | 156 |
| Crude rate∗ | 118.79 | 317.23 | 785.50 | 1179.97 |
| 95% CI | (89.80-157.2) | (262.1-384.0) | (689.6-894.7) | (1009.5-1379.1) |
| Autoimmune | ||||
| Donor-years | 40 855 | 32 915 | 29 105 | 13 690 |
| Events | 137 | 140 | 115 | 57 |
| Crude rate∗ | 335.33 | 425.34 | 395.12 | 416.35 |
| 95% CI | (238.7-396.4) | (360.5-501.8) | (329.2-474.2) | (321.3-539.5) |
| Thrombosis | ||||
| Donor-years | 41 345 | 33 401 | 29 409 | 13 821 |
| Events | 25 | 40 | 60 | 31 |
| Crude rate∗ | 60.47 | 119.76 | 204.02 | 224.29 |
| 95% CI | (40.86-89.48) | (87.86-163.2) | (158.5-262.7) | (157.8-318.8) |
CI, Confidence Interval
Incidence rate per 100 000 person-years.
Incidence rate of MAT events, stratified by age: crude incidence rate of MAT events in BM donors by age
| Age, years . | 18-29 . | 30-39 . | 40-49 . | 50+ . |
|---|---|---|---|---|
| No. of donors | 2542 | 2186 | 1804 | 591 |
| Donor-years | 21 549 | 23 995 | 20 671 | 6428 |
| Myeloid | ||||
| Donor-years | 21 549 | 23 993 | 20 671 | 6428 |
| Events | 0 | 2 | 1 | 0 |
| Crude rate∗ | 0.00 | 8.34 | 4.84 | 0.00 |
| 95% CI | N/A | 2.08-33.33 | 0.68-34.34 | N/A |
| Lymphoid | ||||
| Donor-years | 21 547 | 23 985 | 20 653 | 6387 |
| Events | 1 | 3 | 2 | 4 |
| Crude rate∗ | 4.64 | 12.51 | 9.68 | 62.63 |
| 95% CI | 0.65-32.95 | 4.03-38.78 | 2.42-38.72 | 23.51-166.8 |
| Other malignancies | ||||
| Donor-years | 21 305 | 23 513 | 19 786 | 5949 |
| Events | 41 | 91 | 156 | 80 |
| Crude rate∗ | 192.44 | 387.02 | 788.45 | 1344.74 |
| 95% CI | 141.7-261.3 | 315.3-475.1 | 674.4-921.8 | 1081.7-1671.8 |
| Autoimmune | ||||
| Donor-years | 21 194 | 23 611 | 20 208 | 6257 |
| Events | 73 | 71 | 84 | 34 |
| Crude rate∗ | 344.44 | 300.71 | 415.67 | 543.43 |
| 95% CI | 273.9-433.1 | 238.4-379.3 | 335.8-514.6 | 388.6-759.8 |
| Thrombosis | ||||
| Donor-years | 21 460 | 23 871 | 20 506 | 6335 |
| Events | 20 | 27 | 34 | 14 |
| Crude rate∗ | 93.19 | 113.11 | 165.80 | 221.01 |
| 95% CI | 60.14-144.4 | 77.58-164.9 | 118.5-232.0 | 131.0-372.9 |
| Age, years . | 18-29 . | 30-39 . | 40-49 . | 50+ . |
|---|---|---|---|---|
| No. of donors | 2542 | 2186 | 1804 | 591 |
| Donor-years | 21 549 | 23 995 | 20 671 | 6428 |
| Myeloid | ||||
| Donor-years | 21 549 | 23 993 | 20 671 | 6428 |
| Events | 0 | 2 | 1 | 0 |
| Crude rate∗ | 0.00 | 8.34 | 4.84 | 0.00 |
| 95% CI | N/A | 2.08-33.33 | 0.68-34.34 | N/A |
| Lymphoid | ||||
| Donor-years | 21 547 | 23 985 | 20 653 | 6387 |
| Events | 1 | 3 | 2 | 4 |
| Crude rate∗ | 4.64 | 12.51 | 9.68 | 62.63 |
| 95% CI | 0.65-32.95 | 4.03-38.78 | 2.42-38.72 | 23.51-166.8 |
| Other malignancies | ||||
| Donor-years | 21 305 | 23 513 | 19 786 | 5949 |
| Events | 41 | 91 | 156 | 80 |
| Crude rate∗ | 192.44 | 387.02 | 788.45 | 1344.74 |
| 95% CI | 141.7-261.3 | 315.3-475.1 | 674.4-921.8 | 1081.7-1671.8 |
| Autoimmune | ||||
| Donor-years | 21 194 | 23 611 | 20 208 | 6257 |
| Events | 73 | 71 | 84 | 34 |
| Crude rate∗ | 344.44 | 300.71 | 415.67 | 543.43 |
| 95% CI | 273.9-433.1 | 238.4-379.3 | 335.8-514.6 | 388.6-759.8 |
| Thrombosis | ||||
| Donor-years | 21 460 | 23 871 | 20 506 | 6335 |
| Events | 20 | 27 | 34 | 14 |
| Crude rate∗ | 93.19 | 113.11 | 165.80 | 221.01 |
| 95% CI | 60.14-144.4 | 77.58-164.9 | 118.5-232.0 | 131.0-372.9 |
N/A, not applicable; CI, Confidence Interval.
Incidence rate per 100 000 person-years.
Incidence rate of MAT events, stratified by age: incidence rate ratio of observed MAT events in PBSC donors and BM donors by age
| Age, years . | 18-29 . | 30-39 . | 40-49 . | 50+ . |
|---|---|---|---|---|
| Myeloid | ||||
| Rate ratio | NE | NE | 0.70 | NE |
| 95% CI | 0.04-11.2 | |||
| P value | .80 | |||
| Lymphoid | ||||
| Rate ratio | 2.09 | 1.20 | 1.75 | 0.34 |
| 95% CI | 0.23-18.7 | 0.29-5.00 | 0.34-9.01 | 0.08-1.53 |
| P value | .51 | .81 | .50 | .16 |
| Other malignancies | ||||
| Rate ratio | 0.61 | 0.81 | 0.98 | 0.89 |
| 95% CI | 0.40-0.92 | 0.61-1.07 | 0.80-1.21 | 0.68-1.17 |
| P value | .02∗ | .1344 | .87 | .41 |
| Autoimmune | ||||
| Rate ratio | 0.98 | 1.42 | 0.93 | 0.74 |
| 95% CI | 0.73-1.30 | 1.06-1.89 | 0.70-1.24 | 0.48-1.13 |
| P value | .86 | .02∗ | .62 | .16 |
| Thrombosis | ||||
| Rate ratio | 0.69 | 1.04 | 1.24 | 1.01 |
| 95% CI | 0.38-1.24 | 0.63-1.69 | 0.81-1.88 | 0.54-1.90 |
| P value | .21 | .89 | .32 | .97 |
| Age, years . | 18-29 . | 30-39 . | 40-49 . | 50+ . |
|---|---|---|---|---|
| Myeloid | ||||
| Rate ratio | NE | NE | 0.70 | NE |
| 95% CI | 0.04-11.2 | |||
| P value | .80 | |||
| Lymphoid | ||||
| Rate ratio | 2.09 | 1.20 | 1.75 | 0.34 |
| 95% CI | 0.23-18.7 | 0.29-5.00 | 0.34-9.01 | 0.08-1.53 |
| P value | .51 | .81 | .50 | .16 |
| Other malignancies | ||||
| Rate ratio | 0.61 | 0.81 | 0.98 | 0.89 |
| 95% CI | 0.40-0.92 | 0.61-1.07 | 0.80-1.21 | 0.68-1.17 |
| P value | .02∗ | .1344 | .87 | .41 |
| Autoimmune | ||||
| Rate ratio | 0.98 | 1.42 | 0.93 | 0.74 |
| 95% CI | 0.73-1.30 | 1.06-1.89 | 0.70-1.24 | 0.48-1.13 |
| P value | .86 | .02∗ | .62 | .16 |
| Thrombosis | ||||
| Rate ratio | 0.69 | 1.04 | 1.24 | 1.01 |
| 95% CI | 0.38-1.24 | 0.63-1.69 | 0.81-1.88 | 0.54-1.90 |
| P value | .21 | .89 | .32 | .97 |
NE, not evaluable; CI, Confidence Interval.
Statistically significant result (P < .05).
Lymphoid malignancies
The incidence rate of lymphoid malignancies in PBSC donors was 14.33 (95% CI, 8.91-23.05) and that in BM donors was 13.78 (95% CI, 7.41-25.61) per 100 000 person-years (Table 2). The incidence rate ratio was 1.04 (95% CI, 0.48-2.27; P = .92) (Table 2). The crude incidence rate was highest in donors >50 years of age in both PBSC (21.50; 95% CI, 6.93-66.64) (Table 3) and BM donors (62.63; 95% CI, 23.51-166.8) (Table 4) and the incidence rate ratio for donors >50 years of age was 0.34 (95% CI, 0.08-1.53; P = .16) (Table 5).
Nonhematologic malignancies
The incidence rate of nonhematologic malignancies, as defined in the SEER database, for PBSC donors was 460.36 (95% CI, 423.0-501.0) and in BM donors was 521.59 (95% CI, 471.1-577.6) per 100 000 person-years with an incidence rate ratio of 0.88 (95% CI, 0.77-1.00; P = .05) (Table 2). The crude incidence rates of other malignancies increased as donors aged in both PBSC and BM donors (Tables 3-4). However, in the 18 to 29 age group, there was a statistically significant incidence rate ratio of other malignancies (0.61, 95% CI, 0.40-0.92; P = .02) (Table 5) with the BM group having increased numbers of other types of malignancies, although the numbers were very low. The incidence rate ratio was not statistically significant in the other age groups.
Autoimmune diseases
The incidence rate of autoimmune diseases (eg, rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus, scleroderma, vasculitides, MS, and immune thrombocytopenia, etc) in PBSC donors was 385.19 (95% CI, 351.2-422.4) and in BM donors was 367.62 (95% CI, 325.8-414.9) per 100 000 person-years with an incidence rate ratio of 1.03 (95% CI, 0.89-1.21; P = .66) (Table 2). Interestingly, there was a significant difference in the incidence rate ratio among donors aged 30 to 39 years. The PBSC donors had an incidence rate of 425.3 (95% CI, 360.5-501.8) (Table 3), and the BM donors had an incidence rate of 300.71 (95% CI, 238.4-379.3) (Table 4) and an incidence rate ratio of 1.42 (95% CI, 1.06-1.89; P = .02) (Table 5). This phenomenon was not observed in other age groups.
Thrombotic events
The incidence rate of thrombotic events in PBSC donors was 132.23 (95% CI, 113.0-154.7) and in BM donors it was 131.63 (95% CI, 107.7-160.9) per 100 000 person-years and the incidence rate ratio was 1.01 (95% CI, 0.78-1.30; P = .95). The incidence of thrombotic events was not different between PBSC and BM donors in the different age groups (Tables 3-5).
SEER data comparison
To address the differences between the 2 cohorts and the general population, the SEER database was used, and age-adjusted rates were calculated using the 2000 US Standard Population.45 The general population was more likely to have all types of cancer than PBSC donors (Table 6). The incidence rate of myeloid malignancies in PBSC donors was 2.53 (95% CI, 0.82-7.84) and 17.91 (95% CI, 17.85-17.96) in the general population with a rate ratio of 0.14 (95% CI, 0.05-0.44; P < .001), in lymphoid malignancies the incidence rate in PBSC donors was 14.33 (95% CI, 8.91-23.05) and 33.76 (95% CI, 33.68-33.84) in the general population with a rate ratio of 0.42 (95% CI, 0.26-0.68; P < .001), and in other malignancies the incidence rate in PBSC donors was 460.36 (95% CI, 423.0-501.0) and 568.3 (95% CI, 567.9-568.6) in the general population with a rate ratio of 0.81 (95% CI, 0.74-0.88; P < .001) (Table 6). The incidence rate of myeloid malignancies in BM donors was 4.13 (95% CI, 1.33-12.8) and 17.91 (95% CI, 17.85-17.96) in the general population with a rate ratio of 0.23 (95% CI, 0.07-0.72; P = .011), in lymphoid malignancies the incidence rate in BM donors was 13.78 (95% CI, 7.41-25.61) and 33.76 (95% CI, 33.68-33.84) in the general population with a rate ratio of 0.41 (95% CI, 0.22-0.76; P = .005), and in other malignancies the incidence rate in BM donors was 521.59 (95% CI, 471.1-577.6) and 568.3 (95% CI, 567.9-568.6) in the general population with a rate ratio of 0.92 (95% CI, 0.83-1.02; P = .100) (Table 7).
Age-adjusted incidence rates of malignancies in donors compared with the US general population: PBSC donors vs US general population (SEER)
| Outcomes . | PBSC donors . | US general population (SEER) . | Rate ratio (95% CI) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|
| Person, -years . | No. of events . | Incidence rate∗ (95% CI) . | Person-years . | No. of events . | Incidence rate∗ (95% CI) . | |||
| Myeloid | 118 712 | 3 | 2.53 (0.82-7.84) | 2 211 987 365 | 396 134 | 17.91 (17.85-17.96) | 0.14 (0.05-0.44) | <.001† |
| Lymphoid | 118 649 | 17 | 14.33 (8.91-23.05) | 2 211 987 365 | 746 740 | 33.76 (33.68-33.84) | 0.42 (0.26-0.68) | <.001† |
| Other malignancies | 116 213 | 535 | 460.36 (423.0-501.0) | 2 211 987 365 | 12 569 821 | 568.3 (567.9-568.6) | 0.81 (0.74-0.88) | <.001† |
| Outcomes . | PBSC donors . | US general population (SEER) . | Rate ratio (95% CI) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|
| Person, -years . | No. of events . | Incidence rate∗ (95% CI) . | Person-years . | No. of events . | Incidence rate∗ (95% CI) . | |||
| Myeloid | 118 712 | 3 | 2.53 (0.82-7.84) | 2 211 987 365 | 396 134 | 17.91 (17.85-17.96) | 0.14 (0.05-0.44) | <.001† |
| Lymphoid | 118 649 | 17 | 14.33 (8.91-23.05) | 2 211 987 365 | 746 740 | 33.76 (33.68-33.84) | 0.42 (0.26-0.68) | <.001† |
| Other malignancies | 116 213 | 535 | 460.36 (423.0-501.0) | 2 211 987 365 | 12 569 821 | 568.3 (567.9-568.6) | 0.81 (0.74-0.88) | <.001† |
CI, Confidence Interval
Age-adjusted incidence rate per 100 000 person-years.
Result is statistically significant (P < .05).
Age-adjusted incidence rates of malignancies in donors compared with the US general population: BM donors vs US general population (SEER)
| Outcomes . | BM donors . | US general population (SEER) . | Rate ratio (95% CI) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|
| Person- years . | No. of events . | Incidence rate∗ (95% CI) . | Person-years . | No. of events . | Incidence rate∗ (95% CI) . | |||
| Myeloid | 72 641 | 3 | 4.13 (1.33-12.8) | 2 211 987 365 | 396 134 | 17.91 (17.85-17.96) | 0.23 (0.07-0.72) | .011† |
| Lymphoid | 72 572 | 10 | 13.78 (7.41-25.61) | 2 211 987 365 | 746 740 | 33.76 (33.68-33.84) | 0.41 (0.22-0.76) | .005† |
| Other malignancies | 70 553 | 368 | 521.59 (471.1-577.6) | 2 211 987 365 | 12 569 821 | 568.3 (567.9-568.6) | 0.92 (0.83-1.02) | .100 |
| Outcomes . | BM donors . | US general population (SEER) . | Rate ratio (95% CI) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|
| Person- years . | No. of events . | Incidence rate∗ (95% CI) . | Person-years . | No. of events . | Incidence rate∗ (95% CI) . | |||
| Myeloid | 72 641 | 3 | 4.13 (1.33-12.8) | 2 211 987 365 | 396 134 | 17.91 (17.85-17.96) | 0.23 (0.07-0.72) | .011† |
| Lymphoid | 72 572 | 10 | 13.78 (7.41-25.61) | 2 211 987 365 | 746 740 | 33.76 (33.68-33.84) | 0.41 (0.22-0.76) | .005† |
| Other malignancies | 70 553 | 368 | 521.59 (471.1-577.6) | 2 211 987 365 | 12 569 821 | 568.3 (567.9-568.6) | 0.92 (0.83-1.02) | .100 |
Age-adjusted incidence rate per 100 000 person-years.
Result is statistically significant (P < .05).
Discussion
This is the largest and most comprehensive study on the long-term outcomes in URDs. This provides evidence that filgrastim in the setting of mobilization of PBSC in URDs is not associated with an increase in hematological or nonhematological malignancies, nor is it associated with an increase in autoimmune disorders or thrombotic events.
Concerns were raised about the association between filgrastim and AML due to several reports. Bennett et al26 published 2 cases of AML among 200 individuals who had received filgrastim. In these cases, matched sibling donors, who donated PBSC to their siblings with AML, developed AML ∼4 years after filgrastim exposure. NMDP reviewed their donor outcomes and published on 20 cases of cancer (none of them leukemia or lymphoma) reported among 4015 URDs who had passed their first anniversary of PBSC donation.46 There have been reports from multiple registries that looked at the incidence of cancer after donation during different time frames in PBSC donors. Nacheva et al32 tested 50 PBSC donors for mutations common in hematological malignancies and showed that PBSC samples collected before, on the day of donation, and 90 and 180 days after filgrastim administration had no damaging effects on the genome integrity and did not differ between samples from individuals who donated BM 3 to 5 years prior or healthy people. Additional reports by NMDP showed no increase in cancer, autoimmune diseases, or stroke related to filgrastim in 6768 PBSC donors.17 MD Anderson reported that none of the 343 donors from their institution had been diagnosed with acute or chronic leukemia with a median follow-up of 39 months.47 The Japan Society for Hematopoietic Cell Transplantation reported a long-term follow-up of 1708 PBSC donors and showed no difference in hematological malignancies compared with a retrospective cohort of BM donors.48 A prospective Swedish national cohort study analyzed cancer incidence after donation in 1082 Swedish PBSC donors and did not find differences in the incidence of hematological malignancies or other malignancies between PBSC donors and BM donors or nondonating siblings.49 Notably, different formulations of filgrastim have been used in URDs, such as lenograstim or biosimilars, and data have shown no differences in efficacy or side effects.50-53 Moreover, the World Marrow Donor Association has recommended that biosimilars may be used for mobilization51 and have a mechanism for the ongoing surveillance of donor adverse events.54 Although the PBSC donors in this study received Neupogen, these results could be applicable to donors who received other types of filgrastim. To our knowledge, the study presented here is the first study that analyzed long-term effects of filgrastim on URDs over a 10-year period and confirmed no association between PBSC donors and an increased risk of any type of malignancy, including myeloid, lymphoid, or other types of cancer, compared with BM donors.
Other studies have compared the cancer rates between PBSC donors and the general population. Hölig et11 al published the results of 3928 PBSC donors at the University of Dresen and showed that malignancies were found in 0.3% of the donors; the incidence of Hodgkin lymphoma differed significantly compared with the general population. There was not an increased risk of Hodgkin lymphoma in our study; however, the number of donors was much greater in this study and was more reassuring than in the previous study.11 Halter et al30 published a retrospective multicenter EBMT study covering 51 024 first allogeneic hematopoietic stem cell donations (27 770 BM donors and 23 254 PBSC donors) from predominantly related donors and found that the incidence of hematological malignancies was below the age-specific incidence in the normal population. To elucidate whether PBSC donors were more likely to develop cancer than the general population, we used the SEER database for comparison. URDs that donate BM and PBSC are, in general, healthier than the general population, as they are younger and must also meet the eligibility criteria to donate, and their overall rates of cancer are lower. An important consideration for PBSC and BM donors is that follow-up is shorter than the lifetime follow-up of the general population. However, this comparison suggests that filgrastim did not potentiate cancer risk in this population, and the findings presented here give additional reassurance that both hematological and nonhematological malignancies are not increased in PBSC donors.
One of the secondary objectives of this study was to determine whether filgrastim increased the incidence of autoimmune disorders. Filgrastim has been used in patients with lupus, MS, Felty syndrome, and temporal arteritis to treat neutropenia.35-38,40-42 In URDs who received filgrastim, we did not see an increase in the incidence of the development of autoimmune diseases. However, when the results were stratified based on age, PBSC donors that were 30 to 39 years of age had a higher risk of having autoimmune diseases than BM donors, although the numbers of events were very low (140/3871 [3.6%]). This same phenomenon was not observed in other age ranges. A recent report by Conrad et al55 estimates that 1 of 10 people are affected by autoimmune diseases; the 3.6% observed in the 30 to 39 PBSC age group is less than the expected rate in the general population, giving support that short-term filgrastim use in a donor does not appear to potentiate autoimmune disorders. The low incidence of autoimmune disorders in BM donors could be because BM donors at risk could have been screened out before donation.
Thrombosis has been a concern as a side effect of filgrastim due to reports of arterial thrombosis and a potentially increased prothrombotic state.43,44 This study showed that the incidence of thrombotic events in PBSC donors was not increased compared with BM donors. This confirmed evidence from another report showing stroke was not increased in PBSC donors17 and that short-term-filgrastim use does not appear to be associated with thrombosis.
There are several limitations to this study. BM donors who were later exposed to filgrastim were censored at the time of filgrastim exposure, thus limiting the follow-up time at risk (this issue is addressed to some degree by the very large number of BM donor follow-up years in this trial). Also, donors that had incorrect contact information were not included in this study. Another limitation is that verification could not be performed for all MAT events during medical record review. To address this specific limitation, we attributed unverified events as an event to overestimate, rather than underestimate, the number of late events. Additional limitations include a relatively short follow-up of 7 years for donors enrolled after 2010, self-reporting, and the fact that donors who did not enroll could have increased late effects. Due to the overestimation of late events and the number of donor-years studied, these limitations are not worrisome.
This is the largest prospective study addressing the safety of filgrastim in URDs. These results provide evidence that filgrastim is not associated with an increase in the incidence of myeloid or lymphoid malignancy, other types of cancer, autoimmune diseases, or thrombotic events. Most importantly, this provides URDs with reassurance that in this large study of thousands of healthy donors followed, on average, for >10 years, we saw no adverse effects associated with receiving filgrastim.
Acknowledgments
This study was fully funded by Amgen. The CIBMTR is supported primarily by the US Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; AlloVir, Inc; Amgen, Inc; Angiocrine; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb Co; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; Regimmune; Sanofi; Sarah Cannon; Sobi, Inc; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
Authorship
Contribution: H.E.S designed the research plan, analyzed the data, and wrote the manuscript; M.K and S.B.-S. analyzed the data; H.K. was the project manager in the clinical trial; D.M. gathered data; J.S., D.S., and C.D.O.B. edited the final version of the manuscript; L.J.B. reviewed medical records and critically reviewed and edited the final version of this manuscript; B.E.S and M.A.P critically reviewed and edited the final version of this manuscript; and J.P.M. and S.M.D designed the research plan, reviewed medical records, analyzed the data, and critically reviewed and edited the final version of this manuscript.
Conflict-of-interest disclosure: D.S., C.D.O.B., and J.S. are employed by Amgen and own stocks. B.E.S. reports consultancy from Orca Bio and Mallinkrodt. M.A.P. reports research funding from Adaptive and Miltenyi; reports membership on an entity’s board of directors or advisory committees from GentiBio, Vertex, bluebird, and CARGO; and consultancy from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Heather E. Stefanski, Center for International Blood and Marrow Transplant Research, NMDP, 500 North 5th St, Minneapolis, MN 54001; email: hstefans@nmdp.org.
References
Author notes
J.P.M. and S.M.D. contributed equally to this study.
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research, supporting data are not available. Individual data will not be shared. Aggregated data can be obtained upon reasonable request from the corresponding author, Heather E. Stefanski (hstefans@nmdp.org).
The full-text version of this article contains a data supplement.