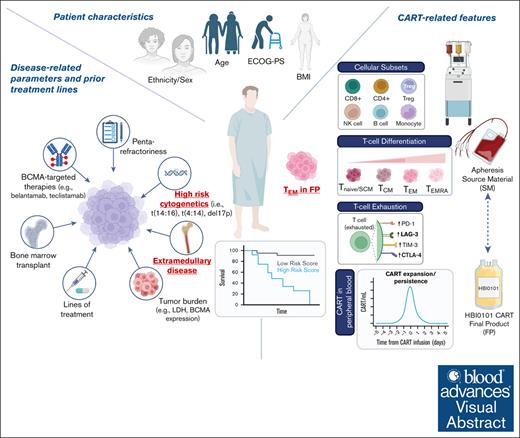
Key Points
HBI0101, an academic BCMA-CART therapy for MM, is a safe, efficient, and available local alternative to commercial drugs.
High-risk cytogenetic, extramedullary disease, and CART differentiation status are predictors of HBI0101 response durability.
Visual Abstract
HBI0101 is an academic chimeric antigen receptor T-cell (CART)–targeted to B-cell maturation antigen (BCMA) for the treatment of relapsed and refractory multiple myeloma (R/RMM) and light chain amyloidosis. Herein, we present the phase 1b/2 results of 50 heavily pretreated patients with R/RMM dosed with 800 × 106 CART cells. Inclusion criteria were relatively permissive (i.e., performance status and baseline organ function) and consequently, approximately half of the enrolled patients would have been ineligible for pivotal clinical trials. The median time elapsed from patient enrollment until CART delivery was 25 days (range, 14-65). HBI0101-related toxicities included grade 1 to 3 cytokine release syndrome, grade 3 to 4 hematologic toxicities, and grade 1 to 2 immune effector cell–associated neurotoxicity syndrome. Responses were achieved in 90% of the patients, 56% achieved stringent and complete response, and 70% reached a minimal residual disease negativity. Within a median follow-up of 12.3 months, the median progression-free survival (PFS) was 11.0 months (95% confidence interval [CI], 6.2-14.6), and the overall survival was not reached (95% CI, 13.3 to not reached). Multivariable analysis on patient/disease and CART-related characteristics revealed that high-risk cytogenetic, extramedullary disease, and increased number of effector-memory T cells in CART products were independently associated with inferior PFS. In conclusion, comprehensive analyses of the parameters affecting the response to CART therapy are essential for improving patients’ outcome. This trial was registered at www.ClinicalTrials.gov as #NCT04720313.
Introduction
The prognosis of patients with multiple myeloma (MM), refractory to the main classes of anti-myeloma therapies (i.e., immunomodulatory agents, proteasome inhibitors, and anti-CD38 monoclonal antibodies), is dismal, with a median progression-free survival (PFS) and overall survival (OS) of 2.8 months and 10.3 months, respectively. The outcome of patients with penta-refractory disease is even worse, with a median PFS and OS of 2.5 and 6.9 months, respectively.1 In the past decade, chimeric antigen receptor T-cell (CART) therapy targeted to the B-cell maturation antigen (BCMA) has emerged as a highly promising immunotherapy for the treatment of relapsed/refractory MM (R/RMM).2 The 2 commercial products idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) have recently joined the ranks of the U.S. Food and Drug Administration (FDA)- and European Medicines Agency (EMA)–approved advanced therapy medicinal products, showing an overall response rate (ORR) of 73% to 84% and 97% to 98%, respectively, and a median PFS of 8.5 to 8.8 months and 27.7 months, respectively.3-7 While the demand for CART-based therapy for R/RMM treatment is constantly increasing, and its introduction into earlier lines of treatment is a hot topic,8-10 a major bottleneck to BCMA-CART therapy remains its limited accessibility worldwide.11 Indeed, the autologous feature of this “living-drug,” together with the logistics and costs12,13 associated with a complex manufacturing process, constrain the supply of CART products, resulting in a growing unmet need. As a result, only very limited number of patients, who clinically fit the drug prescription, are able to fund the expensive treatment, and wait for an available CART production slot, will be offered a chance to receive the drug. Unfortunately, many patients with R/RMM succumb to the disease during this waiting period.11 In addition, in many countries, commercial products are simply not yet available. In this context, decentralized CART production in an academic setting is essential to meet the needs of the local patient population with R/RMM. ARI0002h therapy, developed by academia in Spain to facilitate access and administration of CART products, exemplifies the feasibility of such an approach.14,15
HBI0101 is an academic CART therapy targeted to the BCMA molecule.16 We have recently reported the phase 1a results of the first 20 patients treated with escalating doses of HBI0101 (150 × 106 to 800 × 106 CAR+ cells) and 4 patients with light chain amyloidosis (AL).17,18 Although BCMA–targeted CART therapies have demonstrated unprecedented advances in treating R/RMM, this disease remains incurable, with high rate of relapses. Therefore, it is crucial to identify the determinants of response and mechanisms of resistance to CART therapy, to optimize clinical outcomes. The parameters that could potentially hamper CART efficacy are multifactorial and sometimes interconnected. These include patient (e.g., demographics and performance status) and disease characteristics (e.g., plasma cell cytogenetics, disease presentation, prior treatment lines, and drug refractoriness status) and CART characteristics (e.g., apheresis source material [SM], CART final product [FP], and CART in vivo kinetics).19 A recent meta-analysis pointed out the worsened outcome of patients presenting with high-risk (HR) cytogenetic profile and extramedullary disease (EMD).20 Furthermore, EMD, plasma cell leukemia, lenalidomide-refractoriness, HR cytogenetics, and increased ferritin were independent predictors of early relapse after BCMA-CART commercial and academic therapies.21 Another report on the real-world application of ide-cel revealed a lower PFS in patients with HR cytogenetics, Eastern Cooperative Oncology Group performance status (ECOG-PS) ≥2, younger age, and patients exposed to prior BCMA–targeted therapy (TT).5
In this interim report of our monocentric prospective study, we assessed the safety profile of HBI0101 in the first 50 consecutive patients with R/RMM administered at the therapeutic dose of 800 × 106 CAR+ cells and evaluated its efficacy and durability over time. In addition, we conducted a multivariable analysis of patient/disease and CART characteristics aiming at identifying the potential predictors of durable PFS of patients who received anti–BCMA-CART infusion.
Methods
Study design and patients
The trial NCT04720313 is a single-arm, open-label study evaluating locally produced BCMA-CART HBI0101 and conducted at the Department of Bone Marrow Transplantation and Cancer Immunotherapy, Hadassah Medical Center (Israel). In the phase 1a of the study, 20 patients received infusion with HBI0101 doses ranging from 150 × 106 to 800 × 106 CAR+ cells (150 × 106 CAR+, n = 6; 450 × 106 CAR+, n = 7; 800 × 106, n = 7). In the expansion phase 1b/2, additional patients were enrolled to receive 800 × 106 CAR+ cells. In this study, we report the clinical results of 50 consecutive patients dosed with 800 × 106 CAR+ cells (including the 7 patients from phase 1a) up to 12 March 2023 (at least 3 months of follow-up after CART infusion). Key eligibility criteria of all phases included a diagnosis of R/RMM with ≥3 prior lines of therapy including an immunomodulatory agent, proteasome inhibitor, and an anti-CD38 antibody, age ≥18 years, and a measurable disease, including oligo/nonsecretory myeloma measurable by positron emission tomography/computerized tomography (PET-CT).22 Minimum platelet count was 30 × 109/mL, minimum creatinine clearance was 20 mL per minute, minimal left ventricular ejection fraction was 40%, and the required ECOG-PS was ≤2. A complete description of the study design and eligibility criteria are provided in the study protocol.17,18 For patients requiring disease control between screening and lymphodepletion, bridging antimyeloma therapy was allowed as per clinical protocol. Lymphodepletion regimen included fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 on days –5 to –3 before infusion. Bendamustine 90 mg/m2 and on days –4 and –3 was given to patients with creatinine clearance <30 mL per minute.
Study oversight
The study was designed and approved by the authors and conducted in accordance with the International Council for Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The study protocol was approved by the local institutional review board and the National Ministry of Health. All patients provided written informed consent.
End points and assessments
The primary end point of the phase 1b/2 was to confirm the safety of the highest effective dose of HBI0101, in a larger population. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0, and included incidence and severity of cytokine release syndrome (CRS), neurotoxicity and immune effector cell–associated neurotoxicity syndrome (ICANS; graded according to the 2019 American Society for Transplantation and Cellular Therapy),23 cytopenia, and infection. Secondary end points are evaluating ORR, partial response (PR), very good PR (VGPR), complete response (CR), stringent CR (sCR), PFS, and OS, as well as the rate of minimal residual disease (MRD) negativity (evaluated by flow cytometry, in accordance with the Euroflow standards with a minimum sensitivity of 10–5) and pharmacokinetic and pharmacodynamic markers, according to the International Myeloma Working Group criteria.24
HBI0101 CART manufacture
HBI0101 drug products were manufactured at the Facility for Advanced Cellular Therapy, at Hadassah Medical Center, Israel. HBI0101 was produced either from fresh (n = 45) or cryopreserved (n = 5) peripheral mononuclear cells (PBMCs) within 10 days of manufacture. Details as to HBI0101 manufacture process were reported elsewhere.17
Patient follow-up: sample collection and mononuclear cell isolation and storage
Whole blood samples were collected from patients with R/RMM in heparin and serum collection tubes before (day –10) and after HBI0101 infusion (days +3, +6, +10, +13, and +30 and later on at months 2, 3, etc.). Bone marrow (BM) samples were collected in EDTA tubes at screening and disease assessment and as per physician’s discretion. PBMCs were separated on Ficoll-Paque density gradient (Cytvia) and cryopreserved in 90% fetal bovine serum (Gibco) plus 10% dimethyl sulfoxide (Sigma), until functional/phenotypic characterizations. Sera were collected by centrifugation and frozen at –20°C until further utilization.
CART detection in patients’ peripheral blood
Genomic DNA was isolated from 250 μL whole blood samples collected at the indicated time points using the Maxwell RSC Buffy Coat DNA Kit on the Maxwell instrument (Promega). Real-time PCR for the quantification of the HBI0101 CART cells in 1mL blood was performed as detailed previously.17
Immunophenotyping analyses
The phenotype of premanufacture T cells, CART product, and CART in patients’ peripheral blood were determined using the cellular subset and T-cell subset panels (T-cell differentiation, T-cell exhaustion, and regulatory T cell; supplemental Table 1). Cells were gated either on live cells (SYTOX or DEAD/LIVE-negative, ThermoFisher) or CAR+ (BCMA-Fluorescein isothiocyanate [FITC]+) for T-cell characterization or plasma cells (CD38++CD138++) for BM characterization, as indicated. Samples were acquired on the NAVIOS or DxFlex cytometers (Beckman Coulter) and analyzed using Kaluza software (v2.1).
Soluble BCMA quantification
Soluble BCMA concentration before and after CART infusion was determined using the Human BCMA/TNFRSF17 DuoSet ELISA (DY193, R&D Systems), according to the manufacturer’s instruction.
Statistical analysis
Kaplan-Meier method and log-rank tests were applied to PFS and OS. Variables were categorized into 2 groups of patient/disease and CART characteristics. Given that the ratio of patients to independent variables was low, the analyses proceeded in 3 steps. First, univariate Kaplan-Meier or Cox regression were performed to determine the association between PFS and each single categorical or continuous parameter, respectively. Next, for each group of variables, a multivariate stepwise Cox regression with forward selection was performed with significant univariates (P < .05). Finally, variables that emerged as significant in these analyses were combined in a single Cox regression model that allowed for testing whether any CART characteristic was a significant predictor beyond the patient/disease characteristics. To prevent data loss in multivariate analyses, missing values in CART characteristics were imputed using expectation maximization algorithm. Statistical analyses were performed in SPSS v29, JMP Pro 17 and GraphPad Prism software 9.
The study was approved by the institutional review board of Hadassah Medical Center (Israel) (approval number IRB 0090-20-HMO).
Results
Patient characteristics
Patients with R/RMM who either received or were planned to receive 800 × 106 HBI0101 CART cells between 12 September 2021 and 12 March 2023 were enrolled in this cohort. Of the 59 patients with MM who were screened for eligibility, 51 underwent lymphocyte collection, and 50 of them received HBI0101 infusion (supplemental Figure 1). HBI0101 was generated for all apheresed patients within 10 days of manufacture, with a success rate in delivering the target dose of 800 × 106 of 96% and the 2 products released below the planned dose (230 × 106 and 500 × 106 CAR+). The median age was 65 years (range, 40-84), and 23 (46%) were females. Sixteen patients (32%) had EMD at study entry (includes only patients with lesions outside the bone). Twelve patients (24%) had HR cytogenetics, defined by the occurrence of the following abnormalities: t(4:14), t(14:16), or del(17p); 31 (62%) had 1q gain, and 15 patients (30%) had ECOG-PS of 2 at study entry. The median prior lines of treatment was 4 (range, 3-13), and most patients (46/50 [92%]) were triple refractory. Fifteen patients (30%) were penta-refractory, and 12 (24%) received previous BCMA-TT, mostly belantamab mafodotin. Forty-six patients (92%) were refractory to their last line of therapy. Ten patients (20%) received bridging therapy as detailed in Table 1. Measurable disease after bridging therapy was confirmed in all the patients, except for 2 patients undergoing radiotherapy to solitary sites and for whom disease measurement was questionable. The vast majority of patients (47/50 [94%]) received fludarabine-cyclophosphamide lymphodepletion. Prophylactic immunoglobulin replacement after therapy was given to 18 of 50 patients (36%).
Characteristics of patients at enrollment
| Characteristics . | Total (N = 50) . |
|---|---|
| Median age (range), y | 65 (40-84) |
| Females, n (%) | 23 (46%) |
| Ethnicity, n (%) | |
| Jewish | 40 (80%) |
| Arab | 8 (16%) |
| Others | 2 (4%) |
| Median time since diagnosis (range), y | 5.3 (0.3-24.2) |
| Myeloma subtype, n (%) | |
| Heavy chain involvement | 33 (66%) |
| Light chain involvement | 42 (84%) |
| Nonsecretory | 3 (6%) |
| Myeloma presentation, n (%) | |
| Extramedullary disease | 16 (32%) |
| Plasma cell leukemia | 3 (6%) |
| Comorbidities, n (%) | |
| Cardiac | 16 (32%) |
| Pulmonary | 7 (14%) |
| Renal | 7 (14%) |
| Other malignancies | 5 (10%) |
| Type 2 diabetes | 6 (12%) |
| Amyloid | 1 (2%) |
| Hepatic | 2 (4%) |
| BM involvement, n (%) | |
| All | 29 (58%) |
| Plasma cells ≥60% | 10 (20%) |
| BCMA-expressing plasma cells (total = 47), n (%) | |
| ≥90% | 27 (57%) |
| ECOG-PS, n (%) | |
| 0 | 13 (26%) |
| 1 | 22 (44%) |
| 2 | 15 (30%) |
| R-ISS stage (total = 45), n (%) | |
| I | 13 (29%) |
| II | 26 (58%) |
| III | 6 (13%) |
| HR cytogenetic, n (%) | |
| Not including 1q gain | 12 (24%) |
| Including 1q gain | 34 (68%) |
| Del17p | 9 (18%) |
| t(14;16) | 1 (2%) |
| t(4;14) | 3 (6%) |
| 1q gain | 31 (62%) |
| Double hit (including 1q gain) | 10 (20%) |
| Previous HSCT, n (%) | 34 (68%) |
| Lymphodepletion regimen, n (%) | |
| Fludarabine/cyclophosphamide | 47 (94%) |
| Bendamustine | 3 (6%) |
| Bridging therapy, n (%) | 10 (20%) |
| Radiotherapy | 4 (8%) |
| Chemotherapy | 4 (8%) |
| Plasmapheresis/dexamethasone | 1 (2%) |
| Selenixor/bortezomib | 1 (2%) |
| Median previous lines of treatment (range) | 4 (3-13) |
| Prior BCMA–TT, n (%) | |
| Exposed | 12 (24%) |
| Belantamab mafodotin | 11 (22%) |
| Teclistamab | 2 (17%) |
| BiTE and gamma secretase inhibitor | 1 (8%) |
| Refractory | 10 (20%) |
| Prior proteasome inhibitors, n (%) | |
| Bortezomib | |
| Exposed | 50 (100%) |
| Refractory | 40 (80%) |
| Carfilzomib | |
| Exposed | 33 (66%) |
| Refractory | 28 (56%) |
| Prior IMiDs, n (%) | |
| Lenalidomide | |
| Exposed | 50 (100%) |
| Refractory | 47 (94%) |
| Pomalomide | |
| Exposed | 39 (78%) |
| Refractory | 35 (70%) |
| Prior anti-CD38 monoclonal antibodies (daratumumab), n (%) | |
| Exposed | 50 (100%) |
| Refractory | 50 (100%) |
| Drug refractoriness, n (%) | |
| Triple-class∗ exposed | 50 (100%) |
| Triple-class∗ refractory | 47 (94%) |
| Penta-drug† exposed | 25 (50%) |
| Penta-refractory† | 15 (30%) |
| Refractory to last line therapy | 46 (92%) |
| Characteristics . | Total (N = 50) . |
|---|---|
| Median age (range), y | 65 (40-84) |
| Females, n (%) | 23 (46%) |
| Ethnicity, n (%) | |
| Jewish | 40 (80%) |
| Arab | 8 (16%) |
| Others | 2 (4%) |
| Median time since diagnosis (range), y | 5.3 (0.3-24.2) |
| Myeloma subtype, n (%) | |
| Heavy chain involvement | 33 (66%) |
| Light chain involvement | 42 (84%) |
| Nonsecretory | 3 (6%) |
| Myeloma presentation, n (%) | |
| Extramedullary disease | 16 (32%) |
| Plasma cell leukemia | 3 (6%) |
| Comorbidities, n (%) | |
| Cardiac | 16 (32%) |
| Pulmonary | 7 (14%) |
| Renal | 7 (14%) |
| Other malignancies | 5 (10%) |
| Type 2 diabetes | 6 (12%) |
| Amyloid | 1 (2%) |
| Hepatic | 2 (4%) |
| BM involvement, n (%) | |
| All | 29 (58%) |
| Plasma cells ≥60% | 10 (20%) |
| BCMA-expressing plasma cells (total = 47), n (%) | |
| ≥90% | 27 (57%) |
| ECOG-PS, n (%) | |
| 0 | 13 (26%) |
| 1 | 22 (44%) |
| 2 | 15 (30%) |
| R-ISS stage (total = 45), n (%) | |
| I | 13 (29%) |
| II | 26 (58%) |
| III | 6 (13%) |
| HR cytogenetic, n (%) | |
| Not including 1q gain | 12 (24%) |
| Including 1q gain | 34 (68%) |
| Del17p | 9 (18%) |
| t(14;16) | 1 (2%) |
| t(4;14) | 3 (6%) |
| 1q gain | 31 (62%) |
| Double hit (including 1q gain) | 10 (20%) |
| Previous HSCT, n (%) | 34 (68%) |
| Lymphodepletion regimen, n (%) | |
| Fludarabine/cyclophosphamide | 47 (94%) |
| Bendamustine | 3 (6%) |
| Bridging therapy, n (%) | 10 (20%) |
| Radiotherapy | 4 (8%) |
| Chemotherapy | 4 (8%) |
| Plasmapheresis/dexamethasone | 1 (2%) |
| Selenixor/bortezomib | 1 (2%) |
| Median previous lines of treatment (range) | 4 (3-13) |
| Prior BCMA–TT, n (%) | |
| Exposed | 12 (24%) |
| Belantamab mafodotin | 11 (22%) |
| Teclistamab | 2 (17%) |
| BiTE and gamma secretase inhibitor | 1 (8%) |
| Refractory | 10 (20%) |
| Prior proteasome inhibitors, n (%) | |
| Bortezomib | |
| Exposed | 50 (100%) |
| Refractory | 40 (80%) |
| Carfilzomib | |
| Exposed | 33 (66%) |
| Refractory | 28 (56%) |
| Prior IMiDs, n (%) | |
| Lenalidomide | |
| Exposed | 50 (100%) |
| Refractory | 47 (94%) |
| Pomalomide | |
| Exposed | 39 (78%) |
| Refractory | 35 (70%) |
| Prior anti-CD38 monoclonal antibodies (daratumumab), n (%) | |
| Exposed | 50 (100%) |
| Refractory | 50 (100%) |
| Drug refractoriness, n (%) | |
| Triple-class∗ exposed | 50 (100%) |
| Triple-class∗ refractory | 47 (94%) |
| Penta-drug† exposed | 25 (50%) |
| Penta-refractory† | 15 (30%) |
| Refractory to last line therapy | 46 (92%) |
CHOP, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone; CHOEP, cyclophosphamide, hydroxydaunorubicin, oncovin, etoposide, prednisone; CRCL, creatinine clearance; DCEP, dexamethasone, cyclophosphamide, etoposide, and cisplatin; IMiDs, immunomodulatory agents; LDH, lactate dehydrogenase; PACE, cisplatin, doxorubicin, cyclophosphamide, etoposide; R-ISS, Revised International Staging System; HSCT, hematopoietic stem cell transplantation.
defined as exposed/refractory to 1 immunomodulatory agent, 1 proteasome inhibitor, and anti-CD38 monoclonal antibodies
defined as exposed/refractory to lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab
Safety
The safety profile of HBI0101 is detailed in Table 2. All patients experienced at least 1 grade 3 AE. Early (at or before day +28 after infusion) grade 3 to 4 hematologic toxicities were common (anemia 62%, thrombocytopenia 54%, neutropenia 98%, and lymphopenia 100%). Long-lasting cytopenias of at least grade 3 (at or after day +30 after infusion) were observed in 5 patients with platelet count ≤50 × 109/L (with 3 of 5 with preexisting platelets ≤50 × 109/L) and 7 patients with neutrophil count <1.0 × 109/L (6 of 7 neutropenias resolved by day +90). None of the patients was transfusion dependent or required stem cell infusion.
AEs related to HBI0101 therapy
| Event and grade . | Total (N = 50) . |
|---|---|
| CRS | |
| Any grade, n (%) | 48 (96%) |
| 1 | 17 (34%) |
| 2 | 24 (48%) |
| 3 | 7 (14%) |
| CRS start day, median (range) | 0 (0-4) |
| CRS duration days, median (range) | 1 (1-7) |
| Tocilizumab use, n (%) | 40 (80%) |
| Tocilizumab doses, median (range) | 1 (0-4) |
| Steroids use, n (%) | 8 (16%) |
| Vasopressors, n (%) | 7 (14%) |
| Neurotoxicity | |
| Any, n (%) | 3 (6%) |
| Neurologic deterioration (delirium), n (%) | 1 (2%) |
| ICANS (grade 1-2), n (%) | 2 (4%) |
| ICANS start day, median (range) | 2 (2-2) |
| ICANS duration, median (range), d | 2.5 (2-3) |
| Hematologic toxicity ≤28 d | |
| Neutropenia, n (%) | |
| Any grade | 49 (98%) |
| Grade 3-4 | 49 (98%) |
| Thrombocytopenia, n (%) | |
| Any grade | 36 (72%) |
| Grade 3-4 | 27 (54%) |
| Anemia, n (%) | |
| Any grade | 44 (88%) |
| Grade 3-4 | 31 (62%) |
| Lymphopenia, n (%) | |
| Any grade | 50 (100%) |
| Grade 3-4 | 50 (100%) |
| Febrile neutropenia, n (%) | |
| Any grade | 36 (72%) |
| Grade 3-4 | 36 (72%) |
| Supportive care | |
| G-CSF, n (%) | 32 (64%) |
| Blood transfusion, n (%) | 14 (28%) |
| Platelet transfusion, n (%) | 10 (20%) |
| Nonhematologic toxicity ≤28 d, n (%) | |
| Renal failure | |
| Any grade | 9 (18%) |
| Grade 1 | 8 (16%) |
| Grade 2 | 1 (2%) |
| E/LFT | |
| Any grade | 10 (20%) |
| Grade 1 | 7 (14%) |
| Grade 2 | 2 (4%) |
| Grade 3 | 1 (2%) |
| HLH-like | 4 (8%) |
| Bacteremia | 4 (8%) |
| Other infections | 5 (10%) |
| Hospitalization stay median (d) | 24 (14-55) |
| Event and grade . | Total (N = 50) . |
|---|---|
| CRS | |
| Any grade, n (%) | 48 (96%) |
| 1 | 17 (34%) |
| 2 | 24 (48%) |
| 3 | 7 (14%) |
| CRS start day, median (range) | 0 (0-4) |
| CRS duration days, median (range) | 1 (1-7) |
| Tocilizumab use, n (%) | 40 (80%) |
| Tocilizumab doses, median (range) | 1 (0-4) |
| Steroids use, n (%) | 8 (16%) |
| Vasopressors, n (%) | 7 (14%) |
| Neurotoxicity | |
| Any, n (%) | 3 (6%) |
| Neurologic deterioration (delirium), n (%) | 1 (2%) |
| ICANS (grade 1-2), n (%) | 2 (4%) |
| ICANS start day, median (range) | 2 (2-2) |
| ICANS duration, median (range), d | 2.5 (2-3) |
| Hematologic toxicity ≤28 d | |
| Neutropenia, n (%) | |
| Any grade | 49 (98%) |
| Grade 3-4 | 49 (98%) |
| Thrombocytopenia, n (%) | |
| Any grade | 36 (72%) |
| Grade 3-4 | 27 (54%) |
| Anemia, n (%) | |
| Any grade | 44 (88%) |
| Grade 3-4 | 31 (62%) |
| Lymphopenia, n (%) | |
| Any grade | 50 (100%) |
| Grade 3-4 | 50 (100%) |
| Febrile neutropenia, n (%) | |
| Any grade | 36 (72%) |
| Grade 3-4 | 36 (72%) |
| Supportive care | |
| G-CSF, n (%) | 32 (64%) |
| Blood transfusion, n (%) | 14 (28%) |
| Platelet transfusion, n (%) | 10 (20%) |
| Nonhematologic toxicity ≤28 d, n (%) | |
| Renal failure | |
| Any grade | 9 (18%) |
| Grade 1 | 8 (16%) |
| Grade 2 | 1 (2%) |
| E/LFT | |
| Any grade | 10 (20%) |
| Grade 1 | 7 (14%) |
| Grade 2 | 2 (4%) |
| Grade 3 | 1 (2%) |
| HLH-like | 4 (8%) |
| Bacteremia | 4 (8%) |
| Other infections | 5 (10%) |
| Hospitalization stay median (d) | 24 (14-55) |
CMV, cytomegalovirus; E/LFT, electrolytes or liver function tests; G-CSF, granulocyte colony-stimulating factor; HLH-like, hemophagocytic lymphohistiocytosis-like syndrome; RSV, respiratory syncytial virus.
CRS was observed in 96% of the patients (n = 48), with 14% (n = 7) experiencing a grade 3 CRS. No grade 4 CRS was observed. CRS generally occurred within 24 hours after infusion (43/48), with a median duration of 1 day (range, 1-7). Tocilizumab usage was frequent (40/48) as per clinical protocol, with a median of 1 dose (range, 1-4). Two cases of ICANS, grades 1 and 2, were observed. The case of grade 1 ICANS resolved with steroids after 2 days, whereas the case of grade 2 ICANS improved but persisted with tremor at last follow-up, 3 months after infusion. No Parkinson-like events were observed. Four patients developed CART-associated hemophagocytic lymphohistiocytosis syndrome, which completely resolved with conservative treatment. Febrile neutropenia was also frequent (36/50 [72%]); however, only 4 patients (8%) had documented bacteremia, and 5 other patients (10%) had documented viral infection (respiratory syncytial virus infection, n = 2; parainfluenza, n = 2; cytomegalovirus reactivation, n = 1) before day +28 after infusion. Other early grade ≥3 AEs included ascites (n = 1), pulmonary edema (n = 1), disease-related pelvic hemorrhage (n = 1), and hypercalcemia (n = 2), as well as delirium (n = 1) that was not considered to be ICANS. Kidney impairment was documented in 9 patients (18%; grade 1, n = 8; grade 2, n = 1) and was not related to disease progression. Finally, elevated liver enzymes occurred in 10 patients (20%; grade 1, n = 7; grade 2, n = 2; grade 3, n = 1). No irreversible acute organ toxicities occurred (Table 2).
Two patients were diagnosed with myelodysplastic syndrome at 7 and 12 months after CART transfusion, both retrospectively found to have clonal hematopoiesis in their BMs before CART treatment.25 No other secondary malignancies were observed. Thirteen patients (26%) died at a median of 188 days (range, 86-565) after CART infusion. One patient died of traumatic brain injury at day +166 after infusion while in sCR and MRD-negative status; 1 patient died of myelodysplastic syndrome; all other 11 patients died of disease progression.
Efficacy
Responses to HBI0101 treatment and survival outcomes
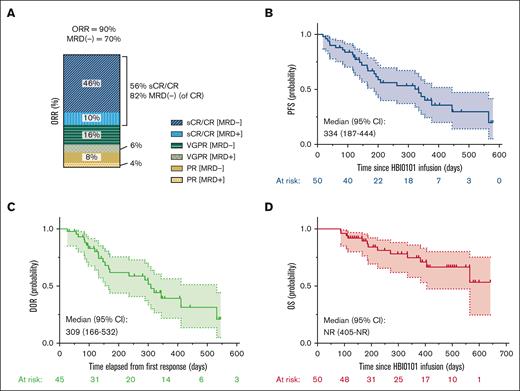
The ORR was 90% (45/50), including 28 patients (56%) with sCR/CR, 11 (22%) with VGPR, and 6 (12%) with PR (Figure 1A). Thirty-five patients (70%) achieved MRD negativity. At a median follow-up of 375 days (range, 18-579), the median PFS was 334 days (95% confidence interval [CI], 187-444), the median duration of response was 309 days (95% CI, 166-532), and the median OS was not reached (NR) (95% CI, 405 to NR; Figure 1B-D). The estimated OS rate at 1 year is 0.75 (95% CI, 0.15-0.39). Patients’ responses over time are summarized in the Swimmer plot (supplemental Figure 2).
Response to HBI0101 therapy. Best ORR (A), PFS (B), DOR in HBI0101 responders (C), and OS (D) were estimated in the first consecutive 50 patients dosed with 800 × 106 CAR+ cells. sCR/CR (dark/light blue); VGPR (dark/light green); PR (dark/light yellow); MRD positive, light colors; MRD negative, dark colors. DOR, duration of response.
Response to HBI0101 therapy. Best ORR (A), PFS (B), DOR in HBI0101 responders (C), and OS (D) were estimated in the first consecutive 50 patients dosed with 800 × 106 CAR+ cells. sCR/CR (dark/light blue); VGPR (dark/light green); PR (dark/light yellow); MRD positive, light colors; MRD negative, dark colors. DOR, duration of response.
Patients with VGPR or better had favorable PFS and OS than patients with PR only or without response; however, similar outcomes were observed in patients with VGPR compared with those with sCR/CR (supplemental Figure 3A-B). MRD negativity was not associated with favorable PFS and OS (supplemental Figure 3C-D). No significant difference in the PFS was observed between patients who achieved a best clinical response 1 month after CART infusion and those who improved their response later on (supplemental Figure 3E), probably reflecting the kinetics of monoclonal protein clearance rather than true clinical difference.
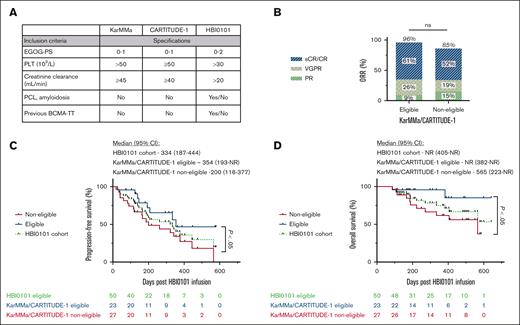
HBI0101 cohort comprises 27 patients (54%) who underwent infusion who would not have met the inclusion criteria for both KarMMa and CARTITUDE-1 studies (Figure 2A), with at least 24% patients presenting with ≥2 exclusion criteria. Stratification of patients who underwent HBI0101 infusion based on their eligibility for commercial trials demonstrates that, despite the high ORR in ineligible patients, these patients display inferior PFS and OS (200 and 565 days, respectively) compared with their eligible counterparts (354 days and NR, respectively; P < .05; Figure 2B-D).
HBI0101 trial permissive inclusion criteria affect patient survival. (A) Major eligibility criteria discriminating HBI0101 trial from the KarMMa/CARTITUDE-1 at study entrance. ORR (B), PFS (C), and OS (D) of patients receiving HBI0101 infusion according to their eligibility to KarMMa/CARTITUDE-1 trial (blue line, eligible, n = 23; red line, noneligible, n = 27; green line, HBI0101 cohort, n = 50). In panel 2B, the percentages for CR, VGPR, and PR do not add up to the percentage atop the bar graph due to rounding. PCL, plasma cell leukemia; PLT, platelet.
HBI0101 trial permissive inclusion criteria affect patient survival. (A) Major eligibility criteria discriminating HBI0101 trial from the KarMMa/CARTITUDE-1 at study entrance. ORR (B), PFS (C), and OS (D) of patients receiving HBI0101 infusion according to their eligibility to KarMMa/CARTITUDE-1 trial (blue line, eligible, n = 23; red line, noneligible, n = 27; green line, HBI0101 cohort, n = 50). In panel 2B, the percentages for CR, VGPR, and PR do not add up to the percentage atop the bar graph due to rounding. PCL, plasma cell leukemia; PLT, platelet.
CART pharmacokinetics
CART in vivo expansion was observed in all response groups (with PR or better) with overlapping peak blood concentrations (supplemental Figure 4A and inset) and persist for a median of 62 days after CART infusion (range, 10-426). This observation is substantiated by the higher concentrations (Cmax) of CART cells detected in the peripheral blood (supplemental Figure 4B; P < .05) and the higher area under the curve (AUC) of patients with a disease response of PR or better (supplemental Figure 4C; P < .01). Both AUC and Cmax of CART cells per mL also positively correlated with a longer PFS (supplemental Figure 4D; P < .01; supplemental Table 4).
Factors predicting survival of HBI0101-treated patients
To identify the potential factors affecting the durability of response to HBI0101 therapy, we performed univariable and multivariable analyses of patient/disease baseline characteristics and CART features.
Among the variables related to patient/disease characteristics, univariate analysis unraveled previous treatment lines (P = .05), penta-refractoriness (P = .02), HR cytogenetic (P = .0001), EMD (P = .002), previous BCMA-TT (P = .01), and LDH (P = .02) as potential predictors of inferior PFS (supplemental Table 2). Multivariable analysis showed that only 2 predictors, HR cytogenetic and EMD, were independently associated with worsened PFS (hazard ratio, 4.55 [95% CI, 1.92-10.78]; P = .001; and hazard ratio, 3.23 [95% CI, 1.45-7.20]; P = .004, respectively).
Univariate analysis of the parameters related to CART features (i.e., apheresis SM, CART FP, and CART in-blood; supplemental Table 3) revealed an association between inferior PFS and the proportion of effector-memory premanufacture T cells (TEM; P = .034), the percentage of transduced HBI0101 T cells (P = .041), and the absolute number of CAR+ TEM in the FP (supplemental Table 4; P = .0008; supplemental Figure 5A for gating strategy for T-cell differentiation status). In contrast, the number of HBI0101-transgene copies per transduced cell (P = .034), the proportion of naïve stem cell–like memory (CAR+ TNAIVE/SCM) and specifically of the CAR+CD8+CCR7+CD27+ subset in the CART product (P = .019), the maximal concentration of CAR+ cell in the blood (CMAX; P = .019), and CAR+ cell persistence until last follow-up (as defined by the AUC; P = .009) were identified as potential predictors of superior PFS (supplemental Table 4). Multivariable analysis showed that only increased number of CAR+ TEM in the FP was significantly associated with inferior PFS (hazard ratio, 1.64; 95% CI, 1.20-2.24; P = .002).
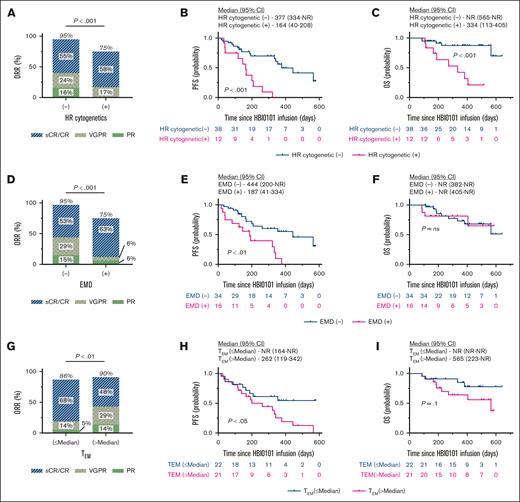
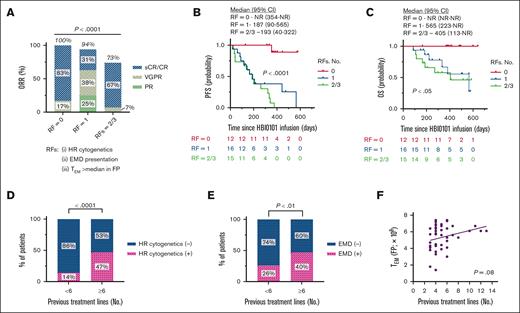
Figure 3 depicts the response to HBI0101 therapy in patients according to the presence of the aforementioned risk factors (RFs), namely, HR cytogenetic (A-C), EMD (D-F), and CAR+ TEM (G-I). A significant decrease in ORR (P < .001) and survival (both PFS and OS) of patients with HR cytogenetic MM was observed (Figure 2A-C; P < .001). The impact of EMD presentation on HBI0101 outcome was reflected in both a reduction in ORR and PFS (Figure 2D-E; P < .01), whereas OS was not affected (Figure 2F). Deeper responses to HBI0101 therapy (Figure 2G; P < .01), and superior PFS and OS were observed in patients infused with CART product displaying reduced proportions of CAR+ TEM (Figure 3H-I; supplemental Figure 5B-C; P < .05). Of note, the acquisition of these RFs was not correlated with CART expansion in vivo (supplemental Figure 6).
Response to HBI0101 therapy by RF groups. ORR (A,D,G), PFS (B,E,H), and OS (C,F,I) were estimated in patients receiving HBI0101 infusion, according to HR cytogenetics (A-C; n = 12), EMD presentation (D-F; n = 16), and the number of CAR+ TEMs in CART FP above the median (>5.7 × 108 CAR+ TEMs) (G-I; n = 23). In panel 3G, the percentages for CR, VGPR, and PR do not add up to the percentage atop the bar graph due to rounding. Pink lines indicate patient group with RF, and blue lines indicate patient group without RF.
Response to HBI0101 therapy by RF groups. ORR (A,D,G), PFS (B,E,H), and OS (C,F,I) were estimated in patients receiving HBI0101 infusion, according to HR cytogenetics (A-C; n = 12), EMD presentation (D-F; n = 16), and the number of CAR+ TEMs in CART FP above the median (>5.7 × 108 CAR+ TEMs) (G-I; n = 23). In panel 3G, the percentages for CR, VGPR, and PR do not add up to the percentage atop the bar graph due to rounding. Pink lines indicate patient group with RF, and blue lines indicate patient group without RF.
Finally, a stepwise Cox regression with forward selection analysis was performed to provide a single model for the prediction of PFS. In this model, CAR+ TEM contributed significantly (Wald χ2 = 5.78; P = .016) to the prediction of PFS beyond HR cytogenetic and EMD. The overall model was significant (Wald χ2 = 27.33; P < .001). Its parameters appear in supplemental Table 5.
We next sought to determine how these RFs, alone or in combination, would affect the PFS. Based on the number of RFs they have acquired, the patients were stratified into 3 risk subgroups: with the absence of RF (RF = 0), 1 RF (RF = 1), and 2 or 3 RFs (RFs = 2/3). Figure 4 indicates that the acquisition of 1 of the aforementioned RFs is sufficient to significantly reduce ORR, PFS, and OS of patients who received HBI0101 infusion (Figure 4A-C; P < .05). Interestingly, the number of previous lines of treatment positively correlated with HR cytogenetic (Figure 4D; P < .001) and EMD (Figure 4E; P < .01) and showed a trend toward increased numbers of CAR+ TEM cells detected in HBI0101 FP (Figure 4F; P = .08). In addition, the proportion of TEM in the FP negatively correlated with the percentage of the CD45RA+CCR7+CD27+CD28+ T-cell subset in the SM (supplemental Figure 5D).
Effect of RFs accumulation on HBI0101 outcome and impact of treatment lines on RFs acquisition. (A-C) Best ORR (A), PFS (B), and OS (C) were estimated in patients receiving HBI0101 infusion, according to their acquisition of RFs, with RF = 0 defined as no RF (red lines); RF = 1 defined as 1 RF (that is, RF HR cytogenetic or extramedullary presentation or the absolute number of CAR+ TEMs detected in HBI0101 FP; blue lines), RF = 2/3, defined as acquisition of 2 or 3 RFs of the formers (green lines). (D-F) Effect of the number of treatment lines endured by patients with myeloma on the acquisition of HR cytogenetic (D), EMD (E), and on the number of CAR+ TEMs in the FP (F). In panel 4A, the percentages for CR, VGPR, and PR do not add up to the percentage atop the bar graph due to rounding.
Effect of RFs accumulation on HBI0101 outcome and impact of treatment lines on RFs acquisition. (A-C) Best ORR (A), PFS (B), and OS (C) were estimated in patients receiving HBI0101 infusion, according to their acquisition of RFs, with RF = 0 defined as no RF (red lines); RF = 1 defined as 1 RF (that is, RF HR cytogenetic or extramedullary presentation or the absolute number of CAR+ TEMs detected in HBI0101 FP; blue lines), RF = 2/3, defined as acquisition of 2 or 3 RFs of the formers (green lines). (D-F) Effect of the number of treatment lines endured by patients with myeloma on the acquisition of HR cytogenetic (D), EMD (E), and on the number of CAR+ TEMs in the FP (F). In panel 4A, the percentages for CR, VGPR, and PR do not add up to the percentage atop the bar graph due to rounding.
Impact of prior exposure to BCMA-TT on PFS
Prior exposure to BCMA-TT was identified as significant univariate but not as significant multivariate of reduced PFS (supplemental Figure 7A; supplemental Table 2). Indeed, previous BCMA-TT did not affect the OS (supplemental Figure 7B). Of note, all the patients in previous BCMA-TT subgroup exhibited at least ≥1 RFs (supplemental Figure 7C), an observation that interfered with our ability to isolate the effect of previous BCMA-TT on PFS by multivariable analysis. However, previous BCMA-TT was not associated with a reduction in the expression level of BCMA at the plasma cell surface of pre-exposed patients (supplemental Figure 7D).
Discussion
We report here the results of the first 50 consecutive patients with myeloma who received infusion with 800 × 106 dose of HBI0101, an academic anti–BCMA-CART therapy, with a median follow-up of 12.3 months. With an ORR of 90%, HBI0101 therapy provides deep (56% of sCR/CR and 70% of MRD negativity) and prolonged responses (median PFS, 11 months) with low-grade, clinically manageable toxicity in patients with R/RMM with dismal prognosis. Two cases (4%) of grade 1 to 2 ICANS were detected, which is significantly lower than the rates previously reported for other commercial products.4,7 The reduced HBI0101-related neurotoxicity reported herein will be the matter of a future investigation. Despite the high rate of MRD negativity reported in our study, this did not translate into prolonged remissions in all responders. This observation probably stems from the fact that many patients had advanced MM with EMD and/or focal bony plasmacytomas before and after CART infusion.
In addition to validating the safety and efficacy of HBI0101 at the therapeutic dose in a larger cohort of patients, this study uncovered several factors influencing long-term patient survival. To this end, our analyses included not only patient and disease characteristics but also CART product–related features. This comprehensive approach (illustrated in Figure 5) yields a deeper understanding of how to improve CART therapy durability and of the patient population who will benefit most. Multivariable analyses of patients receiving HBI0101 infusion unraveled 3 strong independent predictors of inferior PFS: HR cytogenetic, EMD, and increased proportion of CAR+ TEM in HBI0101 FP. Collectively, these 3 factors provide the basis for a simplified prognostic model for PFS prediction of patients receiving HBI0101 infusion, which has yet to be validated in larger cohorts. Our data also indicate that the acquisition of 1 the RFs is sufficient to significantly shorten patient survival.
Determinants of durable response to HBI0101 therapy in patients with infused MM. Numerous variables related to patient characteristics, disease, and CART features (ie, SM, FP, and CART in peripheral blood) were analyzed to determine the potential predictors of PFS in response to HBI0101 therapy. Bold faceted and underlined parameters in red were identified as strong independent determinants of PFS. HR cytogenetic, EMD presentation, and the absolute numbers of CAR+ TEMs (defined as CD3+CAR+CD45RA−CCR7−) in HBI0101 CART FP were identified as predictors of inferior PFS. This illustration was generated with BioRender.com.
Determinants of durable response to HBI0101 therapy in patients with infused MM. Numerous variables related to patient characteristics, disease, and CART features (ie, SM, FP, and CART in peripheral blood) were analyzed to determine the potential predictors of PFS in response to HBI0101 therapy. Bold faceted and underlined parameters in red were identified as strong independent determinants of PFS. HR cytogenetic, EMD presentation, and the absolute numbers of CAR+ TEMs (defined as CD3+CAR+CD45RA−CCR7−) in HBI0101 CART FP were identified as predictors of inferior PFS. This illustration was generated with BioRender.com.
Our results are consistent with emerging real-world retrospective data, shedding light on the clinical outcomes of BCMA-CART therapy in often subrepresented populations in clinical trials.20,21,26 Our data also highlight the tight correlation between the number of treatment lines and the presence of HR cytogenetic and EMD and a trend toward a more differentiated status of CAR+ T cells in the FP. Patients with these RFs had suboptimal responses to HBI0101 but may also have dismal outcomes with other strategies27-29 and hence represent a significant unmet need. Advancing CART therapy to earlier course of the disease may result in lower occurrence of RFs and thus translate into improved outcomes.
Several studies have reported the potential impact of prior exposure to BCMA-TT on BCMA-CART therapy outcome.17,30,31 In our cohort, we were unable to isolate the potential predictive effect of previous exposure to BCMA-TT on HBI0101 outcome. This is probably due to the small sample size and its association with stronger predictors of HBI0101 outcome (ie, HR, EMD, and increased number of CAR+ TEM cells in the FP). Therefore, the optimal therapeutic sequence of BCMA-targeted treatments has yet to be determined to boost all existing BCMA therapies’ outcomes.
We observed a positive correlation between CAR+ TEM in the FP and their presence in the SM. The association of long-term durability of adoptive CART therapies with the presence of less-differentiated T-cell subsets (ie, TNAIVE/SCM and TCM) in the SM is well documented.32-35 Our analyses also pointed toward an increased proportion of TEM in the FP of patients having endured several treatment lines and penta-refractory patients, probably reflecting the impact of lympho-toxic regimens on T-cell fitness at advanced course of myeloma.36 Collectively, our results indicate that HBI0101 product features (i.e., CAR+ TEM) are directly influenced by the composition of the apheresis SM (i.e., naïve-like vs differentiated premanufacture T cells) and further imply that HBI0101 outcome is strongly affected by patient heterogeneity. Therefore, a major challenge for optimizing CART therapy would be the implementation of protocols and manufacture process aiming at preserving specific T-cell subsets even when the SM is not optimal (e.g., lymphocyte collection at earlier time of disease course, selection of less-differentiated T-cell subsets, etc).8,33,37-40
Advantages of academic CART therapies are numerous, because they provide a local solution to CART therapy–reduced accessibility at significantly shorter “brain-to-vein” time (median, 25 days; range, 14-65; supplemental Figure 8) and lower cost than their commercial counterparts. Moreover, in an academic setting, eligibility criteria are often more permissive than those for clinical trials. In this regard, a recent retrospective analysis of ide-cel clinical outcome in real-world patients indicates that 75% of the patients treated with ide-cel in 11 centers in the United States would not have met the eligibility criteria for the KarMMa study.5 By comparison, HBI0101 cohort comprises at least 54% of KarMMa/CARTITUDE-1–ineligible patients, whose PFS and OS were significantly inferior to corresponding eligible patients. Moreover, although direct comparison needs to be done with caution, HBI0101 response rates seem to outperform those of approved bispecific antibodies for MM in a cohort enriched with heavily pretreated and HR patients.27-29 This observation demonstrates HBI0101 efficacy in a real-world population of patients with myeloma but also highlights the importance of point-of-care CART therapy in academia for broadening CART application to patients with limited therapeutic options.
Limitations of our study include the relatively small sample size, which renders very perilous subgroup analyses and correlative evaluations, whose results remain exploratory and require confirmation in larger cohorts. In this respect, and given the exploratory nature of our analyses, we did not adjust for multiple comparisons.
In conclusion, academic anti–BCMA-CART therapy is an available, safe, and efficient alternative to the approved commercial products for real-world patients. The prognostic indicators of long-term durable responses to HBI0101 therapy that were identified hold promise for refining CART treatments and for better targeting of patient population for whom this treatment will benefit the most.
Acknowledgments
The authors thank the patients and their families for participating in the study. The authors also express their gratitude to the management department of the Hadassah Medical Center for the ongoing support and its deep commitment to innovation. The authors also thank the staff in the clinical units for patient care, Avigail Avraham from the apheresis unit, Nassreen Hussein for cell manufacturing, and Gili Gruzman for QC testing and patient follow-up. The authors thank the Manfred Steinfeld family for their ongoing and generous support of the Danny Cunniff Leukemia Research Laboratory. This work is also supported by funding of the General Custodian of Israel.
Authorship
Contribution: S.K.-E. and N.A. designed and performed the experiments, protocol writing, CART production and evaluation, patients’ follow-up, and manuscript writing; E.L., V.V., B.A., S.G., E.Z., and S.E. performed CART treatment and evaluation and manuscript writing; M.A., T.D.-S., N.B., R.A.-S., A.S., A.I., and V.Z., performed CART production and evaluation and patients’ follow-up; M.P. performed CART evaluation and manuscript writing; I.R. and R.S.K. performed statistical analysis and manuscript writing; Y.C. and I.A. performed CART evaluation and manuscript writing; C.J.C. designed HBI0101 CAR construct and performed manuscript writing; M.E.G. performed protocol writing, CART treatment and evaluation, and manuscript preparation; and P.S. designed and supervised the study and performed protocol writing, CART treatment and evaluation, and manuscript preparation.
Conflict-of-interest disclosure: S.K.-E., N.A., C.J.C., and P.S. are inventors on patents on the use of anti–B-cell maturation antigen in CART to target multiple myeloma. The remaining authors declare no competing financial interests.
Correspondence: Nathalie Asherie, Department of Bone Marrow Transplantation and Cancer Immunotherapy, Faculty of Medicine, Hadassah Medical Center, Hebrew University of Jerusalem, Kalman St 1, Jerusalem, 91120, Israel; email: nathaliea@hadassah.org.il.
References
Author notes
S.K.-E., N.A., and E.L. are joint first authors and contributed equally to this study.
M.E.G. and P.S. are joint senior authors and contributed equally to this study.
Original data are available upon request from the corresponding author, Nathalie Asherie (nathaliea@hadassah.org.il).
The full-text version of this article contains a data supplement.