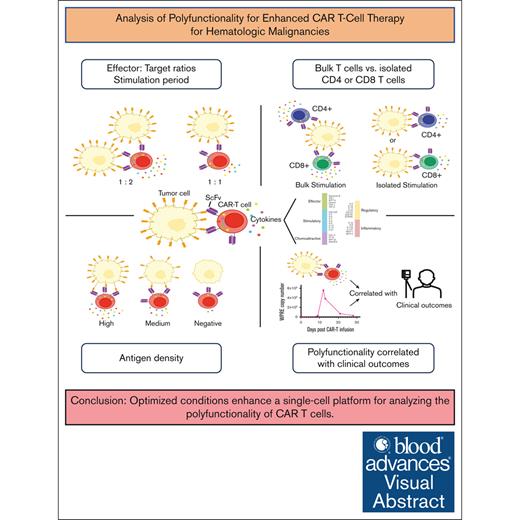
Key Points
The polyfunctionality of CAR T cells is affected by stimulation conditions, effector-to-target ratio, antigen density, and CAR specificity.
Robust indicators of polyfunctionality correlate with successful CAR T-cell expansion after infusion and achievement of durable remission.
Visual Abstract
Chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising immunotherapeutic strategy for eradicating human cancers. Their therapeutic success and durability of clinical responses hinges, in large part, on their functional capacity, including the ability of these engineered cells to simultaneously expand and persist after infusion into patients. CD19 CAR T-cell polyfunctionality, assessing the simultaneous functions of cytokine production, proliferation, and cytotoxicity has been reported to correlate with clinical outcomes. Assay optimization is potentially limited by the heterogeneous nature of CAR T-cell infusion products and target specificity. We optimized a single-cell platform for polyfunctionality using CAR T-cell products manufactured from healthy donors, engineered against a novel target, B-cell–activating factor receptor (BAFF-R) and validated the protocol using CD19 CAR T cells. We observed distinct qualitative differences between BAFF-R and CD19 CAR T cells relative to the proportions of stimulatory vs effector cytokines, based on target antigen density, and, generally, CD19 CAR T cells exhibited lower indices of polyfunctionality. Finally, we applied our assay to the autologous BAFF-R CAR T-cell product generated from the first patient with non-Hodgkin lymphoma treated in an ongoing clinical trial who had progressed after prior CD19 CAR T-cell therapy. We observed robust indicators of polyfunctionality, which correlated with successful CAR T-cell expansion after infusion and achievement of durable complete remission ongoing after 18 months. The precise identification of factors determining the role of BAFF-R CAR T-cell fitness in toxicity and clinical outcome will require the application of this robust assay in the analysis of additional treated patients. This trial was registered at www.ClinicalTrials.gov as #NCT05370430.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a promising immunotherapy approach for the treatment of cancer, particularly hematologic malignancies. Among several factors determining the clinical success of CAR T cells is their ability to expand and persist in the patient, as well as their efficacy after infusion into patients. Previous studies have suggested that the functional capacity of CD19 CAR T cells as measured in the infusion product, particularly the ability of CAR T cells to simultaneously produce multiple cytokines and chemokines in response to antigen stimulation, correlates with their effectiveness in vivo and clinical outcomes.1-3 Optimizing in vitro correlative assays of T-cell polyfunctionality for individual CAR T-cell products is potentially limited by variability in assay conditions and cell handling, which can affect the reliability and reproducibility of results. Furthermore, the complex nature of CAR T-cell products, which contain variable T-cell subsets and proportions of genetically modified cells, can make the assay results challenging. Finally, differences in target specificity of CAR T-cell products may influence optimal assay conditions.
In this study, we describe the optimization of a polyfunctionality assay for CAR T-cell products directed against a novel target, B-cell–activating factor receptor (BAFF-R). We discuss key considerations for assay design, including cell isolation, stimulation conditions, T cell-to-target ratios, antigen density, and CAR T-cell specificity. We also present data that are derived from the autologous CAR T-cell product directed against BAFF-R from a patient with non-Hodgkin lymphoma treated on an active clinical trial, demonstrating the reproducibility of our optimized assay. Overall, our findings provide important insights into the development of a robust and reliable polyfunctional assay for CAR T-cell products, which has the potential to predict patient outcomes and thereby accelerate the development of effective CAR T-cell therapies.
Materials and methods
Cell lines
Nalm-6, Raji, and Z138 cells are commercially available tumor cell lines. Nalm-6 was obtained from The Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (DSMZ) (Brunswick, Germany), and the Raji and Z138 cell lines were purchased from The American Type Culture Collection (ATCC) (Manassas, VA).
Nalm-6 BAFF-R–knockout (KO) and CD19-KO cell lines were developed previously4 using BAFF-R CRISPR–associated protein 9 (Cas9) or CD19 CRISPR-Cas9 and red fluorescent protein reporter genes containing homologous directed repair plasmid systems (Santa Cruz Biotechnology, Dallas, TX) according to the manufacturer’s directions. We transfected plasmids using a Neon Electroporation Transfection System (Thermo Fisher) CD19− and BAFF-R− cells were sorted, and a single-cell clone was expanded. The same approach was used to generate BAFF-R–KO Z138 cells. Stable CD19− and BAFF-R− clones were verified by flow cytometry before banking. CD19 gene was edited by CRISPR-Cas9 technology on Raji wild-type (WT) cells, and Raji CD19-KO cells were established by fluorescence-activated cell sorting of the CD19− population to yield >98% purity. Cell lines expressing low levels of antigen were produced by expressing CD19 and BAFF-R using a phosphoglycerate kinase promoter (PGK100p) on Raji KO and Nalm-6 KO cells by lentiviral transduction system. These low antigen–expressing cells were seeded at a density of 1 cell per well (2 96-well plates). A single-cell–derived clone was screened out and expanded.
Development of BAFF-R and CD19 CAR lentiviral production
Clinical BAFF-R and CD19 single CAR vectors were used in the study. BAFF-R single-chain variable fragment was derived from prior humanized antibody H90,5 and CD19 single-chain variable fragment was from FMC63 monoclonal antibody.6,7 The extracellular domain included immunoglobulin G4 Fc with a double mutation (L235E; N297Q).6 The intracellular costimulatory domain contained 4-1BB with a CD4 transmembrane domain and a CD3ζ motif. The CAR construct sequence was separated from a truncated epidermal growth factor receptor (EGFR) gene (EGFRt) using a T2A ribosomal skip sequence under the control of the EF1a promoter.8 To produce lentivirus for transduction, 293T cells were plated into T-225 flasks with 14 × 106 cells. The next day, the 293T cells were transfected with plasmids encoding the CAR along with packaging and envelop vectors (PCHGP2, pCMV-Rev, and pCMV-G) in the presence of 3 μL of 1 μg/μL polyethylenimine (PEI) (Polysciences) per 1 μg of DNA to the diluted plasmid– Dulbecco modified Eagle medium solution. The lentiviral supernatant was collected, 48 to 96 hours later, and concentrated by centrifuging overnight at 4°C at 6080g (6300 rpm).
Generation of BAFF-R and CD19 CAR T cells with a current good manufacturing practice manufacturing protocol
CAR T cells were produced according to a current good manufacturing practice CAR T-cell production protocol. Leukapheresis products were obtained from healthy donors using protocols approved by the City of Hope institutional review board (no. 15283 for blood collection from healthy donors). Naïve and central memory T cells (Tn/mem) defined as CD62L+CD14−CD25− were isolated on a Miltenyi AutoMACS (Miltenyi Biotec, Inc). Briefly, peripheral blood mononuclear cells were incubated with anti-CD14 and -CD25 microbeads and CD14+ and CD25+ cells were immediately depleted using the AutoMACS. The unlabeled negative fraction was incubated with anti-CD62L microbeads, and CD62L+ cells were purified using positive selection with AutoMACS. After selection, isolated Tn/mem cells were activated, transduced, and expanded for 14 days, as previously described.9,10
Manufacturing of BAFF-R CAR T cells from a patient with mantle cell lymphoma (MCL)
This study examined the efficacy of CAR T cells from distinct Tn/mem populations. The manufacturing methodology has been detailed by Aldoss et al6 and has been successfully applied across various clinical trials. In essence, the approach involved the transduction of Tn/mem cells using a lentiviral vector (BAFF-R(EQ)41BBζ-T2A-EGFRt_epHIV7).9 This vector encoded a BAFF-R CAR featuring a 4-1BB costimulatory domain, alongside a EGFRt intended for tracking purposes.
Cytokine evaluation from CAR T cells for protocol optimization
Cryopreserved BAFF-R CAR T cells were recovered for 3 days with interleukin-2 (IL-2), then CD4+ T and CD8+ T cells were separated from CAR T-cell products and stimulated with either BAFF-R+ or BAFF-R− Nalm-6 target cells for 20 hours at effector-to-target (E:T) ratios of 1:2, 1:1, and 1:0.5, or at ratio of 1:2 for 5 or 20 hours. T-cell subsets were further enriched using anti-CD19–conjugated magnetic beads to deplete Nalm-6 cells. Enriched T cells were cultured for 16 hours and cytokine concentrations in supernatants were then evaluated using a human CD8/natural killer cell panel (BioLegend, catalog no. 741065), a multiplex cytokine bead–based assay, according to the manufacturer’s directions. Results are expressed as mean ± standard deviation.
BAFF-R CAR T- and CD19 CAR T-cell polyfunctionality assay
Cryopreserved BAFF-R CAR T cells were recovered for 3 days with IL-2. For the isolated stimulation: CD4+ T and CD8+ T cells were separated from CAR T-cell products, stimulated with target cells for 5 hours, and tumor cells were removed using anti-CD19–conjugated magnetic beads. For the bulk stimulation: CD4 and CD8 T cells were stimulated in bulk with target cells and then separated into subsets by anti-CD4 and anti-CD8 beads. A certain number of cells were taken to detect CAR (EGFR+) and identity (CD3+) by flow cytometry. The remaining cells were diluted to 1 million cells per mL and ∼30 μL of the cell suspension was loaded onto the single-cell barcode chip (SCBC) microchip for single-cell secretomic evaluation. To apply the optimized protocol to compare BAFF-R and CD19 CAR T cells, BAFF-R and CD19 CARs were expressed in the same lentivirus backbone (4-1BB), and respective CAR T cells were generated from a single healthy donor. Bulk BAFF-R and CD19 CAR T cells were stimulated with Nalm-6 (BAFF-R+ and CD19+) target cells or their respective control Nalm-6 BAFF-R− or Nalm-6 CD19− target cells, at an E:T ratio of 1:2 for 5 hours. To see the influence of antigen density on CAR T cells, BAFF-R and CD19 CAR T cells were stimulated with BAFF-R–high, –low, and –deficient, or with CD19-high, -low, and -deficient target cells at an E:T ratio of 1:2 for 5 hours. After enrichment, the cells were taken to detect CAR (EGFR+) and identity (CD3+) by flow cytometry or were loaded onto the SCBC microchip for polyfunctionality assay. CAR T-cell polyfunctionality values were normalized for the percentage of CAR+ T cells using software provided by the manufacturer (IsoPlexis).
Generation of BAFF-R CAR T cells from a patient with non-Hodgkin lymphoma and assay for polyfunctionality
Clinical BAFF-R CAR T cells were manufactured at the Cell Therapy Production Center. Tn/mem cells were isolated using CliniMACS, then activated and transduced with a lentiviral vector containing a BAFF-R CAR construct. This construct included a 4-1BB costimulatory domain and a EGFRt for T-cell tracking, as outlined in the study by Aldoss et al.6 The product underwent standard quality control testing for clinical use, as previously described.6 An aliquot of the T-cell product was released by the office of quality systems for the studies. Bulk CAR T cells were stimulated with Z138 (BAFF-R+ and CD19+) target cells or Z138 BAFF-R− target cells, at an E:T ratio of 1:2 for 5 hours (Figure 5A-B). After enrichment, the cells were taken to detect purity (EGFR+) and identity (CD3+) by flow cytometry or were loaded onto the SCBC microchip for polyfunctionality assay.
Detection of the WPRE copy number of BAFF-R CAR T cells from a patient
Genomic DNA was prepared from frozen aliquots of blood using the QIAamp DNA mini kit (Qiagen) and then tested for the detection of Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) DNA by quantitative polymerase chain reaction (primers and polymerase chain reaction conditions are available upon request). To estimate the average number of genomic copies of integrated vector per cell, standard curves for WPRE copy numbers were established from serial dilutions of epHIV7 plasmid DNA (10-100 000 copies) using primers specific for the WPRE sequence. Cell number standard curves were established from serial dilutions of a control plasmid containing the human albumin open reading frame11 using primers specific for this endogenous cellular gene (2 alleles per cell).
Statistical analysis
Means ± standard error of the mean of triplicate samples are presented. Experiments were conducted in triplicate and analyzed by a Student t test; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; and ns, not significant.
Results
Optimization of BAFF-R CAR T-cell samples for specific cytokine secretion detection
We first adapted and optimized several key variables from previous protocols of single-cell cytokine profiling of CD19 CAR T-cell products.12 In the studies, 3 BAFF-R CAR T-cell products, manufactured from healthy donors under clinical-grade conditions were used.9 First, CD4+ and CD8+ T cells separated from CAR T-cell products were stimulated with either BAFF-R+– or control BAFF-R−–expressing Nalm-6 human leukemia cells (target cells) at E:T ratios of 1:2, 1:1, or 1:0.5 for 20 hours. T-cell subsets were then further enriched using anti-CD19–conjugated magnetic beads to deplete Nalm-6 cells. After an additional 16-hour culture, supernatants were subjected to multiplex bead–based cytokine analysis. For both CD4+ and CD8+ T cells, specific secretion of 10 of 13 cytokines was observed, and, in almost all cases, cytokine secretion was greatest at the E:T ratio of 1:2 (Figure 1A). Superior secretion at this E:T ratio was most evident for interferon gamma (IFN-γ) and granzyme A and B, with similar trends observed, albeit at lower levels, for 7 other cytokines, particularly for CD8+ T cells. Three cytokines, IL-2, IL-6, and soluble Fas (sFas) were not detectable at any E:T ratio (not shown).
Optimization of BAFF-R CAR T-cell product cytokine profiling. (A) CAR T-cell products were generated from healthy donors, and CD4+ and CD8+ T cells were separated before stimulation with either BAFF-R+ or BAFF-R− Nalm-6 target cells for 20 hours at E:T ratios of 1:2, 1:1, and 1:0.5. The resulting T-cell subsets were further enriched using anti-CD19–conjugated magnetic beads to deplete Nalm-6 cells. Cytokine concentrations in supernatants were then analyzed using a multiplex cytokine bead–based assay. Results are expressed as mean ± standard deviation of triplicate wells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, not significant. Representative data from 2 donors are presented. (B) CD4+ T and CD8+ CAR T cells were stimulated with either BAFF-R+ or BAFF-R− target cells as described above at an E:T ratio of 1:2 for 5 or 20 hours, and cytokine concentrations in supernatants were then analyzed as described above. Representative data from 2 donors are presented. sFasL, sFas ligand; TNF-α, tumor necrosis factor α.
Optimization of BAFF-R CAR T-cell product cytokine profiling. (A) CAR T-cell products were generated from healthy donors, and CD4+ and CD8+ T cells were separated before stimulation with either BAFF-R+ or BAFF-R− Nalm-6 target cells for 20 hours at E:T ratios of 1:2, 1:1, and 1:0.5. The resulting T-cell subsets were further enriched using anti-CD19–conjugated magnetic beads to deplete Nalm-6 cells. Cytokine concentrations in supernatants were then analyzed using a multiplex cytokine bead–based assay. Results are expressed as mean ± standard deviation of triplicate wells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, not significant. Representative data from 2 donors are presented. (B) CD4+ T and CD8+ CAR T cells were stimulated with either BAFF-R+ or BAFF-R− target cells as described above at an E:T ratio of 1:2 for 5 or 20 hours, and cytokine concentrations in supernatants were then analyzed as described above. Representative data from 2 donors are presented. sFasL, sFas ligand; TNF-α, tumor necrosis factor α.
Next, we examined the effect of the duration of CAR T-cell product stimulation on cytokine secretion. BAFF-R CAR T-cell products were stimulated with either BAFF-R+ target cells or BAFF-R− cells at the optimal E:T ratio of 1:2 for either 5 or 20 hours as above (Figure 1B). For CD4+ T cells, significantly greater specific cytokine secretion was associated with the shorter stimulation (5 hour) period for IFN-γ, granzyme B, sFas ligand, IL-2, IL-4, IL-10, and tumor necrosis factor α. We observed a significantly higher secretion of IFN-γ from CD8+ T cells after a shorter 5-hour stimulation period, whereas other cytokines such as granzyme A and B, and perforin were higher after a 20-hour stimulation period, indicating distinct kinetics of cytokine secretion from CD4 and CD8 T cells. Importantly, 5-hour stimulation allowed us to detect significantly higher levels of IL-2, which is a key cytokine of stimulatory cytokine that was missed in the 20-hour stimulation system (Figure 1A).
Stimulation of bulk BAFF-R CAR T cells is more sensitive than stimulation of isolated CD4+ or CD8+ T-cell subsets
Whereas published protocols analyzed CD4+ and CD8+ T-cell subsets isolated from CAR T-cell infusion products before target cell stimulation, we reasoned that the interaction between CD4 and CD8 T cells can influence their cytokine secretion, and result in quantitative and/or qualitative increases in cytokine production. We, therefore, performed a head-to-head comparison of T-cell stimulation in bulk vs isolated T-cell subsets from BAFF-R CAR T-cell products. Either isolated CD4+ and CD8+ T cells or bulk T cells were stimulated with BAFF-R+ or BAFF-R− target cells (control) at an E:T ratio of 1:2 for 5 hours. Bulk-stimulated T cells were then purified using either anti-CD4– or anti-CD8–conjugated magnetic beads, without the need for target cell depletion (Figure 2A). T-cell subsets were loaded onto SCBC microchips for secretomic evaluation using the IsoLight platform, which measures a prespecified panel of 32 key immunologically relevant molecules.
Single-cell cytokine profiling of BAFF-R CAR T-cell products by isolated subset or bulk stimulation. (A) Workflow of CAR T-cell product processing, comparing protocols in which cells were first separated into CD4+ and CD8+ subsets, stimulated with BAFF-R+ target cells, which were subsequently depleted (isolated stimulation), or cells were stimulated in bulk and then separated into subsets without the need for target cell depletion, before analysis (bulk stimulation). (B) Fluorescence-activated cell sorting (FACS) plots showing the frequency of EGFRt+ CAR T cells stimulated with either BAFF-R+ or BAFF-R− targets (control) in cell suspensions prepared either by isolated or bulk stimulation protocols, before loading onto microchips. (C) Heat maps showing frequencies of single CD4+ or CD8+ T cells secreting selected cytokine combinations from isolated or bulk stimulation protocols. Brackets indicate differences observed in specific cytokine profiles between the 2 protocols. (D) Differences between isolated subset and bulk stimulation protocols as measured by PSI. Results are representative of 2 CAR T-cell products generated from healthy donors. Stim, stimulation.
Single-cell cytokine profiling of BAFF-R CAR T-cell products by isolated subset or bulk stimulation. (A) Workflow of CAR T-cell product processing, comparing protocols in which cells were first separated into CD4+ and CD8+ subsets, stimulated with BAFF-R+ target cells, which were subsequently depleted (isolated stimulation), or cells were stimulated in bulk and then separated into subsets without the need for target cell depletion, before analysis (bulk stimulation). (B) Fluorescence-activated cell sorting (FACS) plots showing the frequency of EGFRt+ CAR T cells stimulated with either BAFF-R+ or BAFF-R− targets (control) in cell suspensions prepared either by isolated or bulk stimulation protocols, before loading onto microchips. (C) Heat maps showing frequencies of single CD4+ or CD8+ T cells secreting selected cytokine combinations from isolated or bulk stimulation protocols. Brackets indicate differences observed in specific cytokine profiles between the 2 protocols. (D) Differences between isolated subset and bulk stimulation protocols as measured by PSI. Results are representative of 2 CAR T-cell products generated from healthy donors. Stim, stimulation.
The frequencies of CAR T cells comprising cell suspensions loaded onto microchips (∼40%) were comparable between T cells stimulated with BAFF-R+ or BAFF-R− targets (control) and between the 2 protocols (Figure 2B).
The percentage of single cells producing 1 or multiple cytokines is shown in Figure 2C for selected representative cytokines from the panel. For both CD4+ and CD8+ T cells, both isolated and bulk stimulation protocols induced specific cytokine production against BAFF-R+ target cells at comparable secretion frequencies. However, analysis of bulk-stimulated cells revealed more combinations of multiple cytokine secretion (>3 cytokines) not observed with isolated stimulation. Furthermore, T-cell polyfunctionality was measured by a polyfunctional strength index (PSI), defined as the percentage of single cells that produced ≥2 cytokines in the sample, multiplied by the mean fluorescence intensity of secreted cytokines; the sum of individual cytokine PSI values provided the overall PSI for the sample.13 Bulk-stimulated cells also demonstrated higher PSI values, compared with isolated stimulation (Figure 2D; supplemental Figure 1). PSI was driven mostly by effector, followed by stimulatory, chemotactic (CD8+ T cells), and regulatory cytokines (CD4+ T cells). Taken together, these data suggest that the bulk stimulation protocol is more sensitive to eliciting polyfunctional cytokine secretion by CAR T-cell products in response to BAFF-R+ stimulation, allowing for a more comprehensive evaluation of cytokine secretion and overall CAR T-cell function.
Validation of T-cell polyfunctionality protocol using CD19 CAR T cells
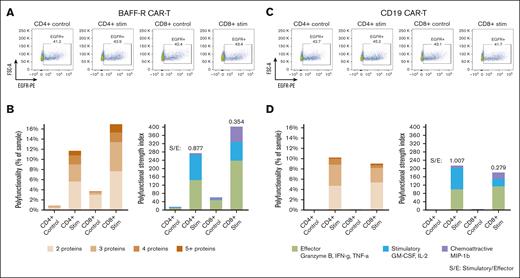
Because the bulk stimulation protocol that we optimized for BAFF-R CAR T cells, as described above, differed from protocols previously described for CD19 CAR T cells, we sought to validate our protocol on CAR T cells directed against CD19. To this end, BAFF-R and CD19 CARs were expressed in the same lentivirus backbone (4-1BB), and respective CAR T cells were generated from the same healthy donor. Bulk BAFF-R and CD19 CAR T cells were stimulated with Nalm-6 (BAFF-R+, CD19+) target cells or their respective control Nalm-6 BAFF-R− or Nalm-6 CD19− target cells, at an E:T ratio of 1:2 for 5 hours. Bulk-stimulated CD4+ and CD8+ T cells were then enriched and were loaded onto microchips for secretomic evaluation.
There were no differences in purity or proportions of CAR-transduced T cells between BAFF-R and CD19 CAR T-cell products. Of enriched cells, >90% were T cells (CD3+) among CD4+ and CD8+ subsets, and residual target cells were <7% (supplemental Figure 2A-B). EGFR+ CAR T cells comprised between 41% to 45% of all T-cell subsets (Figure 3A, C).
Application of the optimized protocol to CD19 CAR T-cell products. BAFF-R and CD19 CARs were expressed in the same lentivirus backbone (4-1BB) and respective CAR T cells were generated from a single healthy donor. Bulk CAR T cells were stimulated with Nalm-6 (BAFF-R+ and CD19+) target cells or their respective control Nalm-6 BAFF-R− or Nalm-6 CD19− target cells at an E:T ratio of 1:2 for 5 hours. Bulk stimulated CD4+ and CD8+ T cells were then enriched, analyzed by FACS for EGFRt+ CAR T cells, or were loaded onto microchips for secretomic evaluation. The proportions of (A) EGFRt+ BAFF-R and (C) CD19 CAR T cells. Polyfunctionality is expressed as the percentage of single cells that released >2 cytokines and as PSI (B and D). S/E, stimulatory-to-effector cytokine ratios.
Application of the optimized protocol to CD19 CAR T-cell products. BAFF-R and CD19 CARs were expressed in the same lentivirus backbone (4-1BB) and respective CAR T cells were generated from a single healthy donor. Bulk CAR T cells were stimulated with Nalm-6 (BAFF-R+ and CD19+) target cells or their respective control Nalm-6 BAFF-R− or Nalm-6 CD19− target cells at an E:T ratio of 1:2 for 5 hours. Bulk stimulated CD4+ and CD8+ T cells were then enriched, analyzed by FACS for EGFRt+ CAR T cells, or were loaded onto microchips for secretomic evaluation. The proportions of (A) EGFRt+ BAFF-R and (C) CD19 CAR T cells. Polyfunctionality is expressed as the percentage of single cells that released >2 cytokines and as PSI (B and D). S/E, stimulatory-to-effector cytokine ratios.
Consistently, specific polyfunctionality was detected in stimulated CD4+ and CD8+ subsets of CD19 CAR T cells (Figure 3D), compared with controls, similar to the specific polyfunctional cytokine secretion observed with BAFF-R CAR T cells (Figure 3B). However, a trend toward greater polyfunctionality was more evident with CD8+ than CD4+ BAFF-R CAR T cells, which was different from the relatively similar polyfunctionality between CD8+ and CD4+ CD19 CAR T cells. These differences may be attributable to differential antigen and CAR engagement resulting from differences in antigen density and CAR affinity.14
The impact of tumor cell antigen density on the polyfunctionality of BAFF-R and CD19 CAR T cells
We then investigated the influence of target cell antigen density on PSI using both BAFF-R and CD19 model systems. Nalm-6 BAFF-R–KO and Raji CD19-KO cell lines and their respective lines expressing low levels of surface antigen were generated as described in “Methods.”
Bulk BAFF-R CAR T cells were stimulated with Nalm-6 BAFF-R–high (WT), BAFF-R–low, or BAFF-R–deficient control target cells (supplemental Figure 3). Polyfunctionality of both CD4+ and CD8+ T cells was substantially increased by the antigen density of the stimulating target cells (Figure 4A-C).
The influence of antigen density on CD19 and BAFF-R CAR T cells. Bulk BAFF-R CAR T cells were stimulated with Nalm-6 BAFF-R–high (WT), BAFF-R–low, or BAFF-R–deficient (control) target cells for 5 hours at an E:T of 1:2. CD4+ and CD8+ T cells were enriched and then loaded onto microchips for secretomic evaluation. (A) FACS showing the proportion of EGFR+ BAFF-R CAR T cells. Polyfunctionality was analyzed either as (B) the percentage of single cells that released >2 cytokines or as (C) PSI. Bulk CD19 CAR T cells were stimulated with Raji-CD19+–high (WT), Raji-CD19+–low, or Raji-CD19–deficient (control) target cells as described above. CD4+ and CD8+ T cells were enriched and then loaded onto microchips for secretomic evaluation. (D) FACS plots showing the proportion of EGFR+ CD19 CAR T cells. Polyfunctionality was analyzed either as (E) the percentage of single cells that released >2 cytokines, or as (F) PSI.
The influence of antigen density on CD19 and BAFF-R CAR T cells. Bulk BAFF-R CAR T cells were stimulated with Nalm-6 BAFF-R–high (WT), BAFF-R–low, or BAFF-R–deficient (control) target cells for 5 hours at an E:T of 1:2. CD4+ and CD8+ T cells were enriched and then loaded onto microchips for secretomic evaluation. (A) FACS showing the proportion of EGFR+ BAFF-R CAR T cells. Polyfunctionality was analyzed either as (B) the percentage of single cells that released >2 cytokines or as (C) PSI. Bulk CD19 CAR T cells were stimulated with Raji-CD19+–high (WT), Raji-CD19+–low, or Raji-CD19–deficient (control) target cells as described above. CD4+ and CD8+ T cells were enriched and then loaded onto microchips for secretomic evaluation. (D) FACS plots showing the proportion of EGFR+ CD19 CAR T cells. Polyfunctionality was analyzed either as (E) the percentage of single cells that released >2 cytokines, or as (F) PSI.
Similarly, we stimulated CD19 CAR T cells with a panel of Raji tumor cells expressing various levels of CD19 (supplemental Figure 4); specifically, CD19-high (unmodified WT Raji cells), CD19-low, and a CD19-deficient control. Bulk CD19 CAR T cells were generated as described and stimulated with each target cell type at an E:T ratio of 1:2 for 5 hours. Product characterization revealed >95% T-cell purity and similar CAR T-cell proportions in CD4 (81.6%, 84.2%, and 75.8%; high, low, and deficient, respectively) and in CD8 T cells (74.1%, 77.3%, and 73.4%, respectively). Overall, we observed greater polyfunctionality in both CD8 and CD4 T-cell fractions with high-density stimulator CD19 (Figure 4D-F). Interestingly, we observed that higher CD19 density led to reduced stimulatory-to-effector cytokine ratios for both CD4 (from 1.9 to 1.4) and CD8 (from 0.5 to 0.3), respectively. In contrast, increased BAFF-R density showed the opposite effect, with enhanced stimulatory-to-effector cytokine ratios for both CD4 (from 0.7 to 1.0) and CD8 (from 0.2 to 0.8), respectively. Combined analysis of overall PSI together with the determination of specific cytokine subsets may provide a more comprehensive and accurate reflection of CAR T-cell function.
Polyfunctionality of the BAFF-R CAR T-cell product generated from, and used to treat, a patient with MCL
Case report
The patient is a 57-year-old Asian-American male with TP53-mutated, relapsed stage IVA MCL with bone marrow involvement diagnosed in 2020. He had experienced transient partial responses to multiple prior therapies, including Rituxan (rituximab) plus bendamustine and cytarabine, acalabrutinib, CD19 CAR T cells on a prior clinical trial (ClinicalTrials.gov identifier: NCT04484012), and acalabrutinib plus venetoclax. He was enrolled and treated as the first patient on our active clinical trial of autologous BAFF-R CAR T cells (ClinicalTrials.gov identifier: NCT05370430) and his leukapheresis product was used to successfully generate BAFF-R CAR T cells. Before starting lymphodepletion there was 10% bone marrow involvement with MCL cells and splenomegaly as the only extramedullary disease documented on pretreatment positron emission tomography and computed tomography scans.
Per protocol, he received lymphodepletion chemotherapy with fludarabine and cyclophosphamide, followed by an infusion of 50 × 106 BAFF-R CAR T cells in November 2022. He experienced grade 1 cytokine release syndrome on days +10 to 12, requiring 1 dose of tocilizumab, and grade 1 immune effector cell–associated neurotoxicity syndrome on day +13, which was self-limited. Response assessments at day +30 revealed complete remission (CR), and the bone marrow was negative for minimal residual MCL by flow cytometry and next-generation sequencing. The patient remains in a minimal residual disease–negative CR at >18 months.
BAFF-R CAR T-cell polyfunctionality and expansion
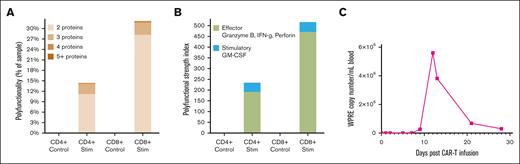
We tested the infusion product for T-cell polyfunctionality using the protocol optimized on healthy donor T cells, as described above. Notably, we observed a significantly higher PSI in the BAFF-R CAR product compared with controls, suggesting BAFF-R antigen-specific cytokine secretion (Figure 5A-B). We also assessed CAR T-cell expansion by measuring the WPRE copy number per mL of blood using genomic DNA extracted from peripheral blood collected at multiple time points from day 0 to day 28 after infusion. Peak expansion was observed on day 12 after infusion (Figure 5C).
Case report: characterization of autologous BAFF-R CAR T-cell product before infusion and CAR T-cell expansion after infusion in a patient with MCL. Autologous CAR T cells were stimulated with Z138 (BAFF-R+ and CD19+) target cells or their control Z138 BAFF-R− target cells at an E:T ratio of 1:2 for 5 hours. Bulk stimulated CD4+ and CD8+ T cells were then enriched and loaded onto microchips for secretomic evaluation. Polyfunctionality was expressed as (A) the percentage of single cells that released >2 cytokines, or as (B) PSI. (C) The WPRE copy number per mL of blood for BAFF-R CAR from the patient with MCL was determined by polymerase chain reaction of genomic DNA extracted from peripheral blood collected at time points from day 0 to day 28 after CAR T-cell infusion.
Case report: characterization of autologous BAFF-R CAR T-cell product before infusion and CAR T-cell expansion after infusion in a patient with MCL. Autologous CAR T cells were stimulated with Z138 (BAFF-R+ and CD19+) target cells or their control Z138 BAFF-R− target cells at an E:T ratio of 1:2 for 5 hours. Bulk stimulated CD4+ and CD8+ T cells were then enriched and loaded onto microchips for secretomic evaluation. Polyfunctionality was expressed as (A) the percentage of single cells that released >2 cytokines, or as (B) PSI. (C) The WPRE copy number per mL of blood for BAFF-R CAR from the patient with MCL was determined by polymerase chain reaction of genomic DNA extracted from peripheral blood collected at time points from day 0 to day 28 after CAR T-cell infusion.
Discussion
T-cell polyfunctionality plays a pivotal role in human immunology by providing a versatile and robust defense mechanism against a wide array of pathogens. This phenomenon refers to the ability of T cells to simultaneously perform multiple functions, such as cytokine production, cytotoxicity, and proliferation, upon encountering an antigen. The assessment of preinfusion CAR T-cell functionality and its correlation with clinical outcomes remains incompletely investigated. One approach to address this is through single-cell analysis of the preinfusion CAR T-cell product using suitable platforms that allow the investigation of multiple immune programs represented by cytokines and chemokines. This analysis can provide valuable insights into the polyfunctionality of CAR T cells.3,12 However, it is important to acknowledge that the polyfunctionality of CAR T cells is a complex trait influenced by various factors. In our study, we aimed to optimize the experimental conditions to facilitate application to current CAR T-cell clinical trials.
BAFF-R is an emerging target for hematologic malignancies.4 The efficacy of both therapeutic antibodies and CAR T cells against this target are being investigated in active clinical trials with encouraging preliminary results15 (ClinicalTrials.gov identifier: NCT03400176, NCT05370430, and NCT04690595). Adapting previously reported protocols for single-cell proteomic analysis of CD19− to BAFF-R CAR T cells from healthy donors yielded several key insights. First, the timeframe for cytokine secretion after antigen stimulation can vary depending on the specific experimental settings, ranging from 6 to 24 hours,16 and different E:T ratios depending on the study settings.12,17,18 Our findings revealed that longer stimulation (20 hours) allowed for the detection of the majority of effector cytokines, including IFN-γ, granzyme A and B, sFas ligand, and perforin. However, we also observed that shorter stimulation (5 hours) facilitated the detection of autocrine IL-2, an important cytokine known to be associated with CAR T-cell survival and persistence.19-21 Noteworthy was the lack of detection of IL-6 levels, a finding that aligns with previous reports that monocytes and macrophages, rather than T cells, are the primary sources of IL-6.22,23
Compared with protocols previously using isolated CD4 and CD8 CAR T-cell stimulation, we observed that bulk stimulation provided a more accurate representation of the polyfunctionality of CAR T-cell products. This may be because in vivo, T cells encounter a complex mixture of antigens and immune cells, and bulk stimulation better mimics this environment compared with isolated cell subtypes in vitro. Additionally, the interaction between CD4 and CD8 T cells can influence their function and cytokine secretion,22-25 making it important to evaluate CAR T cells as a whole population rather than individual subsets.18
We validated the optimized protocol using CD19 CAR T cells generated from healthy donors. However, CD19 CAR T cells exhibited lower PSI values compared with BAFF-R CAR T cells, especially the CD8+ subset, against the same tumor cells (Nalm-6) although there is fivefold higher expression of CD19 antigen density than BAFF-R, indicating that PSI values are likely influenced by multiple factors. Evaluating the T-cell polyfunctionality within the context of different antigens is also challenging because of variations in their engagement with antigens, antigen density, as well as differences in binding affinity and avidity. Studies have demonstrated a positive correlation between higher antigen density and deeper and more sustained responses to CD19 and other CAR T-cell therapy.26-28 We specifically examined 1 variable: antigen density on polyfunctionality of CAR T cells targeting CD19 and BAFF-R. Generally, we observed that higher antigen expression was associated with increased CAR T-cell polyfunctionality. Interestingly, we found distinct differences between CD19 and BAFF-R CAR T cells relative to the proportions of stimulatory vs effector cytokines. Specifically, unlike BAFF-R, higher CD19 density on tumor target cells was not associated with a significant increase in the ratio of stimulatory-to-effector cytokines by their respective CAR T cells. Such qualitative differences may also affect the persistence and/or expansion of CAR T cells. Understanding these relationships between stimulatory-to-effector cytokines can aid in the optimization and development of CAR T-cell therapies for improved tumor recognition and immune response.
Finally, we validated our platform on the autologous BAFF-R CAR T-cell product generated from the first patient with MCL treated in an ongoing clinical trial who had progressed after prior CD19 CAR T-cell therapy. We observed robust indicators of polyfunctionality, which correlated with successful CAR T-cell expansion after infusion and achievement of durable CR.
In summary, we have optimized essential variables to analyze CAR T-cell polyfunctionality within a single-cell platform, which can now be applied to current and future clinical trials of CAR T cells for predictive biomarker discovery. The precise identification of factors determining BAFF-R CAR T-cell fitness, toxicity, and clinical outcome will require the analysis of additional patients treated in the current BAFF-R CAR T-cell clinical trial. In addition, if a particular profile is associated with favorable response and/or lack of toxicity, whether future manufacturing strategies, T-cell genetic programming, or dosing strategies could be manipulated to enrich CAR T cells with these features is a key question and objective of ongoing research.
Acknowledgments
This study was supported by the National Institutes of Health/National Cancer Institute (NIH/NCI; grant R01 CA269569; Multi-PI: L.W.K., I.A., and X.W.) and NCI Lymphoma Specialized Programs of Research Excellence (SPORE) (grant 5P50CA107399-14 [L.W.K. and S.J.F.]). We want to thank Timothy Synold and the Analytical Pharmacology Core Facility at City of Hope for offering support and consultation.
Authorship
Contribution: L.W.K., S.J.F., X.W., and L.E.B. played a role in conceiving the study concept; Z.D., E.O., S.S., A.A., M.D.R., and S.c.C. performed experiments and analyzed the data; L.W.K., X.W., and Z.D. designed the study and wrote the manuscript; and L.W.K., X.W., and S.J.F. were responsible for acquiring funding.
Conflict-of-interest disclosure: L.W.K. reports consulting role with, and equity ownership in, PeproMene Bio. X.W. reports a consulting role with PeproMene Bio. The remaining authors declare no competing financial interests.
Correspondence: Larry W. Kwak, Toni Stephenson Lymphoma Center, Beckman Research Institute, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; email: lkwak@coh.org.
References
Author notes
Data are available on request from the corresponding author, Larry W. Kwak (lkwak@coh.org).
The full-text version of this article contains a data supplement.