Key Points
The human-derived and antibody-like C3b-targeted fusion protein CG001 may simultaneously and effectively block 3 complement pathways.
CG001 holds high potential of further development for clinical study based on the preclinical evaluation.
Visual Abstract
Excessively activated or dysregulated complement activation may contribute to the pathogenesis of a wide range of human diseases, thus leading to a surge in complement inhibitors. Herein, we developed a human-derived and antibody-like C3b-targeted fusion protein (CRIg-FH-Fc) x2, termed CG001, that could potently block all 3 complement pathways. Complement receptor of the immunoglobulin superfamily (CRIg) and factor H (FH) bind to distinct sites in C3b and synergistically inhibit complement activation. CRIg occupancy in C3b prevents the recruitment of C3 and C5 substrates, whereas FH occupancy in C3b accelerates the decay of C3/C5 convertases and promotes the factor I–mediated degradation and inactivation of C3b. CG001 also showed therapeutic effects in alternative pathways–induced hemolytic mouse and classical pathways–induced mesangial proliferative glomerulonephritis rat models. In the pharmacological/toxicological evaluation in rats and cynomolgus monkeys, CG001 displayed an antibody-like pharmacokinetic profile, a convincing complement inhibitory effect, and no observable toxic effects. Therefore, CG001 holds substantial potential for human clinical studies.
Introduction
The complement system, a key part of innate immunity and a bridge to adaptive immunity, can be prototypically activated by 3 distinct pathways: the classical, lectin, and alternative pathways (CP, LP, and AP, respectively). Therefore, it plays an important role in pathogen elimination and immune modulation through its various enzymatic products.1 However, when complement is excessively activated by either autoantibodies in autoimmune diseases such as membranous nephropathy (MN)2 or by invading pathogens such as severe acute respiratory syndrome coronavirus 2,3 or is dysregulated due to defects in regulatory proteins such as paroxysmal nocturnal hemoglobinuria (PNH),4 it turns deleteriously against host tissues. Hyperactive and uncontrolled complement activation may contribute to various human disorders, further leading to the boost of complement-targeted drugs from experimental design to clinical products for unmet clinical needs.5 Currently, the US Food and Drug Administration has approved different complement inhibitors including C1-esterase inhibitor, C5-targeted eculizumab, ravulizumab and avacincaptad pegol, C3-targeted pegcetacoplan, C1s-targeted sutimlimab, C5a-targeted vilobelimab, C5aR1 antagonist avacopan, factor B (FB)-targeted iptacopan, and factor D (FD)-targeted danicopan for the treatment of a variety of human diseases.6-8 It may provide an alternative strategy to develop the complement inhibitors targeting other components, which can block all pathways simultaneously and/or restrain the functions of all active complement products, including C3a/C5a, C3b/iC3b, and C5b-9n (membrane attack complex, MAC).
We have previously developed a C3b-targeted fusion protein, CRIg-factor H (CRIg-FH), which can potently inhibit both CP and AP on the local cell surface and shows high therapeutic potential for PNH ex vivo and in animal models of various diseases, such as mesangial proliferative glomerulonephritis (MsPGN),9 renal ischemia‒reperfusion injury,10 lupus nephritis,11 and experimental autoimmune myasthenia gravis.12 To improve the pharmacological properties and druggability, we herein fused human immunoglobulin G4 (IgG4) fragment crystallizable (Fc) with CRIg-FH to generate (CRIg-FH-IgG4 Fc) x2, termed CG001. Furthermore, we performed a preclinical evaluation of CG001 and tested its potential for treating PNH and MsPGN in animal models. The results demonstrated that CG001 holds substantial potential for future clinical studies.
Methods
Preparation of CG001 and other complement inhibitors
CRIg, FH, CRIg-FH, and FH-CRIg fragments were expressed using the pFUSE-hIgG4-Fc2 vector. CG001 was labeled using a fluorescein isothiocyanate labeling kit (102884, Abcam, Cambridge, United Kingdom) in the cytometry assay. Iptacopan and narsoplimab were obtained from MedChemExpress (Monmouth Junction, NJ). Eculizumab and pegcetacoplan were purchased from manufacturers. Sutimlimab was purchased from Selleck Chemicals (Shanghai, China).
The hemolytic mouse model
C57BL/6 male mice were pretreated with saline or complement inhibitors by tail vein injection, and 20 minutes later, blood was collected from the tail vein as baseline. Then, the mice received 175 μL of normal human serum (NHS; CompTech) via tail vein injection to induce heterologous complement-mediated hemolysis, and blood was collected 1 hour later. Plasma samples were prepared to determine free hemoglobin at an absorbance of 414 nm. Meanwhile, the erythrocyte pellets were used to measure C3b deposition by flow cytometry.
Induction of rat Thy-1 nephritis
Male Sprague-Dawley rats (Vital River Laboratory, Shanghai, China) were randomly divided into control (n = 6) and model groups (n = 6). Anti-rat thymocyte serum (1 mL/100 g body weight) was administered via tail vein injection to induce anti-Thy-1 nephritis. Rats were injected with varying concentrations of CG001 or saline by tail vein injection. The urine collected by metabolic cages was centrifuged, and urine albumin was measured by the Coomassie brilliant blue method (Nanjing Jiancheng Bioengineering Institute, China).
Statistical analysis
All data were analyzed with GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA) and presented as the mean ± standard error of the mean, or mean ± standard deviation. The significance of the inhibitory activity was determined by nonlinear regression (curve fit). Significance was evaluated by an unpaired 2-tailed Student t test between 2 groups, or by 1-way analysis of variance in Wieslab assay. All values for which P was < .05 were regarded as statistically significant, and significance was presented as ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001.
The animal ethics committee at Shanghai Medical School, Fudan University, and the ethics committee of the Fudan University Shanghai Cancer Center approved the study.
Results
Design of CG001
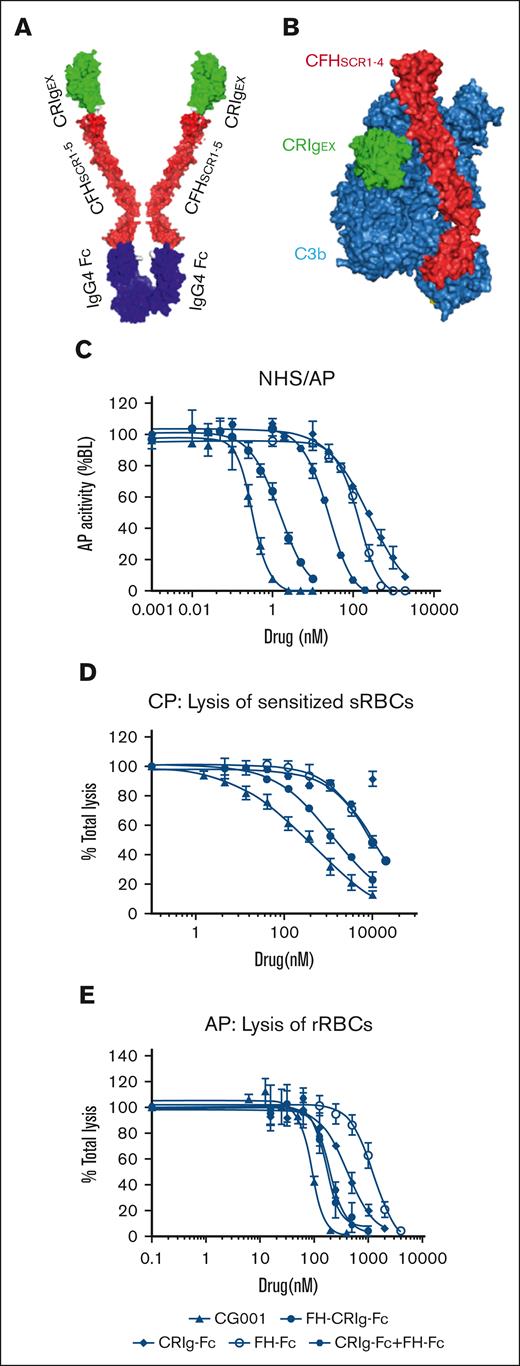
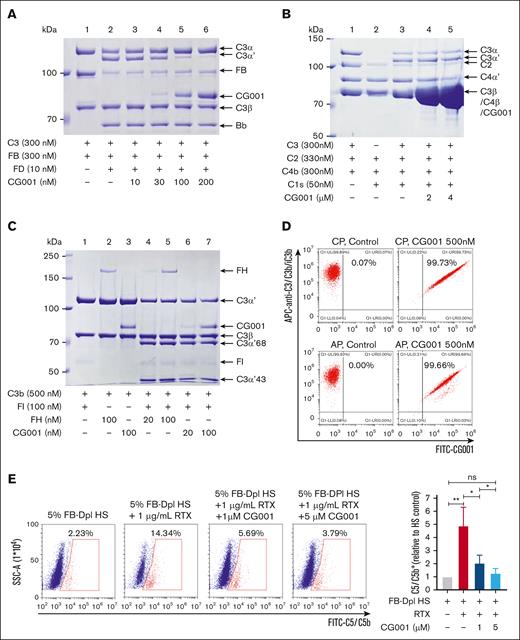
We generated an antibody-like macromolecule (CRIg-FH-IgG4 Fc) ∗2, termed CG001, by conjugating the fusion protein CRIg-FH developed previously9 with human IgG4 Fc (Figure 1A). CRIg binds to the β chain (1-645 amino acids) of C3b via its extracellular domain (Figure 1B), thus inhibiting the AP strongly and the CP marginally.13 FH is a circulatory abundant complement regulator critical for the AP, and its short consensus repeats 1 to 4 are sufficient for AP regulation,16 which results from binding to the C3b α′ chain (727-1641 amino acids) and β chain, which is different from the CRIg binding site (Figure 1B).14 Therefore, CG001 may simultaneously bind to distinct sites in C3b via CRIg and FH (Figure 1B), thus holding the unique trait of powerful complement inhibition. We used the Wieslab AP kit to confirm the improved complement inhibitory ability of CG001 compared with FH-CRIg-Fc, CRIg-Fc, FH-Fc, and CRIg-Fc + FH-Fc. The results showed that CG001 exhibited 1.6-, 288-, 147-, and 30-fold higher activity in inhibiting AP activity than the above fusion proteins (Figure 1C). In hemolysis assays, we also found that CG001 strongly inhibited both the CP and AP compared with FH-CRIg-Fc, CRIg-Fc, FH-Fc, and CRIg-Fc + FH-Fc. (Figure 1D-E). These findings indicate the rationality of the CG001 design, which may also improve the drug pharmacological properties (such as pharmacokinetics [PK]/pharmacodynamics [PD]) and further facilitate drug manufacturing owing to the Fc fragment.
Design of CG001 and its inhibitory effect on complement pathways. (A) Schematic of CG001. It is composed of the extracellular domain of CRIg (green CRIgEX; PDB, 2ICC13), the short consensus repeat (SCR) 1-5 of FH (red FHSCR1-5; image showing SCR1-4 from PDB, 2WII14 in panel B), and the Fc fragment of human IgG4 (purple IgG4 Fc; PDB, 4C5415). (B) Composite image of the binding of CRIgEX (green; PDB, 2ICF13) and FHSCR1-4 (red; PDB, 2WII14) to C3b (sky blue). Diagrams were generated with PyMOL molecular visualization software. (C) Comparison of CG001 with other CRIg- or FH-derived fusion proteins to inhibit AP activation using the Wieslab complement system AP kit. The IC50 was 0.83 ± 0.12 nM for CG001; 1.35 ± 0.49 nM for FH-CRIg-Fc; 239.3 ± 123.5 nM for CRIg-Fc; 121.6 ± 36.2 nM for FH-Fc; and 24.55 ± 0.31 for CRIg-Fc + FH-Fc. (D-E) Effect of CG001 on protecting erythrocytes from complement-mediated hemolysis. (D) CP was activated by 0.2% sheep erythrocyte hemolysin and 1% NHS to induce the hemolysis of sheep erythrocytes (sRBCs), whereas (E) AP was directly activated by 12% NHS to induce the hemolysis of rabbit erythrocytes (rRBCs). The IC50 was 446.2 ± 89.06 nM for CG001; 1083 ± 168.18 nM for FH-CRIg-Fc; 5458 ± 1036.81 nM for FH-Fc; and 9269 ± 307.45 nM for CRIg-Fc + FH-Fc for CP. The IC95 was 34.19 ± 3.77 μM for CG001; 68.66 ± 45.85 μM for FH-CRIg-Fc; 111.07 ± 24.13 μM for FH-Fc; and 176.11 ± 7.15 μM for CRIg-Fc + FH-Fc for CP. CRIg showed little inhibitory activity against CP. In addition, the IC50 was 88.88 ± 8.31 nM for CG001; 167 ± 8.66 nM for FH-CRIg-Fc; 416.1 ± 52.83 nM for CRIg-Fc; 1231 ± 146.83 nM for FH-Fc; and 195.4 ± 16.34 nM for CRIg-Fc + FH-Fc for AP. The IC95 was 206.83 ± 7.83 nM for CG001; 419.23 ± 139.03 nM for FH-CRIg-Fc; 2160 ± 345.98 nM for CRIg-Fc; 5040 ± 1846.65 nM for FH-Fc; and 551.05 ± 158.17 nM for CRIg-Fc + FH-Fc for AP (E). Values represent the mean ± standard deviation (SD), and the experiments were performed at least 3 times.
Design of CG001 and its inhibitory effect on complement pathways. (A) Schematic of CG001. It is composed of the extracellular domain of CRIg (green CRIgEX; PDB, 2ICC13), the short consensus repeat (SCR) 1-5 of FH (red FHSCR1-5; image showing SCR1-4 from PDB, 2WII14 in panel B), and the Fc fragment of human IgG4 (purple IgG4 Fc; PDB, 4C5415). (B) Composite image of the binding of CRIgEX (green; PDB, 2ICF13) and FHSCR1-4 (red; PDB, 2WII14) to C3b (sky blue). Diagrams were generated with PyMOL molecular visualization software. (C) Comparison of CG001 with other CRIg- or FH-derived fusion proteins to inhibit AP activation using the Wieslab complement system AP kit. The IC50 was 0.83 ± 0.12 nM for CG001; 1.35 ± 0.49 nM for FH-CRIg-Fc; 239.3 ± 123.5 nM for CRIg-Fc; 121.6 ± 36.2 nM for FH-Fc; and 24.55 ± 0.31 for CRIg-Fc + FH-Fc. (D-E) Effect of CG001 on protecting erythrocytes from complement-mediated hemolysis. (D) CP was activated by 0.2% sheep erythrocyte hemolysin and 1% NHS to induce the hemolysis of sheep erythrocytes (sRBCs), whereas (E) AP was directly activated by 12% NHS to induce the hemolysis of rabbit erythrocytes (rRBCs). The IC50 was 446.2 ± 89.06 nM for CG001; 1083 ± 168.18 nM for FH-CRIg-Fc; 5458 ± 1036.81 nM for FH-Fc; and 9269 ± 307.45 nM for CRIg-Fc + FH-Fc for CP. The IC95 was 34.19 ± 3.77 μM for CG001; 68.66 ± 45.85 μM for FH-CRIg-Fc; 111.07 ± 24.13 μM for FH-Fc; and 176.11 ± 7.15 μM for CRIg-Fc + FH-Fc for CP. CRIg showed little inhibitory activity against CP. In addition, the IC50 was 88.88 ± 8.31 nM for CG001; 167 ± 8.66 nM for FH-CRIg-Fc; 416.1 ± 52.83 nM for CRIg-Fc; 1231 ± 146.83 nM for FH-Fc; and 195.4 ± 16.34 nM for CRIg-Fc + FH-Fc for AP. The IC95 was 206.83 ± 7.83 nM for CG001; 419.23 ± 139.03 nM for FH-CRIg-Fc; 2160 ± 345.98 nM for CRIg-Fc; 5040 ± 1846.65 nM for FH-Fc; and 551.05 ± 158.17 nM for CRIg-Fc + FH-Fc for AP (E). Values represent the mean ± standard deviation (SD), and the experiments were performed at least 3 times.
Kinetic analysis of the interaction between CG001 and C3 cleavage products
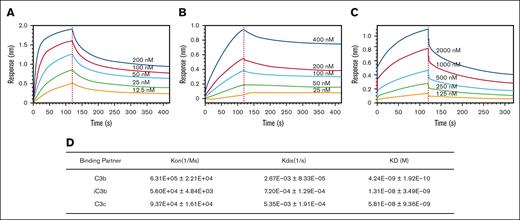
To understand the kinetic binding of CG001 to its targets, we measured the affinity of CG001 for different C3 cleavage products, C3b, iC3b, and C3c. The results showed that C3b exhibited a faster association rate and a slower dissociation rate and thus a stronger binding affinity than iC3b with CG001 (Figure 2A-B,D). In addition, C3c bound to CG001 more slowly than C3b but faster than iC3b, and its dissociation from CG001 displayed a similar pattern (Figure 2C-D). Compared with the original CRIg-FH,9 CG001 exhibited better binding activity, likely because of the addition of IgG4 Fc, thus improving the binding affinity ∼80-fold (CG001, 4.24 nM vs CRIg-FH, 334.6 nM for binding C3b9). These binding kinetic data on CG001 reveal the molecular basis for the binding between CG001 and C3 cleavage products, and more importantly, the importance of conjugating IgG4 Fc to CRIg-FH.
Binding affinity of CG001 to human C3 cleavage products. (A-C) Representative profiles of the interactions between CG001 and human C3 products. (A) C3b, (B) iC3b, and (C) C3c. (D) Binding affinity of CG001 to 3 human C3 cleavage products. The interactions were measured by biolayer interferometry, using 3 batches of CG001 drug substances. Values represent the mean ± SD.
Binding affinity of CG001 to human C3 cleavage products. (A-C) Representative profiles of the interactions between CG001 and human C3 products. (A) C3b, (B) iC3b, and (C) C3c. (D) Binding affinity of CG001 to 3 human C3 cleavage products. The interactions were measured by biolayer interferometry, using 3 batches of CG001 drug substances. Values represent the mean ± SD.
CG001 effectively inhibits all 3 complement pathways
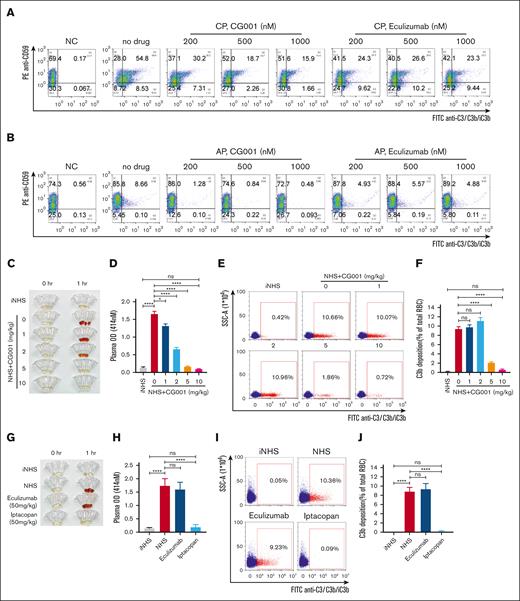
We compared CG001 with eculizumab in inhibiting complement activation. The 50% inhibitory concentration (IC50) values of CG001 were 4.71, 2.52, and 0.83 nM for the CP, LP, and AP, respectively. Furthermore, CG001 was 10-fold stronger than eculizumab for AP inhibition, and 2.5- and 3.1-fold weaker for CP and LP inhibition, respectively (Figure 3A-C). It should be noted that the labeled antibody specific for a neoepitope of MAC in the kit detects the activation of C5 but not C3; thus, the unique characteristics of CG001, but not of eculizumab, in inhibiting C3 activation were concealed by this assay. In FB- and C4-depleted human serum, the IC50 values of CG001 were 10.93 and 0.86 nM for inhibiting the CP and AP, respectively (Figure 3D-E), consistently indicating that CG001 can markedly inhibit both AP and CP activation.
The in vitro complement inhibitory activity of CG001. (A-C) The complement inhibitory effect of CG001 in NHS. The complement was activated via (A) CP, (B) LP, or (C) AP, and CG001 was compared with eculizumab as a control. (A) IC50 = 4.71 ± 0.28 nM (CG001) or 1.54 ± 0.14 nM (eculizumab) for CP, IC95 = 510.95 ± 91.98 nM (CG001) or 5.5 ± 0.29 nM (eculizumab) for CP; (B) 2.52 ± 0.62 nM (CG001) or 1.02 ± 0.10 nM (eculizumab) for LP, and IC95 was 365.73 ± 114.62 nM (CG001) or 5.71 ± 0.48 nM (eculizumab) for LP; and (C) 0.83 ± 0.12 nM (CG001) or 8.56 ± 0.24 nM (eculizumab) for AP and IC95 was 4.0 ± 0.76 nM (CG001) or 22.0 ± 0.93 nM (eculizumab) for AP. (D-E) The inhibitory effect of CG001 on complement component-depleted human serum. (D) IC50 = 10.932 ± 1.629 nM in FB-depleted human serum selective for CP activity, and (E) IC50 = 0.858 ± 0.034 nM in C4-depleted human serum selective for AP activity. (F-I) The complement inhibitory effect of CG001 in normal monkey serum (NMS) and normal rat serum (NRS). (F) IC50 = 9.335 ± 2.328 nM for CP in NMS, (G) 0.455 ± 0.037 nM for AP in NMS, (H) IC50 = 192.5 ± 36.2 nM for CP in NRS, or (I) 96.0 ± 6.8 nM for AP in NRS. Complement activities in human and monkey sera were measured by the Wieslab complement system kit for panels A-G. To evaluate the complement activities in rat serum, (H) CP was activated by 0.2% sheep erythrocyte hemolysin and 1% NRS to induce hemolysis of sRBCs, whereas (I) AP was directly activated by 18% NHS to induce hemolysis of rRBCs. Values represent mean ± SD, and experiments were performed in triplicate.
The in vitro complement inhibitory activity of CG001. (A-C) The complement inhibitory effect of CG001 in NHS. The complement was activated via (A) CP, (B) LP, or (C) AP, and CG001 was compared with eculizumab as a control. (A) IC50 = 4.71 ± 0.28 nM (CG001) or 1.54 ± 0.14 nM (eculizumab) for CP, IC95 = 510.95 ± 91.98 nM (CG001) or 5.5 ± 0.29 nM (eculizumab) for CP; (B) 2.52 ± 0.62 nM (CG001) or 1.02 ± 0.10 nM (eculizumab) for LP, and IC95 was 365.73 ± 114.62 nM (CG001) or 5.71 ± 0.48 nM (eculizumab) for LP; and (C) 0.83 ± 0.12 nM (CG001) or 8.56 ± 0.24 nM (eculizumab) for AP and IC95 was 4.0 ± 0.76 nM (CG001) or 22.0 ± 0.93 nM (eculizumab) for AP. (D-E) The inhibitory effect of CG001 on complement component-depleted human serum. (D) IC50 = 10.932 ± 1.629 nM in FB-depleted human serum selective for CP activity, and (E) IC50 = 0.858 ± 0.034 nM in C4-depleted human serum selective for AP activity. (F-I) The complement inhibitory effect of CG001 in normal monkey serum (NMS) and normal rat serum (NRS). (F) IC50 = 9.335 ± 2.328 nM for CP in NMS, (G) 0.455 ± 0.037 nM for AP in NMS, (H) IC50 = 192.5 ± 36.2 nM for CP in NRS, or (I) 96.0 ± 6.8 nM for AP in NRS. Complement activities in human and monkey sera were measured by the Wieslab complement system kit for panels A-G. To evaluate the complement activities in rat serum, (H) CP was activated by 0.2% sheep erythrocyte hemolysin and 1% NRS to induce hemolysis of sRBCs, whereas (I) AP was directly activated by 18% NHS to induce hemolysis of rRBCs. Values represent mean ± SD, and experiments were performed in triplicate.
CG001 shares 89% and 69% protein sequence identity with its counterparts in cynomolgus monkeys and rats, respectively. Next, we determined the complement inhibitory activity in normal cynomolgus monkeys. It showed that CG001 inhibited the AP more potently than the CP (Figure 3F-G) in monkeys. Because of the species specificity of the kit, we chose the hemolysis assay to assess the complement inhibitory activity of CG001 in rats. The results showed that CG001 could also successfully inhibit rat complement activation in both the CP (Figure 3H) and the AP (Figure 3I). Therefore, these monkey and rodent species can both be used for pharmacological and toxicological evaluation of CG001.
We also measured the complement inhibitory activities of pegcetacoplan, iptacopan, sutimlimab, and narsoplimab. Pegcetacoplan could effectively inhibit not only the AP but also the CP and LP (supplemental Figure 1A). However, iptacopan showed complement inhibitory activity for the AP but not for the CP/LP, sutimlimab for the CP but not for the LP/AP, and narsoplimab for the LP but not for the CP/AP (supplemental Figure 1B-D). Together, the above results demonstrate that CG001 could robustly inhibit the AP, and CP and LP activation, although less potently.
Molecular mechanism by which CG001 inhibits complement activation
The target of CG001, C3b, is cleaved from C3 by various C3 convertases, that is, “tickover” convertase C3(H2O) Bb, amplification loop convertase C3bBb in the AP, and C4bC2a in the CP/LP. In the presence of both FB and FD, the C3 α-chain is cleaved into the α′-chain and released C3a via the AP.17 The addition of CG001 markedly inhibited C3 cleavage and the production of the C3 α′-chain in a dose-dependent manner (Figure 4A), indicating the strong inhibitory activity of CG001 on the AP C3 convertase. Because of the lack of the C3b subunit in the C3 convertase C4bC2a of the CP/LP and no direct binding of CG001 to C3, the addition of CG001 did not affect C3 cleavage in the CP (Figure 4B). These results demonstrate that CG001 inhibited the C3 convertase activity of the AP but not of the CP/LP to produce C3b.
Molecular mechanism by which CG001 inhibits complement activation. (A) CG001 inhibited the C3 convertase in AP. In the presence of FB and FD, the C3α chain of C3 was cleaved to C3α′ and released C3a, which was inhibited by CG001 in a dose-dependent manner. (B) CG001 failed to inhibit the C3 convertase in CP. In the presence of C2, C4b, and C1s, C3α was cleaved to C3α′ and C3a was released; however, CG001 failed to block this process even at high concentrations. (C) CG001 promoted C3b degradation. The C3α′ chain of C3b was proteolyzed to C3α′68 and C3α′43 by FI in the presence of FH or CG001, in which CG001 displayed a similar potency to FH. (D) CG001 bound to the specific cell surface with C3b/iC3b deposition. CP and AP were activated in Raji cells, and the deposition of C3b/iC3b and CG001 was determined by a flow cytometry assay. (E) CG001 impaired C5 binding to the cell surface upon CP activation. After CP activation by rituximab and FB-depleted human serum in Raji cells, C5 was recruited to the membrane-bound C5 convertase C4bC2aC3b, and the resultant proteolytic C5b fragment was inserted into the cell surface, all of which could be detected by anti-C5/C5b antibody with a flow cytometry assay. CG001 reduced C5/C5b staining by preventing C5 recruitment and C5b production by binding to the C3b subunit of C5 convertase. Values represent mean ± SD, and experiments were performed in triplicate. ∗P < .05 and ∗∗P < .01.
Molecular mechanism by which CG001 inhibits complement activation. (A) CG001 inhibited the C3 convertase in AP. In the presence of FB and FD, the C3α chain of C3 was cleaved to C3α′ and released C3a, which was inhibited by CG001 in a dose-dependent manner. (B) CG001 failed to inhibit the C3 convertase in CP. In the presence of C2, C4b, and C1s, C3α was cleaved to C3α′ and C3a was released; however, CG001 failed to block this process even at high concentrations. (C) CG001 promoted C3b degradation. The C3α′ chain of C3b was proteolyzed to C3α′68 and C3α′43 by FI in the presence of FH or CG001, in which CG001 displayed a similar potency to FH. (D) CG001 bound to the specific cell surface with C3b/iC3b deposition. CP and AP were activated in Raji cells, and the deposition of C3b/iC3b and CG001 was determined by a flow cytometry assay. (E) CG001 impaired C5 binding to the cell surface upon CP activation. After CP activation by rituximab and FB-depleted human serum in Raji cells, C5 was recruited to the membrane-bound C5 convertase C4bC2aC3b, and the resultant proteolytic C5b fragment was inserted into the cell surface, all of which could be detected by anti-C5/C5b antibody with a flow cytometry assay. CG001 reduced C5/C5b staining by preventing C5 recruitment and C5b production by binding to the C3b subunit of C5 convertase. Values represent mean ± SD, and experiments were performed in triplicate. ∗P < .05 and ∗∗P < .01.
FH, but not CRIg, is a cofactor for the factor I (FI)–mediated cleavage and inactivation of C3b13,18; thus, we evaluated the cofactor activity of CG001. The results showed that in the presence of FI, both FH and CG001 induced the proteolysis of C3b with a similar potency (Figure 4C).
Circulating C3b is rapidly degraded through proteolysis, whereas membrane-bound C3b appears to be more stable for its functions, including convertases formation and opsonophagocytosis. Next, we evaluated whether CG001 could bind to the membrane-bound C3b. The results showed that all CG001-bound surviving cells were also positive for C3b staining (Figure 4D), suggesting that CG001 binds to specific cells by interacting with C3b.
CG001 inhibited the C3 convertase activity of the AP but not of the CP (Figure 4A-B) but indeed reduced C5 activation via the CP/LP (Figure 3A-B). The interaction of CRIg with C3b prevents the formation of MAC via the AP but not via the CP.13 Therefore, we assume that the conjugation of FH to CRIg in CG001 may radically enhance its binding to the C3b subunit of the CP/LP C5 convertase C4bC2aC3b and then prevent the binding of C5 to C3b, thus leading to the obvious inhibition of C5 activation via the CP/LP. Indeed, we observed the decreased C5/C5b deposition in a subpopulation of surviving Raji cells after CP activation by CG001 treatment in a dose-dependent manner (Figure 4E), indicating that CG001 blocked the recruitment of C5 to the C5 convertase C4bC2aC3b by binding to C3b.
CG001 could anchor to the targeted cell membrane by binding to C3b/iC3b (Figure 4D), which might induce antibody dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) effects via the IgG4 Fc fragment. Rituximab (positive control) showed strong ADCC and ADCP functions as determined by a marked increase in the number of dead Raji cells (supplemental Figure 2A-B) and the number of the phagocytic Raji cells (by over half; supplemental Figure 2C-D), respectively. However, similar to human IgG4-Fc, CG001 had no effects on ADCC and ADCP (supplemental Figure 2A-D), suggesting that CG001 binding to complement-attacked cells may not mediate the clearance of these cells.
Effect of CG001 on preventing PNH erythrocytes from hemolysis
We have previously demonstrated that the CRIg-FH fusion protein could effectively protect mouse and human PNH erythrocytes from complement attacks activated via the CP or AP.9 As expected, CG001 strongly inhibited complement activation regardless of the initiating pathway, thus markedly increasing the proportion of CD59− PNH erythrocytes, promoting C3b degradation, and reducing C3b deposition in PNH cells (Figure 5A-B). It has been reported that rabbit IgG can bind to C3 and C3b19; thus, we observed an obvious CD59+C3/C3b/iC3b+ subpopulation only in the CP activated by rabbit anti-human erythrocyte antibody (Figure 5A). In addition, eculizumab exhibited an effect on inhibiting CP-mediated PNH erythrocyte cytolysis; however, the C3/C3b/iC3b deposition in CD59− PNH erythrocytes even increased with eculizumab treatment (Figure 5A), supporting the previous report that eculizumab treatment may lead to the accumulation of C3b/iC3b/C3d.20 Moreover, eculizumab had little effect on inhibiting AP-mediated PNH erythrocyte cytolysis at the tested dosage (Figure 5B). Therefore, CG001 could effectively protect human PNH erythrocytes from complement attack and reduce C3b deposition during CP or AP activation, thus holding the potential to block both MAC-induced intravascular hemolysis (IVH) and C3b-mediated extravascular hemolysis (EVH).21
CG001 prevented PNH erythrocytes from hemolysis. (A-B) CG001 protected human PNH erythrocytes from CP- and AP-induced hemolysis. Hemolysis and C3b/iC3b deposition (selective for CP) in CD59− PNH erythrocytes were induced by (A) CP or (B) AP activation, which could be effectively prevented by CG001 treatment. However, (A) eculizumab treatment prevented only hemolysis but not C3b/iC3b deposition induced by CP activation and (B) showed little effect on AP-induced hemolysis. (C-J) CG001 blocked both IVH and EVH in the NHS-induced and AP-mediated hemolytic mouse models compared with eculizumab and iptacopan. (C,G) IVH was determined by the plasma images, and (D,H) the quantitative plasma hemoglobin. (E,I) EVH was represented by the deposition of C3b/iC3b in erythrocytes, which is shown as a scatterplot, and (F,J) the related quantitative results. (C-F) CG001, and (G-J) eculizumab and iptacopan. Data are presented as mean ± standard error of the mean (SEM); n = 4; ∗P < .05 and ∗∗∗∗P < .0001.
CG001 prevented PNH erythrocytes from hemolysis. (A-B) CG001 protected human PNH erythrocytes from CP- and AP-induced hemolysis. Hemolysis and C3b/iC3b deposition (selective for CP) in CD59− PNH erythrocytes were induced by (A) CP or (B) AP activation, which could be effectively prevented by CG001 treatment. However, (A) eculizumab treatment prevented only hemolysis but not C3b/iC3b deposition induced by CP activation and (B) showed little effect on AP-induced hemolysis. (C-J) CG001 blocked both IVH and EVH in the NHS-induced and AP-mediated hemolytic mouse models compared with eculizumab and iptacopan. (C,G) IVH was determined by the plasma images, and (D,H) the quantitative plasma hemoglobin. (E,I) EVH was represented by the deposition of C3b/iC3b in erythrocytes, which is shown as a scatterplot, and (F,J) the related quantitative results. (C-F) CG001, and (G-J) eculizumab and iptacopan. Data are presented as mean ± standard error of the mean (SEM); n = 4; ∗P < .05 and ∗∗∗∗P < .0001.
Human serum could induce mouse erythrocyte hemolysis by activating the AP.22 Thus, to detect the therapeutic effect of CG001 in vivo, we injected NHS into mice to develop a hemolytic mouse model induced by alternative pathway. After NHS injection, human complement was strongly activated, whereas the activation of mouse complement was negligible, if any, which was determined by the deposition of C3 cleaved fragments on erythrocytes (supplemental Figure 3). We observed obvious IVH in the collected plasma, which was inhibited by CG001 treatment in a dose-dependent manner (Figure 5C-D). Moreover, EVH, determined by C3b/iC3b deposition, was almost completely abolished by CG001 treatment (Figure 5E-F). In addition, we determined the effects of eculizumab and iptacopan. Most likely because of the low effect and injection volume limitation, eculizumab, even at a dose of 50 mg/kg, failed to block hemolysis and reduce C3b/iC3b deposition (Figure 5G-J). In contrast, 50 mg/kg iptacopan completely blocked hemolysis (Figure 5G-H) and C3b/iC3b deposition (Figure 5I-J). Together, these results demonstrate that complement inhibition at the C3 level is critical for blocking both IVH and EVH in PNH, and CG001 may hold this great potential because of its targeting of C3b/iC3b and blocking all 3 pathways.
Effect of CG001 on experimental MsPGN
We further observed the effect of CG001 in a CP-induced experimental MsPGN rat model by anti-rat thymocyte serum injection.9,23 We observed a segmental increase in the mesangium-intraglomerular matrix and diffuse thickening of the peripheral capillary walls and the glomerular basement membrane, indicating the expansion of the mesangial matrix and proliferation of mesangial and endothelial cells accompanied by extensive complement activation (Figure 6A-D). Furthermore, glomerular injury resulted in the progressive aggravation of proteinuria (Figure 6E-F). However, upon complement inhibition with CG001, hypercellularity, glomerular injury, and resultant proteinuria were potently mitigated in a dose-dependent manner, in which the highest dose of 4.0 mg/kg CG001 even completely reversed these manifestations to the levels of the negative control (Figure 6). Therefore, CG001 may also treat CP-induced, other than AP-induced, complement-related diseases.
CG001 attenuates complement-mediated glomerular lesions in a rat MsPGN model. (A-D) HE, PAS staining, and immunostaining for proliferating cell nuclear antigen (PCNA) and C3d in kidney tissue sections collected from anti-Thy1 nephritic rats on day 7 after treatment with CG001. (A) Representative photomicrographs of glomeruli and (B) quantitative results of total cell counts, (C) PCNA-positive cell counts in the glomeruli and (D) C3d deposition. Arrows indicate the increased mesangial cellularity, and stars indicate the thickened capillary walls and glomerular basement membrane. (E-F) Effect of CG001 treatment on 24-hour urinary protein collected at the indicated time points. Data are presented as (E) individual and (F) combined results (mean ± SEM); n = 4 (negative control, NC), 8 (rabbit anti-Thy1 serum, anti-rat thymocyte serum [ATS] alone treatment), or 6 (additive CG001 treatment). ∗ P < .05, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
CG001 attenuates complement-mediated glomerular lesions in a rat MsPGN model. (A-D) HE, PAS staining, and immunostaining for proliferating cell nuclear antigen (PCNA) and C3d in kidney tissue sections collected from anti-Thy1 nephritic rats on day 7 after treatment with CG001. (A) Representative photomicrographs of glomeruli and (B) quantitative results of total cell counts, (C) PCNA-positive cell counts in the glomeruli and (D) C3d deposition. Arrows indicate the increased mesangial cellularity, and stars indicate the thickened capillary walls and glomerular basement membrane. (E-F) Effect of CG001 treatment on 24-hour urinary protein collected at the indicated time points. Data are presented as (E) individual and (F) combined results (mean ± SEM); n = 4 (negative control, NC), 8 (rabbit anti-Thy1 serum, anti-rat thymocyte serum [ATS] alone treatment), or 6 (additive CG001 treatment). ∗ P < .05, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
PK/PD and toxicity evaluation of CG001 in monkeys and rats
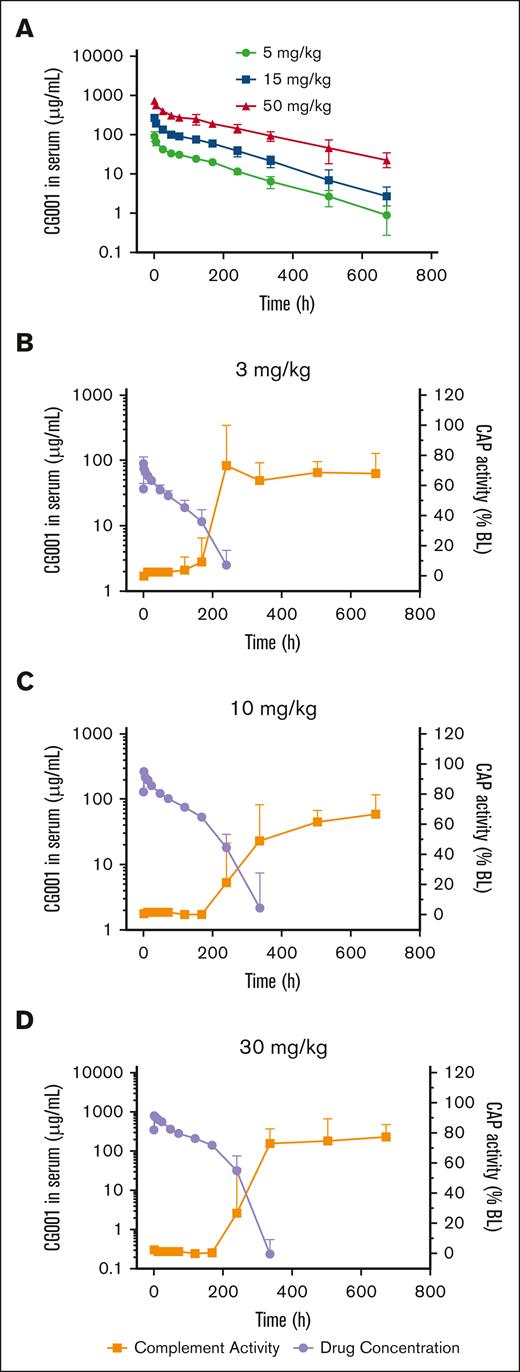
For future potential clinical use, we evaluated the PK features of CG001 in rats by administering 5, 15, or 50 mg/kg CG001 via intravenous infusion. CG001 concentrations reached peak levels at 5 minutes for 91.8, 269.8, and 747.3 μg/mL, and the half-lives were 117.1, 109.5, and 157.1 hours, respectively (Figure 7A).
PK/PD evaluation of CG001 in rats and cynomolgus monkeys. (A-B) PK was evaluated by measuring the serum CG001 concentration in rats. n = 12 per group. (C-D) PK/PD correlation of CG001 in monkeys. Complement activity was measured by the Wieslab complement system AP kit. n = 6 per group. Values represent mean ± SD.
PK/PD evaluation of CG001 in rats and cynomolgus monkeys. (A-B) PK was evaluated by measuring the serum CG001 concentration in rats. n = 12 per group. (C-D) PK/PD correlation of CG001 in monkeys. Complement activity was measured by the Wieslab complement system AP kit. n = 6 per group. Values represent mean ± SD.
Next, we tested the PK/PD of CG001 in cynomolgus monkeys. The results showed that CG001 concentrations reached peak levels at 30 minutes for 90.7, 269.9, and 783.6 μg/mL, and the half-lives were 49.7, 47.6, and 37.1 hours in the 3, 10, or 30 mg/kg CG001 groups, respectively (Figure 7B-D). The shortened half-life in monkeys was most likely attributed to the production of anti-drug antibody (ADA) because of CG001 immunogenicity (all monkeys started to develop ADA on day 15, the first blood sample-collecting time point). Of note, CG001 shares 89% protein sequence identity with its counterpart in cynomolgus monkeys; thus, it was not surprising that CG001 induced ADA in monkeys. However, we expect that CG001 may theoretically not induce such kind of ADA in humans because all fragments of CG001 (ie, CRIg, FH, and IgG4 Fc fragments) are derived from humans, which has been supported by the current clinical results in phase 1 of CG001-treated healthy volunteers and patients with PNH. In addition, the AP activity in the 3, 10, and 30 mg/kg CG001 groups was completely abolished to 0.12%, 0.92%, and 3.93% of the baseline activity, respectively, at the 30-minute time point with the highest CG001 plasma concentration (Figure 7B-D). On day 7 (168 hours), the complement activities recovered to 15.29%, 0.35%, and 1.05%; and on day 10 (240 hours), the complement activities recovered to 105.91%, 36.36%, and 43.00% of the baseline activity (Figure 7B-D) in the 3, 10, and 30 mg/kg CG001 groups, respectively.
In addition, CG001 was injected to evaluate acute and 4-week toxicity in rats and monkeys, respectively. No observable toxic effects were observed in either rats or monkeys after the administration of CG001 at a single dose of 50, 150, and 500 mg/kg. Thus, the maximal tolerance dose of CG001 was 500 mg/kg in rats and monkeys. Furthermore, CG001 was injected into rats (15, 50, and 150 mg/kg) or monkeys (10, 30, and 100 mg/kg) once weekly for 4 weeks. The results showed that the no-observed-adverse-effect level was the highest tested dosage, that is, 150 mg/kg in rats and 100 mg/kg in monkeys. These findings demonstrate the safety of CG001 and warrant further clinical study.
Discussion
Excessive activation or dysregulation of the complement cascade may contribute to various human diseases, including autoimmune, inflammatory, and infectious diseases.24 Therefore, complement-targeted therapeutics have been regarded as promising strategies over the past decades.25 In this study, we developed a C3b-targeted antibody-like fusion protein, CG001. It could specifically deposit on the complement-attacked cell membrane to block all 3 complement pathways at the C3 level, showed a potential therapeutic effect in hemolytic mouse and MsPGN rat models, and had no toxicity in preclinical evaluation in rats and monkeys, thus holding great potential for further clinical studies.
Considering that C3b is also located in the central stage of all 3 pathways and at a much lower level, C3b has been recognized as an optimal complement target.9,26 However, C3b has multiple and complicated active sites, including the Bb- and C3/C5-binding regions; therefore, it is difficult to develop traditional C3b-targeted agents. However, endogenous regulators binding to C3b may hold potential.27 Recombinant CR1 (TP-10),28 full-length FH (GEM103),29,30 minimized FH (miniFH),31-33 and CRIg-Fc fusion protein have been tested in various animal disease models.34 Furthermore, a series of CR2-based complement inhibitors, including CR2-FH (TT30), have been designed to specifically target and protect complement-attacked cells by the binding of CR2 to C3d.26,35,36 However, it was terminated for further development, possibly because of its low potency and short half-life in a phase 1 clinical trial for PNH treatment.26,37,38 In addition, the comparison of IC50 is presented in supplemental Table 1.
To improve the druggability of CRIg-FH,9 we fused it with human IgG4 Fc to develop CG001. Furthermore, we identified the mechanism of CG001 in complement inhibition. The C3b subunit of convertases is responsible for recruitment but not for the proteolysis of C3 and C5 substrates.39,40 Cobra venom factor (CVF), a structural and functional analog of C3b, binds to FB to form CVFBb after cleavage by FD, which is a stable complex and an efficient C5 convertase.41 CRIg interacts with the MG3-MG6 and linker domains of C3b,13 which largely overlaps with the interface of the MG4-MG7 domains of CVF contacting C5.42 Therefore, the binding of CRIg to C3b may structurally interfere with the recruitment of C5 to the C3b-containing C5 convertases, leading to terminal complement inhibition of the CP/LP/AP. The crystal structure of the C3:C3b complex is lacking, so we propose that the binding of CRIg to C3b may prevent the recruitment of C3 to C3b, thus suppressing the C3 convertase activity of the AP, in agreement with a previous report.13 Together, the binding of the CRIg fragment in CG001 to C3b interferes with the recruitment of the substrate C3 to AP C3 convertase and C5 to C5 convertases in both AP and CP/LP. Although CG001 cannot prevent the production of C3b by CP/LP convertase, it may suppress its function and promote its degradation. Unlike CRIg, FH inhibits complement activation via 2 different mechanisms. FH may displace Bb from the C3b in the C3 convertase C3bBb (decay activity) and act as a cofactor for FI to degrade C3b into iC3b (cofactor activity), thus inhibiting complement activation via the AP. Of note, the simultaneous binding of CRIg and FH in CG001 to C3b may synergistically enhance the individual functions of CRIg and FH by increasing the binding affinity, thus significantly potentiating complement inhibition.
In addition to classical proteolysis by trimolecular C5 convertases, C5 can also be cleaved by bimolecular C3 convertases on cell surfaces with densely clustered C3b or C4b, in which C3b or C4b may directly bind to C5 and then prime C5 proteolysis by nearby C3 convertases (supplemental Figure 4).43-45 Therefore, C5 activation in the terminal pathway may be bypassed by diverse anti-C3 or anti-C5 agents when the complement is strongly activated by either the CP/LP or AP, which might weaken the therapeutic efficacy of these drugs.43-45 In the Wieslab assay, we observed that CG001 completely blocked complement activation via the AP but retained residual complement activity (∼12% in the CP, and 7% in the LP), even at high concentrations (Figure 3A-B). The above residual activity of MAC formation in the CP/LP might result from C4bC2a-mediated C5 proteolysis by C4b, which recruits C5. However, CG001 competed with C5 to bind to C3b, effectively blocking the potential C3bBb-mediated C5 proteolysis in the AP.
Comprehensive complement inhibition resulting from CG001 treatment may raise safety concerns regarding infections. However, the long-term clinical use of C3-based pegcetacoplan and C5-based eculizumab has largely addressed this concern,46-48 which may be partially attributed to prophylactic vaccination against encapsulated bacteria such as the deadly Neisseria meningitidis. In addition, 27 patients with primary inherited C3 deficiency in 19 families showed increased susceptibility to opportunistic gram-negative bacterial infections mostly during the first 2 years of life,49 but this was largely alleviated with age because of the gradual maturity of adaptive immunity.50 CG001 treatment may retain the production of C3a and MAC generated by CP/LP-induced C4bC2a. These bioactive fragments may be beneficial for pathogen clearance through C3a-mediated phagocytosis and MAC-mediated direct cytolysis.
Because of the amplification loop, the AP seems essential for total complement activation, regardless of the initiating pathways of the CP/LP.51 However, in real-world studies, it may be difficult to discriminate which pathway contributes the most to distinct human diseases, leading to difficulties in selecting the optimal pathway-specific drug. The suboptimal increase in hemoglobin in patients with PNH after treatment with the AP-specific drugs iptacopan52 and pegcetacoplan47,53,54 might involve additional activation of the CP/LP. Complement activation via the LP/AP has previously been regarded as the major contributor to MN,55 whereas a very recent report demonstrated that CP is the main trigger rather than the LP or AP in patients with MN.2 Therefore, optimal complement inhibitors may need to target ≥2 complement pathways. In addition, any complement inhibitor may have the problem of breakthrough hemolysis (BTH). PK/PD-induced BTH has been clearly discussed in an elegant review.7 Basically, the stronger inhibition of complement, the lower the possibility of BTH. Therefore, CG001 might have a low possibility of BTH because of its complement inhibitory activity for all 3 pathways at the C3 level.
In conclusion, we developed a C3b-targeted complement inhibitor CG001, which can simultaneously inhibit all 3 pathways at the C3 level. CG001 and its prototype CRIg-FH have shown remarkable potential in a number of experimental disease models, including PNH, MsPGN,9 generalized myasthenia gravis,12 lupus nephritis,11 and renal ischemia‒reperfusion injury 10 models. Furthermore, CG001 has finished the phase 1a clinical trial in healthy volunteers, and is being tested in the phase 1b for treating patients with PNH. The current clinical results demonstrate that CG001 displays potent complement inhibitory activity and has no immunogenicity. Therefore, together with preclinical safety evaluation, CG001 warrants further clinical studies in various human diseases.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (82273185, 82121004, 81872354, 81790254, 91629301, and 91029726), the Major State Basic Research Development Program of China (2013CB910802), and the Science and Technology Commission of Shanghai Municipality (21S11909500) to W.H.; the National Natural Science Foundation of China (82370121), the Natural Science Foundation of Beijing Municipality (2023, 7232109), and the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-026) to B.H.; 2023 Students Innovation Training Program of Peking Union Medical College, ID: 2023zglc06062 (XE07230724070349500) to S.S.; the National Natural Science Foundation of China (82373356) to P.D.; and a ComGen research grant (CG201801).
Authorship
Contribution: W.H. conceived and supervised the project; W.H., Ling Li, and P.D. designed the experiments and wrote the manuscript; Ling Li, P.D., and Y.D. performed most of the experiments and analyzed the data, with assistance from X.L., Luying Li, J.Y., J.C., and P.W.; S.S. and B.H. helped to collect the PNH specimens; and T.X. and B.H. contributed the ideas.
Conflict-of-interest disclosure: W.H. is the inventor of CRIg-FH and CG001, and the founder of ComGen Pharmaceutical. X.L., Luying Lo, and J.Y. are employees of ComGen Pharmaceutical. The remaining authors declare no competing financial interests.
Correspondence: Weiguo Hu, Cancer Institute, Fudan University Shanghai Cancer Center, 270 Dong’an Rd, Shanghai 200032, China; email: weiguohu@fudan.edu.cn.
References
Author notes
Ling Li and P.D. contributed equally to this study.
Data are available on request from the corresponding author, Weiguo Hu (weiguohu@fudan.edu.cn).
The full-text version of this article contains a data supplement.


![CG001 attenuates complement-mediated glomerular lesions in a rat MsPGN model. (A-D) HE, PAS staining, and immunostaining for proliferating cell nuclear antigen (PCNA) and C3d in kidney tissue sections collected from anti-Thy1 nephritic rats on day 7 after treatment with CG001. (A) Representative photomicrographs of glomeruli and (B) quantitative results of total cell counts, (C) PCNA-positive cell counts in the glomeruli and (D) C3d deposition. Arrows indicate the increased mesangial cellularity, and stars indicate the thickened capillary walls and glomerular basement membrane. (E-F) Effect of CG001 treatment on 24-hour urinary protein collected at the indicated time points. Data are presented as (E) individual and (F) combined results (mean ± SEM); n = 4 (negative control, NC), 8 (rabbit anti-Thy1 serum, anti-rat thymocyte serum [ATS] alone treatment), or 6 (additive CG001 treatment). ∗ P < .05, ∗∗∗P < .001, and ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2024012874/2/m_blooda_adv-2024-012874-gr6.jpeg?Expires=1765151769&Signature=NldXn-0~Ihk2GwiaZZ0tRFzfGcARIvdiaz-2zkY8nIGX6d8Na7iTvSsn~9CsKRaTs0sHClp~kmWi8CKLYyupukSPzUxUncWFfZCiGIV7edo20vmItVDzSXpAai9sSTLW0UDOnu5eG~s5RG7rZyGlzKOr0cxzaMhGUQy5QkEd4W~WDOjMNLbyCdStImuiLJiyqiJuyXSMWGzzRXPa6o0J~tG1vLqbuDxWJXPkNjmLrA77fS2xVlI3omFQ~OcBGVtiK9Cw-nHckF2XoTEYvS3eHoLbn6rIbHJ9XD9vFhHugjVxTJ3tK8laBJ9FG8JKlT0Andecf37T5GYBzW4r99OtSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)